Abstract
Biota orientalis L. leaf extract (BOLE) is used medically to improve strength and arrest hemorrhage. In China, BOLE has been used in traditional medicine for its antibacterial properties and for hair restoration. In this study, we investigated the mechanism of hair restoration by BOLE from the point of view of the sebum suppressant effect and hair loss prevention. BOLE at 25 or 50 μg/mL final concentrations, a hair growth plant ethanol extract (HGPEE), and a hair growth plant water extract (HGPWE) (the latter two each containing BOLE and other plant compounds), were used to study: (1) the sebum suppressant effect in sebocytes from normal golden hamster ear pinna origin; (2) the effect on the growth of human fetal epidermal keratinocytes; and (3) the effect on gene expression related to hair growth stimulation, with (2) and (3) studied in human fetal epidermal keratinocytes and hair papilla cells. BOLE had a sebum depletion effect in cultured sebocytes; moreover, the amounts of mRNA of the hair growth factors, KGF, VEGF, and G3PDH analyzed by real-time polymerase chain reaction in human hair papilla cells were increased by HGPEE. The amount of mRNA of Wnt10b in cultured epidermal keratinocytes was increased by the addition of BOLE, and the growth of the cultured epidermal keratinocytes was promoted by HGPEE in a two-layer culture system of hair papilla cells and epidermal keratinocytes. HGPEE had a hair growth promotion/hair restoration effect and a sebum suppression effect. Hair restorers containing HGPEE may be useful for stimulating hair growth and suppressing excess scalp sebum in males and females.
1. Introduction
Sometimes the skin excretes oil from pores in excessive amounts. The scalp has a higher quantity of sebum than the face, with excessive oil also excreted from pores. Excessive lipid content on the top of the head (more than on the temporal and occipital regions) contributes to hair loss. The scalp’s sebum, containing triglycerides, fatty acids, squalene and wax esters, are produced by the sebaceous gland [1]. Other lipids on the scalp’s surface, such as cholesterol esters and cholesterol, mainly originate from epidermal cells [1]. There are more wax esters and fatty acids in the scalp than those on facial skin. Triglycerides are broken down by lipase, which results in a fatty acid. Unsaturated fatty acids are easily oxidized in the air or by sun exposure and are converted to peroxides after a few hours, which causes hair loss and thin hair. Therefore, to maintain healthy hair, it is necessary to avoid excessive scalp lipids. Isolation cultures of human sebaceous gland cells (sebocytes) are difficult, so has been performed using hamster sebocytes in biosynthetic research for the study of lipid regulation in sebaceous glands [2]. As hamster sebocytes accumulate lipid droplets through cell differentiation, these cells have been widely used for lipid metabolism research, as well as a cell model system of lipid metabolism using normal cells. Moreover, it is known that the lipids of hamster sebocytes increase with the addition of male hormones (dihydrotestosterone), and the cell characteristics are similar to those of human sebocytes [3]. Therefore, hamster sebocytes have been considered to be useful healthy cells alternatives to human sebocytes in sebaceous gland research.
Hair is an appendage of the skin that is peculiar to mammals. The hair follicle is formed by the interaction between the epidermis and dermis, and hair grows and falls out repeatedly in the hair cycle. In recent years, it has been demonstrated that wingless-type MMTV integration sites (Wnt) are important for hair growth [4]. Wnt10b, which develops in a matrix in the early stage of secondary hair germ, induces formation of secondary hair germ and expression of Sonic hedgehog (Shh); Shh then induces Wnt5a in hair papilla cells [5]. It has also been reported that mature hair follicles are not formed as the expression of Wnt5a in hair papilla cells was not observed in Shh knockout mice [6]. Wnt10b is an important signal protein between cells in the generation of hair follicles and promotes the cell growth of hair follicles, as shown by a study where Wnt10b promoted hair shaft growth in the organ cultures of mice mustache hair follicles [7]. The number of formed hair follicles was shown to decrease to one-third of those observed in lymphoid enhancer-binding factor 1 (Lef 1) (the transcription factor of the Wnt pathway) knockout mice [8]. Moreover, it has been reported that hair in the anagen phase also required hair growth factors such as vascular endothelial growth factor (VEGF) and keratinocyte growth factor (KGF) produced by papilla cells. VEGF has decreased expression in hair tissue in male pattern baldness (androgenetic alopecia). VEGF increases the papilla cells in an autocrine manner [9] and the amount of VEGF expression decreases from the anagen phase to the catagen phase [10,11]. When KGF is injected into nude mice, hair growth is promoted, and the hair follicles develop KGF receptors [12,13].
Biota orientalis (B. orientalis) is an evergreen needle-leaf tree of the Platycladus orientalis genus of the cypress family that originated in China. In Japan, P. orientalis can be planted broadly from southern Hokkaido to Kyushu. The leaf is used medicinally for strength nourishment and hemorrhage arrest [14]. In Japan, a folk medicine extract made by dipping the leaf of dried B. orientalis in white distilled liquor has been used to prevent hair loss and promote hair restoration instead of modern hair tonics. Moreover, in China, B. orientalis has been used for its antibacterial properties and for hair restoration as a tradition medical treatment [14]. It has been reported that a hot water extract of the leaf of Thuja orientalis had a hair restoration effect in mice [15]. Furthermore, the seed of Thuja occidentalis, which is related to B. orientalis, has an inhibitory action of 5α-reductase, and the active ingredients have been reported as flavonoid and diterpene [16].
In this study, the mechanism of hair restoration caused by an ethanol extract of B. orientalis (BOLE) was studied by assessing its sebum-suppressant and hair loss-preventative effects. The results demonstrated that BOLE inhibited lipid production in cultured sebocytes. It was also proven that an ethanol extract of a plant extract mixture that contained B. orientalis leaves inhibited lipid production in cultured sebocytes, increasing the amounts of mRNA in VEGF and KGF, which are hair growth factors in hair papilla cells. In addition, an increase in the amount of mRNA of Wnt10b in cultured epidermal keratinocytes was observed. Furthermore, the growth of epidermal keratinocytes was promoted by a two-layer culture system of hair papilla cells and epidermal keratinocytes.
2. Materials and Methods
2.1. Materials
The following plant extracts were obtained from Yunnan Baiyao Industry Co. Ltd. (Yunnan, China): ethanol extract of B. orientalis L. leaf (Cupressaceae) (BOLE); hair growth plant mixture ethanol extract (HGPEE) consisting of B. orientalis L. (Cupressaceae) leaves, Eclipta thermalis (Compositae), Sophora angustifolia (Leguminosae) root, Cnidium monnieri (Umbelliferae) fruit, Ligusticum chuanxiong (Apiaceae) rhizome, and Panax notoginseng Burk. (Araliaceae); hair growth plant mixture water extract (HGPWE) consisting of B. orientalis L. (Cupressaceae) leaves, E. thermalis (Compositae) grass, S. angustifolia (Leguminosae), Cnidium monnieri (Umbelliferae) fruit, L. chuanxiong (Apiaceae) rhizome, and P. notoginseng Burk. (Araliaceae). Minoxidil was purchased from Sigma-Aldrich Corp. (St. Louis, MO, USA).
2.2. Preparation of Samples
BOLE, HGPEE, and HGPWE were dissolved in dimethyl sulfoxide (DMSO):phosphate-buffered saline (PBS) (1:1) and each diluted to concentrations of 10 mg/mL. These samples were prepared as undiluted solution and as 2-fold, 4-fold, and 8-fold dilutions in DMSO:PBS (1:1) to give 10,000, 5000, 2500, and 1250 μg/mL concentrations, respectively. Minoxidil was prepared at concentrations of 2000, 1000, and 500 μg/mL. DMSO:PBS (1:1) was used as the control (solvent).
2.3. Measurement of Lipids in Sebocytes
Sebocytes obtained from normal golden hamster ear pinna were seeded into a 24-well plate (AGC Techno Glass Co., Ltd., Shizuoka, Japan) at a density of 5.0 x 104 cells/well. The sebocytes were cultured for several days in Dulbecco’s Modified Eagle’s Medium (DMEM):Ham's F12 (1:1) growth culture medium that contained 8% bovine serum, 2% human serum, and 8% 10 ng/mL epidermal growth factor (EGF). Next, the medium was exchanged for a differentiation DMEM:Ham's F12 (1:1) culture medium that contained 8% bovine serum, 2% human serum, and 10 μg/mL insulin (in addition to the BOLE, HGPEE, and HGPWE examination samples), and culturing was continued for 1 week. BOLE was examined at 25 μg/mL and 50 μg/mL final concentrations, and HGPEE and HGPWE were examined at 50 μg/mL final concentrations. In addition, minoxidil was examined at 20 μg/mL final concentration as a positive control. Then, 50 μL of Cell Counting Kit-8 (Dojindo Laboratories, Kumamoto, Japan) was added to each well and incubated at 37 °C for 2 h. The supernatant was measured at a wavelength of 450 nm using a microplate reader (Multi-Detection Microplate POWERSAN HT; BioTek Instruments Inc., Winooski, VT, USA) and the number of viable cells was measured. Furthermore, the cell was fixed at room temperature for 10 min with 4% paraformaldehyde solution after washing each well with PBS. Each well was washed with PBS, then replaced with isopropanol 60% after washing for 1 min, the isopropanol was discarded, 300 μL of filtered 0.3% oil red O stain solution (60% isopropanol) was added, and it was dyed at room temperature for 30 min. Washing with 60% isopropanol was performed once, followed by washing with PBS twice, and observation in PBS under a microscope. The PBS was discarded, and the lipids that were previously dyed were extracted by adding of 400 μL of 100% isopropanol. The lipids were then measured at a wavelength of 520 nm 30 min later, and the amount of lipids was measured.
The following formula was used to calculate the amount of lipids per cell.
where absorbance A (450 nm) reflected the number of cells and absorbance B (520 nm) reflects the amount of lipids.
Amount of lipids per cell = absorbance B/absorbance A
2.4. Effect on Growth of Human Fetal Epidermal Keratinocytes in a Two-Layer Culture System of Human Hair Papilla Cells and Human Epidermal Keratinocytes
Human fetal epidermal keratinocytes were cultured in human epidermal keratinocyte growth medium. The cells were prepared at 60,000 cells/well, and 500 μL/well was seeded into a 24-well plate (Falcon; Thermo Fisher Scientific Inc., Waltham, MA, USA) and cultured for 1 day in the epidermal keratinocyte basic culture medium. Human hair papilla cells (Toyobo Co., Ltd., Osaka, Japan) were seeded into 75 cm2 flasks (3,000,000 cells/flask) and cultured for 1 day in a 10-mL human papilla cell growth culture medium. Next, a 100,000 cells/well, and 100 μL/well were placed in the cell culture insert for a 24-well plate (Falcon) and cultured for 1 day in the human papilla cell basic culture medium. The cells were cultured in human papilla cell basic culture medium containing the control, BOLE (at final concentrations of 12.5, 25, and 50 μg/mL), HGPEE (at final concentrations of 12.5, 25, and 50 μg/mL), HGPWE (at final concentrations of 12.5, 25, and 50 μg/mL), and minoxidil (at final concentrations of 5, 10, and 20 μg/mL) in the cell culture insert for 5 days. The cell culture insert was removed, a 50 μL/well was added to a Cell Counting Kit-8 (Dojindo Laboratories) to the lower layer, culturing proceeded for 2 h, and the number of the human epidermal keratinocytes was measured using a microplate reader (450 nm). Four replicate experiments were performed.
2.5. Influence on Gene Expression Related to Hair Growth/Restoration
Human fetal epidermal keratinocytes (Toyobo) were seeded into a 35-mm dish (300,000 cells/dish) and cultured for 1 day in 1 mL of human epidermal keratinocyte growth medium. An RNA extraction kit (RNeasy Protect Mini Kit; QIAGEN K.K., Tokyo, Japan) was used after culture in the basic culture medium of the control, HGPEE (final concentration of 50 μg/mL), HGPWE (final concentration of 50 μg/mL), and minoxidil (final concentration of 20 μg/mL) for 3 days. The amounts of mRNA of Wnt10b, Lef1, Shh, and G3PDH were analyzed using real-time polymerase chain reaction (RT-PCR) (ABI PRISM 7900HT apparatus) and a TaKaRa One Step SYBR PrimeScript RT-PCR Kit II (Takara Bio Inc., Shiga, Japan). Primers of Wnt10b, Lef1, Shh, and G3PDH were purchased from QIAGEN.
Human hair papilla cells (Toyobo) were seeded into a 35-mm dish (300,000 cells/dish) and cultured for 1 day in 1 mL of human papilla cell growth culture medium. After culture in the basic culture medium of the control, HGPEE (final concentrations of 12.5 and 25 μg/mL), HGPWE (final concentration of 50 μg/mL), and minoxidil (final concentration of 20 μg/mL) for 3 days, an RNA extraction kit (RNeasy Protect Mini Kit, QIAGEN K.K., Tokyo, Japan) was used to perform RT-PCR. The amounts of mRNA of KGF, VEGF, and G3PDH were analyzed using RT-PCR (ABI PRISM 7900HT apparatus) and a TaKaRa One Step SYBR PrimeScript RT-PCR Kit II (Takara Bio Inc.). Primers of KGF, VEGF, and G3PDH were purchased from Qiagen.
2.6. Statistical Analysis
Tests for statistical significance between unpaired groups with normal distribution were performed by using Student’s t-test in the case of homogeneity of variance and with Welch’s t-test in the case of unequal variance, and p < 0.05 indicated statistical significance.
3. Results
3.1. Effects of Biota Orientalis L. Leaf Extract (BOLE), Hair Growth Plant Ethanol Extract (HGPEE), Hair Growth Plant Ethanol Extract (HGPEE), and Minoxidil on the Proliferation of Sebocytes and Amount of Lipid Droplets
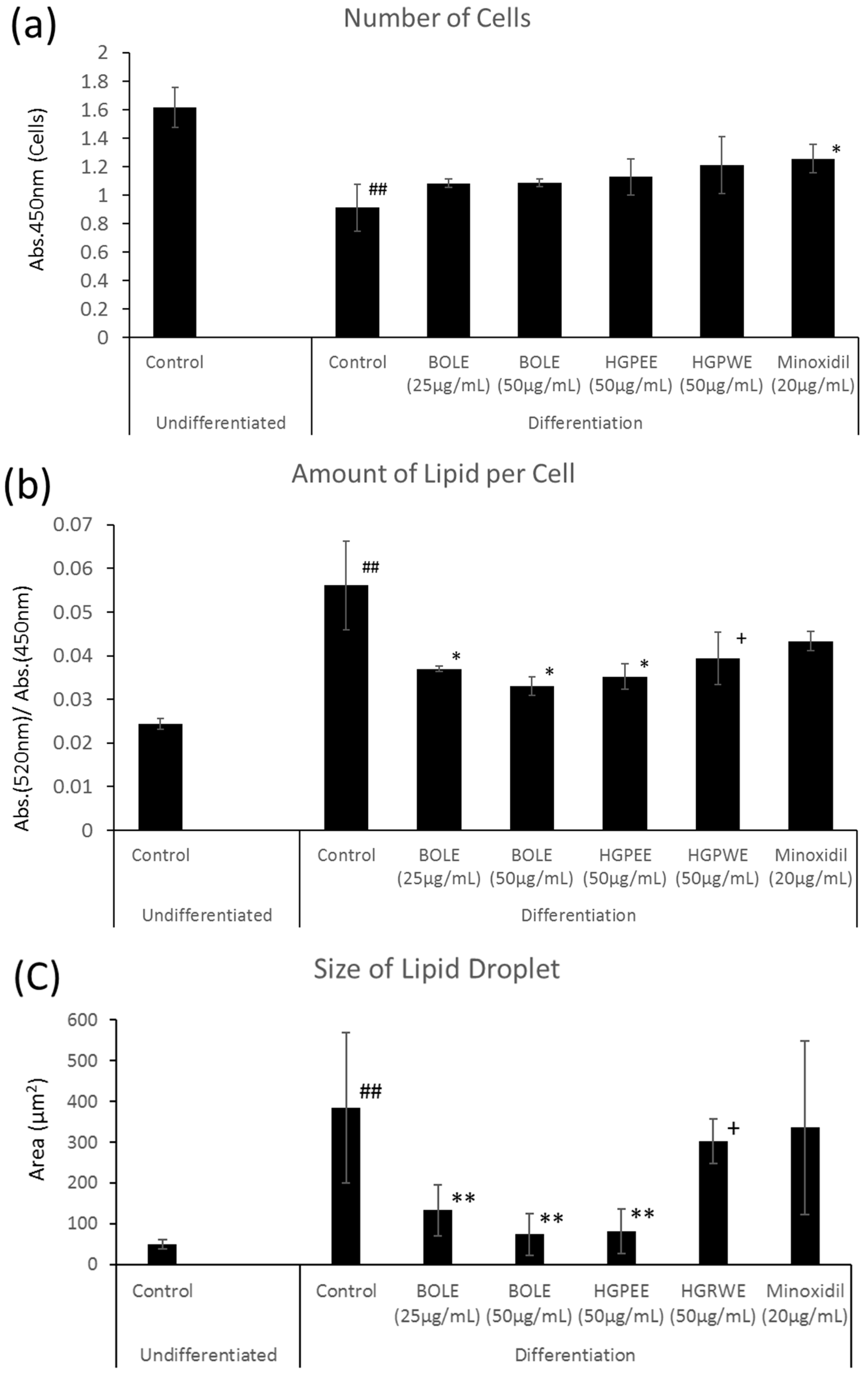
We investigated the mechanism of hair restoration by BOLE on the basis of the sebum suppressant effect. Cell proliferation in the differentiation culture medium was significantly inhibited to three-fifths that of the non-differentiation culture medium (Figure 1). The amount of lipids per cell increased significantly by 2.3-fold and the intracellular lipid droplets also increased significantly by 7.4-fold (Figure 1). There were no significant differences in cell proliferation among the control and BOLE (25, 50 μg/mL), HGPEE (50 μg/mL), and HGPWE (50 μg/mL) in the differentiation culture medium; however, cell proliferation was promoted by minoxidil (20 μg/mL) relative to that of the control (Figure 1).
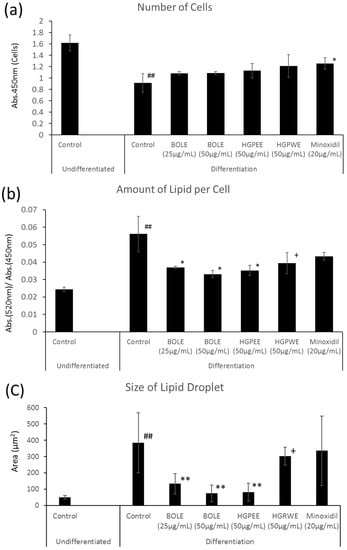
Figure 1.
Effects of Biota orientalis L. leaf extract (BOLE), hair growth plant ethanol extract (HGPEE), hair growth plant ethanol extract (HGPEE), and minoxidil on the number of hamster sebocytes (a); amount of lipids per cell (b); and size of lipid droplets (c). n = 3, mean ± S.D. ## p < 0.01 vs. Control (Undifferentiated); * p < 0.05 vs. Control (Differentiation); ** p < 0.01 vs. Control (Differentiation); + p < 0.1 vs. Control (Differentiation).
The size of the intracellular lipid droplet became significantly small after treatment with BOLE (25, 50 μg/mL) and HGPEE (50 μg/mL) relative to the size after treatment with the control, and the size tended to decrease after treatment with HGPWE (50 μg/mL) in the differentiated culture medium. No significant difference in size was seen between minoxidil (20 μg/mL) and the control in the differentiated culture medium (Figure 1).
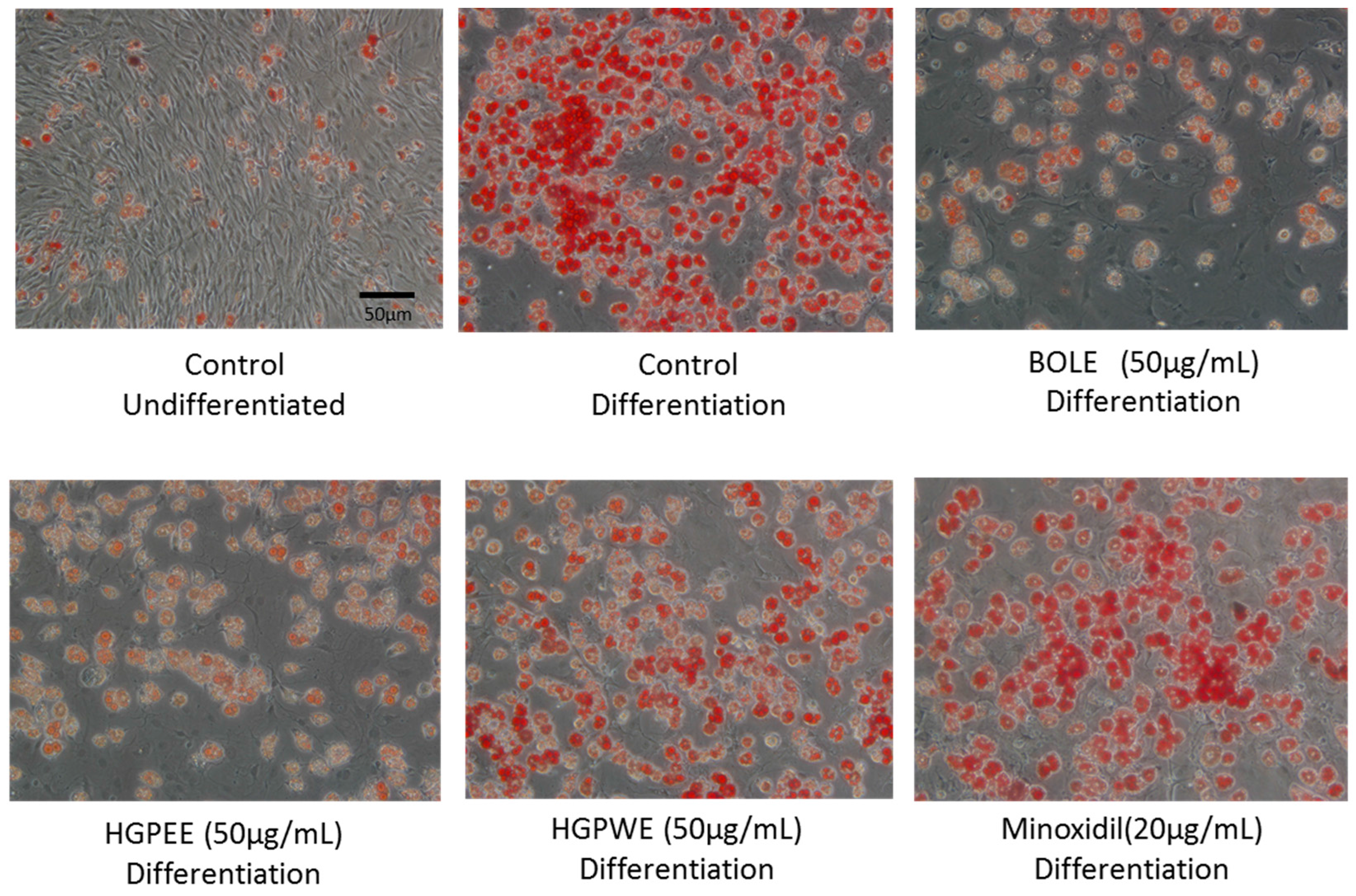
The shape of the sebocytes dyed with oil red O observed under microscope is shown in Figure 2. The cells were undifferentiated sebocytes, differentiated sebocytes, differentiated sebocytes + BOLE (50 μg/mL), differentiated sebocytes + HGPEE (50 μg/mL), differentiated sebocytes + HGPWE (50 μg/mL), and differentiated sebocytes + minoxidil (20 μg/mL).
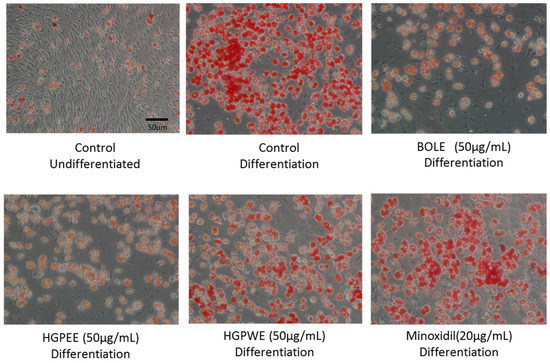
Figure 2.
The shape of microscopic features of sebocytes dyed in oil red O.
3.2. Effects of BOLE, HGPEE, HGPWE, and Minoxidil on the Growth of Human Fetal Epidermal Keratinocytes in a Two-Layer Culture System of Human Hair Papilla Cells and Human Epidermal Keratinocytes
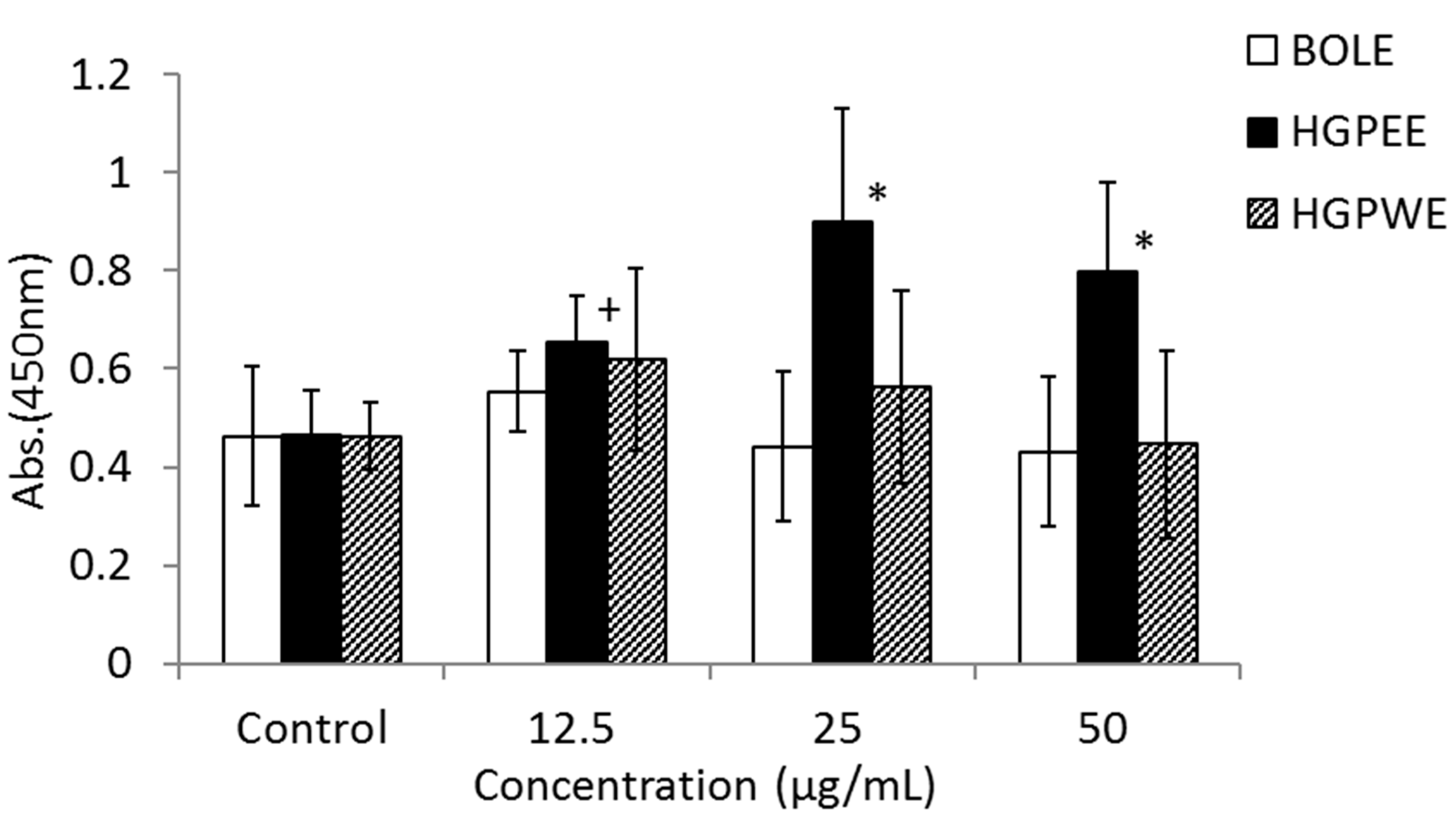
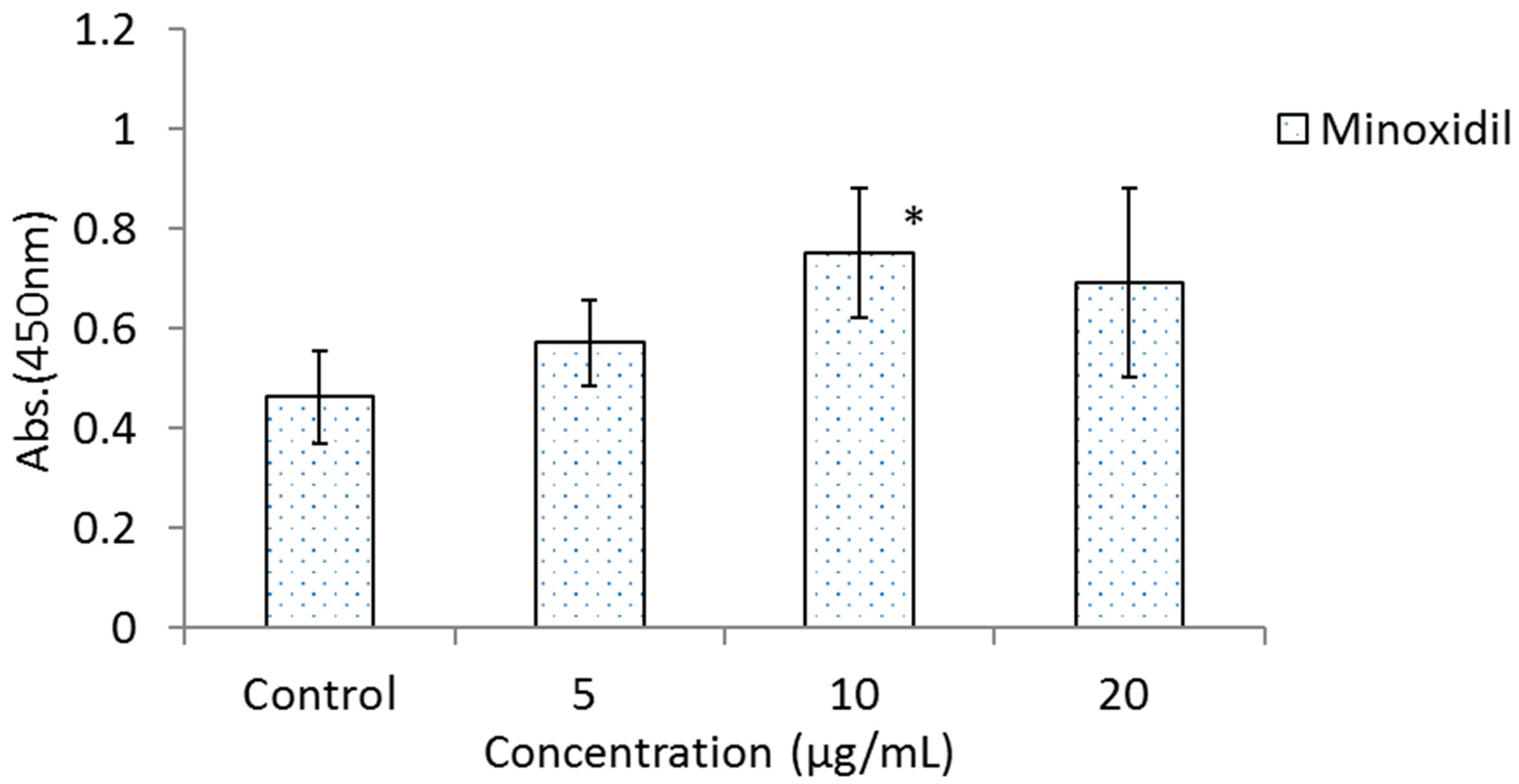
Figure 3 shows the results of the effects of BOLE, HGPEE, HGPWE, and minoxidil on the growth of human epidermal keratinocytes in a two-layer culture system of human hair papilla cells and human fetal epidermal keratinocytes. After 5 days of culture, the number of cells significantly increased after treatment with HGPEE at 12.5, 25, and 50 μg/mL relative to that after treatment with control. Minoxidil also significantly increased the number of human fetal epidermal keratinocytes relative to that of control at 10 μg/mL, but not at 5 or 20 μg/mL. No significant difference was observed in the number of human fetal epidermal keratinocytes between BOLE and the control at 12.5, 25, and 50 μg/mL. Additionally, no significant difference in the number of cells was seen after treatment with HGPWE at 12.5, 25, and 50 μg/mL.
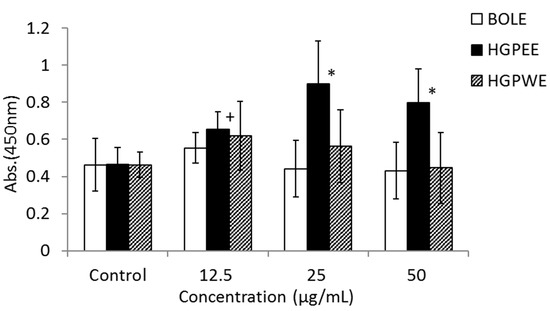
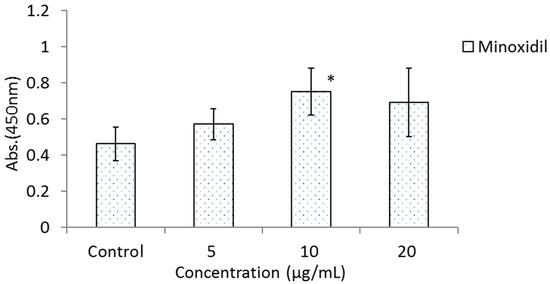
Figure 3.
Effects of BOLE, HGPEE, HGPWE and minoxidil on the number of human fetal epidermal keratinocytes in a two-layer culture of human hair papilla cells and human epidermal keratinocytes. n = 4, mean ± S.D. * p < 0.05 vs. Control, + p < 0.1 vs. Control.
3.3. Effects of HGPEE, HGPWE, and Minoxidil on Expression of mRNA in Human Fetal Epidermal Keratinocytes Related to Hair Growth
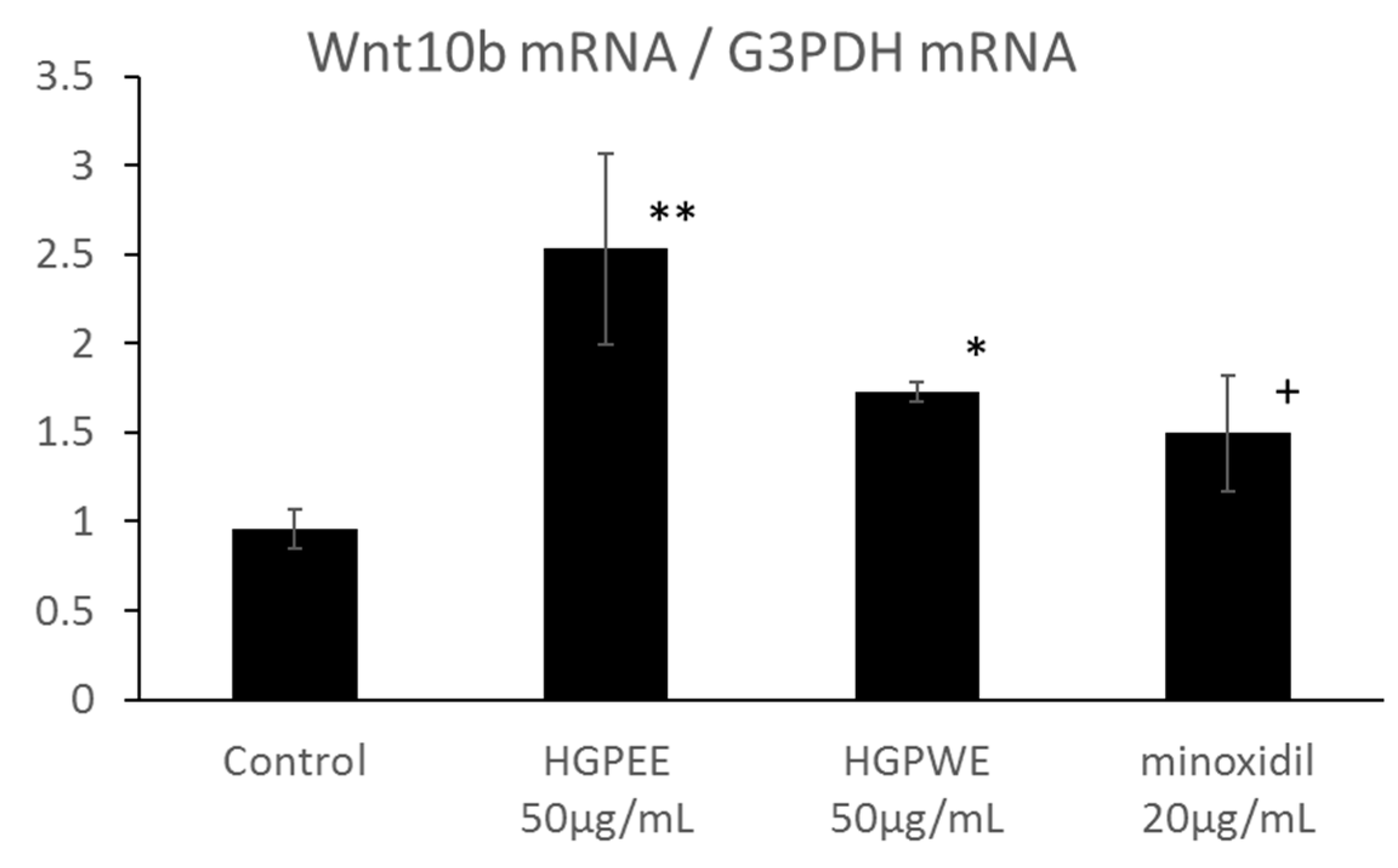
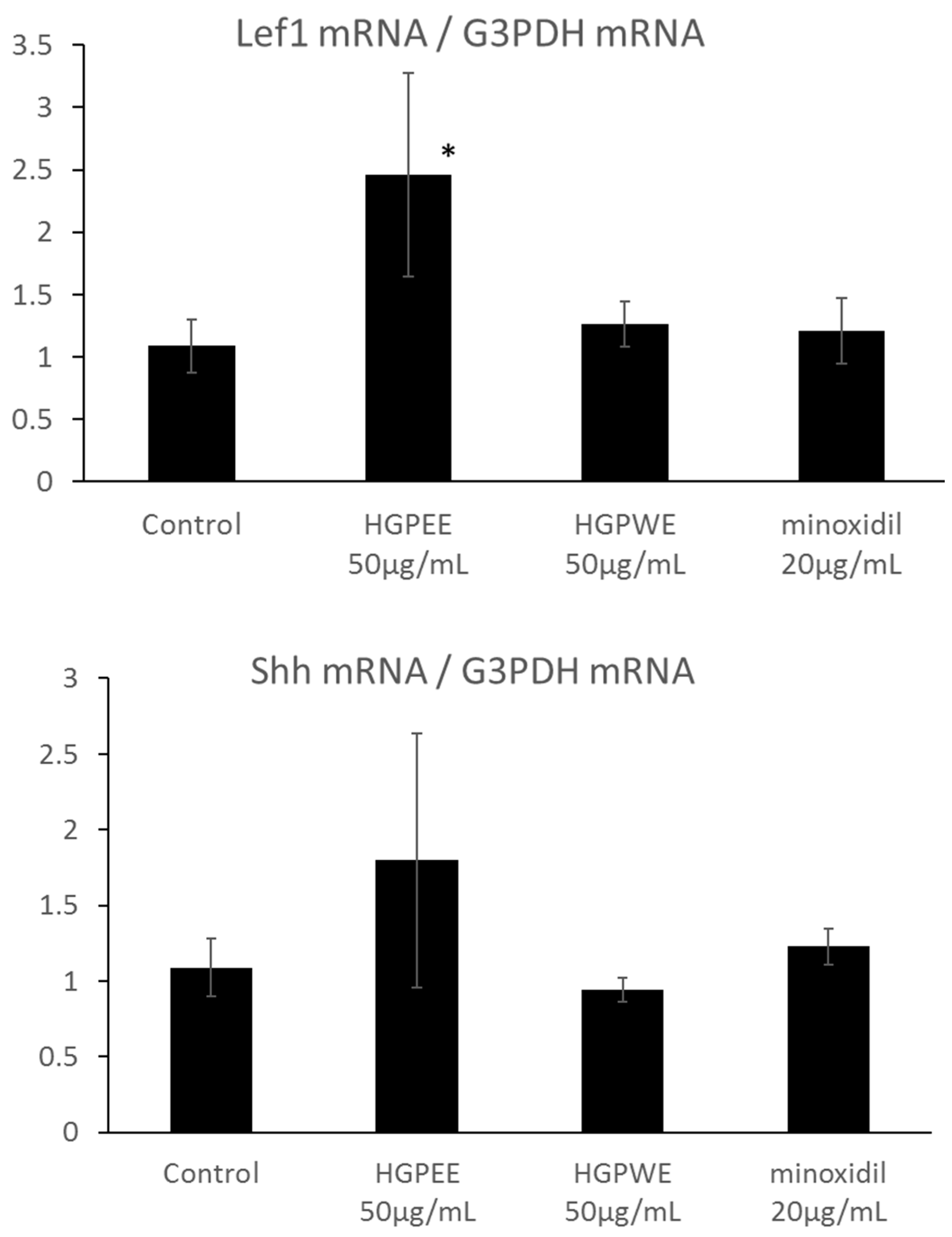
The results of investigating the effect of each sample on the mRNA expressions of Wnt10b, Shh, and Lef1 among hair growth-related factors in the cultured human epidermal keratinocytes are shown in Figure 4. The mRNA expression of Wnt10b increased 2.6-fold, 1.8-fold, and 1.6-fold by HGPEE (50 μg/mL), HGPWE (50 μg/mL), and minoxidil (20 μg/mL), respectively, and the differences were significant between HGPEE (50 μg/mL) or HGPWE (50 μg/mL) and the control. A tendency to increase Wnt10b mRNA expression was observed upon treatment with minoxidil. Wnt10b is expressed by epidermal keratinocytes, and it induces translocation of β-catenin to the nucleus to form a complex with Lef-1 that induces transcription of downstream target genes. Lef1 is a transcription factor that works downstream in the Wnt signaling pathway, and participates in hair follicle regeneration. Lef1 increased 2.3 times by HGPEE (50 μg/mL), which was significantly different from that of the control. No significant difference in Lef1 was seen by HGPWE (50 μg/mL) or minoxidil (20 μg/mL) and the control. Moreover, although expression of Shh increased 1.6 times by HGPEE (50 μg/mL), a significant difference was not observed as the standard deviation was very high. No significant difference in Shh between HGPWE (50 μg/mL) or minoxidil (20 μg/mL) and the control was observed.
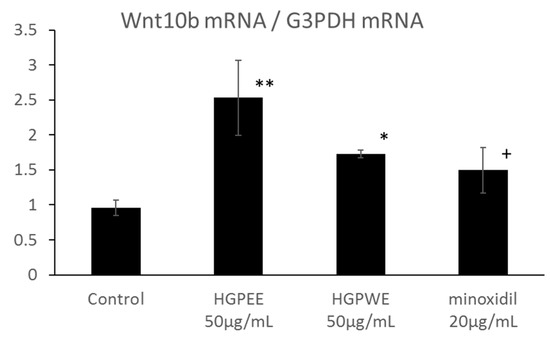
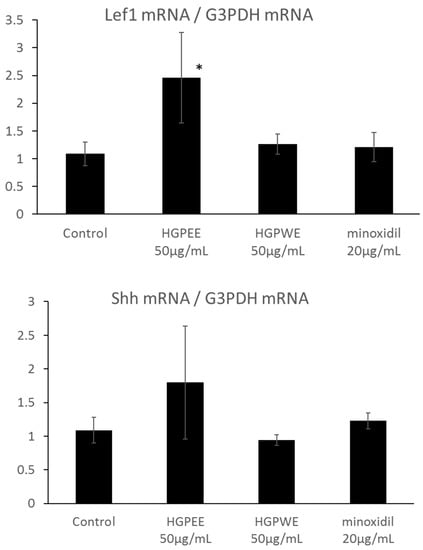
Figure 4.
Effect of HGPEE, HGPWE, and minoxidil on the mRNA expressions of Wnt10b, Lef1, and Shh in cultured human fetal epidermal keratinocytes. n = 3, mean ± S.D., * p < 0.05 vs. Control, ** p < 0.01 vs. Control, + p < 0.1 vs. Control.
3.4. Effects of HGPEE, HGPWE, and Minoxidil on the mRNA Expressions of Hair Growth-Related Factors in Human Hair Papilla Cells
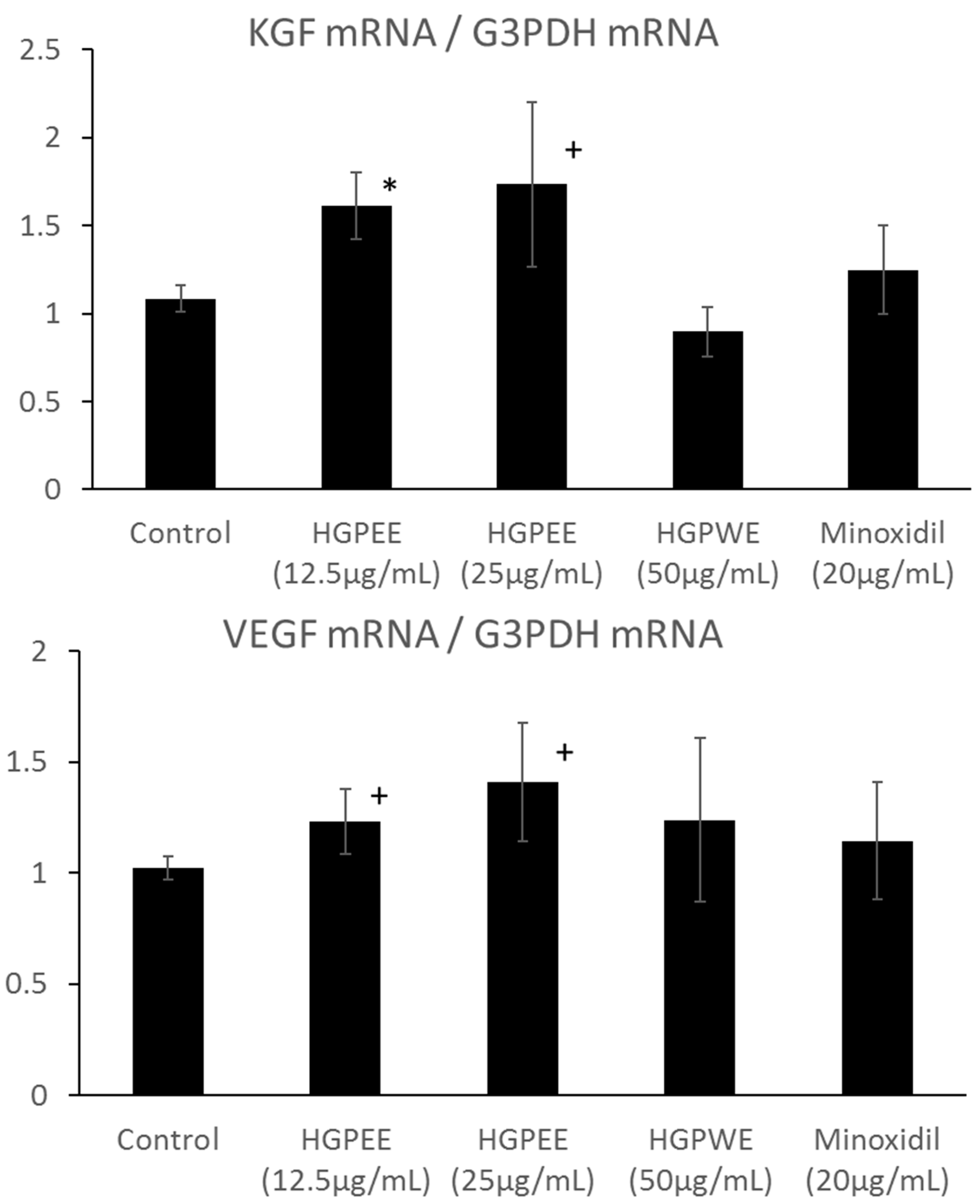
Hair papilla cells play an important role in hair follicle formation. Therefore, we investigated the effects of HGPEE, HGPWE, and minoxidil on the mRNA expressions of hair growth-related factors such as KGF and VEGF in human hair papilla cells. Figure 5 shows the results of investigating the effect of each sample on the mRNA expressions of KGF and VEGF among hair growth-related factors in cultured human papilla cells. The amount of mRNA of KGF was increased by HGPEE (12.5, 25 μg/mL) in a concentration-dependent manner, and a significant difference in the amounts was observed between HGPEE (12.5 μg/mL) and the control. The amount of KGF mRNA was not affected by HGPWE (50 μg/mL) or minoxidil (20 μg/mL). The amount of VEGF mRNA tended to increase in a concentration-dependent manner with treatment by HGPEE (12.5, 25 μg/mL). The amount of VEGF mRNA was not influenced by HGPWE (50 μg/mL) or minoxidil (20 μg/mL).
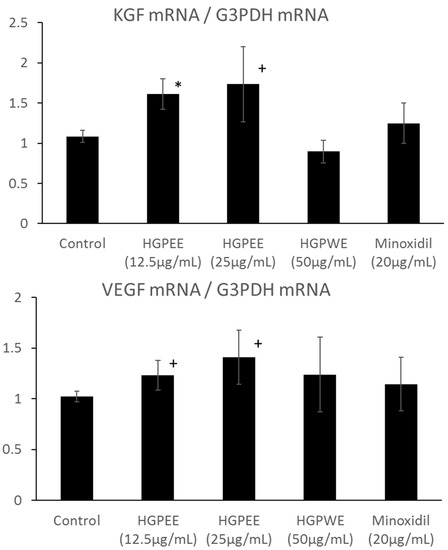
Figure 5.
Effect of HGPEE, HGPWE, and minoxidil on the mRNA expressions of KGF, and VEGF in cultured human hair papilla cells. n = 3, mean ± S.D., * p < 0.05 vs. Control, + p < 0.1 vs. Control.
4. Discussion
The scalp has more sebaceous glands than other parts of the skin, and these glands exhibit a high amount of holocrine secretion of sebum. When a sebaceous gland becomes enlarged and sebum high, hair loss can result in seborrheic alopecia. To close pores, scalp sebum is secreted from sebaceous glands more than is necessary, and inflammation occurs around the hair roots. This can lead to hair loss and seborrheic alopecia. Excessive sebum secretion and indigestion are known causes of hair loss. As BOLE and HGPEE decreased the amount of sebum per cell relative to that by the control, it is thought that they could be useful for preventing hair loss caused by excessive scalp sebum secretion in males and females. HGPEE, especially, strongly decreased the size of lipid droplets, it is necessary to identify the active ingredient in BOLE and HGPEE that prevents depilation caused by an excess of scalp sebum. Sebum mainly consists of a triglyceride (fatty acid ester of glycerin) with lesser amounts of squalene and waxes. When sebum clogs skin pores, fat caps form over the pores, and a true fungus (Malassezia) multiplies by using triglyceride as nutrition and causes inflammation. BOLE can prevent adverse effects caused by excessive sebum in the scalp as it has been reported to have an antibacterial effect [17,18,19,20] and an anti-inflammatory effect of 15-methoxypinusolidic acid is contained in BOLE [21].
Dihydrotestosterone, a male hormone, stimulates a high production of sebum in the sebaceous gland and is secreted in hair pores. As male pattern baldness is a disease caused by excessive sensitivity to dihydrotestosterone, it causes symptoms in young and middle-aged persons similar to sebum-related symptoms of the scalp. In baldness that develops in young people, the period of anagen (hair growth phase) is shortened by male hormones so only thin and very short hair increases instead of thick and long hair. Excessive sebum is considered to be one of the causes of premature balding. The seed of T. occidentalis, which is closely related to B. orientalis L., causes 5α-reductase inhibition [16] via actions of the active ingredients, flavonoid and diterpene, which have been identified in BOLE [22,23,24]. BOLE causes 5α-reductase inhibition and is expected to cause strong inhibition of dihydrotestosterone from testosterone in hair papilla cells and prevent excessive sebum.
On the other hand, hair grows thickly for a long time as the hair follicle cells in the hair root repeat division and push up cells one after another. Hair growth undergoes a cycle (anagen phase→catagen phase→telogen phase), and it is thought that a hair papilla cell present at the root of the hair controls this cycle.
The mechanism of hair growth has not been elucidated in detail, but it is thought that activation of hair papilla cells or hair follicle cells is important. However, if Wnt10b is activated, smaller hair follicles increase in size, and if KGF is promoted, the growth phase of hair will be extended. These two effects should promote hair growth/restoration.
When the organ culture of mice mustache hair follicles was performed, Wnt10b promoted hair shaft growth [7]. Although other Wnt families (Wnt3a, Wnt5a, Wnt11) (except for Wnt10b) have not caused significant extension, Wnt10b is thought to promote the cell growth of hair follicles [7]. Moreover, immunohistochemical analysis of frozen sections of hair follicles has shown that proliferation of lower hair follicle cells was indirectly promoted remarkably by Wnt10b and that this effect involved the β-catenin pathway [25]. Specifically, it was suggested that Wnt10b functions as a specific growth and differentiation-enhancing factor in hair follicles. Thus, the Wnt/β-catenin pathway is closely involved in the formation of hair follicles. The Shh molecule signal has an important role in hair follicle formation; however, how the process is controlled remains unknown. A study has shown that hair did not grow following incomplete development of papilla cells in the skin of Shh mutant mice, which indicated that Shh may control the proliferation of hair follicles [6]. Although investigation of epimorphin, an agonist of Shh, as a hair growth agent has been shown to induce hair follicle formation and promote the growth phase, development was discontinued due to safety concerns [26]. Furthermore, it has been reported that continuous β-catenin signaling may cause hair follicle tumors [27]. HGPEE is a plant mixture ethanol extract that has been used for years, and because it does not activate Shh directly, it is thought that there are no safety problems.
In addition, it is thought that minoxidil suppresses apoptosis of hair follicle cells by improving activation of SUR (sulfonylurea receptor) and mitochondrial ATP-dependent K-channel opening, and also increases the promotion of hair papilla cells and hair organization blood-flow improvement action caused by vascular smooth muscle ATP sensitivity K-channel opening [28]. In mice where the epithelium system strongly expressed VEGF in the presence of a K14 promoter, increases in blood vessels around hair follicles have been observed, and longer than usual thick hair has been recognized [29]. From these observations, it is thought that VEGF produced from hair papilla cells has a hair growth effect via increased action on the blood vessels near the hair follicles, which improves hair organization blood-flow. As the amount of mRNA of Wnt10b increased by HGPEE, as were the amounts of mRNA of KGF and VEGF, prevention of lipid accumulation in hair pores and hair growth promotion and hair restoration effects of hair restorer products containing HGPEE are expected. Furthermore, it has been reported that excessive activation of N-methyl-D-aspartate (NMDA) causes apoptosis of hair cells [30]. 15-Methoxypinusolidic acid (15-MPA) contained in HGPEE has been reported to inhibit glutamate-induced increase in intracellular calcium ([Ca2+] i), which prevented NMDA-induced cytotoxicity [31]. Moreover, 15-MPA has been shown to successfully reduce subsequent overproduction of nitric oxide, reduce cellular peroxide concentrations, and inhibit glutathione depletion and lipid peroxidation induced by glutamate in cultures [31].
Based on previous results, it was possible to conclude that the ethanol extract (HGPEE) was more effective than the aqueous one (HGPWE) in the extraction of hair growth-related factors. Both the leaves and seeds contained an essential oil consisting of borneol, bornyl acetate, thujone, camphor, and sesquiterpenes [32]. The leaves also contained rhodoxanthin, amentoflavone, quercetin, myricetin, carotene, xanthophyll, and ascorbic acid [32]. Many fatty acids and nonpolar substances were present in the ethanol plant mixture extract. Further research and clinical tests are required to identify which ingredients in HGPEE contributed to the hair growth/restoration functionality demonstrated in this study.
5. Conclusions
In conclusion, the results from this study showed that BOLE reduced generation of sebum from sebocytes and decreased the size of lipid droplets. Moreover, an ethanol extract containing B. orientalis and other plant materials (HGPEE) increased the amounts of mRNA of Wnt10b and Lef1 in cultured human fetal epidermal keratinocytes, as well as the amounts of mRNA of VEGF and KGF in cultured human hair papilla cells. These results indicate that future studies should focus on the timing of hair cycle phases, specifically to learn if HPGEE lengthens the anagen phase in specific hair follicle cells to promote hair growth.
Acknowledgments
The author gratefully acknowledges the technical assistance of Rin Kouriki, Yuuki Matsuzaki and Kyouhei Igarashi.
Author Contributions
H.Z., L.G. and K.M. performed the experiments. K.M. designed the study and performed the analysis. H.Z., L.G., and K.M. interpreted the data and drafted the manuscript. K.M. supervised the progress and critically revised the manuscript. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare that they have no competing interests.
Ethics Approval and Consent to Participate
Not applicable.
Funding
This study was supported by a grant from Asian Scalp Healthy Research Center in Kobe, Japan.
References
- Kellum, R.E. Human sebaceous gland lipids. Analysis by thin-layer chromatography. Arch. Dermatol. 1967, 95, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Sakiguchi, T.; Kitamura, K.; Akamatsu, H.; Horio, T. Establishment of a tissue culture system for hamster sebaceous gland cells. Dermatology 1988, 197, 238–244. [Google Scholar] [CrossRef]
- Sato, T.; Imai, N.; Akimoto, N.; Sakiguchi, T.; Kitamura, K.; Ito, A. Epidermal growth factor and 1alpha,25-dihydroxyvitamin D3 suppress lipogenesis in hamster sebaceous gland cells in vitro. J. Investig. Dermatol. 2001, 117, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Millar, S.E. Molecular mechanisms regulating hair follicle development. J. Investig. Dermatol. 2002, 118, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.; Andl, T.; Bagasra, A.; Lu, M.M.; Epstein, D.J.; Morrisey, E.E.; Millar, S.E. Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mech. Dev. 2001, 107, 69–82. [Google Scholar] [CrossRef]
- Chiang, C.; Swan, R.Z.; Grachtchouk, M.; Bolinger, M.; Litingtung, Y.; Robertson, E.K.; Cooper, M.K.; Gaffield, W.; Westphal, H.; Beachy, P.A.; et al. Essential role for Sonic hedgehog during hair follicle morphogenesis. Dev. Biol. 1999, 205, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ouji, Y.; Yoshikawa, M.; Moriya, K.; Nishiofuku, M.; Matsuda, R.; Ishizaka, S. Wnt-10b, uniquely among Wnts, promotes epithelial differentiation and shaft growth. Biochem. Biophys. Res. Commun. 2008, 367, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Van Genderen, C.; Okamura, R.M.; Fariñas, I.; Quo, R.G.; Parslow, T.G.; Bruhn, L.; Grosschedl, R. Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev. 1994, 8, 2691–2703. [Google Scholar] [CrossRef] [PubMed]
- Goldman, C.K.; Tsai, J.C.; Soroceanu, L.; Gillespie, G.Y. Loss of vascular endothelial growth factor in human alopecia hair follicles. J. Investig. Dermatol. 1995, 104, 18S–20S. [Google Scholar] [CrossRef] [PubMed]
- Lachgar, S.; Moukadiri, H.; Jonca, F.; Charveron, M.; Bouhaddioui, N.; Gall, Y.; Bonafe, J.L.; Plouët, J. Vascular endothelial growth factor is an autocrine growth factor for hair dermal papilla cells. J. Investig. Dermatol. 1996, 106, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Lachgar, S.; Charveron, M.; Ceruti, I.; Lagarde, J.M.; Gall, Y.; Bonafe, J.L. VEGF mRNA expression in different stages of the human hair cycle, analysis by confocal laser microscopy. In Hair Research for the Next Millennium; Van Neste, D., Randall, V.A., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1996; p. 407. [Google Scholar]
- Guo, L.; Degenstein, L.; Fuchs, E. Keratinocyte growth factor is required for hair development but not for wound healing. Genes Dev. 1996, 10, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Danilenko, D.M.; Ring, B.D.; Yanagihara, D.; Benson, W.; Wiemann, B.; Starnes, C.O.; Pierce, G.F. Keratinocyte growth factor is an important endogenous mediator of hair follicle growth, development, and differentiation. Normalization of the nu/nu follicular differentiation defect and amelioration of chemotherapy-induced alopecia. Am. J. Pathol. 1995, 147, 145–154. [Google Scholar] [PubMed]
- Yeung, H.C. Handbook of Chinese Herbs and Formulas; Institute of Chinese Medicine: Los Angeles, CA, USA, 1985. [Google Scholar]
- Zhang, N.N.; Park, D.K.; Park, H.J. Hair growth-promoting activity of hot water extract of Thuja orientalis. BMC Complement Altern. Med. 2013, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Park, W.S.; Lee, C.H.; Lee, B.G.; Chang, I.S. The extract of Thujae occidentalis semen inhibited 5alpha-reductase and androchronogenetic alopecia of B6CBAF1/j hybrid mouse. J. Dermatol. Sci. 2003, 31, 91–98. [Google Scholar] [CrossRef]
- Jain, R.K.; Garg, S.C. Antimicrobial activity of the essential oil of Thuja orientalis L. Ancient. Sci. Life 1997, 16, 186–189. [Google Scholar]
- Hassanzadeh, M.K.; Rahimizadeh, M.; Fazly Bazzaz, B.S.; Emami, S.A.; Asili, J. Chemical and antimicrobial studies of Platycladus orientalis essential oils. Pharm. Biol. 2001, 5, 388–390. [Google Scholar] [CrossRef]
- Duhan, J.S.; Saharan, P.; Surekha; Kumar, A. Antimicrobial potential of various fractions of Thuja orientalis. Afr. J. Microbiol. Res. 2013, 7, 3179–3186. [Google Scholar]
- Jasuja, N.D.; Sharma, S.K.; Saxena, R.; Choudhary, J.; Sharma, R.; Joshi, S.C. Antibacterial, antioxidant and phytochemical investigation of Thuja orientalis leaves. J. Med. Plants Res. 2013, 7, 1886–1893. [Google Scholar]
- Choi, Y.; Moon, A.; Kim, Y.C. A pinusolide derivative, 15-methoxypinusolidic acid from Biota orientalis inhibits inducible nitric oxide synthase in microglial cells, implication for a potential anti-inflammatory effect. Int. Immunopharmacol. 2008, 8, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.X.; Wang, Y.; Kong, L.D.; Yang, C.; Zhang, X. Effects of Biota orientalis extract and its flavonoid constituents, quercetin and rutin on serum uric acid levels in oxonate-induced mice and xanthine dehydrogenase and xanthine oxidase activities in mouse liver. J. Ethnopharmacol. 2004, 93, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, Z.; Wang, Z.; Wei, D. Quality evaluation of Platycladus orientalis (L.) Franco through simultaneous determination of four bioactive flavonoids by high-performance liquid chromatography. J. Pharm. Biomed. Anal. 2006, 4, 1186–1190. [Google Scholar] [CrossRef] [PubMed]
- Duhan, J.S.; Saharan, P.; Gahlawat, S.K. Antioxidant potential of various extracts of stem of Thuja orientalis, in vitro study. Intern. J. App. Bio Pharma. Technol. 2013, 3, 264–271. [Google Scholar]
- Zhang, Y.; Xing, Y.; Guo, H.; Ma, X.; Li, Y. Immunohistochemical study of hair follicle stem cells in regenerated hair follicles induced by Wnt10b. Int. J. Med. Sci. 2016, 13, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Takebe, K.; Oka, Y.; Radisky, D.; Tsuda, H.; Tochigui, K.; Koshida, S.; Kogo, K.; Hirai, Y. Epimorphin acts to induce hair follicle anagen in C57BL/6 mice. FASEB J. 2003, 17, 2037–2047. [Google Scholar] [CrossRef] [PubMed]
- Lo Celso, C.; Prowse, D.M.; Watt, F.M. Transient activation of beta-catenin signalling in adult mouse epidermis is sufficient to induce new hair follicles but continuous activation is required to maintain hair follicle tumours. Development 2004, 131, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Marubayashi, A.; Nakaya, Y.; Fukui, K.; Arase, S. Dermal papilla cells possess sulfonylurea receptor 2B and adenosine receptors which are the possible mediators of minoxidil-induced VEGF productions. J. Investig. Dermatol. 2001, 117, 1594–1600. [Google Scholar] [PubMed]
- Yano, K.; Brown, L.F.; Detmar, M. Control of hair growth and follicle size by VEGF-mediated angiogenesis. J. Clin. Investig. 2001, 107, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Sheets, L. Excessive activation of ionotropic glutamate receptors induces apoptotic hair-cell death independent of afferent and efferent innervation. Sci. Rep. 2017, 7, 41102. [Google Scholar] [CrossRef] [PubMed]
- Koo, K.A.; Kim, S.H.; Lee, M.K.; Kim, Y.C. 15-Methoxypinusolidic acid from Biota orientalis attenuates glutamate-induced neurotoxicity in primary cultured rat cortical cells. Toxicol. In Vitro 2006, 20, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Bown, D. Encyclopaedia of Herbs and Their Uses; Dorling Kindersley: London, UK, 1995. [Google Scholar]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).