Abstract
Background: Melanins are high molecular weight pigments responsible for the mammalian skin and hair colour and play a key role in skin protection from UV radiation; however, their overproduction and excessive accumulation lead to pigmentation problems including melasma, freckles, uneven colouring, and age spots. Therefore, the modulation of melanin synthesis represents a critical issue in medicine and cosmetology. In the present study, an innovative polymeric antioxidant to be used as skin whitening agent is developed by the conjugation of dextran with rosmarinic acid. Methods: Dextran-rosmarinic acid conjugates (DEX-RA) were synthesized in a one-pot method starting from Origanum vulgare aqueous leaf extract and dextran. The total polyphenol content and the antioxidant activity were assessed by Folin-Ciocalteau assay and 2,2-diphenyl-1-picrylhydrazyl radical (DPPH) and bleaching tests, respectively. The efficacy of DEX-RA was evaluated by inhibition of tyrosinase activity, in vitro diffusion and stability studies and in vivo studies. The biocompatibility of the conjugates was investigated by 3-[4,5-Dimethylthiaoly]-2,5-diphenyltetrazoliumbromide (MTT) and EPISKIN™ model. Results: Efficacy and safety studies confirmed the antioxidant and tyrosinase inhibitory activities and the biocompatibility of the synthesized conjugates. Conclusion: The polymeric conjugates, comparing to the free antioxidant, show a long-lasting efficacy combined to an enhanced stability resulting in an improved performance of the cosmetic formulations prepared using this innovative whitening agent as a bioactive ingredient.
1. Introduction
Melanins are coloured polymorphous biopolymers, which represent the main determinant of mammalian skin and hair colour [1]. These pigments are synthesized within melanosomes by a combination of chemical and enzyme-catalysed reactions involving the amino acid l-tyrosine and called melanogenesis [2]. This biosynthetic pathway includes two main processes, eumelanogenesis and pheomelanogenesis, with the production of eumelanin and pheomelanin, respectively. Tyrosinase is the copper-containing monooxygenase involved in the first step of melanogenesis [3,4], which is the only rate-limiting one. This enzyme catalyses the hydroxylation of tyrosine to 3,4-dihydroxyphenylalanine (DOPA) and the oxidation of DOPA to dopaquinone [5,6]. Then, this compound is converted to dopachrome and DOPA, through auto-oxidation, and the last one is oxidized to dopaquinone again by tyrosinase. During pheomelanogenesis, dopaquinone reacts with glutathione (GSH) or cysteine leading to the formation of yellow-red pheomelanin while, in eumelanogenesis pathway, it is initially converted to dopachrome and, then, to different intermediates with the final production of brown-black eumelanin [7]. Melanin exerts a key role in protecting skin against several types of ionizing radiations, such as ultraviolet (UV) light, and radical species produced within cytoplasm and inhibits photocarcinogenesis [8]. However, its abnormal accumulation can lead to aesthetic problems related to hyperpigmentation, including melasma, freckles, ephelides, uneven colouring, and age spots [9,10]. These hyperpigmented skin regions are not considered cosmetically attractive in today’s society [11,12]; therefore, the development and screening for novel effective and safe skin whitening agents, which act by modulating melanin synthesis, is attracting significant attention both in cosmetic and medical fields. The inhibition of tyrosinase activity represents an interesting strategy to achieve important goals in this research area. Several tyrosinase inhibitors, including hydroquinone [13], kojic acid [14], azelaic acid [15], and arbutin [16], have been employed for cosmetic purposes. However, these compounds present topical as well as systemic side effects consisting of irritant dermatitis, leukoderma, ochronosis, post-inflammatory hyperpigmentation and kidney and liver diseases [17,18]. For these reasons, and taking into account that the use of cosmetic formulations is widespread and involves a repeated and long-term exposure without any medical supervision, some of these active molecules, such as hydroquinone, are forbidden in the EU for cosmetic applications [19]. Therefore, safer products have great potential as whitening agents in skin care. In this way, the use of natural ingredients can meet the needs of the market and consumers in terms of “green cosmetics”. The growing request for natural bioactive molecules, which can be employed as bleaching agents, has led to extensive research and, among polyphenols, rosmarinic acid (RA) has reached great attention due to its reported health benefits. This compound is an ester of 3,4-dihydroxyphenyllactic acid and caffeic acid and it is usually found in different species belonging to Boraginaceae. Rosmarinic acid is characterized by several interesting biological properties including antibacterial, antimutagenic, anti-allergenic, antiviral, antioxidant and anti-inflammatory activities [20,21,22,23]. Moreover, the inhibitory effects of RA on tyrosinase was also reported in literature [22,24]. UV radiations and oxidative stress, due to an excessive level of free radicals, are related to skin disorders, thus, antioxidant molecules may exert a beneficial effect on skin health. Reactive species play also a crucial role in the modulation of melanocyte proliferation and melanogenesis; on the other hand, inhibitors of the generation of free radicals and scavengers of reactive species may down-regulate hyperpigmentation and prevent UV-induced melanogenesis [25]. Therefore, compounds that combined antioxidant and tyrosinase inhibitory activities could be very promising skin whitening agents, even if the use of antioxidants could represent a challenge due to their poor stability. Phenolic acids including RA are, indeed, characterized by high sensitivity to different factors such as exposure to UV light or air oxygen [26]. In this context, the present work aims to develop an innovative polymeric antioxidant to be used as skin whitening agent by the conjugation of dextran (DEX), a natural biodegradable and biocompatible polysaccharide widely used in cosmetic and pharmaceutical formulations [27,28], with rosmarinic acid.
The polymeric bioactive was designed on the basis of an idea born in 2009 and according to the European Patent EP 2535087A2 developed by Macrofarm s.r.l. [29], a spin-off company of the University of Calabria, which focuses its activity on research, development of advanced technologies, and industrial innovation in cosmetic, pharmaceutical and nutraceutical fields. The dextran-rosmarinic acid conjugate was synthesized in a one-pot way starting from Origanum vulgare leaf extract polyphenols, titled in rosmarinic acid, by using a water-soluble initiator system. This innovative synthetic strategy allows to obtain a fully biocompatible polymeric conjugate in which the active molecule does not suffer of the commonly known problems of instability, migration, and blooming often found in formulations. Moreover, comparing to the free antioxidant, the conjugate shows a long-lasting efficacy improving the performance of the cosmetic formulations.
2. Materials and Methods
2.1. Materials
Dextran from Leuconostoc spp (DEX), hydrogen peroxide (H2O2), ascorbic acid (AA), 2,2-diphenyl-1-picrylhydrazyl radical (DPPH), Folin-Ciocalteu reagent, sodium carbonate, rosmarinic acid (RA), β-carotene, linoleic acid, Tween 20, tyrosinase from mushroom (EC 1.14.18.1), l-tyrosine, disodium hydrogen phosphate (Na2HPO4), sodium dihydrogen phosphate (NaH2PO4), l-glutamine, penicillin-streptomycin, 3-[4,5-Dimethylthiaoly]-2,5-diphenyltetrazoliumbromide (MTT) and sodium hydroxide (NaOH) were obtained from Sigma-Aldrich (Milano, Italy).
Origanum vulgare leaf extract was prepared by aqueous extraction following a specific temperature gradient. The plants were obtained from a local store.
Solvents were purchased from Carlo Erba Reagents (Milano, Italia).
Strat-M® membranes (25 mm discs, Cat. No. SKBM02560) were purchased from Merck Millipore (Darmstadt, Germany).
The EPISKIN™ RHE/L/13 human skin equivalent kit was obtained from SkinEthic Laboratories (Lyon, France).
2.2. Cell Lines and Culture Conditions
HaCaT keratinocytes were provided by Cell Line Services. Cells were grown in DMEM/F12 medium (Invitrogen, Waltham, MA, USA) supplemented with 2 mM l-glutamine (1%), penicillin-streptomycin (1%), and heat-inactivated foetal calf serum (10%, Biosera, Nuaille, France). HaCaT keratinocytes were maintained as a monolayer culture in a humidified incubator at 37 °C and 5% CO2.
2.3. Instrumentation
Absorption spectra were recorded with a Jasco V-530 UV-VIS spectrometer (Jasco, Oklahoma City, OK, USA).
In vitro diffusion studies were performed using Franz diffusion cells (Disa, Milan, Italy; permeation area 0.4614 cm2).
The stability studies were carried out in a photo-reactor equipped with a mercury lamp (photochemical reactor with a high pressure mercury lamp TQ 718 of 250 W, from Heraeus Noblelight Company, Hanau, Germany).
The intensity of dark spot, the red component of the skin colour and skin lightness/radiance were evaluated by using a spectrophotometer/colorimeter CM-700D (Konica Minolta, Cinisello Balsamo MI, Italy).
2.4. Synthesis of the Antioxidant Polymeric Conjugates
Dextran-rosmarinic acid conjugates (DEX-RA) were synthesized in a one-pot way according to the European Patent EP 2535087A2 [29].
The polymeric active was prepared starting from Origanum vulgare aqueous leaf extract and different amount of dextran (5%, 10%, 15%, and 30% w/w) by using a water-soluble initiator system consisting of a redox pair.
2.5. Folin-Ciocalteu Assay
The total polyphenols content of DEX-RA conjugates was evaluated by performing the Folin-Ciocalteau colorimetric assay with slight modifications [30]. For this purpose, all the tested items were previously diluted in distilled water (4% v/v). One millilitre of each item was mixed with 1 mL of the 0.2 N Folin-Ciocalteu reagent and 1 mL of a sodium carbonate solution (7.5% w/v). The obtained samples were incubated in dark conditions at room temperature and, after 2 h, the absorbance was recorded at 760 nm.
The same experimental conditions were adopted for the starting Origanum vulgare leaf extract and on a control sample (Blank), consisting of a dextran aqueous solution (30% w/w), in the aim to investigate the interference of polymeric material in the Folin-Ciocalteu assay.
The amount of total phenolic groups was expressed as mg equivalent of rosmarinic acid per gram of sample (mg eq RA/g) by using the equation obtained from the calibration curve of the antioxidant.
The experiments were conducted in triplicate and data were expressed as means.
2.6. DPPH Assay
In the aim to explore the antioxidant activity of DEX-RA conjugates, their scavenging properties toward a stable free radical, such as 2,2-diphenyl-1-picrylhydrazyl radical (DPPH), were investigated according to literature with slight modification [31]. For this purpose, all the tested samples were previously diluted in distilled water (4% v/v). One millilitre of each item was mixed with 4 mL of an ethanol DPPH solution (188 μM) and 5 mL of ethanol. The obtained samples were incubated in dark conditions and, after 15 min, the absorbance was measured at 517 nm.
The same conditions were adopted for the starting Origanum vulgare leaf extract and on a control sample (Blank), consisting of a dextran aqueous solution (30% w/w), in the aim to assess the interference of dextran in the DPPH test.
The scavenging activity was measured as the decrease in DPPH absorbance and it was expressed as inhibition percentage.
Each experiment was performed in triplicate and data were expressed as means.
2.7. β-Carotene Bleaching Test
The antioxidant activity of DEX-RA conjugates was also investigated by measuring the ability to inhibit peroxidation processes in a system consisting of β-carotene and linoleic acid [32]. For this purpose, all the tested items were previously diluted in distilled water (1% v/v). Firstly, β-carotene was dissolved in chloroform reaching a concentration of 0.2 mg/mL and, then, 0.2 mL of Tween 20 and 0.02 mL of linoleic acid were added to 1 mL of the previously-prepared β-carotene solution. Chloroform was removed by evaporation and 100 mL of distilled water was added under stirring in order to obtain an emulsion.
DEX-RA samples (0.5 mL) were introduced into test tubes containing 5 mL of the emulsion and incubated for 60 min at 45 °C. The absorbance of the tested items and control was measured at 470 nm against a blank, consisting of an emulsion prepared in the absence of β-carotene. The measurement was performed at the initial time (t = 0) and after 60 min.
The same experimental conditions were adopted for the starting Origanum vulgare leaf extract and for the control sample (Blank), consisting of a dextran aqueous solution (30% w/w), with the aim to evaluate the interference of dextran in the performed test.
The antioxidant properties were evaluated in terms of β-carotene bleaching and expressed as the inhibition percentage.
Each test was conducted in triplicate and data were expressed as means.
2.8. Inhibition of Tyrosinase Activity
Inhibition of tyrosinase activity was determined by using l-tyrosine as substrate according to literature with slight modifications [33,34]. Reaction mixtures containing 1.5 mL of phosphate buffer (pH 6.8), 0.6 mL of a 0.3 mg/mL tyrosinase solution and 0.6 mL of tested item were incubated at 37 ± 0.5 °C in the presence or not of 0.6 mL of a 10 mM l-tyrosine solution (alternatively, 0.6 mL of water). After 10 min, the absorbance was determined at 475 nm.
The same conditions were adopted for the starting Origanum vulgare leaf extract and for the control sample (Blank), consisting of a dextran aqueous solution (30% w/w), in the aim to assess the interference of dextran in the tyrosinase assay.
The inhibition of tyrosinase activity was measured as the decrease in absorbance and it was expressed as percent.
The experiments were performed in triplicate and data were expressed as means.
2.9. In Vitro Diffusion Studies by Vertical Franz Cells
The in vitro diffusion studies were carried out at 37 ± 0.5 °C using Franz diffusion cells. For this purpose, Strat-M® membrane was positioned between the donor and receptor sections of the diffusion cells and, then, the two chambers were fixed together [35]. The receptor compartment was filled with 5.5 mL of phosphate buffer at pH 7.4 (10−3 M) and, after 20–30 min when the receptor solution reached 37 °C, 0.5 mL of the tested DEX-RA sample were added to the donor compartment, which was covered with ParafilmTM (Sigma-Aldrich, Milan, Italy). The content of the receptor chamber was removed at 1, 2, 4, 6, 8 and 24 h, for UV-VIS analysis, and replaced with fresh phosphate buffer.
The same conditions were applied on the starting Origanum vulgare leaf extract.
The in vitro diffusion studies were carried out in triplicate and the obtained results were expressed as diffused amount (%) of the tested items.
2.10. Stability Studies
Stability studies were carried out according to literature with slight modification [36]. For this purpose, all the DEX-RA samples were placed into a photo-reactor and irradiated for 12 h, employing a mercury lamp (emission in the range between 200 and 600 nm), in the presence of H2O2. The stability of each sample was assessed at predetermined time intervals (0, 3, 6, 12, and 24 h) by spectrophotometric analysis and data were expressed as degradation percentage. The same conditions were applied on the starting Origanum vulgare leaf extract.
The experiments were conducted in triplicate and data were expressed as means.
2.11. Statistical Analysis
All the measurements for the in vitro assays, including Folin-Ciocalteu and DPPH assays, β-carotene bleaching test, inhibition of tyrosinase activity and diffusion and stability studies, were means of triplicate measurements (n = 3) including standard deviation (±SD).
Data were performed using a Microsoft Excel worksheet.
2.12. In Vivo Studies for Clinical-Instrumental Assessment of the Whitening and Lightening Efficacy
In order to evaluate the whitening and lightening efficacy of DEX-RA as cosmetic ingredient, a placebo-controlled clinical-instrumental study was carried out on 20 female subjects clinically showing a dull skin complexion and dark spots on the face due both to chrono or photo-aging. The study protocol was carried out according to the ethical principles for the medical research (Ethical Principles for Medical Research Involving Human Subjects, adopted by the 18th WMA General Assembly Helsinki, Finland, June 1964, and successive amendments). All of the subjects participating in the study were healthy volunteers at least 18 years old and signed informed consent was obtained from each of them. Volunteers, over 30 years old and showing a dull skin complexion and dark spots on the face, were selected under the supervision of a dermatologist from a panel of healthy female subjects. During all the study period, participants did not use any topic products with a similar effect to the tested item. Pregnant or nursing women, subjects that have shown allergies to cosmetic products, with skin disorders on the test area and/or under pharmacological treatment were excluded. The 20 volunteers participating in the study were divided into two groups: 10 volunteers tested a cream formulation containing DEX-RA as the bioactive ingredient, and 10 volunteers tested the placebo formulation, which had the same composition but without the polymeric conjugate. Products efficacy was evaluated after 30 and 60 days of treatment, which consisted of a once-a-day application on clean face in the evening, by means of non-invasive bioengineering technique that allows to quantify parameters related to skin colour and lightness. The instrumental analysis was integrated with the clinical analysis carried out by the dermatologist.
2.12.1. Evaluation of the Dark Spots Intensity and Calculation of ITA° (Individual Typology Angle)
L* and b* values, which characterize the dark spot, define the color brightness and saturation, respectively. These values were registered and the data were, then, interpolated using a mathematical formula that allows the calculation of the ITA°. This parameter was used to evaluate the intensity of the colour of the stain inside the dark spot. Low ITA° values indicate a brown pigmentation, while high ITA° values indicate a very light pigmentation.
2.12.2. Evaluation of the Red Component of the Skin Colour (a*)
The a* value that is related with the red (+a*)/green (−a*) component of the skin colour was analysed. In this study it is desirable to record a decrease of a* values that indicates a reduction of the red component of the skin colour.
2.12.3. Evaluation of the Skin Lightness/Radiance
Skin lightness/radiance was investigated by employing a spectrophotometer/colorimeter CM-700D (Konica Minolta, Cinisello Balsamo MI, Italy). The instrument evaluates the colour in accordance with a standardized international method developed by the International Commission on Illumination (CIE). The gloss parameter is related with the ability of the skin to reflect the light; therefore, the gloss value measures the specular component of the reflected light and correlates with the gloss/shine perception.
2.12.4. Clinical Evaluation of the Reduction of Dark Spots Appearance and Skin Complexion Brightness
The clinical evaluation of the reduction of dark spots appearance and skin brightness was performed by the dermatologist.
2.12.5. Statistical Analysis for the In Vivo Studies
The instrumental data were submitted to the two-way Student’s t-test for paired (intra-group statistic) or unpaired (inter-group statistic) data. Variation is considered statistically significant when p < 0.05.
2.13. Cell Viability by MTT Assay
In the present study, the colorimetric MTT assay was employed to detect cell viability [37]. For this purpose, DEX-RA was dissolved in medium and cells were exposed to the polymeric conjugate at different concentrations for 24 h at 37 °C under 5% CO2. After the treatment, the DEX-RA containing medium was aspirated out and cells were rinsed with phosphate buffer at pH 7.4. Fresh MTT, re-suspended in phosphate buffer solution (PBS), was added to each well reaching a final concentration of 0.33 mg/mL. After 2 h incubation at 37 °C in an atmosphere containing 5% CO2, culture media were removed and replaced with 1 mL of DMSO for formazan crystal solubilization. The plates were incubated in dark conditions and, after 10 min, the absorbance of reduced MTT was read at 570 nm.
Cell viability was expressed as a percentage compared to control wells.
The same experimental conditions were adopted for the starting Origanum vulgare leaf extract and for a dextran aqueous solution.
Each experiment was repeated in triplicate and data were expressed as mean values ± standard deviation (SD).
2.14. Assessment of Skin Irritation: EPISKIN™ Model
In the aim to investigate the cutaneous irritant potential of the developed dextran-based conjugates, the EPISKIN™ RHE/L/13 human skin was treated with DEX-RA (0.1 nM), PBS without Ca2+ and Mg2+ as a negative control and 5% (w/v) SDS as a positive control according to the protocol reported in the literature [38].
3. Results and Discussion
3.1. Synthesis of the Polymeric Skin Whitening Agents
Dextran is a natural hydrophilic, biodegradable, and biocompatible polysaccharide mainly based on α-1-6-linked d-glucose units and produced from Leuconostoc, Lactobacillus, Streptococcus, and Weissella species [39]. Due to its features, this polymer is widely used in cosmetic and pharmaceutical formulations [27,28].
In the present study, DEX-RA conjugates were prepared by following a one-pot approach, which involves a grafting reaction carried out in aqueous media and at room temperature. The adopted experimental protocol involves the use of a biocompatible redox pair, hydrogen peroxide and ascorbic acid, as the initiator system and allows for avoiding the use of organic solvents and the formation of toxic by-products.
The reaction mechanism consists of the oxidation of ascorbic acid by H2O2 with the formation of hydroxyl radicals, which attack the hydrogen atoms in the α-methylene and hydroxyl positions of the polymeric backbone leading to the formation of reactive sites involved in the reaction for rosmarinic acid insertion [40].
3.2. Efficacy Assessment
3.2.1. Evaluation of the Total Polyphenols Content: Folin-Ciocalteu Assay
The Folin-Ciocalteu assay represents a conventional method, which is described in several pharmacopoeias, for the quantification of the total phenolic content.
The redox reaction, which occurs between phenolic molecules and the Folin-Ciocalteu reagent, leads to the formation of a blue complex that is measured by visible-light spectrophotometry (V-530 UV-VIS spectrometer, Jasco, Oklahoma City, OK, USA) [41].
This assay involves the ability of polyphenols to reduce the phosphomolybdic/phosphotungstic acid complexes present in the reagent with the production of chromogens, in which the metals have lower valence, and, therefore, it is also a useful method for the evaluation of antioxidant activity [42].
The polyphenols content of the tested DEX-RA conjugates was expressed as the mg equivalent of rosmarinic acid per gram of sample (mg eq RA/g) by employing the equation obtained from the calibration curve of the antioxidant. The obtained results were reported in Table 1.

Table 1.
Total polyphenols content expressed as mg equivalent of rosmarinic acid per gram of sample and DPPH, lipid peroxidation and tyrosinase inhibition expressed as percentage (mean value ± st. dev.).
According to the applied experimental protocol and the obtained results, it is possible to conclude that the variation of dextran amount, employed in the preparation of the polymeric conjugate, does not affect the total phenolic content of the final product.
3.2.2. Evaluation of the Scavenging Activity on DPPH Radicals
The DPPH assay is designed for the estimation of the antioxidant properties and it is based on the measurement of the scavenging capacity of antioxidants towards 2,2-diphenyl-1-picrylhydrazyl radicals, which are stable organic free radicals characterized by an absorption maximum band around 515–528 nm [31].
In the present method, the antioxidant molecule reduces the radical to a yellow-coloured compound, diphenylpicrylhydrazine, and the extent of the reaction will depend on the hydrogen-donating ability of the antioxidant.
The scavenging abilities of the tested items were evaluated in terms of DPPH reduction and data were expressed as inhibition percent calculated according to Equation (1):
where A0 is the absorbance of a standard prepared in the same conditions, but without any sample, and A1 is the absorbance of the tested item.
According to the applied experimental protocol and the obtained results (Table 1), it is possible to conclude that all the tested items are characterized by good antioxidant properties in terms of DPPH scavenging activity. The Origanum vulgare leaf extract and the polymeric conjugates, indeed, have been shown inhibition percentages around 40%.
3.2.3. Evaluation of the Antioxidant Activity: β-Carotene Bleaching Test
The lipid peroxidation represents a key step in the pathogenesis of several disease states and it is due to the effect of different reactive oxygen species, including hydroxyl radical, hydrogen peroxide etc., which attack the polyunsaturated fatty acids of the fatty acid membrane starting a self-propagating chain reaction.
The inhibition of lipid peroxidation by DEX-RA conjugates was investigated by carrying out the β-carotene bleaching test [32].
In the absence of an antioxidant agent, linoleic acid oxidation produces radical species that react with β-carotene molecules, which lose their conjugation leading to a discoloration of the test solution. On the contrary, the presence of an antioxidant compound allows to avoid the damage of the β-carotene conjugate system and, therefore, the colour of the test solution is preserved.
The antioxidant activity (AoxA) was evaluated in terms of β-carotene discoloration and expressed as inhibition percentage according to the Equation (2):
where A0 and A0c represent the absorbance at the initial time for samples and control, respectively, while A60 and A60c represent the absorbance after 60 min of incubation for the samples and control, respectively.
The obtained results (Table 1) underlined the relevant antioxidant power of the synthesized conjugates, with inhibition values higher than 70%.
3.2.4. Inhibition of Tyrosinase Activity
Tyrosinase is a copper-containing monooxygenase involved in melanogenesis and in the first step of this process, which is the only rate-limiting one, this enzyme catalyses tyrosine oxidation to dopaquinone. This molecule is auto-oxidized to form dopachrome and dopa; this last one also represents a tyrosinase substrate.
In the present assay, the inhibition of tyrosinase activity was evaluated by the quantification of the amount of produced dopachrome [33,34], and it was expressed as percent calculated according to Equation (3):
in which A0 represents the absorbance of a standard prepared in the same conditions, but without any sample, A1 the absorbance of the tested item, and Aint the absorbance of a sample prepared in the absence of the substrate.
The obtained data were reported in Table 1 and indicated the ability of all the tested polymeric conjugates to inhibit tyrosinase reaching inhibition percentages higher than 95% and suggesting the application of DEX-RA as a skin-whitening agent.
3.2.5. In Vitro Diffusion Studies by Vertical Franz Cells: Long-Lasting Effect
The in vitro diffusion studies, performed by employing Franz diffusion cells, represent a highly useful method for the development of new formulations [43], but also for quality control [44,45,46].
In the present study, Strat-M® membrane was employed as synthetic membrane alternative to human skin. The use of this kind of membrane, indeed, allows to obtain data that are closely related to the diffusion processes which occur through human skin [35].
The obtained results were expressed as cumulative diffused amount (%) of the tested items and the achieved diffusion profiles were reported in Figure 1.
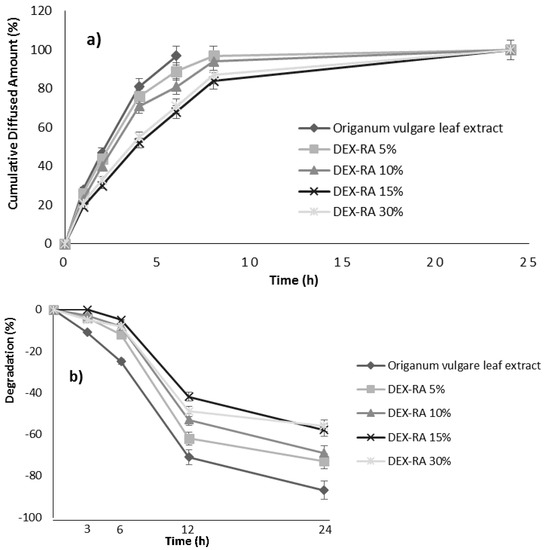
Figure 1.
(a) In vitro diffusion profiles; and (b) stability studies: degradation percentages.
In the case of Origanum vulgare leaf extract, the diffused amount is equal to 97% within the first 6 h while, at the same time, the observed values for DEX-RA 5%, DEX-RA 10%, DEX-RA 15%, and DEX-RA 30% were 89%, 81%, 68%, and 71%, respectively.
The obtained results indicated a slower and prolonged diffusion process for the polymeric conjugates, which is more evident for DEX-RA 15% and DEX-RA 30% items.
3.2.6. Stability Studies
The present study aims to investigate the stability of the tested items involving oxidation processes, such as UV/H2O2 [47].
The tested samples were irradiated with UV light in the presence of hydrogen peroxide. The photolysis of H2O2 generates hydroxyl radicals, which are responsible for the degradation process, through its homolytic cleavage as reported in Equation (4) [48]:
The stability of the different samples was evaluated by recording the evolution of the UV-VIS absorption spectra for 24 h.
The obtained results were expressed as degradation percentages and reported in Figure 1.
According to the applied experimental protocol and the obtained results, it is possible to conclude that the polymeric conjugates are characterized by a higher stability compared to the Origanum vulgare leaf extract. This behaviour is more evident in the case of DEX-RA 15% and DEX-RA 30% items. At the time point of 24 h, indeed, the degradation percentage for Origanum vulgare leaf extract is equal to 86%, while the observed values for DEX-RA 5%, DEX-RA 10%, DEX-RA 15%, and DEX-RA 30% were 73%, 69%, 58%, and 56%, respectively.
3.2.7. In Vivo Studies for Clinical-Instrumental Assessment of the Whitening and Lightening Efficacy
For the in vivo studies, the polymeric active synthesized starting from Origanum vulgare aqueous leaf extract and 15% by weight of dextran, which showed the best in vitro results, was employed.
In order to evaluate the whitening and lightening efficacy of DEX-RA as cosmetic ingredient, a placebo-controlled clinical-instrumental study was carried out on 20 female subjects clinically showing a dull skin complexion and dark spots on the face due both to chrono- or photo-aging.
The mean ITA° values, the mean a* values related to the red component of the skin colour (skin redness) and the mean GLOSS values correlated with the ability of the skin to reflect the light (skin radiance/brightness) recorded at each experimental time (after 30 and 60 days of products use) for each tested formulation were reported in Figure 2.
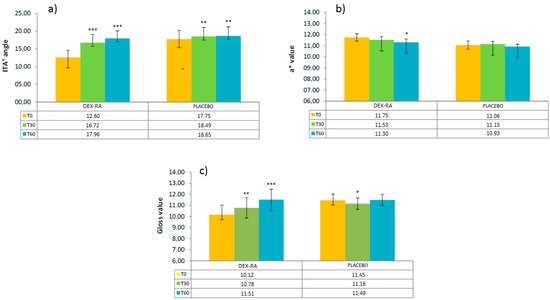
Figure 2.
(a) ITA° values; (b) a* values related to the red component of the skin colour (skin redness) and (c) GLOSS values correlated with the ability of the skin to reflect the light (skin radiance/brightness) recorded at each experimental time for each tested formulation. Note: the asterisks above the error bars are referred to the intra-group statistical analysis (vs T0); the inter-group statistical analysis performed by means of t-test for unpaired data is reported in Table 2.
Low ITA° values are ascribable to a brown pigmentation, while high ITA° values indicate a very light pigmentation; therefore, in this study, it is desirable to record an increase of ITA° that indicates a reduction of the dark spot intensity.
After 30 and 60 days of use, the efficacy of the tested formulation with DEX-RA in reducing the dark spot staining and in increasing the skin radiance resulted greater, in a statistically significant way, than the efficacy of the PLACEBO formulation at each experimental time.
Furthermore, the formulation with DEX-RA determined a reduction of the skin redness statistically significant compared to T0.
Figure 3 reports the percentage of subjects who showed/did not show an improvement after 30 and 60 days of products use in terms of reduction of dark spots appearance and skin complexion brightness.
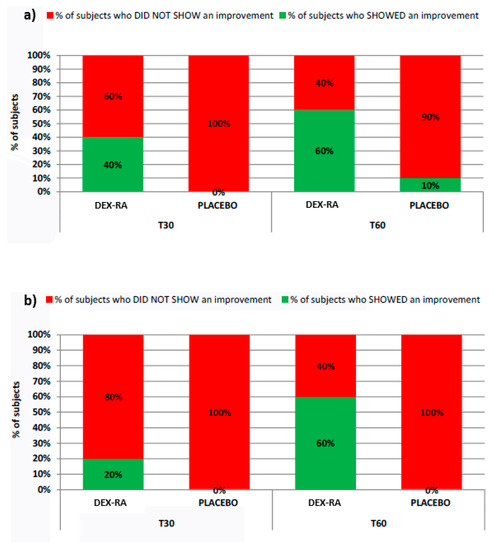
Figure 3.
Subjects who showed/did not show an improvement after 30 and 60 days of products use in terms of (a) reduction of dark spots appearance and (b) skin complexion brightness.
As it is possible to note, in both cases the 60% of the subjects have shown an improvement after 60 days of treatment with formulation containing DEX-RA.
3.3. Safety Assessment
3.3.1. Cell Viability by MTT Assay
The MTT assay represents a well-known colorimetric assay for the identification of cytotoxic and adverse cellular effects. This test allows to evaluate the activity of mitochondrial enzymes in healthy cells by measuring the absorbance of purple formazan. This MTT reduction product, indeed, is formed through an NADP-dependent reaction catalysed by succinate dehydrogenase in metabolically active cells [49].
Keratinocytes were incubated in the presence of DEX-RA, a dextran aqueous solution (DEX) and Origanum vulgare leaf extract at various doses for 24 h and, as shown in Figure 4, the polymeric conjugate had any effect on cell viability at the concentrations used during this study. These data confirmed the non-cytotoxic effect of the synthesized polymeric material.
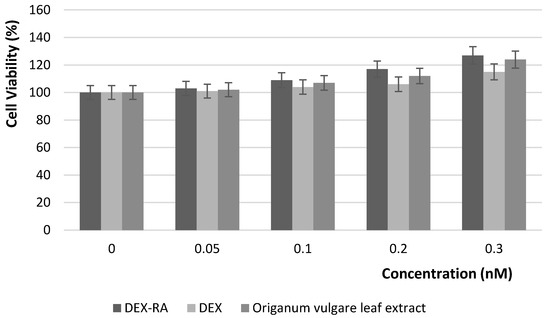
Figure 4.
Effect of Dex-RA, DEX and Origanum vulgare leaf extract on keratinocytes viability.
3.3.2. Assessment of Skin Irritation: EPISKIN™ Model
The cutaneous irritant potential of the synthesized conjugates was assessed by using the EPISKIN prediction model. For this purpose, the in vitro reconstructed human epidermis was treated with DEX-RA (0.1 nM) according to the “42 bis” procedure, which involves a 42 min exposure time followed by a rinsing step and a 42 h post-incubation period. Then, cell viability was quantify by MTT reduction and the obtained results are reported in Figure 5.
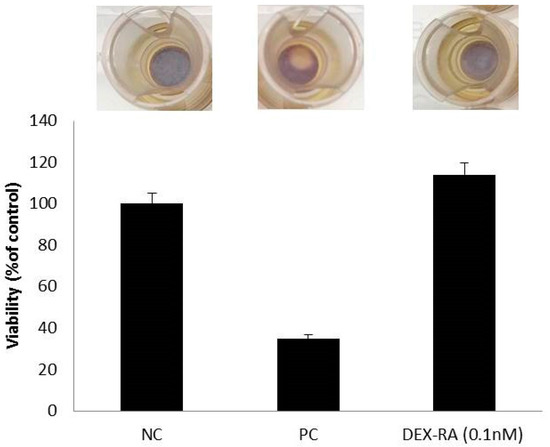
Figure 5.
Effect of synthesized DEX-RA conjugates on EPISKIN™ RHE/L/13 human skin.
The developed skin whitening agent did not show irritation effects in EPISKIN™ RHE/L/13 human skin equivalent.
4. Conclusions
The development and screening for novel effective and safe skin whitening agents have received considerable attention due to different reasons, such as cultural, aesthetic, and therapeutic motives.
This kind of products can be applied to a limited skin surface, such as neck or face, as well as to a larger body area in the aim to mitigate the effect of an irregular skin tone through the local depigmentation of dark spots or a generalized reduction of the skin tone.
In the present study, an innovative polymeric antioxidant to be used as a skin whitening agent was successfully developed by the conjugation of dextran, a natural biodegradable and biocompatible polysaccharide widely used in cosmetic and pharmaceutical formulations, with rosmarinic acid.
In vitro and in vivo studies confirmed the antioxidant and tyrosinase inhibitory activities, the whitening and lightening efficacy and the biocompatibility of the synthesized dextran-rosmarinic acid conjugates. Furthermore, the active polymer, compared to the free antioxidant, shows a long-lasting efficacy combined to an enhanced stability resulting in an improved performance of the cosmetic formulations prepared using this innovative whitening agent as a bioactive ingredient.
Acknowledgments
The authors thank the University of Calabria for the financial support of the work.
Author Contributions
Francesco Puoci and Vincenzo Pezzi designed and supervised the research; Fabio Amone, Mariarosa Ruffo, and Luca Scrivano performed the synthesis and tests; Rocco Malivindi performed the MTT and Episkin assays; Vincenzo Nobile performed the in vivo tests; Ortensia I. Parisi analysed the data and wrote the manuscript; and Rosella Malanchin, Cristiana Piangiolino, and Federica Carlomagno contributed to the study design.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lee, H.E.; Kim, E.H.; Choi, H.R.; Sohn, U.D.; Yun, H.Y.; Baek, K.J.; Kwon, N.S.; Park, K.C.; Kim, D.S. Dipeptides inhibit melanin synthesis in Mel-Ab cells through down-regulation of tyrosinase. Korean J. Physiol. Pharmacol. 2012, 16, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.S. Natural melanogenesis inhibitors acting through the down-regulation of tyrosinase activity. Materials 2012, 5, 1661–1685. [Google Scholar] [CrossRef]
- Parveen, I.; Threadgill, M.D.; Moorby, J.M.; Winters, A. Oxidative phenols in forage crops containing polyphenol oxidase enzymes. J. Agric. Food Chem. 2010, 58, 1371–1382. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.H.; Lin, R.D.; Hsu, F.L.; Huang, Y.H.; Chang, H.C.; Huang, C.Y.; Lee, M.H. Cosmetic applications of selected traditional Chinese herbal medicines. J. Ethnopharmacol. 2006, 106, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Passi, S.; Nazzaro-Porro, M. Molecular basis of substrate and inhibitory specificity of tyrosinase: Phenolic compounds. Br. J. Dermatol. 1981, 104, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.S.; Tsai, K.C.; Chen, W.C.; Wang, Y.T.; Lee, Y.C.; Lu, C.K.; Don, M.J.; Chang, C.Y.; Lee, C.H.; Lin, H.H. Discovery of Potent Cysteine-Containing Dipeptide Inhibitors against Tyrosinase: A Comprehensive Investigation of 20 × 20 Dipeptides in Inhibiting Dopachrome Formation. J. Agric. Food Chem. 2015, 63, 6181–6188. [Google Scholar] [CrossRef] [PubMed]
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, A.; Devkota, H.; Takano, A.; Masuda, K.; Nakane, T.; Basnet, P.; Skalko-Basnet, N. Screening of Nepalese crude drugs traditionally used to treat hyperpigmentation: In vitro tyrosinase inhibition. Int. J. Cosmet. Sci. 2008, 30, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Tai, S.S.K.; Lin, C.G.; Wu, M.H.; Chang, T.S. Evaluation of depigmenting activity by 8-hydroxydaidzein in mouse B16 melanoma cells and human volunteers. Int. J. Mol. Sci. 2009, 10, 4257–4266. [Google Scholar] [CrossRef] [PubMed]
- Ando, H.; Ichihashi, M.; Hearing, V.J. Role of the ubiquitin proteasome system in regulating skin pigmentation. Int. J. Mol. Sci. 2009, 10, 4428–4434. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, T.; Arndt, K.; El Mofty, A.; Pathak, M. Hydroquinone and psoralens in the therapy of hypermelanosis and vitiligo. Arch. Dermatol. 1966, 93, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.M.; Rho, H.S.; Baek, H.S.; Joo, Y.H.; Hong, Y.D.; Shin, S.S.; Park, Y.H.; Park, S.N. Inhibitory activity of novel kojic acid derivative containing trolox moiety on melanogenesis. Bioorg. Med. Chem. Lett. 2011, 21, 7466–7469. [Google Scholar] [CrossRef] [PubMed]
- Rigoni, C.; Toffolo, P.; Serri, R.; Caputo, R. Use of a cream based on 20% azelaic acid in the treatment of melasma. G. Ital. Dermatol. Venereol. 1989, 124, I–VI. [Google Scholar]
- Germanas, J.P.; Wang, S.; Miner, A.; Hao, W.; Ready, J.M. Discovery of small-molecule inhibitors of tyrosinase. Bioorg. Med. Chem. Lett. 2007, 17, 6871–6875. [Google Scholar] [CrossRef] [PubMed]
- Karamagi, C.; Owino, E.; Katabira, E. Hydroquinone neuropathy following use of skin bleaching creams: Case report. East Afr. Med. J. 2001, 78, 223–224. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Dreher, F.; Chew, A.; Zhai, H.; Levin, C.; Stern, R.; Maibach, H. Cutaneous bioassay of salicylic acid as a keratolytic. Int. J. Pharm. 2005, 292, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Mahe, A.; Ly, F.; Aymard, G.; Dangou, J.M. Skin diseases associated with the cosmetic use of bleaching products in women from Dakar, Senegal. Br. J. Dermatol. 2003, 148, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Campillo, M.; Gabaldon, J.; Castillo, J.; Benavente-García, O.; Del Baño, M.; Alcaraz, M.; Vicente, V.; Alvarez, N.; Lozano, J. Rosmarinic acid, a photo-protective agent against UV and other ionizing radiations. Food Chem. Toxicol. 2009, 47, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Casanova, F.; Estevinho, B.; Santos, L. Preliminary studies of rosmarinic acid microencapsulation with chitosan and modified chitosan for topical delivery. Powder Technol. 2016, 297, 44–49. [Google Scholar] [CrossRef]
- Ding, H.Y.; Chou, T.H.; Liang, C.H. Antioxidant and antimelanogenic properties of rosmarinic acid methyl ester from Origanum vulgare. Food Chem. 2010, 123, 254–262. [Google Scholar] [CrossRef]
- Fujimoto, A.; Masuda, T. Antioxidation mechanism of rosmarinic acid, identification of an unstable quinone derivative by the addition of odourless thiol. Food Chem. 2012, 132, 901–906. [Google Scholar] [CrossRef]
- Kang, H.S.; Byun, D.S.; Choi, J.S. Rosmarinic acid as a tyrosinase inhibitors from Salvia miltiorrhiza. Nat. Prod. Sci. 2004, 10, 80–84. [Google Scholar]
- Funasaka, Y.; Komoto, M.; Ichihashi, M. Depigmenting Effect of α-Tocopheryl Ferulate on Normal Human Melanocytes. Pigment Cell Res. 2000, 13, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Boyadzhiev, L.; Dimitrova, V. Extraction and liquid membrane preconcentration of rosmarinic acid from lemon balm (Melissa officinalis L.). Sep. Sci. Technol. 2006, 41, 877–886. [Google Scholar] [CrossRef]
- Sutherland, I.W. Novel and established applications of microbial polysaccharides. Trends Biotechnol. 1998, 16, 41–46. [Google Scholar] [CrossRef]
- Peppas, N.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Puoci, F.; Iemma, F.; Pezzi, V.; Picci, N.; Cirillo, G.; Curcio, M.; Parisi, O.I. Cosmetic, Pharmaceutical or Nutraceutical Formulations Containing Antioxidant Molecules and Polymeric Materials. Patent EP2535087A2, 19 December 2012. [Google Scholar]
- Pagliarulo, C.; De Vito, V.; Picariello, G.; Colicchio, R.; Pastore, G.; Salvatore, P.; Volpe, M.G. Inhibitory effect of pomegranate (Punica granatum L.) polyphenol extracts on the bacterial growth and survival of clinical isolates of pathogenic Staphylococcus aureus and Escherichia coli. Food Chem. 2016, 190, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Puoci, F.; Morelli, C.; Cirillo, G.; Curcio, M.; Parisi, O.I.; Maris, P.; Sisci, D.; Picci, N. Anticancer activity of a quercetin-based polymer towards HeLa cancer cells. Anticancer Res. 2012, 32, 2843–2847. [Google Scholar] [PubMed]
- Vittorio, O.; Cirillo, G.; Iemma, F.; Di Turi, G.; Jacchetti, E.; Curcio, M.; Barbuti, S.; Funel, N.; Parisi, O.I.; Puoci, F. Dextran-catechin conjugate: A potential treatment against the pancreatic ductal adenocarcinoma. Pharm. Res. 2012, 29, 2601–2614. [Google Scholar] [CrossRef] [PubMed]
- Rangkadilok, N.; Sitthimonchai, S.; Worasuttayangkurn, L.; Mahidol, C.; Ruchirawat, M.; Satayavivad, J. Evaluation of free radical scavenging and antityrosinase activities of standardized longan fruit extract. Food Chem. Toxicol. 2007, 45, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Iwai, K.; Kishimoto, N.; Kakino, Y.; Mochida, K.; Fujita, T. In vitro antioxidative effects and tyrosinase inhibitory activities of seven hydroxycinnamoyl derivatives in green coffee beans. J. Agric. Food Chem. 2004, 52, 4893–4898. [Google Scholar] [CrossRef] [PubMed]
- Millipore, M. Substituting Strat-M® Membrane for Human Skin in Evaluating Effect of Encapsulation on Diffusion of Sunscreen Formulations; Application Note [Online]; EMD Millipore: Billerica, MA, USA, 2013. [Google Scholar]
- Zanta, C.; Martínez-Huitle, C. Degradation of 2-hydroxybenzoic acid by advanced oxidation processes. Braz. J. Chem. Eng. 2009, 26, 503–513. [Google Scholar] [CrossRef]
- Frost, L.; Suryadevara, P.; Cannell, S.J.; Groundwater, P.W.; Hambleton, P.A.; Anderson, R.J. Synthesis of diacylated γ-glutamyl-cysteamine prodrugs, and in vitro evaluation of their cytotoxicity and intracellular delivery of cysteamine. Eur. J. Med. Chem. 2016, 109, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Parisi, O.I.; Aiello, D.; Casula, M.F.; Puoci, F.; Malivindi, R.; Scrivano, L.; Testa, F. Mesoporous nanocrystalline TiO2 loaded with ferulic acid for sunscreen and photo-protection: Safety and efficacy assessment. RSC Adv. 2016, 6, 83767–83775. [Google Scholar] [CrossRef]
- Yang, Y.; Peng, Q.; Guo, Y.; Han, Y.; Xiao, H.; Zhou, Z. Isolation and characterization of dextran produced by Leuconostoc citreum NM105 from manchurian sauerkraut. Carbohydr. Polym. 2015, 133, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Parisi, O.I.; Puoci, F.; Iemma, F.; Curcio, M.; Cirillo, G.; Spizzirri, U.G.; Picci, N. Flavonoids preservation and release by methacrylic acid-grafted (N-vinyl-pyrrolidone). Pharm. Dev. Technol. 2013, 18, 1058–1065. [Google Scholar] [CrossRef] [PubMed]
- Iemma, F.; Puoci, F.; Curcio, M.; Parisi, O.I.; Cirillo, G.; Spizzirri, U.G.; Picci, N. Ferulic acid as a comonomer in the synthesis of a novel polymeric chain with biological properties. J. Appl. Polym. Sci. 2010, 115, 784–789. [Google Scholar] [CrossRef]
- Della Pelle, F.; Vilela, D.; González, M.C.; Sterzo, C.L.; Compagnone, D.; Del Carlo, M.; Escarpa, A. Antioxidant capacity index based on gold nanoparticles formation. Application to extra virgin olive oil samples. Food Chem. 2015, 178, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.F.; Rouse, J.; Sanderson, D.; Eccleston, G. A comparative study of transmembrane diffusion and permeation of ibuprofen across synthetic membranes using Franz diffusion cells. Pharmaceutics 2010, 2, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Siewert, M.; Dressman, J.; Brown, C.K.; Shah, V.P.; Aiache, J.-M.; Aoyagi, N.; Bashaw, D.; Brown, C.; Brown, W.; Burgess, D. FIP/AAPS guidelines to dissolution/in vitro release testing of novel/special dosage forms. AAPS PharmSciTech. 2003, 4, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.P.; Elkins, J.S.; Williams, R.L. Evaluation of the test system used for in vitro release of drugs for topical dermatological drug products. Pharm. Dev. Technol. 1999, 4, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Ueda, C.T.; Shah, V.P.; Derdzinski, K.; Ewing, G.; Flynn, G.; Maibach, H.; Marques, M.; Rytting, H.; Shaw, S.; Thakker, K. Topical and transdermal drug products. Pharm. Forum 2009, 35, 750–764. [Google Scholar] [CrossRef]
- Legrini, O.; Oliveros, E.; Braun, A. Photochemical processes for water treatment. Chem. Rev. 1993, 93, 671–698. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Liu, Y.; Nair, M.G. An efficient and economical MTT assay for determining the antioxidant activity of plant natural product extracts and pure compounds. J. Nat. Prod. 2010, 73, 1193–1195. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).