Seaweeds as Source of Bioactive Substances and Skin Care Therapy—Cosmeceuticals, Algotheraphy, and Thalassotherapy
Abstract
1. Introduction
Taxonomy of Algae and Their Bioactivities
- (a)
- red algae are included in the Rhodophyta phylum, and their photosynthetic pigments are chlorophyll a, phycobilins (r-phycocyanin and r-phycoerythrin), and carotenoids (lutein, zeaxanthin, β-carotene);
- (b)
- brown algae are included in the Ochrophyta (or Heterokontophyta) phylum, Phaeophyceae class, and their pigments include chlorophylls a, c, and carotenoids (fucoxanthin); and
- (c)
- green algae are included in the Chlorophyta phylum and their pigmentation is identical to that of land plants (chlorophyll a, b, and carotenoids).
2. Bioactive Compounds Used in Cosmeceuticals
2.1. Hydrocolloids and Other Seaweed Polysaccharides
2.1.1. Agar
2.1.2. Alginic Acid
2.1.3. Carrageenans
2.1.4. Porphyran
2.1.5. Laminaran
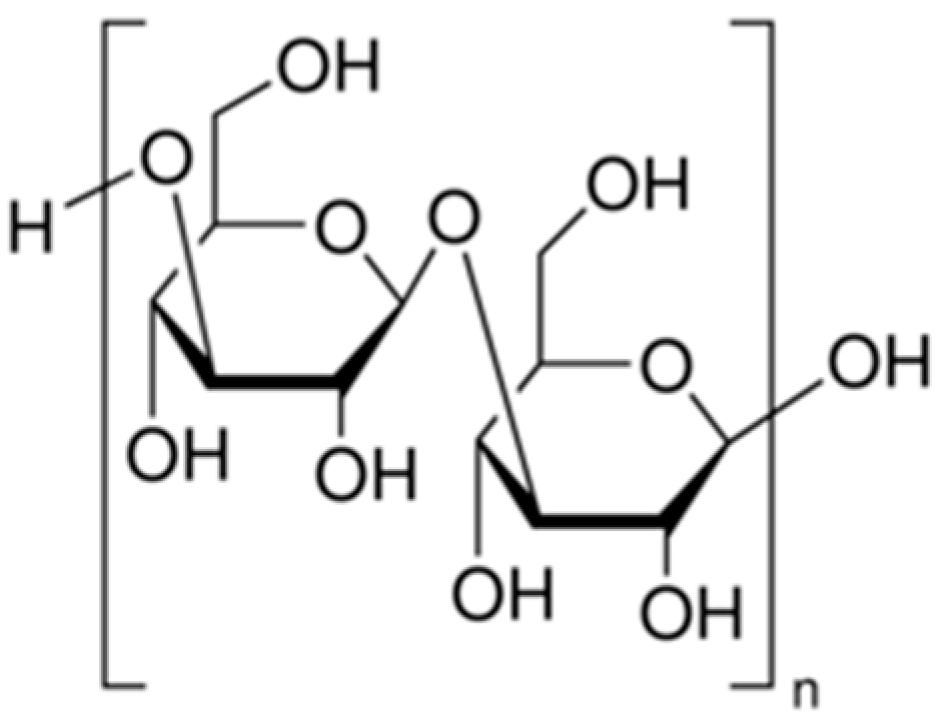
2.1.6. Fucoidan
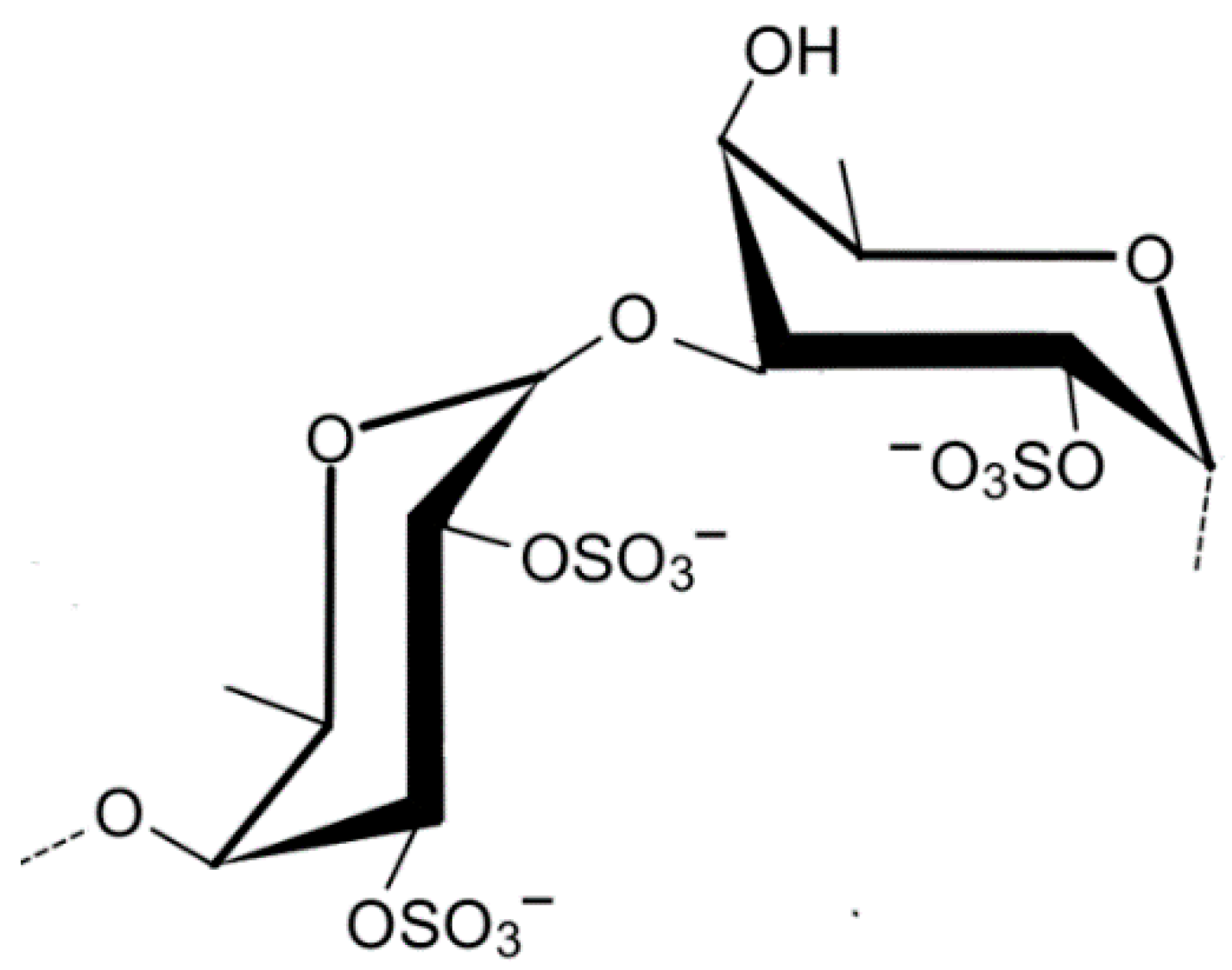
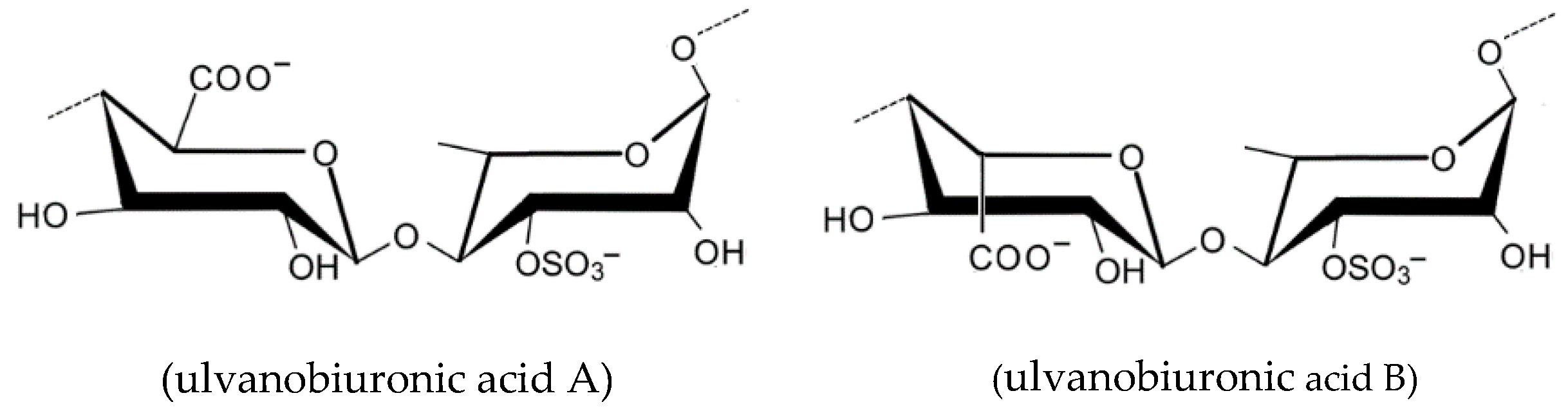
2.1.7. Ulvan
2.2. Proteins and Amino Acids
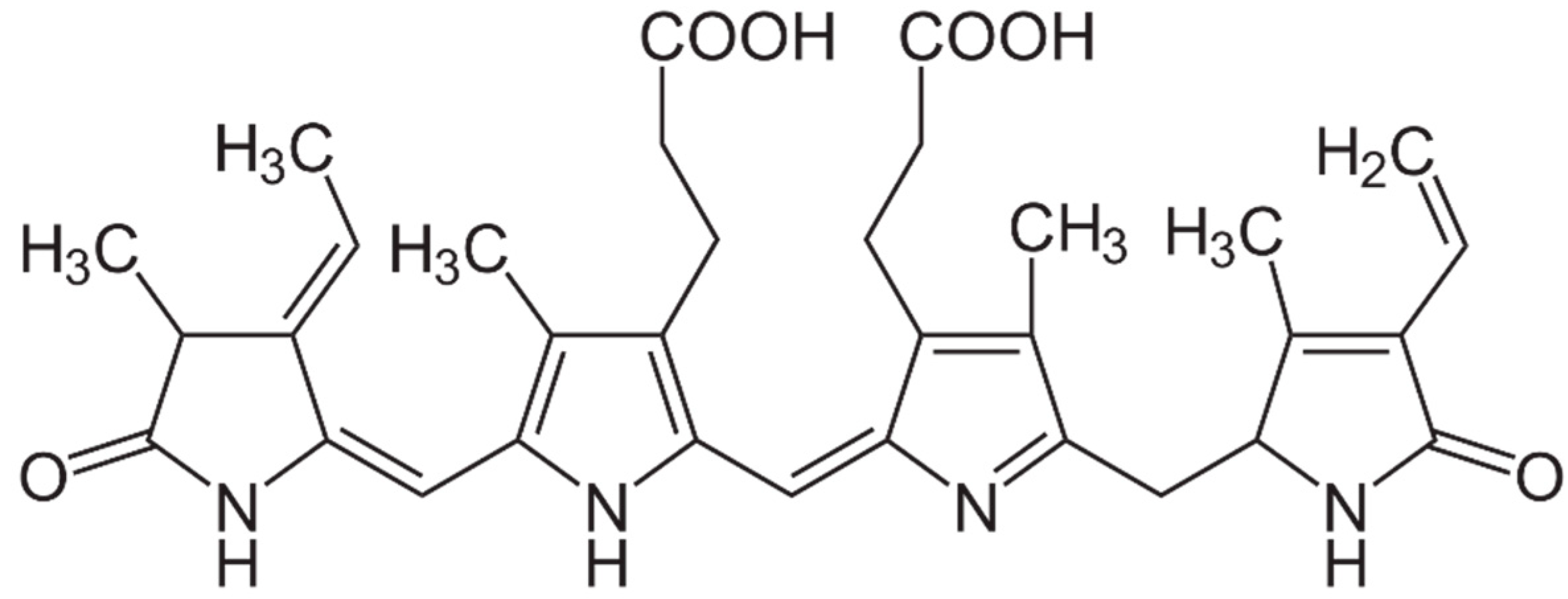
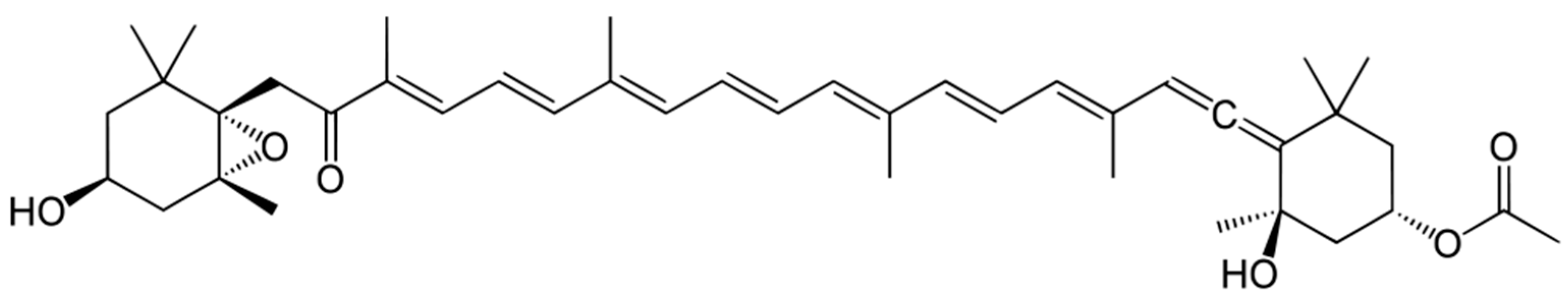
2.3. Pigments
2.4. Lipids
2.5. Phenolic Compounds
2.6. Vitamins and Minerals
3. Algotherapy—Herbal Medicine and Algae-Based Phytochemistry
4. Therapies Coming from the Sea
4.1. Seawater
4.2. Thalassotherapy
- -
- Increased skin permeability;
- -
- their ionic characteristics, when interacting with the skin, facilitate the penetration of the cosmetic compounds during or after the immediate to these treatments;
- -
- regulates the organic functions through the neuro-endocrine system, as enzymatic cofactor;
- -
- relaxes tight muscles, giving a rested appearance to the skin;
- -
- tends to normalize seborrheic secretion;
- -
- reduces hyperhidrosis (excessive perspiration, including feet);
- -
- promotes organic remineralization at the cutaneous tissue level; and
- -
- activates the cutaneous metabolism and locally stimulates the blood circulation, indirectly promoting the oxygenation and nutrition of these tissues.
4.3. Cosmeceuticals and Cosmetics
4.3.1. Skin: Anti-Wrinkling and Whitening
4.3.2. Skin Aging
4.3.3. Skin Whitening
4.3.4. Wound Healing of the Skin
4.3.5. Sliming and Anti-Cellulite Properties of Seaweed Extracts
- -
- stimulate tissue metabolism and blood circulation in the area of application, which helps to mobilize the fat installed in the subcutaneous tissue, as is the case of the unsightly cellulite, in which algae treatment has had very positive results;
- -
- tonify skin tissues by moisturizing, re-firming and hardening them, to prevent and attenuate wrinkles, delaying the aging of the skin, contributing aesthetically to a more luminous, firm and youthful skin;
- -
- they give back to the demineralized, brittle or devitalized hair its natural brightness, flexibility and texture, while giving it softness; and
- -
- promote the proper functioning of the sebaceous glands and regulate the water content of the skin tissue, facilitating the elimination of toxins.
4.3.6. Hair Growth Activities
4.4. Industrial Processes Used in the Elaboration of Cosmetic Products
- -
- Drying in air or in industrial ventilated ovens or chambers, for the removal of water from algae tissues (dehydration), is promoted by a controlled environment process where the temperature must not exceed 40 °C to avoid the destruction of the active elements (for example, protein molecules, such as enzymes, which are, thus, not denatured) present in algae. This is, of all the methods mentioned here, not only the simplest, but the most cost-effective. On the other hand, it is the least direct, and often incorporates other methods as a process step.
- -
- In the extraction by liquid phase, the compounds are extracted with different solvents, aqueous or organic (water, glycerin, ethyl alcohol, etc.) that allow the separation and isolation of the metabolites with different bioactive functions, depending on their chemical affinity with the solvent, in different phases, some of which are discarded successively for purification and concentration. Depending on the solvents used, temperature, pH, and duration of extraction, different extraction efficiencies can be achieved. In the case of water and other polar solvents, extracts are rich in polysaccharides, proteins, and other bioactive water-soluble compounds. In extractions with non-polar solvents the extracts obtained are rich in phenolic compounds, fatty acids, pigments, and other lipophilic compounds.
- -
- Enzyme assisted extraction (EAE) has attracted considerable interest because its hydrolytic action on algae structures and compounds can weaken or disrupt the cell wall structure and also break down complex internal storage compounds, releasing polysaccharides, proteins, and peptides or amino acids. Algal cell walls are typically composed of fibrous composites of microfibrillar polysaccharides (cellulose) embedded in a matrix of sulfated polysaccharides and proteoglycans. The efficiency of extracting marine macroalgae compounds is limited due to the presence of these complex cell walls, with mixtures of branched sulfated polysaccharides associated with proteins and various bound ions, such as calcium, sodium and potassium. Ultrasonic assisted extraction (UAE), an alternative energy assisted extraction method, is based on the migration of sound waves, which creates cavitation that grow and collapse, leading to rupture of the cells and their walls. UAE and EAE have been reported as alternative approaches with great potential for extracting bioactive substances from seaweed.
- -
- Lyophilization: the dehydration process is carried out at low temperature and in vacuum; the dried product thus obtained is then milled to the most suitable granulometry for a given application: creams, lotions, facial masks, bath gels, etc.
- -
- Cell microcracking: a process where cells rupture (lysis by disruption) when subjected to a grinding process, by compression and decompression (resorting to the use of mechanical disruptors-disruptors beads), releasing their content (trace elements, vitamins, and other metabolites) and from which the water is subsequently eliminated; the obtained powder contains all the active elements that normally are inside the cells, and, in addition, it has a very fine granulometry that allows a more effective transcutaneous penetration, in a topical application. In the cryo-microcracking technique, algae are milled at very low temperatures (well below freezing temperature, using liquid nitrogen), resulting in a fluid extract capable of being integrated into wet preparations, especially suitable for thalassotherapy treatments.
- -
- Fresh algae suspensions: extremely homogeneous cell suspensions obtained at low temperature, are stabilized in ethyl alcohol to avoid degradation; these suspensions recover their activity when diluted in water at the time of use.
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pomponi, S.A. The bioprocess-technological potential of the sea. J. Biotechnol. 1999, 70, 5–13. [Google Scholar] [CrossRef]
- Joshi, S.; Kumari, R.; Upasani, V.N. Applications of algae in cosmetics: An overview. Int. J. Innov. Res. Sci. Eng. Technol. 2018, 7, 1269–1278. [Google Scholar]
- Schrammek. Beauty News: Algae in Cosmetics—The All-Round Talents. Available online: https://www.schrammek.com/beautynews/algae-in-cosmetics/ (accessed on 22 September 2018).
- Pereira, L. Algae. Litoral of Viana do Castelo; Câmara Municipal de Viana do Castelo: Viana do Castelo, Portugal, 2010; pp. 7–8. ISBN 978-972-588-217-7. [Google Scholar]
- Pereira, L.; Correia, F. Algas Marinhas da Costa Portuguesa—Ecologia, Biodiversidade e Utilizações; Nota de Rodapé Editores: Paris, France, 2015; p. 341. ISBN 978-989-20-5754-5. [Google Scholar]
- Pereira, L. Guia Ilustrado das Macroalgas—Conhecer e Reconhecer Algumas Espécies da Flora Portuguesa; University de Coimbra Press: Coimbra, Portugal, 2009; p. 91. ISBN 978-989-26-0002-4. [Google Scholar]
- Pereira, L. Chapter 4—Cytological and cytochemical aspects in selected carrageenophytes (Gigartinales, Rhodophyta). In Advances in Algal Cell Biology; Heimann, K., Katsaros, C., Eds.; De Gruyter: Berlin, Germany, 2012; pp. 81–104. ISBN 978-3-11-022960-8. [Google Scholar] [CrossRef]
- González-Minero, F.J.; Bravo-Díaz, L. The Use of Plants in Skin-Care Products, Cosmetics and Fragrances: Past and Present. Cosmetics 2018, 5, 50. [Google Scholar] [CrossRef]
- Pereira, L. Therapeutic and Nutritional Uses of Algae, 1st ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 2–64. ISBN 9781498755382. [Google Scholar]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Microalgae as sources of pharmaceuticals and other biologically active compounds. J. Appl. Phycol. 1995, 7, 3–15. [Google Scholar] [CrossRef]
- Metting, B.; Pyne, J.W. Biologically active compounds from microalgae. Enzyme Microb. Technol. 1986, 8, 386–394. [Google Scholar] [CrossRef]
- Cannell, R.J.P. Algae as a source of biologically active products. Pestic. Sci. 2006, 39, 147–153. [Google Scholar] [CrossRef]
- Paul, C.; Pohnert, G. Production and role of volatile halogenated compounds from marine algae. Nat. Prod. Rep. 2011, 28, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Ibañez, E.; Herrero, M.; Mendiola, J.A.; Castro-Puyana, M. Extraction and characterization of bioactive compounds with health benefits from marine resources: Macro and micro algae, Cyanobacteria, and invertebrates. In Marine Bioactive Compounds; Haves, M., Ed.; Springer: New York, NY, USA, 2012; pp. 55–98. ISBN 978-1-4614-1246-5. [Google Scholar]
- Vo, T.S.; Ngo, D.H.; Kim, S.K. Marine algae as a potential pharmaceutical source for anti-allergic therapeutics. Process. Biochem. 2012, 47, 386–394. [Google Scholar] [CrossRef]
- Thomas, N.V.; Kim, S.-K. Beneficial effects of marine algal compounds in cosmeceuticals. Mar. Drugs 2013, 11, 146–164. [Google Scholar] [CrossRef] [PubMed]
- Indergaard, M. The aquatic resource. In Biomass Utilization; Côté, W.A., Ed.; Springer: Boston, MA, USA, 1983; pp. 137–168. ISBN 978-1-4757-0835-6. [Google Scholar]
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal chemodiversity and bioactivity: Sources of natural variability and implications for commercial application. Biotechnol. Adv. 2011, 29, 483–501. [Google Scholar] [CrossRef] [PubMed]
- Mafinowska, P. Algae extracts as active cosmetic ingredients. Zeszy. Naukowe 2011, 212, 123–129. [Google Scholar]
- Majmudar, G. Compositions of Marine Botanicals to Provide Nutrition to Aging and Environmentally Damaged Skin. US Patent 8318178 B2, 2012. Available online: https://patentimages.storage.googleapis.com/1f/41/48/4dfcee08799f34/US8318178.pdf (accessed on 22 September 2018).
- Wang, H.-M.; Chou, Y.-T.; Wen, Z.-H.; Wang, C.-Z.; Wang, C.-H.; Ho, M.-L. Novel biodegradable porous scaffold applied to skin regeneration. PLoS ONE 2013, 8, e56330. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jin, W.; Hou, Y.; Niu, X.; Zhang, H.; Zhang, Q. Chemical composition and moisture-absorption/retention ability of polysaccharides extracted from five algae. Int. J. Biol. Macromol. 2013, 57, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Thalgo. La Beaute Marine, 2018. 2018. Available online: http://www.thalgo.com/ (accessed on 22 September 2018).
- La-Mer. My Skin—And What It Needs, 2018. Available online: https://www.la-mer.com/en (accessed on 22 September 2018).
- Goldberg, S.L. The use of water soluble chlorophyll in oral sepsis: An experimental study of 300 cases. Am. J. Surg. 1943, 62, 117–123. [Google Scholar] [CrossRef]
- Spears, K. Developments in food colorings: The natural alternatives. Trends Biotechnol. 1988, 6, 283–288. [Google Scholar] [CrossRef]
- Lanfer-Marquez, U.M.; Barros, R.M.C.; Sinnecker, P. Antioxidant activity of chlorophylls and their derivatives. Food Res. Int. 2005, 38, 885–891. [Google Scholar] [CrossRef]
- Borowitzka, M.A. High-value products from microalgae—Their development and commercialization. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Christaki, E.; Bonos, E.; Giannenas, I.; Florou-Paneri, P. Functional properties of carotenoids originating from algae. J. Sci. Food Agric. 2013, 93, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Norzagaray-Valenzuela, C.D.; Valdez-Ortiz, A.; Shelton, L.M.; Jiménez-Edeza, M.; Rivera-López, J.; Valdez-Flores, M.A.; Germán-Báez, J. Residual biomasses and protein hydrolysates of three green microalgae species exhibit antioxidant and anti-aging activity. J. Appl. Phycol. 2017, 29, 189–198. [Google Scholar] [CrossRef]
- Huangfu, J.; Liu, J.; Sun, Z.; Wang, M.; Jiang, Y.; Chen, Z.Y.; Chen, F. Antiaging effects of astaxanthin-rich alga Haematococcus pluvialis on fruit flies under oxidative stress. J. Agric. Food Chem. 2013, 61, 7800–7804. [Google Scholar] [CrossRef] [PubMed]
- Houston, M.C. Nutraceuticals, vitamins, antioxidants, and minerals in the prevention and treatment of hypertension. Prog. Cardiovasc. Dis. 2005, 47, 396–449. [Google Scholar] [CrossRef] [PubMed]
- Ziaja. Focus on Skin, 2018. Available online: http://ziaja.co.uk/ (accessed on 22 September 2018).
- Delaunay, D.; Voile, I. Composition Dermatologique et/ou Cosmétique Utilisée Pour la Régénération de la Peau, 2011. European Patent EP2488149 B1. Available online: https://data.epo.org/publication-server/rest/v1.0/publication-dates/20131002/patents/EP2488149NWB1/document.html (accessed on 22 September 2018).
- Nurjanah; Nurilmala, M.; Hidayat, T.; Sudirdjo, F. Characteristics of seaweed as raw materials for cosmetics. Aquat. Procedia 2016, 7, 177. [Google Scholar] [CrossRef]
- Demais, H.; Brendle, J.; Le Deit, H.; Laza Anca, L.; Lurton, L.; Brault, D. Argiles Intercalés, 2007. European Patent EP1786862 A1, 2007. Available online: https://patents.google.com/patent/EP1786862A1 (accessed on 27 September 2018).
- Olmix. Specialist of Natural Algae-Based Solutions, 2017. Available online: https://www.olmix.com/ (accessed on 27 September 2018).
- Lahaye, M.; Robic, A. Structure and functional properties of ulvan, a polysaccharide from green seaweeds. Biomacromolecules 2007, 8, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Robic, A.; Rondeau-Mouro, C.; Sassi, J.F.; Lerat, Y.; Lahaye, M. Structure and interactions of ulvan in the cell wall of the marine green algae Ulva rotundata (Ulvales, Chlorophyceae). Carbohydr. Polym. 2009, 77, 206–216. [Google Scholar] [CrossRef]
- Guglielmo, M.; Montanari, D. Cosmetic Composition with A Lifting Effect for Sustaining Relaxed Tissues. Patent WO2008146116 A2, 2008. Available online: https://patents.google.com/patent/WO2008146116A3/tr (accessed on 27 September 2018).
- Wang, R.; Paul, V.J.; Luesch, H. Seaweed extracts and unsaturated fatty acid constituents from the green alga Ulva lactuca as activators of the cytoprotective Nrf2-ARE pathway. Free Radic. Biol. Med. 2013, 57, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Lusalgae. We Innovate in Marine Biotechnology. Research Activities, Wholesale and Retail Trade of Cosmetic and Hygiene Products, 2018. Available online: http://www.lusalgae.pt/lusalgae_en.html (accessed on 27 September 2018).
- Samarakoon, K.; Jeon, Y.J. Bio-functionalities of proteins derived from marine algae—A review. Food Res. Int. 2012, 48, 948–960. [Google Scholar] [CrossRef]
- Verdy, C.; Branka, J.-E.; Mekideche, N. Quantitative assessment of lactate and progerin production in normal human cutaneous cells during normal ageing: Effect of an Alaria esculenta extract. Int. J. Cosmet. Sci. 2011, 33, 462–466. [Google Scholar] [CrossRef]
- Chizhov, A.O.; Dell, A.; Morris, H.R.; Haslam, S.M.; McDowell, R.A.; Shashkov, A.S.; Nifantev, N.E.; Khatuntseva, E.A.; Usov, A.I. A study of fucoidan from the brown seaweed Chorda filum. Carbohydr. Res. 1999, 320, 108–119. [Google Scholar] [CrossRef]
- Wijesinghea, W.A.J.P.; Jeona, Y.-J. Biological activities and potential industrial applications of fucose rich sulfated polysaccharides and fucoidans isolated from brown seaweeds: A review. Carbohydr. Polym. 2012, 88, 13–20. [Google Scholar] [CrossRef]
- Senni, K.; Gueniche, F.; Foucault-Bertaud, A.; Igondjo-Tchen, S.; Fioretti, F.; Colliec-Jouault, S.; Durand, P.; Guezennec, J.; Godeau, G.; Letourneur, D. Fucoidan a sulfated polysaccharide from brown algae is a potent modulator of connective tissue proteolysis. Arch. Biochem. Biophys. 2006, 445, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Ryu, B.; Li, Y.; Qian, Z.J.; Kim, M.M.; Kim, S.K. Exhibitory effects of compounds from brown alga Ecklonia cava on the human osteoblasts. J. Biotech. 2008, 136, 577–588. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Dai, Y.-C.; Zhong, W.; Tan, M.; Lv, Z.-P.; Zhou, Y.-C.; Jiang, X. Tannic acid inhibited norovirus binding to HBGA receptors, a study of 50 Chinese medicinal herbs. Bioorg. Med. Chem. 2012, 20, 1616–1623. [Google Scholar] [CrossRef] [PubMed]
- Codif. Technologie Naturelle, 2018. Available online: http://www.codif-tn.com/ (accessed on 28 September 2018).
- Dermika. Awakens Your Beauty, 2018. Available online: http://dermika.pl/en/ (accessed on 28 September 2018).
- Ferreres, F.; Lopes, G.; Gil-Izquierdo, A.; Andrade, P.B.; Sousa, C.; Mouga, T.; Valentão, P. Phlorotannin extracts from fucales characterized by HPLC-DAD-ESI-MSn: Approaches to hyaluronidase inhibitory capacity and antioxidant properties. Mar. Drugs 2012, 10, 2766–2781. [Google Scholar] [CrossRef] [PubMed]
- Tonnesen, H.H.; Karlsen, J. Alginate in drug delivery systems. Drug. Dev. Ind. Pharm. 2002, 2, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, Y.; Takeuchi, Y.; Kakinuma, M.; Amano, H. Inhibitory effects of seaweeds on histamine release from rat basophile leukemia cells (RBL-2H3). Fish. Sci. 2006, 72, 1286–1291. [Google Scholar] [CrossRef]
- Heo, S.J.; Lee, K.W.; Song, C.B.; Jean, Y.J. Antioxidant activity of enzymatic extracts from brown seaweeds. Algae 2003, 18, 71–81. [Google Scholar] [CrossRef]
- Ahn, M.J.; Yoon, K.D.; Min, S.Y.; Lee, J.S.; Kim, J.H.; Kim, T.G.; Kim, S.H.; Kim, N.G.; Huh, H.; Kim, J. Inhibition of HIV-1 reverse transcriptase and protease by phlorotannins from the brown alga Ecklonia cava. Biol. Pharm. Bull. 2004, 27, 544–547. [Google Scholar] [CrossRef] [PubMed]
- Freurence, J. Seaweed proteins, Chapter 9. In Proteins in Food Processing, 2nd ed.; Yada, R.Y., Ed.; Woodhead Publishing Limited: Boca Raton, FL, USA; CRC Press LLC: Boca Raton, FL, USA, 2018; pp. 245–262. ISBN 978-0-08-100722-8. [Google Scholar]
- Le, Q.T.; Li, Y.; Qian, Z.J.; Kim, M.M.; Kim, S.K. Inhibitory effects of polyphenols isolated from marine alga Ecklonia cava on histamine release. Process. Biochem. 2009, 44, 168–176. [Google Scholar] [CrossRef]
- Yoon, N.Y.; Eom, T.-K.; Kim, M.-M.; Kim, S.-K. Inhibitory effect of phlorotannins isolated from Ecklonia cava on mushroom tyrosinase activity and melanin formation in mouse B16F10 melanoma cells. J. Agric. Food Chem. 2009, 57, 4124–4129. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.-J.; Ko, S.-C.; Cha, S.-H.; Kang, D.-H.; Park, H.-S.; Choi, Y.-U.; Kim, D.; Jung, W.-K.; Jeon, Y.-J. Effect of phlorotannins isolated from Ecklonia cava on melanogenesis and their protective effect against photo-oxidative stress induced by UV-B radiation. Toxicol. In Vitro 2009, 23, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.-S.; Kim, J.-A.; Ahn, B.-N.; Kim, S.-K. Potential effect of phloroglucinol derivatives from Ecklonia cava on matrix metalloproteinase expression and the inflammatory profile in lipopolysaccharide-stimulated human THP-1 macrophages. Fish. Sci. 2011, 77, 867–873. [Google Scholar] [CrossRef]
- Kang, J.-I.; Kim, S.-C.; Kim, M.-K.; Boo, H.-J.; Jeon, Y.-J.; Koh, Y.-S.; Yoo, E.-S.; Kang, S.-M.; Kang, H.-K. Effect of dieckol, a component of Ecklonia cava, on the promotion of hair growth. Int. J. Mol. Sci. 2012, 13, 6407–6423. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.-C.; Cha, S.-H.; Heo, S.-J.; Lee, S.-H.; Kang, S.-M.; Jeon, Y.-J. Protective effect of Ecklonia cava on UVB-induced oxidative stress: In vitro and in vivo zebrafish model. J. Appl. Phycol. 2011, 23, 697–708. [Google Scholar] [CrossRef]
- Kim, K.C.; Piao, M.J.; Zheng, J.; Yao, C.W.; Cha, J.W.; Kumara, M.H.S.R.; Han, X.; Kang, H.K.; Lee, N.H.; Hyun, J.W. Fucodiphlorethol G purified from Ecklonia cava suppresses ultraviolet B radiation-induced oxidative stress and cellular damage. Biomol. Ther. 2014, 22, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Bak, S.S.; Ahn, V.; Kim, J.A.; Shin, S.H.; Kim, J.C.; Kim, M.K.; Sung, Y.K.; Kim, S.K. Ecklonia cava promotes hair growth. J. Clin. Exp. Dermatol. Res. 2013, 38, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.C.; Myoungsook, L.; Ji-Hyeok, L.; Seung-Hong, L.; Yunsook, L.; Jeon, Y.J. Dieckol, a phlorotannin isolated from a brown seaweed, Ecklonia cava, inhibits adipogenesis through AMP-activated protein kinase (AMPK) activation in 3T3-LI preadipocytes. Environ. Toxicol. Pharmacol. 2013, 36, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Kang, S.-M.; Sok, C.H.; Hong, J.T.; Oh, J.-Y.; Jeon, Y.-J. Cellular activities and docking studies of eckol isolated from Ecklonia cava (Laminariales, Phaeophyceae) as potential tyrosinase inhibitor. Algae 2015, 30, 163–170. [Google Scholar] [CrossRef]
- Shibata, T.; Fujimoto, K.; Nagayama, K.; Yamaguchi, K.; Nakamura, T. Inhibitory activity of brown algal phlorotannins against hyaluronidase. Int. J. Food Sci. Tech. 2002, 37, 703–709. [Google Scholar] [CrossRef]
- Kang, H.; Kim, H.; Byun, D.; Son, B.; Nam, T.; Choi, J. Tyrosinase inhibitors isolated from the edible brown alga Ecklonia stolonifera. Arch. Pharm. Res. 2004, 27, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Joe, M.-J.; Kim, S.-N.; Choi, H.-Y.; Shin, W.-S.; Park, G.-M.; Kang, D.-W.; Kim, Y.K. The inhibitory effects of eckol and dieckol from Ecklonia stolonifera on the expression of matrix metalloproteinase-1 in human dermal fibroblasts. Biol. Pharm. Bull. 2006, 29, 1735–1739. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.R.; Lee, M.-S.; Shin, T.-S.; Hua, H.; Jang, B.-C.; Choi, J.-S.; Byun, D.-S.; Utsuki, T.; Ingram, D.; Kim, H.-R. Phlorofucofuroeckol A inhibits the LPS-stimulated iNOS and COX-2 expressions in macrophages via inhibition of NF-κB, Akt, and p38 MAPK. Toxicol. In Vitro 2011, 25, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Rupérez, P. Mineral content of edible marine seaweeds. Food Chem. 2002, 79, 23–26. [Google Scholar] [CrossRef]
- Je, J.-Y.; Park, P.-J.; Kim, E.-K.; Park, J.-S.; Yoon, H.-D.; Kim, K.-R.; Ahn, C.-B. Antioxidant activity of enzymatic extracts from the brown seaweed Undaria pinnatifida by electron spin resonance spectroscopy. LWT-Food Sci. Technol. 2009, 42, 874–878. [Google Scholar] [CrossRef]
- Al-Bader, T.; Byrne, A.; Gillbro, J.; Mitarotonda, A.; Metois, A.; Vial, F.; Rawlings, A.V.; Laloeuf, A. Effect of cosmetic ingredients as anticellulite agents: Synergistic action of actives with in vitro and in vivo efficacy. J. Cosmet. Dermatol. 2012, 11, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Al-Bader, T.; Davis, M.; Laloeuf, A.; Rawlings, A.V. Topical Cosmetic Compositions for Treating or Preventing Cellulite. European Patent 2414047 B1, 2013. Available online: https://patents.google.com/patent/EP2414047B1 (accessed on 29 September 2018).
- Oriflame. Oriflame Cosmetics SA, 2018. Available online: https://oriflame.com/ (accessed on 29 September 2018).
- Fitton, J.H.; Dell’Acqua, G.; Gardiner, V.-A.; Karpiniec, S.S.; Stringer, D.N.; Davis, E. Topical benefits of two fucoidan-rich extracts from marine macroalgae. Cosmetics 2015, 2, 66–81. [Google Scholar] [CrossRef]
- Jones, A. CBI Product Factsheet: Natural Ingredients for Cosmetics in Europe; CBI Market Intelligence: The Hague, The Netherlands, 2016; p. 14. Available online: https://www.cbi.eu/sites/default/files/market_information/researches/channels-segments-europe-natural-ingredients-cosmetics-2016.pdf (accessed on 30 September 2018).
- Bielenda. Institute Bielenda Professional, 2018. Available online: http://bielenda.pl/en/series/professional-formula-series (accessed on 30 September 2018).
- Plaza, M.; Santoyo, S.; Jaime, L.; Garcia-Blairsy, R.G.; Herrero, M.; Senorans, F.J.; Ibãnez, E. Screening for bioactive compounds from algae. J. Pharm. Biomed. Anal. 2010, 51, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-N.; Yang, H.-M.; Kang, S.-M.; Kim, D.; Ahn, G.; Jeon, Y.-J. Octaphlorethol A isolated from Ishige foliacea inhibits α-MSH-stimulated induced melanogenesis via ERK pathway in B16F10 melanoma cells. Food Chem. Toxicol. 2013, 59, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.-J.; Ko, S.-C.; Kang, S.-M.; Cha, S.-H.; Lee, S.-H.; Kang, D.-H.; Jung, W.-K.; Affan, A.; Oh, C.; Jeon, Y.-J. Inhibitory effect of diphlorethohydroxycarmalol on melanogenesis and its protective effect against UV-B radiation-induced cell damage. Food Chem. Toxicol. 2010, 48, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Gedouin, A.; Valle, R.; Morvan, P.Y. Use of Algae Extract to Stimulate the Oxygen Uptake by the Cells Having Lipolytic Effect to Produce ATP Molecules, 2006. Patent FR2879098 A1. Available online: https://www.lens.org/lens/patent/FR_2879098_A1 (accessed on 1 October 2018).
- Kordjazi, M.; Shabanpour, B.; Zabihi, E.; Faramarzi, M.A.; Feizi, F.; Gavlighi, H.A.; Feghhi, M.A.; Hosseini, S.A. Sulfated polysaccharides purified from two species of Padina improve collagen and epidermis formation in the Rat. Int. J. Mol. Cell. Med. 2013, 2, 156–163. [Google Scholar] [PubMed]
- Saidani, K.; Bedjou, F.; Benabdesselam, F.; Touati, N. Antifungal activity of methanolic extracts of four Algerian marine algae species. Afr. J. Biotechnol. 2012, 39, 9496–9500. [Google Scholar] [CrossRef]
- Gutiérrez, G. Compositions of Padina Algae or Their Extracts, and Their Pharmaceutical, Food Compositions, or Use for the Culture of Molluscs or Arthropods. European Patent EP 0655250 Al, 1995. Available online: https://patents.google.com/patent/EP0655250A1/en (accessed on 1 October 2018).
- Texinfine. Laboratoires ICP-Texinfine, 2018. Available online: http://www.icp-texinfine.com/ (accessed on 1 October 2018).
- Hupel, M.; Lecointre, C.; Meudec, A.; Poupart, N.; Ar Gall, E. Comparison of photoprotective responses to UV radiation in the brown seaweed Pelvetia canaliculata and the marine angiosperm Salicornia ramosissima. J. Exp. Mar. Biol. Ecol. 2011, 401, 36–47. [Google Scholar] [CrossRef]
- Jang, W.S.; Choung, S.Y. Antiobesity effects of the ethanol extract of Laminaria japonica Areshoung in high-fat-diet-induced obese rat. Evid. Based Complement. Alternat. Med. 2013, 2013, 492807. Available online: https://www.ncbi.nlm.nih.gov/pubmed/23365609 (accessed on 1 October 2018). [CrossRef] [PubMed]
- Shimoda, H.; Tanaka, J.; Shan, S.J.; Maoka, T. Anti-pigmentary activity of fucoxanthin and its influence on skin mRNA expression of melanogenic molecules. J. Pharm. Pharmacol. Res. 2010, 62, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Rioux, L.E.; Moulin, V.; Beaulieu, M.; Turgeon, S.L. Human skin fibroblast response is differentially regulated by galactofucan and low molecular weight galactofucan. Bioact. Carbohydr. Diet. Fibre 2013, 1, 105–110. [Google Scholar] [CrossRef]
- Mizutani, S.; Deguchi, S.; Kobayashi, E.; Nishiyama, E.; Sagawa, H.; Kato, I. Fucoidan Containing Cosmetics. Patent US7678368 B2, 2010. Available online: https://patents.google.com/patent/US20060093566 (accessed on 1 October 2018).
- TaKaRa. Available online: http://www.takara-bio.com/ (accessed on 1 October 2018).
- Liu, C.L.; Chiu, Y.T.; Hu, M.L. Fucoxanthin enhances HO-1 and NQOI expression in murine hepatic BNL CL2 cells through activation of the Nrf2/ARE system partially by its pro-oxidant activity. J. Agric. Food Chem. 2011, 59, 11344–11351. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.S.; Park, H.-Y.; Nam, K.-H. Whitening effects of 4-hydroxyphenethyl alcohol isolated from water boiled with Hizikia fusiformis. Food Sci. Biotechnol. 2014, 23, 555–560. [Google Scholar] [CrossRef]
- Kamei, Y.; Sueyoshi, M.; Hayashi, K.; Terada, R.; Nozaki, H. The novel anti-Propionibacterium acnes compound, Sargafuran, found in the marine brown alga Sargassum macrocarpum. J. Antibiot. 2009, 62, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Song, T.Y.; Chen, C.; Yang, N.C.; Fu, C.S.; Chang, Y.T.; Chen, C.L. The correlation of in vitro mushroom tyrosinase activity with cellular tyrosinase activity and melanin formation in melanoma cells A2058. J. Food Drug Anal. 2009, 17, 156–162. [Google Scholar]
- Chan, Y.Y.; Kim, K.H.; Cheah, S.H. Inhibitory effects of Sargassum polycystum on tyrosinase activity and melanin formation in B16F1O murine melanoma cells. J. Ethnopharmacol. 2011, 137, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.H.; Ko, S.C.; Kim, D.; Jeon, Y.J. Screening of marine algae for potential tyrosinase inhibitor: Those inhibitors reduced tyrosinase activity and melanin synthesis in zebra fish. J. Dermatol. 2011, 38, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Rozkin, M.; Levina, M.N.; Efimov, V.S.; Usov, A.I. The anticoagulant and lipolysis-stimulating activity of polysaccharides from marine brown algae. Farmakol. Toksicol. 1991, 54, 40–42. [Google Scholar]
- Kang, J.-I.; Kim, M.-K.; Lee, J.-H.; Jeon, Y.-J.; Hwang, E.-K.; Koh, Y.-S.; Hyun, J.-W.; Kwon, S.-Y.; Yoo, E.-S.; Kang, H.-K. Undariopsis peterseniana promotes hair growth by the activation of Wnt/β-Catenin and ERK pathways. Mar. Drugs 2017, 15, 130. [Google Scholar] [CrossRef] [PubMed]
- Quindere, A.L.G.; Fontes, B.P.; Vanderlei, E.D.O.; de Queiroz, I.N.L.; Rodrigues, J.A.G.; de Araujo, I.W.F.; Jorge, R.J.B.; de Menezes, D.B.; e Silva, A.A.R.; Chaves, H.V.; et al. Peripheral antinociception and anti-edematogenic effect of a sulfated polysaccharide from Acanthophora muscoides. Pharmacol. Rep. 2013, 65, 600–613. [Google Scholar] [CrossRef]
- Gurgel-Rodrigues, J.A.; Freire-Tovar, A.M.; Gomes-Quinderé, A.L.; de Souza Mourão, P.A.; Nilo Lino de Queiroz, I.; Barros Benevides, N.M. Extraction and structural properties of Acanthophora muscoides (Rhodophyceae) extracellular matrix sulfated polysaccharides and their effects on coagulation. Acta Sci-Technol. 2016, 38, 273–282. [Google Scholar] [CrossRef]
- Gurgel-Rodrigues, J.A.; de Queiroz, I.N.L.; Quinderé, A.L.G.; Benevides, N.M.B.; Tovar, A.M.F.; de Souza Mourão, P.A. Mild-acid hydrolysis of a native polysulfated fraction from Acanthophora muscoides generates sulfated oligosaccharides displaying in vitro thrombin generation inhibition. Acta Sci. Biol. Sci. 2016, 38, 7–15. [Google Scholar] [CrossRef]
- Al-Fadhli, A.; Wahidulla, S.; D’Souza, L. Glycolipids from the red alga Chondria armata. Glycobiology 2006, 16, 902–915. [Google Scholar] [CrossRef] [PubMed]
- Fabrowska, J.; Łęska, B.; Schroeder, G.; Messyasz, B.; Pikosz, M. Biomass and extracts of algae as material for cosmetics. In Marine Algae Extracts; Kim, S.-K., Chojnacka, K., Eds.; Wiley-VCH, Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 681–706. ISBN 9783527337088. [Google Scholar] [CrossRef]
- Sanghvi, A.M.; Lo, Y.M. Present and potential industrial applications of macro- and microalgae. Recent Pat. Food Nutr. Agric. 2010, 2, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Stengel, D.B.; Connan, S. Marine algae: A source of biomass for biotechnological applications. Methods Mol. Biol. 2015, 1308, 1–37. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, D.; Wu, J.; Chen, Y.; Wang, S. In vitro antioxidant activities of sulfated polysaccharide fractions extracted from Corallina officinalis. Int. J. Biol. Macromol. 2011, 49, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Ryu, B.; Qian, Z.J.; Kim, N.M.; Nam, K.W.; Kim, S.K. Anti-photoaging activity and inhibition of matrix metalloproteinase (MMP)by marine red alga, Corallina pilulifera methanol extract. Radiat. Phys. Chem. 2009, 78, 98–105. [Google Scholar] [CrossRef]
- Iberagar. Agar-agar, poderoso espessante e gelificante natural. Food Ingred. Bras. 2010, 14, 49–50. Available online: http://www.revista-fi.com/materias/146.pdf (accessed on 2 October 2018). (In Portuguese).
- Pereira, L. Biological and therapeutic properties of the seaweed polysaccharides. Int. Biol. Rev. 2018, 2, 1–50. [Google Scholar] [CrossRef]
- Hanan, A.; Gowsaly, P.; Elakkiya, V.T.; Shankar, K.R. Exploration of beneficial activities of marine algae for cosmeceuticals. Seaweed Res. Utiln. 2016, 38, 133–137. [Google Scholar]
- Kang, J.I.; Kim, S.C.; Han, S.C.; Hong, H.J.; Jeon, Y.J.; Kim, B.; Koh, Y.S.; Yoo, E.S.; Kang, H.K. Hair-loss preventing effect of Grateloupia elliptica. Biomol. Ther. 2012, 20, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Fard, S.M.; Shamsabadi, F.T.; Emadi, M.; Meng, G.Y.; Muhammad, K.; Mohamed, S. Ethanolic extract of Eucheuma cottonii promotes in vivo hair growth and wound healing. J. Anim. Vet. Adv. 2011, 10, 601–605. [Google Scholar] [CrossRef]
- Dixit, D.; Reddy, C.R.K. Non-targeted secondary metabolite profile study for deciphering the cosmeceutical potential of red marine macro alga Jania rubens—An LCMS-based approach. Cosmetics 2017, 4, 45. [Google Scholar] [CrossRef]
- Fenical, W. Chemical variation in a new bromochamigrene derivative from the red seaweed Laurencia pacifica. Phytochemistry 1976, 15, 511–512. [Google Scholar] [CrossRef]
- Liu, M.; Hansen, P.L.; Lin, X. Bromophenols in marine algae and their bioactivities. Mar. Drugs 2011, 9, 1273–1292. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.V.; Westcott, N.D.; Hu, C.; Kitts, D.D. Mycosporine-like amino acid composition of edible red alga Palmaria palmata (Dulse) harvested from the west and east coasts of Grand Manan Island, New Brunswick. Food Chem. 2009, 12, 321–328. [Google Scholar] [CrossRef]
- Carreto, J.I.; Carignan, M.O.; Montoya, N.G. Comparative studies on mycosporine-like amino acids, paralytic shellfish toxins and pigment profiles of the toxic dinoflagellates Alexandrium tamarense, A. catenella and A. minutum. Mar. Ecol. Prog. Ser. 2001, 223, 49–60. [Google Scholar] [CrossRef]
- Mercurio, D.G.; Wagemaker, T.A.L.; Alves, V.M.; Benevenuto, C.G.; Gaspar, L.R.; Maia Campos, P.M.B.G. In vivo photoprotective effects of cosmetic formulations containing UV filters, vitamins, Ginkgo biloba and red algae extracts. J. Photochem. Photobiol. B Biol. 2015, 153, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Kazlowska, K.; Lin, H.T.; Chang, S.H.; Tsai, G.J. In vitro and in vivo anticancer effects of sterol fraction from red algae Porphyra dentata. Evid. Based. Complement. Alternat. Med. 2013, 2013, 493869. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yu, P.; Li, Z.; Zhang, H.; Xu, Z.; Li, P. Antioxidant activities of sulfated polysaccharide fractions from Porphyra haitanesis. J. Appl. Phycol. 2003, 15, 305–310. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, N.; Lin, X.; Zhao, Z.; Li, Z.; Xu, Z. The structure of a sulfated galactan from Porphyra haitanensis and its in vivo antioxidant activity. Carbohydr. Res. 2004, 339, 105–111. [Google Scholar] [CrossRef]
- Senevirathne, M.; Ahn, C.-B.; Je, J.-Y. Enzymatic extracts from edible red algae, Porphyra tenera, and their antioxidant, anti-acetylcholinesterase, and anti-inflammatory activities. Food Sci. Biotechnol. 2010, 19, 1551–1557. [Google Scholar] [CrossRef]
- Pimentel, F.B.; Alves, R.C.; Rodrigues, F.; Oliveira, M.B.P.P. Macroalgae-derived ingredients for cosmetic industry—An Update. Cosmetics 2018, 5, 2. [Google Scholar] [CrossRef]
- Yun, E.J.; Lee, S.; Kim, J.H.; Kim, B.B.; Kim, H.T.; Lee, S.H.; Pelton, J.G.; Kang, N.J.; Choi, I.G.; Kim, K.H. Enzymatic production of 3,6-anhydro-L-galactose from agarose and its purification and in vitro skin whitening and anti-inflammatory activities. Appl. Microbiol. Biotechnol. 2013, 97, 2961–2970. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Chen, Y.; Wang, W.; Cui, B.; Wan, N. Synthesis of superparamagnetic carboxymethyl chitosan/sodium alginate nanosphere and its application for immobilizing α-amylase. Carbohydr. Polym. 2016, 151, 600–605. [Google Scholar] [CrossRef] [PubMed]
- ACTISEANE. A Unique Combination of Natural Algal Growth Substances. Gelyma, 2018. Available online: http://www.biosiltech.com/pdf/gelyma/ACTISEANE%20-%20LEAFLET.pdf (accessed on 3 October 2018).
- Andre, G.; Pellegrini, M.; Pellegrini, M. Cosmetic or Dermatological Compositions Especially Useful as Antiaging and Antiwrinkle Products Comprise Extracts of Ascophyllum and Halopteris Seaweeds (Gelyma). Patent FR2837386, 2003. Available online: https://worldwide.espacenet.com/publicationDetails/biblio?II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20030926&CC=FR&NR=2837386A1&KC=A1 (accessed on 3 October 2018).
- Carvalho, L.G.; Pereira, L. Review of marine algae as source of bioactive metabolites, chapter 6. In Marine Algae—Biodiversity, Taxonomy, Environmental Assessment and Biotechnology, 1st ed.; Pereira, L., Neto, J.M., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 192–224. ISBN 9781466581678. [Google Scholar]
- Pereira, L. Carrageenans—Sources and Extraction Methods, Molecular Structure, Bioactive Properties and Health Effects; Nova Science Publishers: Hauppauge, NY, USA, 2016; p. 293. ISBN 1634855035. [Google Scholar]
- Colwell, R.R. Marine polysaccharides for pharmaceutical and microbiological applications. In Biotechnology of Marine Polysaccharides; Colwell, R.R., Pariser, E.R., Sinskey, J., Eds.; McGraw-Hill: New York, NY, USA, 1985; pp. 363–376, ISBN-13 978-0891164333. [Google Scholar]
- Pereira, L.; Ribeiro-Claro, P.J.A. Analysis by vibrational spectroscopy of seaweed with potential use in food, pharmaceutical and cosmetic. In Marine Algae—Biodiversity, Taxonomy, Environmental Assessment and Biotechnology, 1st ed.; Pereira, L., Neto, J.M., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 225–247. ISBN 9781466581678. [Google Scholar]
- De Philippis, R.; Sili, C.; Paperi, R.; Vincenzini, M. Exopolysaccharide-producing cyanobacteria and their possible exploitation: A review. J. Appl. Phycol. 2001, 13, 293–299. [Google Scholar] [CrossRef]
- Arad, S.; Levy-Ontman, O. Red microalgal cell-wall polysaccharides: Biotechnological aspects. Curr. Opin. Biotechnol. 2010, 21, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Yun, E.J.; Choi, I.-G.; Kim, K.H. Red macroalgae as a sustainable resource for bio-based products. Trends Biotechnol. 2015, 33, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Balboa, E.M.; Conde, E.; Soto, M.L.; Pérez-Armada, L.; Domínguez, H. Cosmetics from marine sources. In Springer Handbook of Marine Biotechnology; Kim, S.-K., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1015–1042. ISBN 978-3-642-53970-1. [Google Scholar]
- Ouyang, Q.-Q.; Hu, Z.; Li, S.-D.; Quan, W.-Y.; Wen, L.-L.; Yang, Z.; Pu-Wang, L. Thermal degradation of agar mechanism and toxicity of products. Food Chem. 2018, 264, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.P.S.; Sanjeewa, K.K.A.; Kim, S.-Y.; Lee, J.S.; Jeon, Y.J. Reduction of heavy metal (Pb2+) biosorption in zebrafish model using alginic acid purified from Ecklonia cava and two of its synthetic derivatives. Int. J. Biol. Macromol. 2018, 106, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Lahaye, M. Chemistry and physico-chemistry of phycocolloids. Cah. Biol. Mar. 2001, 42, 137–157. [Google Scholar] [CrossRef]
- Pereira, L.; Gheda, S.F.; Ribeiro-Claro, P.J. Analysis by vibrational spectroscopy of seaweed polysaccharides with potential use in food, pharmaceutical, and cosmetic industries. Int. J. Carbohydr. Chem. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Rinaudo, M. Seaweed polysaccharides. In Comprehensive Glycoscience, Vol. 2, Analysis of Glycans; Polysaccharide Functional, Properties, Kamerling, J.P., Eds.; Elsevier: Oxford, UK, 2007; pp. 691–735. ISBN 978-0-444-51967-2. [Google Scholar] [CrossRef]
- Pereira, L.; Amado, A.M.; Critchley, A.T.; van de Velde, F.; Ribeiro-Claro, P.J.A. Identification of selected seaweed polysaccharides (Phycocolloids) by vibrational spectroscopy (FTIR-ATR and FT-Raman). Food Hydrocoll. 2009, 23, 1903–1909. [Google Scholar] [CrossRef]
- Hotchkiss, S.; Campbell, R.; Hepburn, C. Carrageenan: Sources and extraction methods. In Carrageenans: Sources and Extraction Methods, Molecular Structure, Bioactive Properties and Health Effects; Pereira, L., Ed.; Nova Science Publishers: New York, NY, USA, 2016; pp. 1–16. ISBN 978-1-63485-503-7. [Google Scholar]
- Munaf, E.; Zein, R.; Dharma, A.; Indrawati; Lim, L.H.; Takeuchi, T. Optimation study of carrageenan extraction from red algae (Eucheuma cottonii). Jurnal Riset Kimia 2015, 2, 120–2126. [Google Scholar] [CrossRef]
- Charlier, R.H.; Chaineux, M.-C.P. The healing sea: A sustainable coastal ocean resource: Thalassotherapy. J. Coast. Res. 2009, 25, 838–856. [Google Scholar] [CrossRef]
- Pereira, L. Algae. Litoral of Viana do Castelo: Uses in Agriculture, Gastronomy and Food Industry (Bilingual); Câmara Municipal de Viana do Castelo: Viana do Castelo, Portugal, 2010; pp. 7–8. ISBN 978-972-588-218-4. [Google Scholar]
- Villarroel, L.H.; Zanlungo, A.B. Structural studies on the Porphyran from Porphyra columbina. Carbohydr. Res. 1981, 88, 139–145. [Google Scholar] [CrossRef]
- Bhatia, S.; Sharma, A.; Sharma, K.; Kavale, M.; Chaugule, B.B.; Dhalwal, K.; Namdeo, A.G.; Mahadik, K.R. Novel algal polysaccharides from marine source: Porphyran. Pharmacogn. Rev. 2008, 2, 271–276. [Google Scholar]
- Zhang, Q.B.; Qi, H.M.; Zhao, T.T.; Deslandes, E.; Ismaeli, N.M.; Molloy, F.; Critchley, A.T. Chemical characteristics of a polysaccharide from Porphyra capensis (Rhodophyta). Carbohydr. Res. 2005, 340, 2447–2450. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Oyamada, S.; Matsushima, R.; Murata, M.; Muraoka, T. Inhibitory effect of porphyran, prepared from dried Nori, on contact hypersensitivity in mice. Biosci. Biotechnol. Biochem. 2005, 69, 1824–1830. [Google Scholar] [CrossRef] [PubMed]
- Kylin, H. Biochemistry of sea algae. Z. Phys. Chem. 1913, 83, 171–197. [Google Scholar] [CrossRef]
- Chevolot, L.; Mulloy, B.; Ratiskol, J.; Foucault, A.; Colliec-Jouault, S. A disaccharide repeat unit is the major structure in fucoidans from two species of brown algae. Carbohydr. Res. 2001, 330, 529–535. [Google Scholar] [CrossRef]
- Fitton, J.H. Therapies from fucoidan; multifunctional marine polymers. Mar. Drugs 2011, 9, 1731–1760. [Google Scholar] [CrossRef] [PubMed]
- Lorbeer, A.J.; Tham, R.; Zhang, W. Potential products from the highly diverse and endemic macroalgae of southern Australia and pathways for their sustainable production. J. Appl. Phycol. 2013, 25, 717–732. [Google Scholar] [CrossRef]
- Kuznetsova, T.A.; Besednova, N.N.; Mamaev, A.N.; Momot, A.P.; Shevchenko, N.M.; Zvyagintseva, T.N. Anticoagulant activity of fucoidan from brown algae Fucus evanescens of the Okhotsk sea. Bull. Exp. Biol. Med. 2013, 136, 471–473. [Google Scholar] [CrossRef]
- Wu, L.; Sun, J.; Su, X.; Yu, Q.; Yu, Q.; Zhang, P. A review about the development of fucoidan in antitumor activity: Progress and challenges. Carbohydr. Polym. 2016, 154, 96–111. [Google Scholar] [CrossRef] [PubMed]
- Saravana, P.S.; Cho, Y.-N.; Patil, M.P.; Cho, Y.-J.; Kim, G.-D.; Park, Y.B.; Woo, H.-C.; Chun, B.-S. Hydrothermal degradation of seaweed polysaccharide: Characterization and biological activities. Food Chem. 2018, 268, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lu, F.; Wei, X.J.; Zhao, R.X. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [PubMed]
- Holtkamp, A.D.; Kelly, S.; Ulber, R.; Lang, S. Fucoidans and fucoidanases-focus on techniques for molecular structure elucidation and modification of marine polysaccharides. Appl. Microbiol. Biotechnol. 2009, 82, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jiao, G.L.; Yu, G.L.; Zhang, J.Z.; Ewart, S.H. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef] [PubMed]
- Thanh-Sang, V.; Kim, S.-K. Fucoidans as a natural bioactive ingredient for functional foods. J. Funct. Foods. 2013, 16–27. [Google Scholar] [CrossRef]
- Cardoso, S.M.; Carvalho, L.G.; Silva, P.J.; Rodrigues, M.S.; Pereira, O.R.; Pereira, L. Bioproducts from seaweeds: A review with special focus on the Iberian Peninsula. Curr. Org. Chem. 2014, 18, 896–917. [Google Scholar] [CrossRef]
- Cardoso, S.M.; Pereira, O.R.; Seca, A.M.; Pinto, D.; Silva, A.M. Seaweeds as preventive agents for cardiovascular diseases: From nutrients to functional Foods. Mar. Drugs 2015, 13, 6838–6865. [Google Scholar] [CrossRef] [PubMed]
- Zenmony. Fucoidan in Cosmetics, 2018. Available online: http://www.zenmony.com/fucoidan-in-cosmetics-a25/ (accessed on 23 September 2018).
- Moon, H.E.; Islam, N.; Ahr, B.R.; Chowdhury, S.S.; Sohn, H.S.; Jung, H.A.; Choi, J.S. Protein tyrosine phosphatase 1B and α-glucosidase inhibitory phlorotannins from edible brown algae Ecklonia stolonifera and Eisenia bicyclis. Biosci. Biotechnol. Biochem. 2011, 75, 1472–1480. [Google Scholar] [CrossRef] [PubMed]
- Narayanaswamy, R.; Jo, B.W.; Choi, S.-K.; Ismail, I.S. Fucoidan: Versatile cosmetic ingredient. An overview. J. Appl. Cosmetol. 2013, 31, 131–138. [Google Scholar]
- Usov, A.I.; Zelinsky, N.D. Chapter 2—Chemical structures of algal polysaccharides. In Functional Ingredients from Algae for Foods and Nutraceuticals; Domínguez, H., Ed.; Woodhead Publishing, Elsevier: London, UK, 2013; pp. 23–86. ISBN 978-0-85709-512-1. [Google Scholar] [CrossRef]
- Gesztesi, J.L.; Silva, L.V.N.; Robert, L.; Robert, A. Cosmetic Composition of Two Polysaccharides Based on Fucose and Rhamnose. US Patent US20060115443A1, 2003. Available online: https://patents.google.com/patent/US20060115443 (accessed on 23 September 2018).
- Yaich, H.; Amira, A.B.; Abbes, F.; Bouaziz, M.; Besbes, S.; Richel, A.; Blecker, C.; Attia, H.; Garna, H. Effect of extraction procedures on structural, thermal and antioxidant properties of ulvan from Ulva lactuca collected in Monastir coast. Int. J. Biol. Macromol. 2017, 105, 1430–1439. [Google Scholar] [CrossRef] [PubMed]
- Sekar, S.; Chandramohan, M. Phycobiliproteins as a commodity: Trends in applied research, patents and commercialization. J. Appl. Phycol. 2008, 20, 113–136. [Google Scholar] [CrossRef]
- Galland-Irmouli, A.V.; Fleurence, J.; Lamghari, R.; Lun, M.; Rouxel, C.; Barbaroux, O.; Bronowicki, J.-P.; Villaume, C.; Guéant, J.-L. Nutritional value of proteins from edible seaweed, Palmaria palmata (dulse). J. Biol. Chem. 1999, 10, 353–359. [Google Scholar] [CrossRef]
- WHO. Iodine Status Worldwide WHO Global Database on Iodine Deficiency; World Health Organization: Geneva, Switzerland, 2004. Available online: http://apps.who.int/iris/bitstream/handle/10665/43010/9241592001.pdf?sequence=1 (accessed on 26 September 2018).
- Pereira, L. Edible Seaweeds of the World, 1st ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2016; p. 453. ISBN 9781498730471. [Google Scholar]
- Bedoux, G.; Hardouin, K.; Burlot, A.S.; Bourgougnon, N. Bioactive components from seaweeds: Cosmetic applications and future development. Adv. Bot. Res. 2014, 71, 345–378. [Google Scholar] [CrossRef]
- Reef, R.; Kaniewska, P.; Hoegh-Guldberg, O. Coral skeletons defend against ultraviolet radiation. PLoS ONE 2009, 4, e7995. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L. Chapter 6—Seaweed Flora of the European North Atlantic and Mediterranean. In Springer Handbook of Marine Biotechnology; Se-Kwon, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 65–178. ISBN 978-3-642-53971-8. [Google Scholar] [CrossRef]
- Hosikian, A.; Lim, S.; Halim, R.; Danquah, M.K. Chlorophyll extraction from microalgae: A review on the process engineering aspects. Int. J. Chem. Eng. 2010, 391632. [Google Scholar] [CrossRef]
- Horwitz, B. Role of chlorophyll in proctology. Am. J. Surg. 1951, 81, 81–84. [Google Scholar] [CrossRef]
- Vílchez, C.; Forján, E.; Cuaresma, M.; Bédmar, F.; Garbayo, I.; Vega, J.M. Marine carotenoids: Biological functions and commercial applications. Mar. Drugs 2011, 9, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Mata-Gómez, L.C.; Montañez, J.C.; Méndez-Zavala, A.; Aguilar, C.N. Biotechnological production of carotenoids by yeasts: An overview. Microb. Cell. Fact. 2014, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Yuan, J.-P.; Wu, C.-F.; Wang, J.-H. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: Metabolism and bioactivities relevant to human health. Mar. Drugs 2011, 9, 1806–1828. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, N.; Gemello, E.; Gammone, M.A.; Girolamo, M.; Ficoneri, C.; Riccioni, G. Fucoxantin: A treasure from the sea. Mar. Drugs 2012, 10, 604–616. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, A.M.R.; Rodríguez, M.L.G. Lipids in pharmaceutical and cosmetic preparations. Grasas Aceites 2000, 51, 74–96. [Google Scholar] [CrossRef]
- Singh, J.; Gu, S. Commercialization potential of microalgae for biofuels production. Renew. Sustain. Energy Rev. 2010, 14, 2596–2610. [Google Scholar] [CrossRef]
- Pereira, L.; Soares, F.; Freitas, A.C.; Duarte, A.C.; Ribeiro-Claro, P. Extraction, characterization and use of carrageenans, Chapter 3. In Industrial Applications of Marine Biopolymers, 1st ed.; Sudha, P.N., Ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2017; p. 472. ISBN 9781315313528. [Google Scholar]
- Harborne, J.B. Phytochemical Methods—A Guide to Modern Techniques of Plant. Analysis; Springer Science & Business Media: Berlin, Germany, 1998; p. 310. ISBN 978-0-412-57260-9. [Google Scholar]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [PubMed]
- Tückmantel, W.; Kozikowski, A.P.; Romanczyk, L.J. Studies in polyphenol chemistry and bioactivity. 1. Preparation of building blocks from (+)-catechin. Procyanidin formation. Synthesis of the cancer cell growth inhibitor, 3-O-galloyl(2R,3R)-epicatechin-4β,8-[3-O-galloyl-(2R,3R)-epicatechin]. J. Am. Chem. Soc. 1999, 121, 12073–12081. [Google Scholar] [CrossRef]
- Urquiaga, I.; Leighton, F. Plant polyphenol antioxidants and oxidative stress. Biol. Res. 2000, 33, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Gu, L. Phlorotannins from brown algae (Fucus vesiculosus) inhibited the formation of advanced glycation endproducts by scavenging reactive carbonyls. J. Agric. Food Chem. 2012, 60, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, X.; Gao, L.; Cui, C.; Li, C.; Li, J.; Wang, B. Extraction and PTP1B inhibitory activity of bromophenols from the marine red alga Symphyocladia latiuscula. Chin. J. Oceanol. Limnol. 2011, 29, 686–690. [Google Scholar] [CrossRef]
- Target, N.M.; Arnold, T.M. Effects of secondary metabolites on digestion in marine herbivores. In Marine Chemical Ecology, 1st ed.; McClintock, J.B., Baker, B.J., Eds.; CRC Press: Boca Raton, FL, USA, 2001; pp. 391–411. ISBN 9780849390647. [Google Scholar]
- Sanjeewa, K.K.A.; Kim, E.A.; Son, K.T.; Jeon, Y.J. Bioactive properties and potentials cosmeceutical applications of phlorotannins isolated from brown seaweeds: A review. J. Photochem. Photobiol. B 2016, 162, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L. A review of the nutrient composition of selected edible seaweeds, Chapter 2. In Seaweed: Ecology, Nutrient Composition and Medicinal Uses; Pomin, V.H., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2011; pp. 15–47. ISBN 9781614708780. [Google Scholar]
- Wang, J.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef] [PubMed]
- De Benoist, B.; McLean, E.; Andersson, M.; Rogers, L. Iodine Deficiency in 2007: Global Progress Since 1993. Food Nutr. Bull. 2008, 29, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B. Iodine requirements and the risks and benefits of correcting iodine deficiency in populations. J. Trace Elem. Med. Biol. 2008, 22, 81–92. [Google Scholar] [CrossRef] [PubMed]
- ATSDR. Agency for Toxic Substances and Disease Registry; Division of Toxicology: Atlanta, GA, USA, 2004. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp162.pdf (accessed on 26 September 2018).
- Westby, T.; Cadogan, A.; Duignan, G. In vivo uptake of iodine from a Fucus serratus Linnaeus seaweed bath: Does volatile iodine contribute? Environ. Geochem. Health 2018, 40, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Gomez, C.G.; Lambrecht, M.V.P.; Lozano, J.E.; Rinaudo, M.; Villar, M.A. Influence of the extraction-purification conditions on final properties of alginates obtained from brown algae (Macrocystis pyrifera). Int. J. Biol. Macromol. 2009, 44, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Quinton, R. L’Eau de Mer—Milieu organique, 1st ed.; Masson et Cie, Éditeurs: Paris, France, 1904; p. 497, ISBN-10: 1295831783. [Google Scholar]
- Barata, E.A.F. A Cosmetologia—Princípios Básicos; Tecnopress: São Paulo, Brasil, 1995; p. 176. ISBN 8586543039. [Google Scholar]
- Westby, T.; Duignan, G.; Smyth, T.; Cadogan, A. Method validation and determination of total iodine in seaweed bathwater. Bot. Mar. 2016, 59, 241–249. [Google Scholar] [CrossRef]
- Goldsmith, L.A. Biochemistry and Physiology of the Skin, 2nd ed.; Oxford University Press: Oxford, UK; Oxford Medicine Publications: Oxford, UK, 1983; p. 1364, ISBN-13 978-0192612533. [Google Scholar]
- Andrade, S.C.D.; Carvalho, R.; Soares, A.S.; Vilar, M.J. Benefícios da talassoterapia e balneoterapia na fibromialgia. Rev. Bras. Reumatol. 2008, 48, 94. [Google Scholar] [CrossRef]
- Kim, S.K.; Ravichandran, Y.D.; Khan, S.B.; Kim, Y.T. Prospective of the cosmeceuticals derived from marine organisms. Biotechnol. Bioprocess. Eng. 2008, 13, 511. [Google Scholar] [CrossRef]
- FDA—Cosmetics and Cosmeceuticals, 2018. Available online: https://www.fda.gov/cosmetics/labeling/ (accessed on 22 November 2018).
- Buschmann, A.H.; Camus, C.; Infante, J.; Neori, A.; Israel, Á.; Hernández-González, M.C.; Pereda, S.V.; GomezPinchetti, J.L.; Golberg, A.; Tadmor-Shalev, N.; et al. Seaweed production: Overview of the global state of exploitation, farming and emerging research activity. Eur. J. Phycol. 2017, 52, 391–406. [Google Scholar] [CrossRef]
- Netalgae. Inter-Regional Network to Promote Sustainable Development in the Marine Algal Industry, 2017. Available online: http://www.netalgae.eu/index-en.php (accessed on 5 October 2018).
- Mesnildrey, L.; Jacob, C.; Frangoudes, K.; Reunavot, M.; Lesueur, M. Seaweed industry in France. Report. Interreg program NETALGAE. Les publications du pôle halieutique Agrocampus Ouest 2012, 9, 34. [Google Scholar]
- Ferdouse, F.; Holdt, S.L.; Murúa, P.; Yang, Z. The Global Status of Seaweed Production, Trade and Utilization; FAO Globefish Research Programme: Rome, Italy, 2018; p. 120. ISBN 978-92-5-130870-7. Available online: http://www.fao.org/3/CA1121EN/ca1121en.pdf (accessed on 5 October 2018).
- Brenner, M.; Hearing, J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2007, 84, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Wollenberg, A.; Kraft, S.; Hanau, D.; Bieber, T. Immunomorphological and ultrastructural characterization of langerhans cells and a novel, inflammatory dendritic epidermal cell (IDEC) population in lesional skin of atopic eczema. J. Investig. Dermatol. 1996, 106, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C. Acne and sebaceous gland function. Clin. Dermatol. 2004, 22, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Denda, M.; Sokabe, T.; Fukumi-Tominaga, T.; Tominaga, M. Effects of skin surface temperature on epidermal permeability barrier homeostasis. J. Investig. Dermatol. 2007, 127, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.C.; Chimeh, F.; Lu, Z.; Mathieu, J.; Person, K.S.; Zhang, A.; Kohn, N.; Martinello, S.; Berkowitz, R.; Holick, M.F. Factors that influence the cutaneous synthesis and dietary sources of vitamin D. Arch. Biochem. Biophys. 2007, 460, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Wickett, R.R.; Visscher, M.O. Structure and function of the epidermal barrier. Am. J. Infect. Control. 2006, 34, S98–S110. [Google Scholar] [CrossRef]
- Benbow, M. Maintaining skin integrity and preventing pressure damage. Nurs. Resid. Care 2009, 11, 44–450. [Google Scholar] [CrossRef]
- Tsukahara, K.; Takema, Y.; Moriwaki, S.; Tsuji, N.; Suzuki, Y.; Fujimura, T.; Imokawa, G. Selective inhibition of skin fibroblast elastase elicits a concentration-dependent prevention of ultraviolet B-induced wrinkle formation. J. Investig. Dermatol. 2001, 117, 671–677. [Google Scholar] [CrossRef] [PubMed]
- McCullough, J.L.; Kelly, K.M. Prevention and treatment of skin aging. Ann. N.Y. Acad. Sci. 2006, 1067, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Park, S.-J.; Kim, I.-H.; Choi, Y.H.; Nam, T.-J. Protective effect of Porphyra-334 on UVA-induced photoaging in human skin fibroblasts. Int. J. Mol. Med. 2014, 34, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, H.; Hanada, K.; Hashimoto, I. Correlation of skin phototype with facial wrinkle formation. Photodermatol. Photoimmunol. Photomed. 1999, 15, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Magnenat-Thalmann, N.; Kalra, P.; Lévêque, J.L.; Bazin, R.; Batisse, D.; Querleux, B. A computational skin model: Fold and wrinkle formation. IEEE. Trans. Inf. Technol. Biomed. 2002, 6, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.F.; Hussey, P.S. Population aging: A comparison among industrialized countries. Health Aff. 2000, 19, 191–203. [Google Scholar] [CrossRef]
- Tsuji, N.; Moriwaki, S.; Suzuki, Y.; Takema, Y.; Imokawa, G. The role of elastases secreted by fibroblasts in wrinkle formation: Implication through selective inhibition of elastase activity. Photochem. Photobiol. 2001, 74, 283–290. [Google Scholar] [CrossRef]
- Kim, M.M.; Ta, Q.V.; Mendis, E.; Rajapakse, N.; Jung, W.K.; Byun, H.G.; Jeon, Y.J.; Kim, S.K. Phlorotannins in Ecklonia cava extract inhibit matrix metalloproteinase activity. Life Sci. 2006, 79, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Chung, C.B.; Kim, J.G.; Ko, K.I.; Park, S.H.; Kim, J.-H.; Eom, S.Y.; Kim, Y.S.; Hwang, Y.-I.; Kim, K.H. Anti-wrinkle activity of Ziyuglycoside I isolated from a Sanguisorba officinalis root extract and its application as a cosmeceutical ingredient. Biosci. Biotechnol. Biochem. 2008, 72, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Arica, Ş.Ç.; Ozyilmaz, A.; Demirci, A. A study on the rich compounds and potential benefits of algae: A review. Pharm. Innov. 2017, 6, 42–51. [Google Scholar]
- German-Báez, L.J.; Valdez-Flores, M.A.; Félix-Medina, J.V.; Norzagaray-Valenzuela, C.D.; Santos-Ballardo, D.U.; Reyes-Moreno, C.; Shelton, L.M.; Valdez-Ortiz, A. Chemical composition and physicochemical properties of Phaeodactylum tricornutum microalgal residual biomass. Food Sci. Technol. Int. 2017, 23, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.D.; Chen, C.C.; Huynh, P.; Chang, J.S. Exploring the potential of using algae in cosmetics. Bioresour. Technol. 2015, 184, 355. [Google Scholar] [CrossRef] [PubMed]
- Ariede, M.B.; Candido, T.M.; Jacome, A.L.M.; Velasco, M.V.R.; de Carvalho, J.C.M.; Baby, A.R. Cosmetic attributes of algae—A review. Algal Res. 2017, 25, 483–487. [Google Scholar] [CrossRef]
- De Roeck-Holtzhauer, Y. Uses of seaweeds in cosmetics. In Seaweed Resources in Europe: Uses and Potential; Guiry, M., Blunden, G., Eds.; John Wiley & Sons: Chichester, UK, 1991; pp. 83–94. [Google Scholar]
- Fujimura, T.; Tsukahara, K.; Moriwaki, S.; Kitahara, T.; Sano, T.; Takema, Y. Treatment of human skin with an extract of Fucus vesiculosus changes its thickness and mechanical properties. J. Cosmet. Sci. 2002, 53, 1–9. [Google Scholar] [PubMed]
- Boonme, P.; Junyaprasert, V.B.; Suksawad, N.; Songkro, S. Microemulsions and nanoemulsions: Novel vehicles for whitening cosmeceuticals. J. Biomed. Nanotechnol. 2009, 5, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Gilchrest, B.A.; Eller, M.S. DNA photodamage stimulates melanogenesis and other photoprotective responses. J. Investig. Dermatol. Symp. Proc. 1999, 4, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.C. Skin of color: Biology, structure, function, and implications for dermatologic disease. J. Am. Acad. Dermatol. 2002, 46, S41–S62. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Esparza, M.; Jiménez-Cervantes, C.; Solano, F.; Lozano, J.A.; García-Borrón, J.C. Mechanisms of melanogenesis inhibition by tumor necrosis factor-α in B16/F10 mouse melanoma cells. Eur. J. Biochem. 1998, 255, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Solano, F.; Briganti, S.; Picardo, M.; Ghanem, G. Hypopigmenting agents: An updated review on biological, chemical and clinical aspects. Pigment. Cell. Res. 2006, 19, 550–571. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.I. Skin whitening effect. In New Perspectives on Aloe; Park, Y.I., Lee, S.K., Eds.; Springer: New York, NY, USA, 2006; pp. 127–135. ISBN 978-0-387-31799-1. [Google Scholar]
- Silab. WHITONYL® and the Complexion Becomes Porcelain, 2018. Available online: https://www.silab.fr/produit-55-whitonyl_usa.html (accessed on 20 October 2018).
- Dae, H.P.; Won, S.C.; Sean, H.Y.; Jung, S.S.; Chul, H.S. A developmental study of artificial skin using the alginate dermal substrate. Key Eng. Mater. 2007, 342, 125–128. [Google Scholar] [CrossRef]
- Gong, T.F.; Fang, C.Y.; Chen, W.; Wang, P.N. Physiochemical properties of alginate in tissue engineering research and its clinical application. J. Clin. Rehabil. Tissue Eng. Res. 2007, 11, 3613–3616. [Google Scholar]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in chronic wounds. Int. J. Mol. Sci. 2016, 17, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ha, W.H.; Park, D.H. Effect of seaweed extract on hair growth promotion in experimental study of C57BL/6 mice. Arch. Craniofac. Surg. 2013, 14, 1–10. [Google Scholar] [CrossRef]
- Zikeli, S. Production process of a new cellulosic fiber with antimicrobial properties. Curr. Probl. Dermatol. 2006, 33, 110–126. [Google Scholar] [CrossRef] [PubMed]
- Fluhr, J.W.; Breternitz, M.; Kowatzki, D.; Bauer, A.; Bossert, J.; Elsner, P.; Hipler, U.C. Silver-loaded seaweed-based cellulosic fiber improves epidermal skin physiology in atopic dermatitis: Safety assessment, mode of action and controlled, randomized single-blinded exploratory in vivo study. Exp. Dermatol. 2010, 19, 1600–1625. [Google Scholar] [CrossRef] [PubMed]
- Isolauri, E.; Turjanmaa, K. Combined skin prick and patch testing enhances identification of food allergy in infants with atopic dermatitis. J. Allergy Clin. Immunol. 1996, 97, 9–15. [Google Scholar] [CrossRef]
- Novak, N.; Bieber, T. Allergic and nonallergic forms of atopic diseases. J. Allergy Clin. Immunol. 2003, 112, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Shakespeare, P. Burn wound healing and skin substitutes. Burns 2001, 27, 517–522. [Google Scholar] [CrossRef]
- Matou, S.; Helley, D.; Chabut, D.; Bros, A.; Fischer, A.-M. Effect of fucoidan on fibroblast growth factor-2-induced angiogenesis in vitro. Thromb. Res. 2002, 106, 213–221. [Google Scholar] [CrossRef]
- Leoni, G.; Neumann, P.-A.; Sumagin, R.; Denning, T.; Nusrat, A. Wound repair: Role of immune–epithelial interactions. Mucosal Immunol. 2015, 8, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Rahimpour, Y.; Hamishehkar, H. Liposomes in cosmeceutics. Expert Opin. Drug Deliv. 2012, 9, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Sezer, A.D.; Erdal, C.; Fatih, H.P.; Zeki, O.; Ahmet, L.B.; Julide, A. Preparation of fucoidan-chitosan hydrogel and its application as burn healing accelerator on rabbits. Biol. Pharm. Bull. 2008, 31, 2326–2333. [Google Scholar] [CrossRef] [PubMed]
- Sasikala, L.; Durai, B.; Rathinamoorthy, R. Manuka honey loaded chitosan hydrogel films for wound dressing applications. Int. J. PharmTech Res. 2013, 5, 1774–1785. [Google Scholar]
- Kang, J.-I.; Yoo, E.-S.; Hyun, J.-W.; Koh, Y.-S.; Lee, N.H.; Ko, M.-H.; Ko, C.-S.; Kang, H.-K. Promotion effect of Apo-9’-fucoxanthinone from Sargassum muticum on hair growth via the activation of Wnt/β-Catenin and VEGF-R2. Biol. Pharm. Bull. 2016, 39, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Balboa, E.; Conde, E.; Constenla, A.; Falqué, E.; Domínguez, H. Sensory evaluation and oxidative stability of a suncream formulated with thermal spring waters from Ourense (NW Spain) and Sargassum muticum extracts. Cosmetics 2017, 4, 19. [Google Scholar] [CrossRef]
- Couteau, C.; Coiffard, L. Seaweed Application in Cosmetics. In Seaweed in Health and Disease Prevention; Fleurence, J., Levine, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 423–441. ISBN 9780128027936. [Google Scholar]
- Berardesca, E.; Abril, E.; Rona, C.; Vesnaver, R.; Cenni, A.; Oliva, M. An effective night slimming topical treatment. Int. J. Cosmet. Sci. 2012, 34, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Trüeb, R.M. The value of hair cosmetics and pharmaceuticals. Dermatology 2001, 202, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Weitz, R. Women and their hair: Seeking power through resistance and accommodation. Gend. Soc. 2001, 15, 667–686. [Google Scholar] [CrossRef]
- Qiu, Y. Cosmeceutical Formulations of Natural Ingredients for Hair Growth. US Patent US20150297502A1, 2015. Available online: https://patents.google.com/patent/US20150297502 (accessed on 25 September 2018).
- Cotsarelis, G.; Millar, S.E. Towards a molecular understanding of hair loss and its treatment. Trends Mol. Med. 2001, 7, 293–301. [Google Scholar] [CrossRef]
- Price, V.H. Treatment of hair loss. N. Engl. J. Med. 1999, 341, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, K.D.; Olsen, E.A.; Whiting, D.; Savin, R.; DeVillez, R.; Bergfeld, W.; Price, V.H.; van Neste, D.; Roberts, J.L.; Hordinsky, M.; et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J. Am. Acad. Dermatol. 1998, 39, 578–589. [Google Scholar] [CrossRef]
- Burton, J.L.; Marshall, A. Hypertrichosis due to minoxidil. Br. J. Dermatol. 1979, 101, 593–595. [Google Scholar] [CrossRef] [PubMed]
- Gromley, G.J. Finasteride: A clinical review. Biomed. Pharmacother. 1995, 49, 319–324. [Google Scholar] [CrossRef]
- McClellan, K.J.; Markham, A. Finasteride: A review of its use male pattern hair loss. Drugs 1999, 57, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.K.; Mamdani, B.; Shansky, R.M.; Mahurkar, S.D.; Dunea, G. Severe hypertension. Treatment with minoxidil. J. Am. Med. Assoc. 1975, 233, 249–252. [Google Scholar] [CrossRef]
- Tanigaki-Obana, N.; Ito, M. Effects of cepharanthine and minoxodil on proliferation, differentiation and keratinozation of cultured cells from the murine hair apparatus. Arch. Dermatol. Res. 1992, 284, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Kwon, O.S.; Chung, J.H.; Cho, K.H.; Eun, H.C.; Kim, K.H. Effect of minoxodil on proliferation and apoptosis in dermal papilla cells of human hair follicle. J. Dermatol. Sci. 2004, 34, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Hamaoka, H.; Minakuchi, K.; Miyoshi, H.; Arase, S.; Chen, C.H.; Nakaya, Y. Effect of K+ channel openers on K+ channel in cultured human dermal papilla cells. J. Med. Invest. 1997, 44, 73–77. [Google Scholar] [PubMed]
- Shorter, K.; Farjo, N.P.; Picksley, S.M.; Randall, V.A. Human hair follicles contain two forms of ATP-sensitive potassium channels, only one of which is sensitive to minoxidil. Faseb. J. 2008, 22, 1725–1736. [Google Scholar] [CrossRef] [PubMed]
- Lachgar, S.; Moukadiri, H.; Jonca, F.; Charveron, M.; Bouhaddioui, N.; Gall, Y.; Bonafe, J.L.; Plouet, J. Vascular endothelial growth factor is an autocrine growth factor for hair dermal papilla cells. J. Investig. Dermatol. 1996, 106, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Kwack, M.H.; Kang, B.M.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Minoxidil activates beta-catenin pathway in human dermal papilla cells: A possible explanation for its anagen prolongation effect. J. Dermatol. Sci. 2011, 62, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Bak, S.-S.; Sung, Y.; Kim, S.-K. 7-Phloroeckol promotes hair growth on human follicles in vitro. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2014, 387, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Olaizola, M. Commercial development of microalgal biotechnology: From the test tube to the marketplace. Biomol. Eng. 2003, 20, 459–466. [Google Scholar] [CrossRef]
- Huang, C.C.; Chen, Y.F.; Wang, C.C.R. Effects of micronization on the physicochemical properties of peels of three root and tuber crops. J. Sci. Food Agric. 2010, 90, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Dyminiska, L.; Szatkowski, M.; Wróbel-Kwiatkowska, M.; Kurzawa, A.; Syska, W.; Ggot, A.; Zawadzki, M.; Ptak, M.; Maczka, M.; Hanuza, J.; et al. Improved properties of micronized genetically modified flax fibers. J. Biotechnol. 2012, 164, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.; Mendiola, J.A.; Cifuentes, A.; Ibáñe, E. Supercritical fluid extraction: Recent advances and applications. J. Chromatogr. A 2010, 1217, 2495–2511. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jónsdóttir, R.; Kristinsson, H.G.; Hreggvidsson, G.O.; Jónsson, J.Ó.; Thorkelsson, G.; Ólafsdóttir, G. Enzyme-enhanced extraction of antioxidant ingredients from red algae Palmaria palmata. LWT Food. Sci. Technol. 2010, 43, 1387–1393. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Park, S.; Kim, M.H.; Choi, Y.-K.; Yang, Y.-H.; Kim, H.J.; Kim, H.; Songd, K.-G.; Lee, S.H. Ultrasound-assisted extraction of lipids from Chlorella vulgaris using [Bmim][MeSO4]. Biomass Bioenergy 2013, 56, 99–103. [Google Scholar] [CrossRef]
- Rodrigues, D.; Sousa, S.; Silva, A.; Amorim, M.; Pereira, L.; Rocha-Santos, T.A.; Gomes, A.M.; Duarte, A.C.; Freitas, A.C. Impact of enzyme and ultrasound assisted extraction methods on biological properties of red, brown and green seaweeds from the Central West Coast of Portugal. J. Agric. Food. Chem. 2015, 63, 3177–3188. [Google Scholar] [CrossRef] [PubMed]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Breithaupt, D.E. Simultaneous HPLC determination of carotenoids used as food coloring additives: Applicability of accelerated solvent extraction. Food Chem. 2004, 86, 449–456. [Google Scholar] [CrossRef]
- Chen, K.-T.; Cheng, C.-H.; Wu, Y.-H.; Lu, Y.-C.; Lin, Y.-H.; Lee, H.-T. Continuous lipid extraction of microalgae using high-pressure carbon. Bioresour. Technol. 2013, 146, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Esthetic World. Termalismo y Estética II, 2008. Available online: https://www.balneariosurbanos.es/termalismo-y-estetica-ii/ (accessed on 20 October 2018).
- Pereira, L. (Ed.) Algal Biofuels, 1st ed.; Science Publishers’ (SP), An Imprint of CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2017; p. 232. ISBN 9781498752312. [Google Scholar]
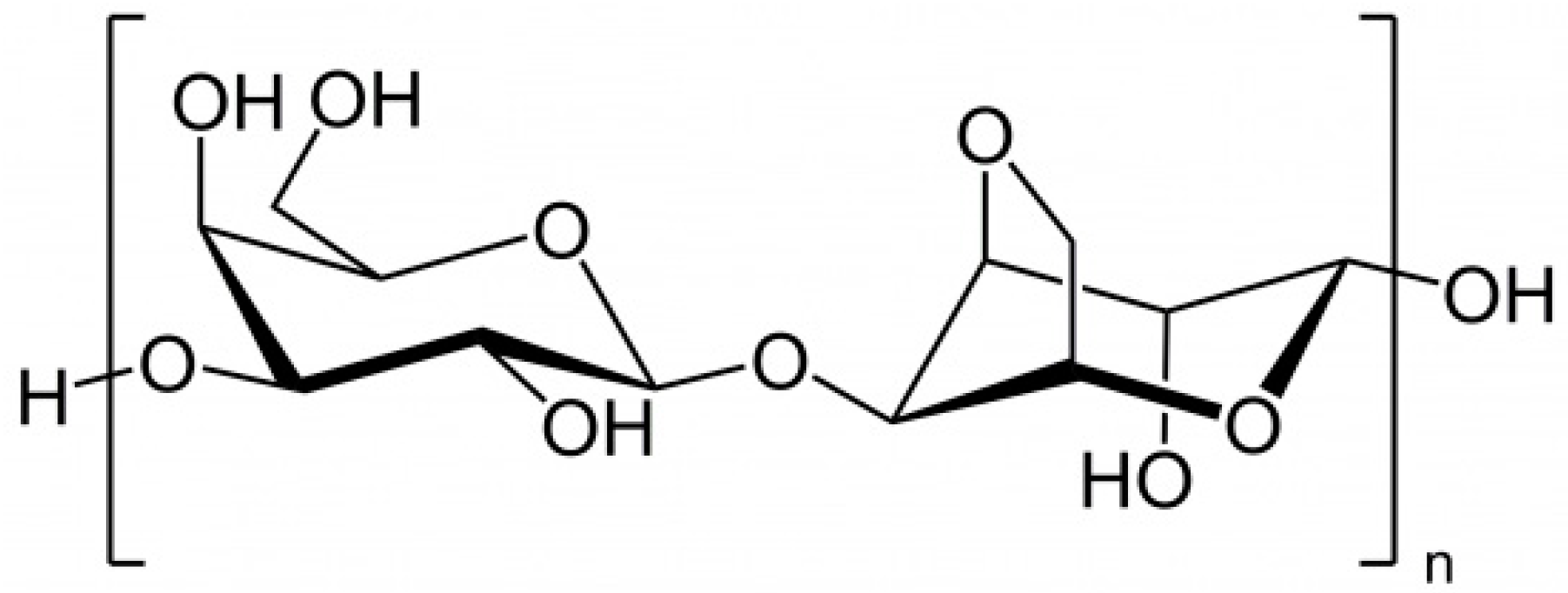
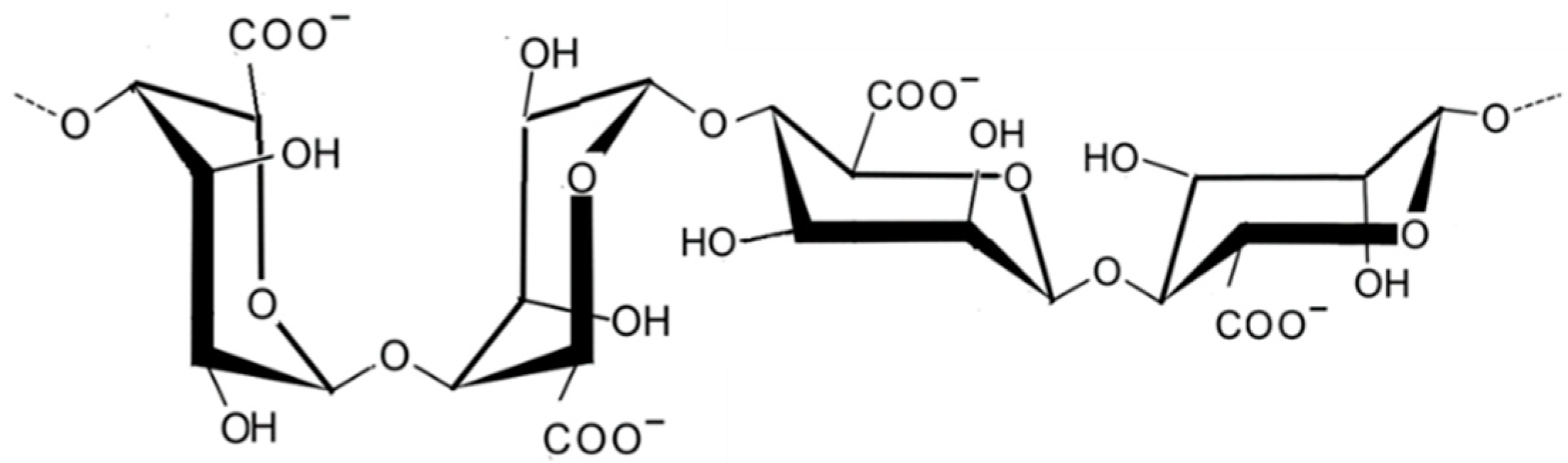
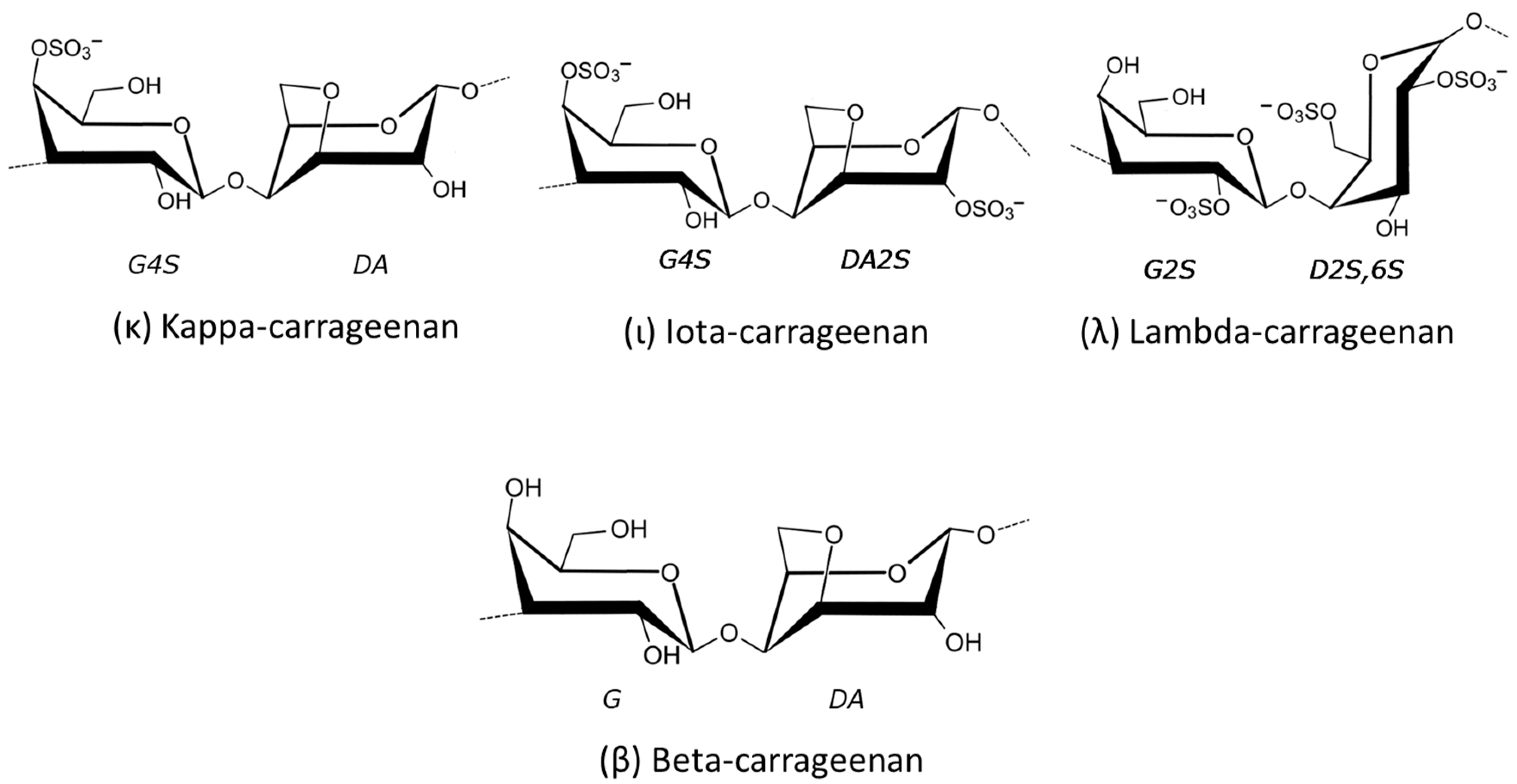
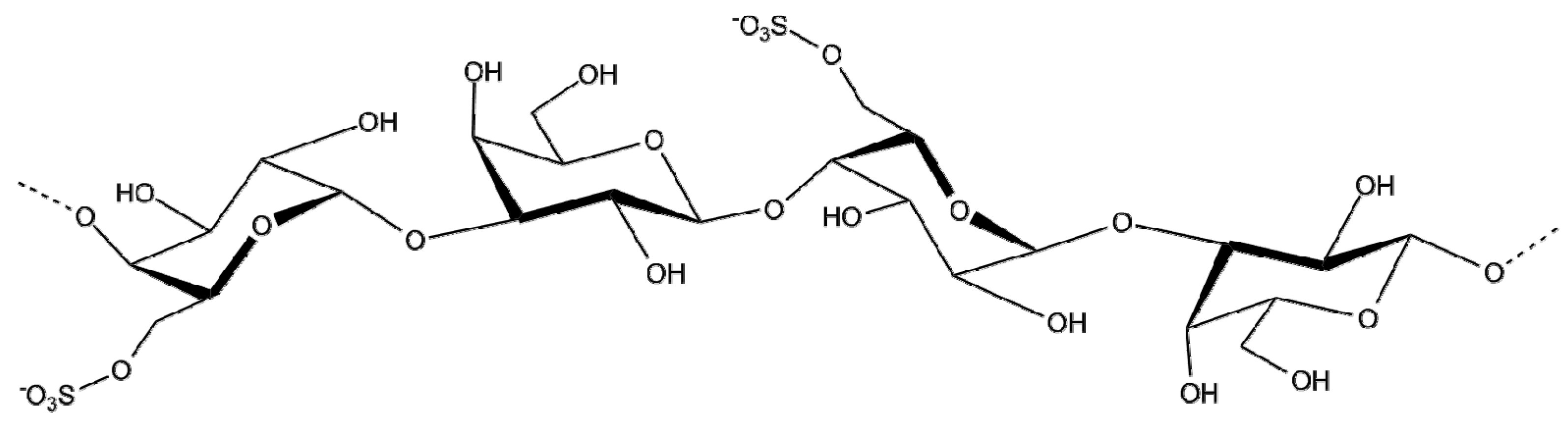
| Species | Extract/Compound | Cosmetics Properties and/or Products | References |
|---|---|---|---|
| Chlorophyta (green algae) | |||
| Codium tomentosum | Extract 3 | Moisturizing | [21] |
| C. tomentosum | Extract | Skin moisturization and protection | [22,23] |
| Chlorella vulgaris * | Extracts | Anti-stretch marks creams, body lotions, eye creams, face masks, shower gels | [24,25] |
| Cladophora glomerata | Chlorophylls (a, b, c, d) | Antibacterial, antioxidant, coloring, antibacterial, deodorizing, tissue growth stimulating agents | [26,27,28] |
| Caulerpa sp. | Extracts: steroids, flavonoids, phenols hydroquinone and saponin | Tyrosinase inhibitor to inhibit melanin pigment | [37] |
| Dunaliella salina * | Carotenoids (astaxanthin, β-carotene, fucoxanthin, lutein) | Tyrosinase inhibitors, antiaging, anti-inflammatory, antioxidant, adical scavengers, antiphotoaging agents, and colorants | [27,29,30] |
| D. tertiolecta * | Phenolic compund | Anti-aging | [31] |
| Tetraselmis suecica * | Phenolic compund | Anti-aging | [31] |
| Haematococcus lacustris (as H. pluvialis) * | Carotenoids (astaxanthin, β-carotene, fucoxanthin, lutein) | Antioxidant, anti-inflammatory, antiaging, antiphotoaging agents, radical scavengers, colorants, and tyrosinase inhibitors | [27,29,30] |
| H. lacustris (as H. pluvialis) * | Astaxantin | Anti-aging | [32] |
| Ulva australis (as Ulva pertusa) | Proteins (amino acids) | Moisturizers, antioxidants, and natural sunscreens | [29,33] |
| Ulva compressa (as Enteromorpha compressa) | Micronized algae | Body scrubs, face peelings, antiaging and smoothing face creams, firming body lotions | [24,34] |
| U. compressa (as E. compressa) | Extracts | Body lotions, cleansing gels, face masks, fluids, tonics, hair shampoos, day and night face creams, eye creams | [24,25] |
| U. lactuca | Chlorophylls (a, b, c, d) | Antibacterial, antioxidant, coloring, antibacterial, deodorizing, tissue growth stimulating agents | [26,27,28] |
| U. lactuca | Seaweed lipopeptide mixed with clay | Anti-elastase, collagen synthesis stimulation | [35] |
| U. lactuca | Seaweed polysaccharide mixed with clay 1 | anti-aging, antioxidant activity, anti-elastase, collagen synthesis stimulation | [37,38] |
| U. lactuca | Sulfated polysaccharide (ulvan) | Antioxidative, chelating, gelling, moisturizing, and protective agents | [39,40] |
| U. lactuca | Tripeptide: arginine, glycine, aspartic acid | Stimulation of collagen production via TGF-β, elastine, increase in the biosynthesis of collagen I | [41] |
| U. lactuca | Carotenoids (astaxanthin, β-carotene, fucoxanthin, lutein) | Anti-inflammatory, antiaging, antioxidant, tyrosinase inhibitors, antiphotoaging agents, radical scavengers, colorants | [27,29,30] |
| U. lactuca | Fatty acids | Antioxidant, cytoprotective Nrf2-ARE pathway | [42] |
| U. lactuca | Extracts | Exfoliating gel, body mask, bath salts, moisturizing cream (components of the thalassotherapy kit 2) | [43] |
| U. rigida (as U. armoricana) | Sulfated polysaccharide (ulvan) | Antioxidative, chelating, gelling, moisturizing, and protective agents | [39,40] |
| U. rigida | Sulfated polysaccharide (ulvan) | Antioxidative, chelating, gelling, moisturizing, and protective agents | [39,40] |
| U. rotundata | Sulfated polysaccharide (ulvan) | Antioxidative, chelating, gelling, moisturizing, and protective agents | [39,40] |
| Ulva sp. | Lectins | Antiadhesive agents, antibacterial, anti-inflammatory, antiviral | [44] |
| Ochrophyta (Phaeophyceae, brown algae) | |||
| Alaria esculenta | Extract | Skin anti-ageing | [45] |
| Ascophyllum nodosum | Sulfated polysaccharide (fucoidan) | Antioxidant, anticellulite, antiviral, anti-inflammatory, anti-aging, antiphotoaging agents, elastase, tyrosinase inhibitors | [46,47] |
| A. nodosum | Acid hydrolyzed fucoidan | Protective effects of elastin degradation by downregulating elastase activity; in vitro stimulation of dermal fibroblast proliferation; in vivo inhibition of gelatinase A secretion and interleukin-1β in dermal fibroblast cells. | [48] |
| A. nodosum | Phlorotannins: eckols, fucols, fucophlorethols, fuhalols, phlorethols | Tyrosinase and hyaluronidase inhibitors, anti-inflammatory, antioxidants, antiaging, antiphotoaging, antiallergic, chelating agents, UV screens, histamine | [17,19,29,49] |
| A. nodosum | Extract | Skin moisturization and protection | [22,23] |
| A. nodosum | Extract | Anti-free-radical, tyrosinase inhibiting 4 | [51] |
| A. nodosum | Extract | Skin conditioning regenerating and sebum regulating agent 5 | {51] |
| A. nodosum | Micronized algae | Anticellulite and face creams, slimming creams, and serum | [24,52] |
| A. nodosum | Aqueous extracts rich in phytohormone: abscisic acid, auxins, betaines, cytokines, gibberellins | Antiaging, antiwrinkle activities 6 | [132,133] |
| Bifurcaria bifurcata | Extracts | Exfoliating gel, body mask, bath salts, moisturizing cream (components of the thalassotherapy kit 2) | [43] |
| Cladosiphon okamuranus | Extract | Skin moisturization and protection | [22,23] |
| Cystoseira nodicaulis | Phlorotannins: fucophloroethol, bieckol, phlorofucofuroeckol, 7-phloroeckol | Anti-inflammatory, antioxidant, anti-skin aging, anti-wrinkling (hyaluronidase Inhibition), lipid peroxidation inhibition | [53] |
| C. tamariscifolia | Phlorotannins: fucophloroethol, bieckol, phlorofucofuroeckol, 7-phloroeckol | Anti-inflammatory, antioxidant, anti-skin aging, anti-wrinkling (hyaluronidase Inhibition), lipid peroxidation inhibition | [53] |
| C. usneoides | Phlorotannins: fucophloroethol, bieckol, phlorofucofuroeckol, 7-phloroeckol | Anti-inflammatory, antioxidant, anti-skin aging, anti-wrinkling (hyaluronidase Inhibition), lipid peroxidation inhibition | [53] |
| Durvillaea antarctica | Extract | Skin moisturization and protection | [22,23] |
| Durvillea spp. | Alginates | Emulsion stabilizers, chelating agents, colloids, gelling, immunostimulating agents, moisturizing, protective colloids | [20,54] |
| Ecklonia arborea (formerly Eisenia arborea) | Methanol extracts rich in phlorotannins | Potentiality to treat histamine-related inflammatory diseases that include atopic dermatitis (AD) | [55] |
| Ecklonia bicyclis (as Eisenia bicyclis) | Phlorotannins (Phlorofucofuroeckol A, dieckol, eckol, phloroglucinol, 8.8′-bieckol) | Inhibition of hyaluronidase, anti-wrinkling | [55] |
| Ecklonia cava | Proteins (amino acids) | Radical scavengers, antioxidant, chelating agents | [56,57,58] |
| E. cava | Phlorotannins: dieckol and phloroglucinol | Histamine release, anti-allergic | [59] |
| E. cava | Phlorotannins (eckol, dieckol, dioxinodehydroeckol, 7-phloroeckol, phloroglucinol, phloroglucinol) | Tyrosinase inhibition with whitening effect | [60,61,68] |
| E. cava | Phlorotannins (eckol, dieckol, dioxinodehydroeckol, 7-phloroeckol, phloroglucinol, phloroglucinol) | Phlorotannin’s effect on melanogenesis and their protective effect against photo-oxidative stress induced by UV-B radiation | [62] |
| E. cava | Phlorotannins: 6,6′-bieckol and dioxinodehydroeckol | Matrix metalloproteinases (MMPs) inhibition, anti-wrinkling | [50,62] |
| E. cava | Sulfated polysaccharide (fucoidan) | Antiviral, anticellulite, antioxidant, anti-inflammatory, antiphotoaging agents, elastase, inhibitors of tyrosinase, antiaging, | [46,47] |
| E. cava | Phlorotannins: dieckol | Hair growth | [63] |
| E. cava | Phlorotannins: eckols, fucols, fucophlorethols, fuhalols, phlorethols | Antiaging, antiphotoaging, anti-inflammatory, antioxidant, histamine, tyrosinase, antiallergic, chelating agents, antioxidants, UV screens, inhibitors of hyaluronidase | [17,19,29,49] |
| E. cava | Phlorotannins (eckol, eckstolonol, dieckol, triphlorethol A, fucodiphlorethol G, phloroglucinol) | Antioxidant, UV protection | [61,64,65] |
| E. cava | Phlorotannins: dioxinodehy-droeckol | Hair growth | [66] |
| E. cava | Phlorotannins: dieckol | Adipogenesis inhibitory effect | [67] |
| E. cava | Phlorotannins (eckol, 7-phloroeckol, dieckol, dioxinodehydroeckol, phloroglucinol, phloroglucinol) | Tyrosinase inhibition, whitening effect | [68] |
| E. kurome | Phlorotannins: dieckol, eckol, phloroglucinol, phlorofucofuroeckol A and 8.8′-bieckol | Hyaluronidase inhibition, anti-wrinkling | [69] |
| E. stolonifera | Phlorotannins (eckol, phlorofucofuroeckol A, dieckol, eckstolonol, phloroglucinol) | Tyrosinase inhibition, whitening effect | [70] |
| E. stolonifera | Phlorotannins: eckol and dieckol | Matrix metalloproteinases (MMPs) inhibition, anti-wrinkling | [71] |
| E. stolonifera | Phlorotannins: phlorofucofuroeckol A | Anti-inflammatory | [72] |
| Fucus spiralis | Phlorotannins: phlorofucofuroeckol, bieckol, fucophloroethol, 7-phloroeckol | Anti-inflammatory, antioxidant, anti-skin aging, anti-wrinkling (hyaluronidase Inhibition), lipid peroxidation inhibition | [53] |
| F. vesiculosus | Sulfated polysaccharide (fucoidan) | Antiphotoaging agents, antioxidant, antiaging, elastase, anticellulite, antiviral, anti-inflammatory, inhibitors of tyrosinase | [46,47] |
| F. vesiculosus | Sulfated polysaccharide (fucoidan) | Anticoagulant, antioxidant, skin fibroblast stimulation | [73] |
| F. vesiculosus | Phlorotannins: eckols, fucols, fucophlorethols, fuhalols, phlorethols | Antiphotoaging, antiaging, anti-inflammatory, antiallergic, antioxidants, chelating agents, UV screens, inhibitors of histamine, tyrosinase, and hyaluronidase | [17,19,29,49] |
| F. vesiculosus | Fucoidan and alginate | Antioxidative properties, prevent skin aging and cutaneous disorders | [74] |
| F. vesiculosus | Micronized algae | Topical cosmetic compositions for treating or preventing cellulite | [75,76,77] |
| F. vesiculosus | Fucoidan-rich extracts with high polyphenol content | Increased brightness and skin age spot reduction, UV radiation protection and soothing | [79] |
| F. vesiculosus | Extract | Emollient, humectant, masking, oral care, skin conditioning | [81] |
| F. vesiculosus | Micronized algae | Slimming, antiaging, and anticellulite creams, body scrubs | [24,52,80] |
| Halopteris scoparia | Aqueous extracts: phytohormones (e.g., gibberellins, auxins, betaines, cytokines, abscisic acid) | Antiaging and antiwrinkle activities 6 | [130,131] |
| Himanthalia elongata | Fatty acids and volatile compounds | Antioxidant and antimicrobial activity | [81] |
| Ishige foliacea | Phlorotannins: octaphlorethol A | Tyrosinase inhibition, whitening effect | [82] |
| I. okamurae | Phlorotannins: diphlorethohydroxycarmalol | Antioxidant, UV protection | [83] |
| Laminaria digitata | Carbohydrates (69%), minerals (20%), proteins (11%) | Lipolytic 5 | [51,84] |
| L. digitata | Micronized algae | Antiaging, antiacne, slimming and anticellulite creams and lotions, peelings, moisturizing face creams | [24,52,80] |
| L. hyperborea | Extracts | Antiaging, and antiacne creams, face masks, tonics, fluids, moisturizing, tonics | [24,25] |
| L. ochroleuca | Extracts | Antiacne creams, antiaging creams and serums, cleansing gels, day and night face creams, fluids, tonics, hair shampoos and conditioners, sun protection creams | [24,25] |
| Laminaria sp. | Laminarans | Antioxidant, anticellulite and anti-inflammatory agents | [19] |
| Laminaria sp. | Alginates | Gelling colloids, emulsion stabilizers, immunostimulating agents, moisturizing, protective phycocolloids | [20,54] |
| Lessonia sp. | Alginates | Emulsion stabilizers, chelating agents, colloids, gelling, immunostimulating agents, moisturizing, protective colloids | [20,54] |
| Macrocystis sp. | Alginates | Emulsion stabilizers, chelating agents, colloids, gelling, immunostimulating agents, moisturizing, protective colloids | [20,54] |
| Padina boergesenii | Sulfated polysaccharides | Collagen formation and epidermal regeneration | [85] |
| P. pavonica | Methanolic extract | Antifungal and antibacterial, maintaining skin flora in state of equilibrium | [86] |
| P. pavonica | Extract | Keratinocytes differentiation, protein synthesis activation | [87,88] |
| P. tetrastromatica | Sulfated polysaccharides | Collagen formation and epidermal regeneration | [85] |
| Pelvetia canaliculata | Ethanol extract: alginic acid, amino acids, flavonoids, fucoidans, polyols | Antioxidant, collagen synthesis stimulation, proteoglycans synthesis stimulation. Anti-obesity effects | [89,90] |
| Saccharina japonica (as Laminara japonica) | Fucoxanthin | Anti-tyrosinase activity in guinea pig UVB irradiated, and melanogenesis in mice UVB irradiated | [91] |
| S. japonica (as Laminara japonica) | Carotenoids: Astaxanthin, β-carotene, fucoxanthin, lutein | Anticellulite, antioxidant, antiaging, anti-inflammatory, antiviral, antiphotoaging agents, elastase, and inhibitors of tyrosinase | [46,47] |
| S. japonica | Polysaccharides | Skin moisturization and protection | [22,23] |
| S. longicruris | Galactofucan (638 and 1529 kDa) | Synthesis of matrix metalloproteinase and collagen-I, fibroblasts growth rate | [92] |
| S. sculpera (as Kjellmaniella crassifolia) | Fucoidan | Antiaging, antiwrinkle | [93,94] |
| Sargassum fusiforme (as Hijikia fusiformis) | Fucoxanthin | In vivo inducer of the Nrf2-ARE | [95] |
| S. fusiforme (as H. fusiformis) | Phlorotannins: 4-hydroxyphenethyl alcohol | Tyrosinase inhibition, whitening effect | [96] |
| S. macrocarpum | Sargafuran | Anti-acne cosmetics | [97] |
| S. polycystum | Flavonoids, tannins, terpenoids, phenols, saponins | Anti-melanogenesis or skin-whitening effect | [98,99] |
| S. siliquastrum | Water (20 °C) extract | Skin-whitening agent | [100] |
| Scytosiphon lomentaria | Proteins (amino acids) | Radical scavengers, antioxidant, chelating agents | [56,57,58] |
| Silvetia babingtonii (as Pelvetia wrightii) | Polysaccharides | Anticellulite | [101] |
| Turbinaria conoides | Fucoidan and alginate | Antioxidative properties, prevent skin aging and cutaneous disorders | [74] |
| Undaria peterseniana (as Undariopsis peterseniana) | Extract with apo-9′-fucoxanthinone | Treatment with extract ex vivo for 21 days significantly increased the hair-fiber lengths | [102] |
| Undaria pinnatifida | Sulfated polysaccharide (fucoidan) | Antioxidant, anti-inflammatory, antiphotoaging, anticellulite, antiviral, and antiaging compounds, elastase, inhibitors of tyrosinase | [46,47] |
| U. pinnatifida | Extract | Skin moisturization and protection | [22,23] |
| U. pinnatifida | Fucoidan-rich extracts | Skin immunity, soothing and UV protection | [78] |
| Rhodophyta (red algae) | |||
| Acanthophora muscoides | Sulfated polysaccharide (carrageenan) | Anticoagulant, antinociceptive and anti-inflammatory, gelling agents | [103,104,105] |
| A. nayadiformis (as A. delilei) | Proteins (amino acids) | Radical scavengers, antioxidant, chelating agents | [56,57,58] |
| Chondria armata | Galactoglycerolipids | Antimicrobial activity (see also Chapters 7 and 8) | [106,107] |
| Chondrus crispus | Fatty acids (EPA, AA, DHA, GLA, ALA, LA, palmitic acid, oleic acid) | Antiallergic, antiaging, anti-inflammatory, antiaging, antiwrinkle, antimicrobial, antioxidant, emollients, regenerating compounds, used in the treatment of eczema and psoriasis | [108,109] |
| C. crispus | Sulfated polysaccharide (carrageenan) | Gelling agents, protective colloids, thickeners | [109] |
| C. crispus | Polysaccharides | Skin moisturization and protection | [22,23] |
| C. crispus | Extracts | Exfoliating gel, body mask, bath salts, moisturizing cream (components of the thalassotherapy kit 2) | [43] |
| C. crispus | Extracts | Body lotions, fluids, face creams, make-up removers, hair conditioners, and shampoos | [24,25] |
| Corallina officinalis | Sulfated polysaccharides | Antioxidant | [110] |
| C. pilulifera | Phlorotannins: eckols, fucols, fucophlorethols, fuhalols, phlorethols | Antiaging, antiphotoaging, anti-inflammatory, antioxidants, antiallergic, chelating agents, UV screens, inhibitors of the tyrosinase, histamine, and hyaluronidase | [17,19,29,49] |
| Eucheuma serra | Lectins | Antiadhesive agents, antibacterial, anti-inflammatory, antiviral | [44] |
| Furcellaria lumbricalis | Micronlzed algae | Topical cosmetic compositions for treating or preventing cellulite | [75,76,77] |
| Gelidium sp. | Agar | Emulsion stabilizers, gelling agents, thickeners | [112,113] |
| Gracilaria sp. | Agar | Emulsion stabilizers, gelling agents, thickeners | [112,113] |
| Gracilariopsis longissima (as Gracialaria verrucosa) | Crude algal extract | Antioxidant and anti-inflammatory properties. The cosmetic cream produced was also found to be cytotoxicity free. So, this species is recommended for usage in cosmetic industry | [114] |
| Grateloupia elliptica | Extract | Prevention of hair loss | [115] |
| Kappaphycus alvarezii (as Eucheuma cottonii) | Ethanolic and aqueous extracts | Hair growth | [116] |
| Jania rubens | The alga was macerated using liquid N2, with the addition of chilled aqueous methanol (70%, v/v) | Extract is a rich source of essential macro- as well as microminerals, natural antioxidants, and bioactive metabolites with cosmeceutical potential. Promising candidate for anti-ageing, skin whitening, skin conditioning, skin polishing cosmetics | [117] |
| Laurencia pacifica | Laurinterol | Can kill bad bacteria (Staphylococcus aureus), maintaining skin flora in state of balance | [118] |
| Laurencia sp. | Bromophenols | Antioxidant, antimicrobial, antithrombotic agents | [119] |
| Palmaria palmata | MAAs | Anti-UV | [120] |
| P. palmata | Proteins (amino acids) | Moisturizers, antioxidants, natural sunscreens | [29,33] |
| Porphyra umbilicalis | MAAs | Anti-UVA | [121] |
| P. umbilicalis | Fatty acids (EPA, AA, DHA, GLA, ALA, LA, palmitic acid, oleic acid) | Antiallergic, antiaging, antiaging, anti-inflammatory, antiwrinkle, antioxidant, antimicrobial, emollients, regenerating agents, used in the treatment of eczema and psoriasis | [19,108] |
| P. umbilicalis | Proteins (amino acids) | Moisturizers, antioxidants, and natural sunscreens | [29,33] |
| P. umbilicalis | Extract | Skin conditioning | [79] |
| P. umbilicalis | Extract | Sunscreen formulation with red algae extract; photoprotective formulation with anti-aging properties | [122] |
| Portieria spp. | Phycobiliproteins: Allophycocyanin, phycoerythrin, phycocyanin | Antioxidant, anti-inflammatory, colorants, radical scavenging agents | [19,29] |
| Pyropia dentata (as Porphyra dentata) | Phytosterols: brassicasterol, ergosterol, fucosterol, ergosterol, 3-sitosterol | Antiallergic, anti-inflammatory agents, antioxidants, radical scavengers | [19,29,123] |
| Pyropia haitanensis (as Porphyra haitanesis) | Porphyran, shinorine | Anti-aging | [124] |
| Pyropia haitanensis (as Porphyra haitanensis) | Sulfated galactans | In vivo antioxidant activity | [123] |
| Pyropia tenera (as Porphyra tenera) | Source of anti-inflammatory and antioxidants compounds | Ingredients for cosmetic uses | [126,127] |
| Pyropia yezoensis (as Porphyra yezoensis) | Porphyran | Antioxidant and anti-inflammatory | [128] |
| Rhodella spp.* | Phycobiliproteins: Allophycocyanin, phycoerythrin, phycocyanin | Antioxidant, anti-inflammatory, colorants, radical scavenging agents | [19,29] |
| Rhodomela confervoides | Methanolic extracts | Antifungal and antibacterial, maintaining skin flora in state of stability | [86] |
| Vertebrata lanosa (as Polysiphonia lanosa) | Extract | Skin moisturization and protection | [22,23] |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, L. Seaweeds as Source of Bioactive Substances and Skin Care Therapy—Cosmeceuticals, Algotheraphy, and Thalassotherapy. Cosmetics 2018, 5, 68. https://doi.org/10.3390/cosmetics5040068
Pereira L. Seaweeds as Source of Bioactive Substances and Skin Care Therapy—Cosmeceuticals, Algotheraphy, and Thalassotherapy. Cosmetics. 2018; 5(4):68. https://doi.org/10.3390/cosmetics5040068
Chicago/Turabian StylePereira, Leonel. 2018. "Seaweeds as Source of Bioactive Substances and Skin Care Therapy—Cosmeceuticals, Algotheraphy, and Thalassotherapy" Cosmetics 5, no. 4: 68. https://doi.org/10.3390/cosmetics5040068
APA StylePereira, L. (2018). Seaweeds as Source of Bioactive Substances and Skin Care Therapy—Cosmeceuticals, Algotheraphy, and Thalassotherapy. Cosmetics, 5(4), 68. https://doi.org/10.3390/cosmetics5040068





