GHK-Cu may Prevent Oxidative Stress in Skin by Regulating Copper and Modifying Expression of Numerous Antioxidant Genes
Abstract
:1. Introduction
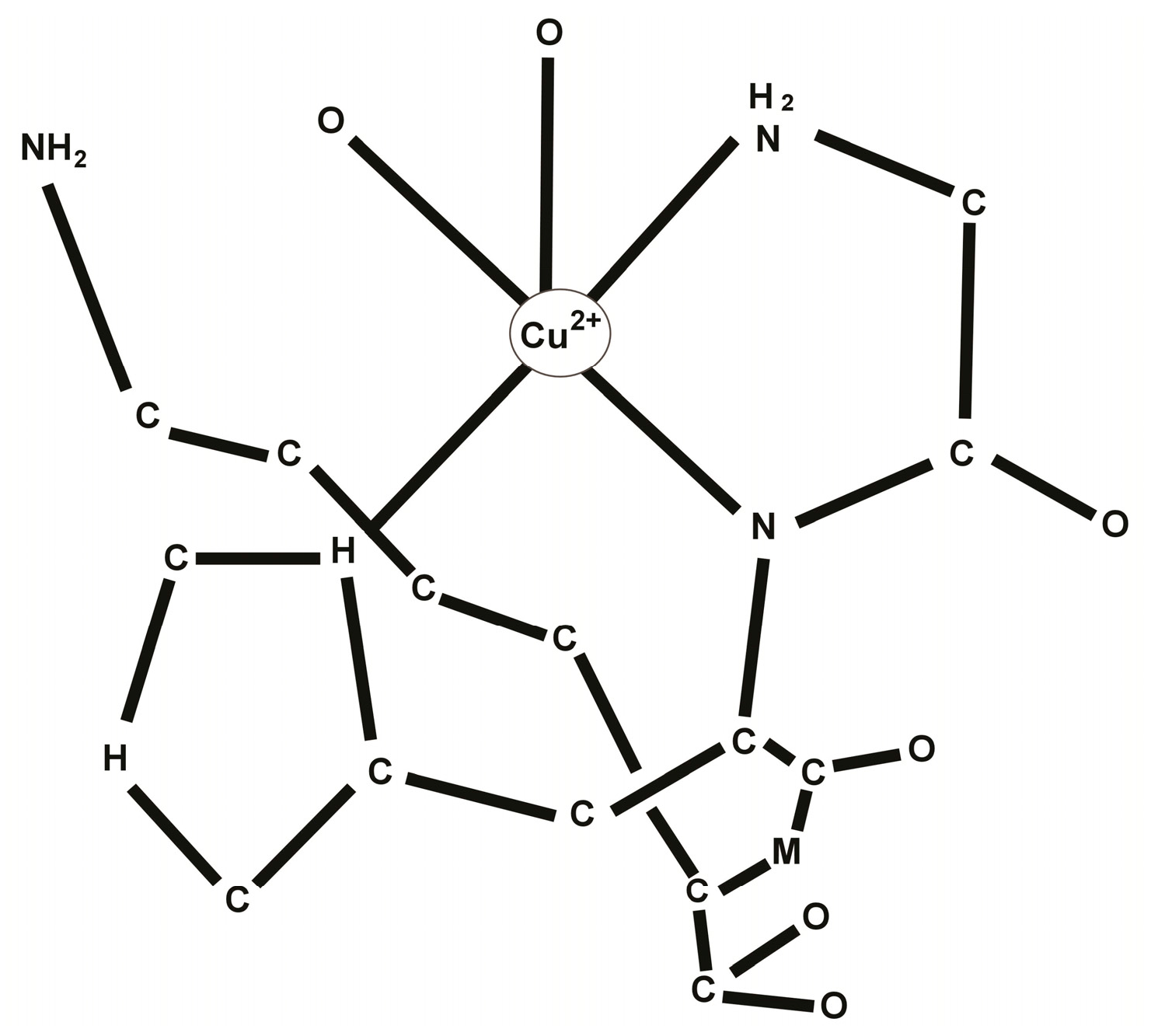
2. Chemistry of the GHK-Cu Complex
3. Biology of the GHK-Cu Complex
4. GHK as a Gene Expression Modifier
5. Wound Healing and Tissue Remodeling Activity of GHK-Cu
6. Antioxidant Activity of GHK-Cu
6.1. Synthesis of GHK-Cu Analogs with Higher Anti-ROS Activity
| Chemical Structure | SOD-Mimetic Activity Compared to GHK:Cu(2+) |
|---|---|
| GHK:Cu(2+) | 100 (base line) |
| KHG-Amide:Cu(2+) | 21 |
| GHKAFA:Cu(2+) | 561 |
| AHK:Cu(2+) | 563 |
| GHK-Octyl Ester:Cu(2+) | 810 |
| GHCaprolactam:Cu(2+) | 4500 |
| HGK:Cu(2+) | 22,300 |
6.2. Antioxidant Gene Expression Analysis
| Percent Change | Genes Stimulated | Genes Suppressed |
|---|---|---|
| 50%–99% | 1569 | 583 |
| 100%–199% | 646 | 469 |
| 200%–299 | 227 | 196 |
| 300%–599% | 196 | 207 |
| 600%–899% | 39 | 42 |
| 900%–1199% | 8 | 7 |
| 1200% or more | 2 | 4 |
| Genes | Percent Change in Gene Expression * | Comments |
|---|---|---|
| TLE1 | 762 | Inhibits the oxidative/inflammatory gene NF-κB [43]. |
| SPRR2C | 721 | This proline-rich, antioxidant protein protects outer skin cells from oxidative damage from ROS. When the ROS level is low, the protein remains in the outer cell membrane, but when the ROS level is high, the protein clusters around the cell’s DNA to protect it [44,45]. |
| ITGB4 | 609 | Up-regulation of ITGB4 promotes wound repair ability and antioxidative ability [46]. |
| APOM | 403 | Binds oxidized phospholipids and increases the antioxidant effect of high-density lipoproteins (HDL) [47]. |
| PON3 | 319 | Absence of PON3 (paraoxonase 3) in mice resulted in increased rates of early fetal and neonatal death. Knockdown of PON3 in human cells reduced cell proliferation and total antioxidant capacity [48]. |
| IL18BP | 295 | The protein encoded by this gene is an inhibitor of the pro-inflammatory cytokine IL18. IL18BP abolished IL18 induction of interferon-gamma (IFNgamma), IL8, and activation of NF-κB in vitro. Blocks neutrophil oxidase activity [49]. |
| HEPH | 217 | Inhibits the conversion of Fe(2+) to Fe(3+). HEPH increases iron efflux, lowers cellular iron levels, suppresses reactive oxygen species production, and restores mitochondrial transmembrane potential [50]. |
| GPSM3 | 193 | Acts as a direct negative regulator of NLRP3. NLRP3 triggers the maturation of the pro-inflammatory cytokines IL-1β and IL-18 [51]. |
| FABP1 | 186 | Reduces intracellular ROS level. Plays a significant role in reduction of oxidative stress [52,53]. |
| PON1 | 149 | PON1 (paraoxonase 1) is a potent antioxidant and a major anti-atherosclerotic component of HDL [54]. |
| MT3 | 142 | Metallothioneins (MTs) display in vitro free radical scavenging capacity, suggesting that they may specifically neutralize hydroxyl radicals. Metallothioneins and metallothionein-like proteins isolated from mouse brain act as neuroprotective agents by scavenging superoxide radicals [55,56]. |
| PTGS2 | 120 | Produces cyclooxygenase-II (COX-II), which has antioxidant activities [57]. |
| SLC2A9 | 117 | The p53-SLC2A9 pathway is a novel antioxidant mechanism. During oxidative stress, SLC2A9 undergoes p53-dependent induction, and functions as an antioxidant by suppressing ROS, DNA damage, and cell death [58]. |
| NFE2L2 | 56 | Nuclear respiratory factor 2 helps activate antioxidant responsive element-regulated genes which contribute to the regulation of the cellular antioxidant defense systems [59]. |
| PTGS1 | 50 | Produces cyclooxygenase-I (COX-I), which has antioxidant activity [57]. |
| TNF | −115 | GHK suppresses this pro-oxidant TNF gene [60]. |
| IL17A | −1018 | This cytokine can stimulate the expression of IL6 and cyclooxygenase-2 (PTGS2/COX-2), as well as enhance the production of nitric oxide (NO). High levels of this cytokine are associated with several chronic inflammatory diseases including rheumatoid arthritis, psoriasis, and multiple sclerosis (http://www.ncbi.nlm.nih.gov/gene/). |
7. Conclusions
Author Contributions
Conflicts of Interest
References
- Young, I.S.; Woodside, J.V. Antioxidants in health and disease. J. Clin. Pathol. 2001, 54, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Godic, A.; Poljsak, B.; Adamic, M.; Dahmane, R. The role of antioxidants in skin cancer prevention and treatment. Oxid. Med. Cell. Longev. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Wen, L.L.; Huang, Y.N.; Chen, Y.T.; Ku, M.C. Dual effects of antioxidants in neurodegeneration: Direct neuroprotection against oxidative stress and indirect protection via suppression of glia-mediated inflammation. Curr. Pharm. Des. 2006, 12, 3521–3533. [Google Scholar] [CrossRef] [PubMed]
- Pickart, L. The human tri-peptide GHK and tissue remodeling. J. Biomater. Sci. Polym. Ed. 2008, 19, 969–988. [Google Scholar] [CrossRef] [PubMed]
- Pickart, L.; Pickart, F. A possible mechanism whereby skin remodeling may suppress cancer metastasis genes. In Wound Repair and Regeneration; Wiley-Blackwell: Malden, MA, USA, 2011; Volume 19, p. A62. [Google Scholar]
- Pickart, L. A Tripeptide in Human Plasma that Increases the Survival of Hepatocytes and the Growth of Hepatoma Cells. Ph.D. Thesis, University of California, San Francisco, CA, USA, 1973. [Google Scholar]
- Pickart, L.; Thaler, M.M. Growth-modulating tripeptide (glycylhistidyllysine): Association with copper and iron in plasma, and stimulation of adhesiveness and growth of hepatoma cells in culture by tripeptide-metal ion complexes. J. Cell. Physiol. 1980, 102, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, D.; Pickart, L.; Thaler, M. Growth-modulating serum tripeptide is glycyl-histidyl-lysine. Cell. Mol. Life Sci. 1977, 33, 324–325. [Google Scholar] [CrossRef]
- Lane, T.; Iruela-Arispe, M.; Johnson, R.; Sage, E. SPARC is a source of copper-binding peptides that stimulate angiogenesis. J. Cell. Biol. 1994, 125, 929–943. [Google Scholar] [CrossRef] [PubMed]
- Pickart, L.; Freedman, J.; Loker, W.; Peisach, J.; Perkins, C.; Stenkamp, R.; Weinstein, B. Growth-modulating plasma tripeptide may function by facilitating copper uptake into cells. Nature 1980, 288, 715–717. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.; Sarkar, B. The interaction of copper(II) and glycyl-l-histidyl-l-lysine, a growth-modulating tripeptide from plasma. Biochem. J. 1981, 199, 649–656. [Google Scholar] [PubMed]
- Pickart, L. The use of glycylhistidyllysine in culture systems. In Vitro 1981, 17, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.V.; Cheng, Y. Glycyl-l-histidyl-l-lysine, a growth promoting factor for human cells. Cytobios 1979, 27, 19–25. [Google Scholar]
- Barra, R. Effects of glycyl-histidyl-lysine on Morris hepatoma 7777 cells. Cytobios 1986, 52, 99–107. [Google Scholar]
- Pollard, J.; Quan, S.; Kang, T.; Koch, R. Effects of copper tripeptide on the growth and expression of growth factors by normal and irradiated fibroblasts. Arch. Facial Plast. Surg. 2005, 7, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Lamb, J. The connectivity map: A new tool for biomedical research. Nat. Rev. Cancer 2007, 7, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Iorio, F.; Bosotti, R.; Scacheri, E.; Belcastro, V.; Mithbaokar, P.; Ferriero, R.; Murino, L.; Tagliaferri, R.; Brunetti-Pierri, N.; Isacchi, A.; et al. Discovery of drug mode of action and drug repositioning from transcriptional responses. Proc. Natl. Acad. Sci. 2010, 107, 14621–14626. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Downey, T.; Eu, K.; Koh, P.; Cheah, P. A “metastasis-prone” signature for early-stage mismatch-repair proficient sporadic colorectal cancer patients and its implications for possible therapeutics. Clin. Exp. Metastasis 2010, 27, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.D.; McDonough, J.E.; Zeskind, J.E.; Hackett, T.L.; Pechkovsky, D.V.; Brandsma, C.A.; Suzuki, M.; Gosselink, J.V.; Liu, G.; Alekseyev, Y.O.; et al. A gene expression signature of emphysema-related lung destruction and its reversal by the tripeptide GHK. Genome Med. 2012, 4, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Pickart, L.; Vasquez-Soltero, J.M.; Margolina, A. GHK and DNA: Resetting the human genome to health. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Pickart, L. Use of GHL-Cu as a Wound-Healing and Anti-Inflammatory Agent. U.S. Patent No. 4,760,051, 26 July 1988. [Google Scholar]
- Downey, D.; Larrabee, W.; Voci, V.; Pickart, L. Acceleration of wound healing using glycyl-histidyl-lysine copper (II). Surg. Forum 1985, 25, 573–575. [Google Scholar]
- Maquart, F.; Pickart, L.; Laurent, M.; Gillery, P.; Monboisse, J.; Borel, J. Stimulation of collagen synthesis in fibroblast cultures by the tripeptide-copper complex glycyl-l-histidyl-l-lysine-Cu2+. FEBS Lett. 1988, 238, 343–346. [Google Scholar] [CrossRef]
- Maquart, F.; Bellon, G.; Chaqour, B.; Wegrowski, J.; Patt, L.M.; Trachy, R.; Monboisse, J.; Chastang, F.; Birembaut, P.; Gillery, P.; et al. In vivo stimulation of connective tissue accumulation by the tripeptide-copper complex glycyl-l-histidyl-l-lysine-Cu2+ in rat experimental wounds. J. Clin. Invest. 1993, 92, 2368–2376. [Google Scholar] [CrossRef] [PubMed]
- Wegrowski, Y.; Maquart, F.; Borel, J. Stimulation of sulfated glycosaminoglycan synthesis by the tripeptide-copper complex glycyl-l-histidyl-l-lysine-Cu2+. Life Sci. 1992, 51, 1049–1056. [Google Scholar] [CrossRef]
- Simeon, A.; Emonard, H.; Hornebeck, W.; Maquart, F. The tripeptide-copper complex glycyl-l-histidyl-l-lysine-Cu2+ stimulates matrix metalloproteinase-2 expression by fibroblast cultures. Life Sci. 2000, 67, 2257–2265. [Google Scholar] [CrossRef]
- Simeon, A.; Wegrowski, Y.; Bontemps, Y.; Maquart, F. Expression of glycosaminoglycans and small proteoglycans in wounds: Modulation by the tripeptide-copper complex glycyl-l-histidyl-l-lysine-Cu(2+). J. Invest. Dermatol. 2000, 115, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Gul, N.Y.; Topal, A.; Cangul, I.T.; Yanik, K. The effects of topical tripeptide copper complex and helium-neon laser on wound healing in rabbits. Vet. Dermatol. 2008, 19, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Cangul, I.T.; Gul, N.Y.; Topal, A.; Yilmaz, R. Evaluation of the effects of topical tripeptide-copper complex and zinc oxide on open-wound healing in rabbits. Vet. Dermatol. 2006, 17, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Pickart, L. Method of Using Copper(II) Containing Compounds to Accelerate Wound Healing. U.S. Patent No. 5,164,367, 17 November 1992. [Google Scholar]
- Arul, V.; Kartha, R.; Jayakumar, R. A therapeutic approach for diabetic wound healing using biotinylated GHK incorporated collagen matrices. Life Sci. 2007, 80, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Canapp, S.J.; Farese, J.; Schultz, G.; Gowda, S.; Ishak, A.; Swaim, S.; Vangilder, J.; Lee-Ambrose, L.; Martin, F. The effect of topical tripeptide-copper complex on healing of ischemic open wounds. Vet. Surg. 2003, 32, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Swaim, S.; Vaughn, D.; Kincaid, S.; Morrison, N.; Murray, S.; Woodhead, M.; Hoffman, C.; Wright, J.; Kammerman, J. Effect of locally injected medications on healing of pad wounds in dogs. Am. J. Vet. Res. 1996, 57, 394–399. [Google Scholar] [PubMed]
- Thomas, C.E. The influence of medium components on Cu2+-dependent oxidation of low-density lipoproteins and its sensitivity to superoxide dismutase. Biochim. Biophys. Acta 1992, 1128, 50–57. [Google Scholar] [CrossRef]
- Beretta, G.; Arlandini, E.; Artali, R.; Anton, J.M.; Maffei Facino, R. Acrolein sequestering ability of the endogenous tripeptide glycyl-histidyl-lysine (GHK): Characterization of conjugation products by ESI-MSn and theoretical calculations. J. Pharm Biomed. Anal. 2008, 47, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Beretta, G.; Artali, R.; Regazzoni, L.; Panigati, M.; Facino, R.M. Glycyl-histidyl-lysine (GHK) is a quencher of α,β-4-hydroxy-trans-2-nonenal: A comparison with carnosine. Insights into the mechanism of reaction by electrospray ionization mass spectrometry, 1H NMR, and computational techniques. Chem. Res. Toxicol. 2007, 20, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Cebrian, J.; Messeguer, A.; Facino, R.; Garcia Anton, J. New anti-RNS and -RCS products for cosmetic treatment. Int. J. Cosmet. Sci. 2005, 27, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Smakhtin, M.; Konoplia, A.; Sever’ianova, L.; Shveinov, I. [Pharmacological correction of immuno-metabolic disorders with the peptide Gly-His-Lys in hepatic damage induced by tetrachloromethane]. Patol. Fiziol. Eksp. Ter. Russ. 2003, 2, 19–21. [Google Scholar]
- Cherdakov, V.Y.; Smakhtin, M.Y.; Dubrovin, G.M.; Dudka, V.T.; Bobyntsev, I.I. Synergetic antioxidant and reparative action of thymogen, dalargin and peptide Gly-His-Lys in tubular bone fractures. Exp. Biol. Med. 2010, 4, 15–20. [Google Scholar]
- Miller, D.M.; DeSilva, D.; Pickart, L.; Aust, S.D. Effects of glycyl-histidyl-lysyl chelated Cu(II) on ferritin dependent lipid peroxidation. Adv. Exp. Med. Biol. 1990, 264, 79–84. [Google Scholar] [PubMed]
- Pickart, L. Anti-Oxidative and Anti-Inflammatory Metal:Peptide Complexes and Uses Thereof. U.S. Patent No. 07/478,091, 2 June 1992. [Google Scholar]
- Pickart, L.; Vasquez-Soltero, J.M.; Pickart, F.D.; Majnarich, J. GHK, the human skin remodeling peptide, induces anti-cancer expression of numerous caspase, growth regulatory, and DNA repair genes. J. Anal. Oncol. 2014, 3, 79–87. [Google Scholar] [CrossRef]
- Mariappan, N.; Elks, C.M.; Sriramula, S.; Guggilam, A.; Liu, Z.; Borkhsenious, O.; Francis, J. NF-kappaB-induced oxidative stress contributes to mitochondrial and cardiac dysfunction in type II diabetes. Cardiovasc. Res. 2010, 85, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Vermeij, W.P.; Florea, B.I.; Isenia, S.; Alia, A.; Brouwer, J.; Backendorf, C. Proteomic identification of in vivo interactors reveals novel function of skin cornification proteins. J. Proteome Res. 2012, 11, 3068–3076. [Google Scholar] [CrossRef] [PubMed]
- Vermeij, W.P.; Alia, A.; Backendorf, C. ROS quenching potential of the epidermal cornified cell envelope. J. Invest. Dermatol. 2011, 131, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, H.J.; Xiang, Y.; Tan, Y.R.; Zhu, X.L.; Qin, X.Q. Wound repair and anti-oxidative capacity is regulated by ITGB4 in airway epithelial cells. Mol. Cell. Biochem. 2010, 341, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Elsoe, S.; Ahnstrom, J.; Christoffersen, C.; Hoofnagle, A.N.; Plomgaard, P.; Heinecke, J.W.; Binder, C.J.; Bjorkbacka, H.; Dahlback, B.; Nielsen, L.B. Apolipoprotein M binds oxidized phospholipids and increases the antioxidant effect of HDL. Atherosclerosis 2012, 221, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Kempster, S.L.; Belteki, G.; Licence, D.; Charnock-Jones, D.S.; Smith, G.C. Disruption of paraoxonase 3 impairs proliferation and antioxidant defenses in human A549 cells and causes embryonic lethality in mice. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E103–E107. [Google Scholar] [CrossRef] [PubMed]
- Novick, D.; Kim, S.H.; Fantuzzi, G.; Reznikov, L.L.; Dinarello, C.A.; Rubinstein, M. Interleukin-18 binding protein: A novel modulator of the Th1 cytokine response. Immunity 1999, 10, 127–136. [Google Scholar] [CrossRef]
- Song, N.; Wang, J.; Jiang, H.; Xie, J. Ferroportin1 and hephaestin overexpression attenuate iron-induced oxidative stress in MES23.5 dopaminergic cells. J. Cell. Biochem. 2010, 110, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Giguere, P.M.; Gall, B.J.; Ezekwe, E.A., Jr.; Laroche, G.; Buckley, B.K.; Kebaier, C.; Wilson, J.E.; Ting, J.P.; Siderovski, D.P.; Duncan, J.A. G Protein signaling modulator-3 inhibits the inflammasome activity of NLRP3. J. Biol. Chem. 2014, 289, 33245–33257. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Wang, G.; Gong, Y.; Yan, J.; Chen, Y.; Burczynski, F.J. Hepatoprotective role of liver fatty acid binding protein in acetaminophen induced toxicity. BMC Gastroenterol. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Gong, Y.; Anderson, J.; Sun, D.; Minuk, G.; Roberts, M.S.; Burczynski, F.J. Antioxidative function of L-FABP in L-FABP stably transfected Chang liver cells. Hepatology 2005, 42, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Rosenblat, M.; Karry, R.; Aviram, M. Paraoxonase 1 (PON1) is a more potent antioxidant and stimulant of macrophage cholesterol efflux, when present in HDL than in lipoprotein-deficient serum: Relevance to diabetes. Atherosclerosis 2006, 187, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Slikker, W., Jr.; Ali, S.F. Role of metallothionein and other antioxidants in scavenging superoxide radicals and their possible role in neuroprotection. Neurochem. Int. 1996, 29, 145–152. [Google Scholar] [CrossRef]
- Viarengo, A.; Burlando, B.; Ceratto, N.; Panfoli, I. Antioxidant role of metallothioneins: A comparative overview. Cell. Mol. Biol. 2000, 46, 407–417. [Google Scholar] [PubMed]
- Henry, G.E.; Momin, R.A.; Nair, M.G.; Dewitt, D.L. Antioxidant and cyclooxygenase activities of fatty acids found in food. J. Agric. Food Chem. 2002, 50, 2231–2234. [Google Scholar] [CrossRef] [PubMed]
- Itahana, Y.; Han, R.; Barbier, S.; Lei, Z.; Rozen, S.; Itahana, K. The uric acid transporter SLC2A9 is a direct target gene of the tumor suppressor p53 contributing to antioxidant defense. Oncogene 2015, 34, 1799–1810. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Kunsch, C. Induction of cytoprotective genes through Nrf2/antioxidant response element pathway: A new therapeutic approach for the treatment of inflammatory diseases. Curr. Pharm. Des. 2004, 10, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Finkley, M.; Appa, Y.; Bhandarkar, S. Copper peptide and skin. In Cosmeceuticals and Active Cosmetics: Drugs vs. Cosmetics; Elsner, P., Maibach, H., Eds.; Marcel Dekker: New York, NY, USA, 2005; pp. 549–563. [Google Scholar]
- Mariappan, N.; Soorappan, R.N.; Haque, M.; Sriramula, S.; Francis, J. TNF-alpha-induced mitochondrial oxidative stress and cardiac dysfunction: Restoration by superoxide dismutase mimetic Tempol. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H2726–H2737. [Google Scholar] [CrossRef] [PubMed]
- Hostynek, J.; Dreher, F.; Maibach, H. Human skin penetration of a copper tripeptide in vitro as a function of skin layer. Inflamm. Res. 2011, 60, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Nielsen, H.M.; Mullertz, A. Oral delivery of peptides and proteins using lipid-based drug delivery systems. Expert Opin. Drug Deliv. 2012, 9, 1289–1304. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, J.; Ehrhardt, C. Liposomal delivery of proteins and peptides. Expert Opin. Drug Deliv. 2012, 9, 1489–1503. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pickart, L.; Vasquez-Soltero, J.M.; Margolina, A. GHK-Cu may Prevent Oxidative Stress in Skin by Regulating Copper and Modifying Expression of Numerous Antioxidant Genes. Cosmetics 2015, 2, 236-247. https://doi.org/10.3390/cosmetics2030236
Pickart L, Vasquez-Soltero JM, Margolina A. GHK-Cu may Prevent Oxidative Stress in Skin by Regulating Copper and Modifying Expression of Numerous Antioxidant Genes. Cosmetics. 2015; 2(3):236-247. https://doi.org/10.3390/cosmetics2030236
Chicago/Turabian StylePickart, Loren, Jessica Michelle Vasquez-Soltero, and Anna Margolina. 2015. "GHK-Cu may Prevent Oxidative Stress in Skin by Regulating Copper and Modifying Expression of Numerous Antioxidant Genes" Cosmetics 2, no. 3: 236-247. https://doi.org/10.3390/cosmetics2030236
APA StylePickart, L., Vasquez-Soltero, J. M., & Margolina, A. (2015). GHK-Cu may Prevent Oxidative Stress in Skin by Regulating Copper and Modifying Expression of Numerous Antioxidant Genes. Cosmetics, 2(3), 236-247. https://doi.org/10.3390/cosmetics2030236






