Evaluating Efficacy and Tolerability of a New Intradermal Biorejuvenation with Free Hyaluronic Acid and Glycerol in Photoaging: A Retrospective Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Methodology
2.2. Study Population
2.3. Objectives
2.3.1. Primary Objective
2.3.2. Secondary Objectives
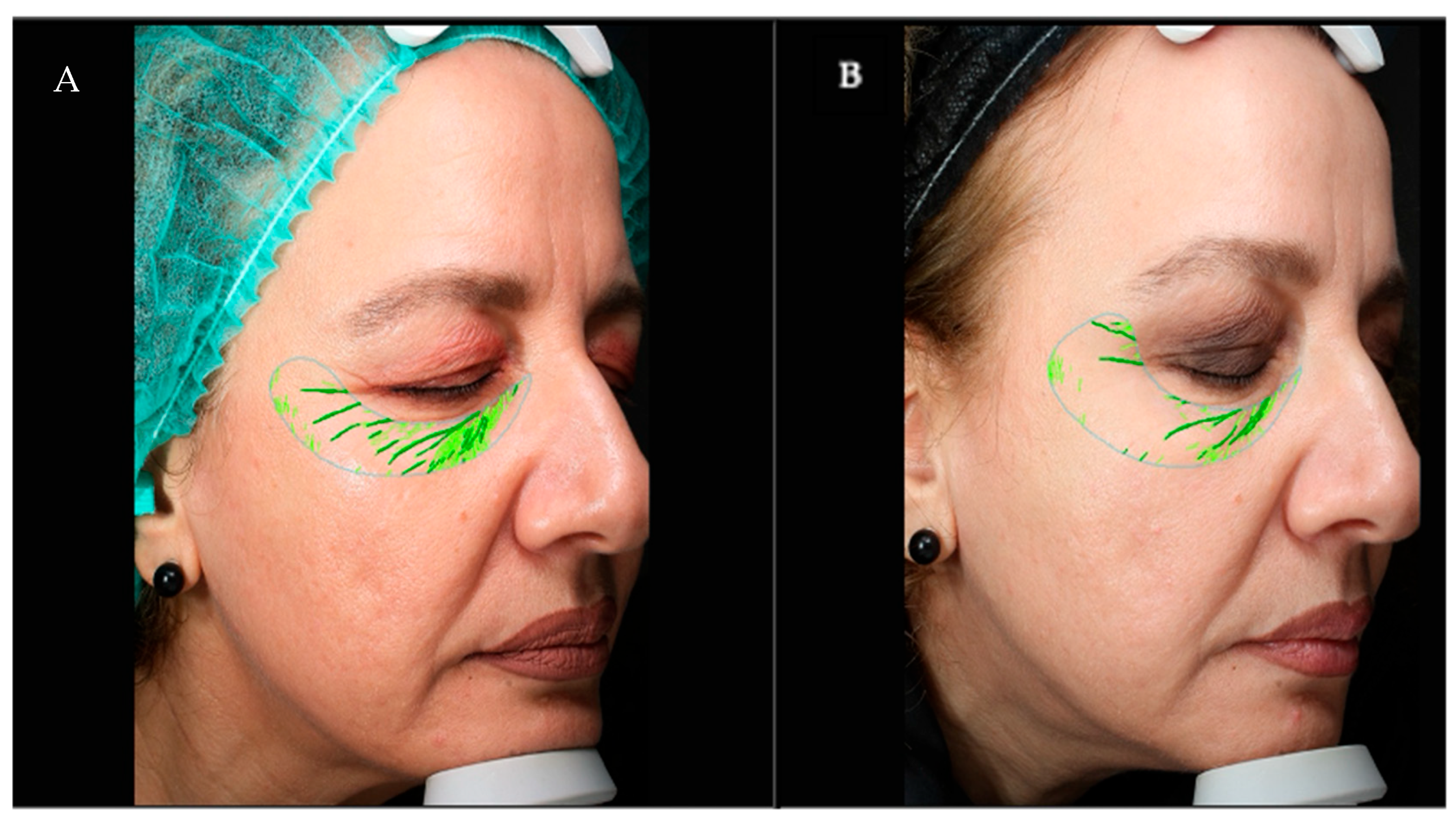
2.4. Procedures
2.4.1. Injection Protocols
- Protocol A (Standard): Three treatment sessions were carried out at two-week intervals. The traditional picotage technique was used, involving approximately 50 microinjections (0.02 mL per injection) evenly distributed across the treatment area. This approach is well established in clinical biorejuvenation and is associated with significant regenerative effects, although it may involve a slightly greater procedural complexity and recovery time compared to less intensive techniques [41].
- Protocol B (Customized): Two treatment sessions were conducted, also spaced two weeks apart. Each session consisted of four injection points in total (two per hemiface), with 0.25 mL of product administered per point. Injection sites were customized based on individual photoaging patterns to optimize diffusion and reduce procedural invasiveness. Following injection, a gentle manual massage was applied to promote homogeneous intradermal diffusion of the product.
2.4.2. Instrumental Assessment
2.4.3. Standardized Home Skincare Routine
2.5. Data Analysis and Statistical Methods
Exploratory Morphological Classification
- Cluster 1: centrally distributed, relatively homogeneous depressions with preserved facial symmetry;
- Cluster 2: diffuse or peripheral irregularities with heterogeneous morphology;
- Cluster 3: multiple persistent irregular depressions.
2.6. Ethical Considerations
3. Results
3.1. Visia® CR—Skin Quality Parameters
3.2. PRIMOS—Volumetric Analysis
3.3. PRIMOS—Roughness Metrics
3.4. PRIMOS—Color by Distance
3.5. PRIMOS—Exploratory Morphological Classification
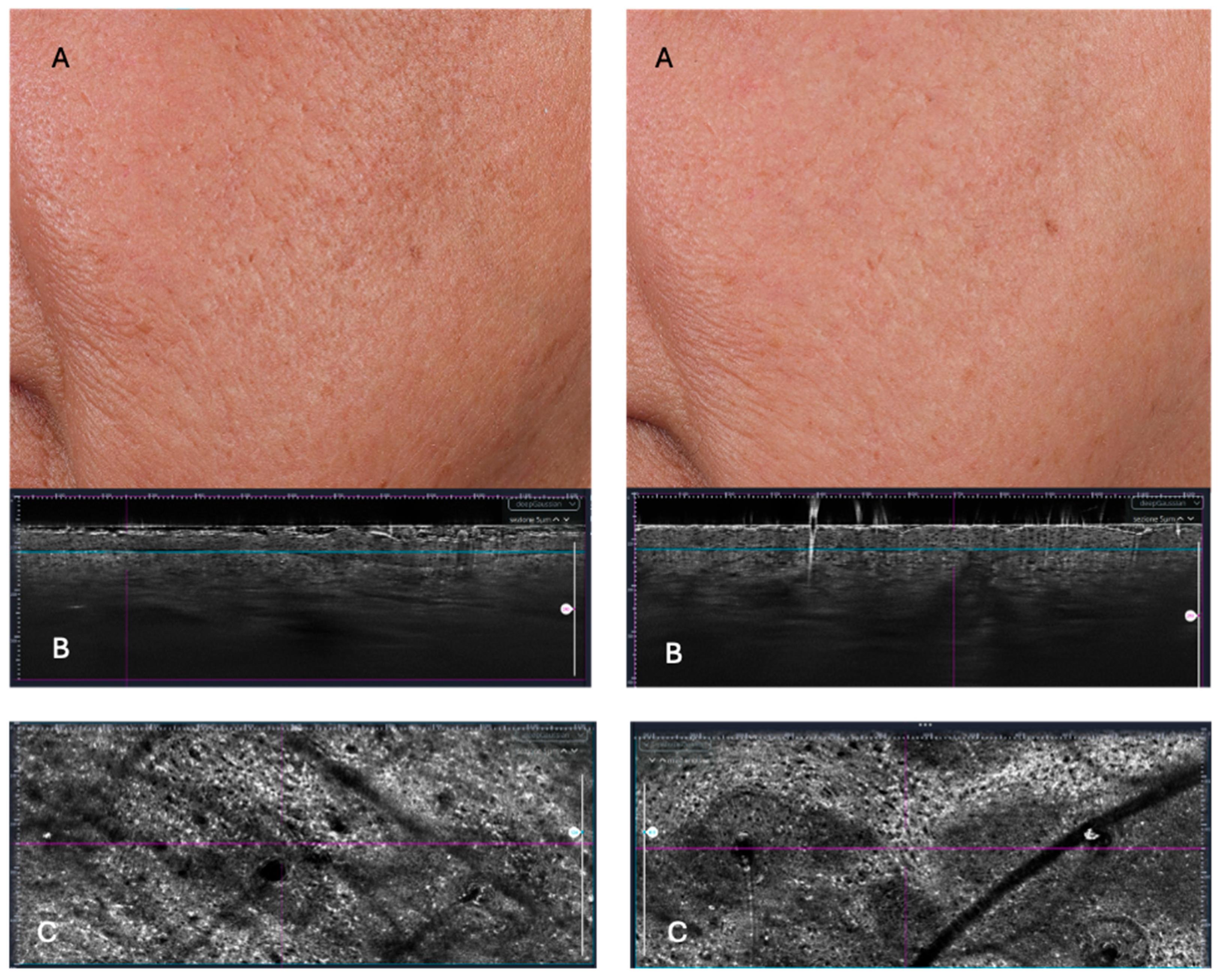
3.6. LC-OCT Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sachs, D.L.; Varani, J.; Chubb, H.; Fligiel, S.E.; Cui, Y.; Calderone, K.; Voorhees, J.J. Atrophic and hypertrophic photoaging: Clinical, histologic, and molecular features of 2 distinct phenotypes of photoaged skin. J. Am. Acad. Dermatol. 2019, 81, 480–488. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef] [PubMed]
- Khavkin, J.; Ellis, D.A. Aging skin: Histology, physiology, and pathology. Facial Plast. Surg. Clin. 2011, 19, 229–234. [Google Scholar] [CrossRef]
- Guida, S.; Pellacani, G.; Ciardo, S.; Longo, C. Reflectance Confocal Microscopy of Aging Skin and Skin Cancer. Dermatol. Pract. Concept. 2021, 11, e2021068. [Google Scholar] [CrossRef]
- Longo, C.; Casari, A.; Beretti, F.; Cesinaro, A.M.; Pellacani, G. Skin aging: In vivo microscopic assessment of epidermal and dermal changes by means of confocal microscopy. J. Am. Acad. Dermatol. 2013, 68, e73–e82. [Google Scholar] [CrossRef]
- Longo, C. Well-Aging: Early Detection of Skin Aging Signs. Dermatol. Clin. 2016, 34, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Langton, A.K.; Ayer, J.; Griffiths, T.W.; Rashdan, E.; Naidoo, K.; Caley, M.P.; Birch-Machin, M.A.; O’Toole, E.A.; Watson, R.E.B.; Griffiths, C.E.M. Distinctive clinical and histological characteristics of atrophic and hypertrophic facial photoageing. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 762–768. [Google Scholar] [CrossRef]
- Grandahl, K.; Olsen, J.; Friis, K.B.E.; Mortensen, O.S.; Ibler, K.S. Photoaging and actinic keratosis in Danish outdoor and indoor workers. Photodermatol. Photoimmunol. Photomed. 2019, 35, 201–207. [Google Scholar] [CrossRef]
- Berneburg, M.; Plettenberg, H.; Krutmann, J. Photoaging of human skin. Photodermatol. Photoimmunol. Photomed. 2000, 16, 239–244. [Google Scholar] [CrossRef]
- Kutlu Haytoglu, N.S.; Gurel, M.S.; Erdemir, A.; Falay, T.; Dolgun, A.; Haytoglu, T.G. Assessment of skin photoaging with reflectance confocal microscopy. Ski. Res. Technol. 2014, 20, 363–372. [Google Scholar] [CrossRef]
- Bosset, S.; Bonnet-Duquennoy, M.; Barre, P.; Chalon, A.; Kurfurst, R.; Bonte, F.; Nicolas, J.F. Photoageing shows histological features of chronic skin inflammation without clinical and molecular abnormalities. Br. J. Dermatol. 2003, 149, 826–835. [Google Scholar] [CrossRef]
- Sander, C.S.; Chang, H.; Salzmann, S.; Müller, C.S.; Ekanayake-Mudiyanselage, S.; Elsner, P.; Thiele, J.J. Photoaging is associated with protein oxidation in human skin in vivo. J. Investig. Dermatol. 2002, 118, 618–625. [Google Scholar] [CrossRef]
- Contet-Audonneau, J.L.; Jeanmaire, C.; Pauly, G. A histological study of human wrinkle structures: Comparison between sun-exposed areas of the face, with or without wrinkles, and sun-protected areas. Br. J. Dermatol. 1999, 140, 1038–1047. [Google Scholar] [CrossRef]
- El-Domyati, M.; Attia, S.; Saleh, F.; Brown, D.; Birk, D.E.; Gasparro, F.; Ahmad, H.; Uitto, J. Intrinsic aging vs. photoaging: A comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp. Dermatol. 2002, 11, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.E.; Wang, T.S.; Hamilton, T.A.; Voorhees, J.J.; Ellis, C.N. A photonumeric scale for the assessment of cutaneous photodamage. Arch. Dermatol. 1992, 128, 347–351. [Google Scholar] [PubMed]
- Helfrich, Y.R.; Maier, L.E.; Cui, Y.; Fisher, G.J.; Chubb, H.; Fligiel, S.; Voorhees, J. Clinical, histologic, and molecular analysis of differences between erythematotelangiectatic rosacea and telangiectatic photoaging. JAMA Dermatol. 2015, 151, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Saurat, J.H. Dermatoporosis: The functional side of skin aging. Dermatology 2007, 215, 271–272. [Google Scholar] [CrossRef]
- Vierkötter, A.; Krutmann, J. Environmental influences on skin aging and ethnic-specific manifestations. Dermato-Endocrinology 2012, 4, 227–231. [Google Scholar] [CrossRef]
- Craven, N.M.; Watson, R.E.B.; Jones, C.J.P.; Shuttleworth, C.A.; Kielty, C.M.; Griffiths, C.E.M. Clinical features of photodamaged human skin are associated with a reduction in collagen VII. Br. J. Dermatol. 1997, 137, 344–350. [Google Scholar] [CrossRef]
- Wurm, E.M.T.; Longo, C.; Curchin, C.; Soyer, H.P.; Prow, T.W.; Pellacani, G. In vivo assessment of chronological ageing and photoageing in forearm skin using reflectance confocal microscopy. Br. J. Dermatol. 2012, 167, 270–279. [Google Scholar] [CrossRef]
- Longo, C.; Casari, A.; De Pace, B.; Simonazzi, S.; Mazzaglia, G.; Pellacani, G. Proposal for an in vivo histopathologic scoring system for skin aging by means of confocal microscopy. Ski. Res. Technol. 2013, 19, e167–e173. [Google Scholar] [CrossRef]
- Longo, C.; Zalaudek, I.; Argenziano, G.; Pellacani, G. New Directions in Dermatopathology: In Vivo Confocal Microscopy in Clinical Practice. Dermatol. Clin. 2012, 30, 799–814. [Google Scholar] [CrossRef]
- Guida, S.; De Pace, B.; Ciardo, S.; Farnetani, F.; Pellacani, G. Non-invasive Imaging for Skin Cancers—The European Experience. Curr. Dermatol. Rep. 2019, 8, 172–181. [Google Scholar] [CrossRef]
- Goberdhan, L.T.; Pellacani, G.; Ardigo, M.; Schneider, K.; Makino, E.T.; Mehta, R.C. Assessing changes in facial skin quality using noninvasive in vivo clinical skin imaging techniques after use of a topical retinoid product in subjects with moderate-to-severe photodamage. Skin Res. Technol. 2022, 28, 604–613. [Google Scholar] [CrossRef]
- Dan, X.; Li, S.; Chen, H.; Xue, P.; Liu, B.; Ju, Y.; Fan, X. Tailoring biomaterials for skin anti-aging. Mater. Today Bio 2024, 28, 101210. [Google Scholar] [CrossRef]
- Csekes, E.; Račková, L. Skin Aging, Cellular Senescence and Natural Polyphenols. Int. J. Mol. Sci. 2021, 22, 12641. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Gao, X.; Xie, W. Research Progress in Skin Aging, Metabolism, and Related Products. Int. J. Mol. Sci. 2023, 24, 15930. [Google Scholar] [CrossRef] [PubMed]
- Michelini, S.; Greco, M.E.; Vespasiani, G.; Trovato, F.; Chello, C.; Musolff, N.; Pellacani, G. Non-Invasive Imaging for the Evaluation of a New Oral Supplement in Skin Aging: A Case-Controlled Study. Ski. Res. Technol. 2025, 31, e70171. [Google Scholar] [CrossRef] [PubMed]
- Trovato, F.; Ceccarelli, S.; Michelini, S.; Vespasiani, G.; Guida, S.; Galadari, H.I.; Pellacani, G. Advancements in Regenerative Medicine for Aesthetic Dermatology: A Comprehensive Review and Future Trends. Cosmetics 2024, 11, 49. [Google Scholar] [CrossRef]
- Giordano, V.; Federica, T.; Giuseppina, R.; Simone, M.; Giovanni, P. Hyaluronic Acid Fillers in Reconstructive Surgery. J. Cosmet. Dermatol. 2025, 24, e16693. [Google Scholar] [CrossRef]
- Park, J.Y.; Youn, C.; Lee, C.; Lee, K.C.; Shin, H.; Yeom, K.B.; Hong, W. Facial Skin Quality Improvement After Treatment with CPM-HA20G: Clinical Experience in Korea. J. Cosmet. Dermatol. 2025, 24, e16795. [Google Scholar] [CrossRef]
- Baspeyras, M.; Rouvrais, C.; Liégard, L.; Delalleau, A.; Letellier, S.; Bacle, I.; Schmitt, A.M. Clinical and biometrological efficacy of a hyaluronic acid-based mesotherapy product: A randomised controlled study. Arch. Dermatol. Res. 2013, 305, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Rho, N.K.; Kim, H.S.; Kim, S.Y.; Lee, W. Injectable “Skin Boosters” in Aging Skin Rejuvenation: A Current Overview. Arch. Plast Surg. 2024, 51, 528–541. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fluhr, J.W.; Mao-Qiang, M.; Brown, B.E.; Wertz, P.W.; Crumrine, D.; Sundberg, J.P.; Elias, P.M. Glycerol regulates stratum corneum hydration in sebaceous gland deficient (asebia) mice. J. Investig. Dermatol. 2003, 120, 728–737. [Google Scholar] [CrossRef]
- Sagrafena, I.; Morin, M.; Paraskevopoulos, G.; Nilsson, E.J.; Hrdinová, I.; Kováčik, A.; Björklund, S.; Vávrová, K. Structure and function of skin barrier lipids: Effects of hydration and natural moisturizers in vitro. Biophys. J. 2024, 123, 3951–3963. [Google Scholar] [CrossRef]
- Kleine-Börger, L.; Hofmann, M.; Kerscher, M. Microinjections with hyaluronic acid in combination with glycerol: How do they influence biophysical viscoelastic skin properties? Ski. Res. Technol. 2022, 28, 633–642. [Google Scholar] [CrossRef]
- Succi, I.B.; da Silva, R.T.; Orofino-Costa, R. Rejuvenation of periorbital area: Treatment with an injectable nonanimal non-crosslinked glycerol added hyaluronic acid preparation. Dermatol. Surg. 2012, 38, 192–198. [Google Scholar] [CrossRef]
- Carruthers, J.; Carruthers, A. Hyaluronic acid gel in skin rejuvenation. J. Drugs Dermatol. 2006, 5, 959–964. [Google Scholar]
- Guida, S.; Galadari, H.; Vespasiani, G.; Pellacani, G. Skin biostimulation and hyaluronic acid: Current knowledge and new evidence. J. Cosmet. Dermatol. 2024, 23, 701–703. [Google Scholar] [CrossRef] [PubMed]
- Monheit, G.D.; Coleman, K.M. Hyaluronic acid fillers. Dermatol. Ther. 2006, 19, 141–150. [Google Scholar] [CrossRef]
- Iranmanesh, B.; Khalili, M.; Mohammadi, S.; Amiri, R.; Aflatoonian, M. Employing hyaluronic acid-based mesotherapy for facial rejuvenation. J. Cosmet. Dermatol. 2022, 21, 6605–6618. [Google Scholar] [CrossRef]
- Witmer, W.K.; Lebovitz, P.J. Clinical Photography in the Dermatology Practice. Semin. Cutan. Med. Surg. 2012, 31, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Linming, F.; Wei, H.; Anqi, L.; Yuanyu, C.; Heng, X.; Sushmita, P.; Li, L. Comparison of two skin imaging analysis instruments: The VISIA® from Canfield vs the ANTERA 3D®CS from Miravex. Ski. Res. Technol. 2018, 24, 3–8. [Google Scholar] [CrossRef]
- Ogien, J.; Tavernier, C.; Fischman, S.; Dubois, A. Line-field confocal optical coherence tomography (LC-OCT): Principles and practical use. Ital. J. Dermatol. Venereol. 2023, 158, 171–179. [Google Scholar] [CrossRef]
- Chauvel-Picard, J.; Bérot, V.; Tognetti, L.; Orte Cano, C.; Fontaine, M.; Lenoir, C.; Suppa, M. Line-field confocal optical coherence tomography as a tool for three-dimensional in vivo quantification of healthy epidermis: A pilot study. J. Biophotonics 2022, 15, e202100236. [Google Scholar] [CrossRef]
- Bonnier, F.; Pedrazzani, M.; Fischman, S.; Viel, T.; Lavoix, A.; Pegoud, D.; Korichi, R. Line-field confocal optical coherence tomography coupled with artificial intelligence algorithms to identify quantitative biomarkers of facial skin ageing. Sci. Rep. 2023, 13, 13881. [Google Scholar] [CrossRef]
- Santoprete, R.; Hourblin, V.; Foucher, A.; Dufour, O.; Bernard, D.; Domanov, Y.; Potter, A. Reduction of wrinkles: From a computational hypothesis to a clinical, instrumental, and biological proof. Ski. Res. Technol. 2023, 29, e13267. [Google Scholar] [CrossRef]
- Krueger, N.; Luebberding, S.; Oltmer, M.; Streker, M.; Kerscher, M. Age-related changes in skin mechanical properties: A quantitative evaluation of 120 female subjects. Ski. Res. Technol. 2011, 17, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Pawlaczyk, M.; Lelonkiewicz, M.; Wieczorowski, M. Age-dependent biomechanical properties of the skin. Postepy Dermatol. Alergol. 2013, 30, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.S.; Joo, Y.H.; Kim, S.O.; Park, K.C.; Youn, S.W. Influence of age and regional differences on skin elasticity as measured by the Cutometer®. Ski. Res. Technol. 2008, 14, 354–358. [Google Scholar] [CrossRef]
- Caubet, C.; Jonca, N.; Brattsand, M.; Guerrin, M.; Bernard, D.; Schmidt, R.; Serre, G. Degradation of corneodesmosome proteins by two serine proteases of the kallikrein family, SCTE/KLK5/hK5 and SCCE/KLK7/hK7. J. Investig. Dermatol. 2004, 122, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- King, I.A.; Tabiowo, A.; Fryer, P.R. Evidence that major 78-44 kDa concanavalin A-binding glycopolypeptides in pig epidermis arise from the degradation of desmosomal glycoproteins during terminal differentiation. J. Cell Biol. 1987, 105, 3053–3063. [Google Scholar] [CrossRef]
- Ernst, N.; Yay, A.; Bíró, T.; Tiede, S.; Humphries, M.; Paus, R.; Kloepper, J.E. β1 integrin signaling maintains human epithelial progenitor cell survival in situ and controls proliferation, apoptosis and migration of their progeny. PLoS ONE 2013, 8, e84356. [Google Scholar] [CrossRef]
- Kowalczyk, A.P.; Bornslaeger, E.A.; Norvell, S.M.; Palka, H.L.; Green, K.J. Desmosomes: Intercellular adhesive junctions specialized for attachment of intermediate filaments. Int. Rev. Cytol. 1999, 185, 237–302. [Google Scholar]
- Niiyama, S.; Yoshino, T.; Yasuda, C.; Yu, X.; Izumi, R.; Ishiwatari, S.; Mukai, H. Galectin-7 in the stratum corneum: A biomarker of the skin barrier function. Int. J. Cosmet. Sci. 2016, 38, 487–495. [Google Scholar] [CrossRef]
- Jonca, N.; Guerrin, M.; Hadjiolova, K.; Caubet, C.; Gallinaro, H.; Simon, M.; Serre, G. Corneodesmosin, a component of epidermal corneocyte desmosomes, displays homophilic adhesive properties. J. Biol. Chem. 2002, 277, 5024–5029. [Google Scholar] [CrossRef]
- Chen, H.L.; Lo, C.H.; Huang, C.C.; Lu, M.P.; Hu, P.Y.; Chen, C.S.; Liu, F.T. Galectin-7 downregulation in lesional keratinocytes contributes to enhanced IL-17A signaling and skin pathology in psoriasis. J. Clin. Investig. 2021, 131, e130740. [Google Scholar] [CrossRef]
- Ohman, H.; Vahlquist, A. In vivo studies concerning a pH gradient in human stratum corneum and upper epidermis. Acta Derm. Venereol. 1994, 74, 375–379. [Google Scholar] [CrossRef]
- Suzuki, Y.; Nomura, J.; Hori, J.; Koyama, J.; Takahashi, M.; Horii, I. Detection and characterization of endogenous protease associated with desquamation of stratum corneum. Arch. Dermatol. Res. 1993, 285, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Fujimoto, E.; Fujimoto, N.; Akiyama, M.; Satoh, T.; Maeda, H.; Tajima, S. In vitro amyloidogenic peptides of galectin-7: Possible mechanism of amyloidogenesis of primary localized cutaneous amyloidosis. J. Biol. Chem. 2014, 289, 29195–29207. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Chiang, P.C.; Lo, C.H.; Lo, Y.H.; Hsu, D.K.; Chen, H.Y.; Liu, F.T. Galectin-7 regulates keratinocyte proliferation and differentiation through JNK-miR-203-p63 signaling. J. Investig. Dermatol. 2016, 136, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Meunier, M.; De Tollenaere, M.; Jarrin, C.; Chapuis, E.; Bracq, M.; Lapierre, L.; Zanchetta, C.; Tiguemounine, J.; Scandolera, A.; Reynaud, R. Bacterial porphyrins in healthy skin: Microbiota components impact melanogenesis and age-related processes leading to Porphyr’ageing. Int. J. Cosmet. Sci. 2025; Early View. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Clinical Relevance |
|---|---|
| Visible spots | Quantification of superficial pigmentary dyschromia |
| Wrinkles | Detection of fine lines and epidermal folds |
| Texture uniformity | Assessment of skin tone and grain homogeneity |
| Pores | Measurement of follicular opening size and visibility |
| UV spots | Identification of subclinical photodamage |
| Porphyrins | Detection of microbial accumulations at the follicular level |
| Parameter | Description |
|---|---|
| P (Profile Line Measurement) | Linear surface profile acquisition |
| R (Roughness Line Measure) | High-frequency microscopic roughness |
| W (Waviness Line Measure) | Low-frequency surface undulations (macrotexture) |
| Color by Distance | Chromatic 3D mapping of depressions and elevations |
| Parameter | Measurement Focus |
|---|---|
| Stratum corneum thickness | Average thickness of stratum corneum |
| Viable epidermis thickness | Avere thickness of epidermis (excluded stratum corneum |
| Dermo-epidermal junction (DEJ) undulation | Interface morphology |
| Keratinocyte density, volume, compactness | Cellular morphology |
| Cellular atypia | Variations in nuclear size |
| Dermal fibers | Quantification of collagen |
| Melanin distribution | Pigmentation analysis |
| Microvasculature | Vascular density and pattern |
| Parameter | Protocol A—Standard (3 Sessions) | p-Value (Within-Group) | Protocol B—Simplified (2 Sessions) | p-Value (Within-Group) |
|---|---|---|---|---|
| Visible spots | Baseline: 0.4035 Post: 0.3543 | 0.008 | Baseline: 0.3868 Post: 0.3759 | 0.557 |
| Wrinkles | Baseline: 0.1561 Post: 0.1232 | 0.054 | Baseline: 0.1828 Post: 0.1896 | 0.711 |
| Evenness | Baseline: 0.2299 Post: 0.2590 | 0.082 | Baseline: 0.2368 Post: 0.2139 | 0.236 |
| Pores | Baseline: 0.1485 Post: 0.1270 | 0.184 | Baseline: 0.0750 Post: 0.0721 | 0.874 |
| UV spots | Baseline: 0.4584 Post: 0.4253 | 0.052 | Baseline: 0.3411 Post: 0.3152 | 0.186 |
| Porphyrins | Baseline: 0.1866 Post: 0.1519 | 0.044 | Baseline: 0.1293 Post: 0.0861 | 0.044 |
| Baseline Variable | Protocol A (3 Sessions) (n = 10) | Protocol B (2 Sessions) (n = 7) | p-Value |
|---|---|---|---|
| Age (years) | 53.6 ± 6.1 | 54.7 ± 5.3 | 0.72 |
| Fitzpatrick phototype | I: 3 (30%) II: 5 (50%) III: 2 (20%) | I: 2 (28.6%) II: 3 (42.9%) III: 2 (28.6%) | — |
| VISIA Visible spots (baseline) | 0.4035 | 0.3868 | 0.78 |
| VISIA Wrinkles (baseline) | 0.1561 | 0.1828 | 0.59 |
| VISIA Evenness (baseline) | 0.2299 | 0.2368 | 0.84 |
| VISIA Pores (baseline) | 0.1485 | 0.0750 | 0.17 |
| VISIA UV spots (baseline) | 0.4584 | 0.3411 | 0.21 |
| VISIA Porphyrins (baseline) | 0.1866 | 0.1293 | 0.34 |
| Parameter | Protocol A—Standard (3 Sessions) | Protocol B—Simplified (2 Sessions) | p-Value (Between Groups) |
|---|---|---|---|
| Rising Volume (Δ mm3) | Mean Δ: −2.1 ± 4.9 | Mean Δ: −1.4 ± 5.3 | 0.661 |
| Mean Projection Rising (Δ mm) | Mean Δ: −1.2 ± 2.7 | Mean Δ: −1.0 ± 2.9 | 0.469 |
| Parameter | Protocol A—Standard (3 Sessions) | p-Value (Within-Group) | Protocol B—Simplified (2 Sessions) | p-Value (Within-Group) |
|---|---|---|---|---|
| SPp (µm) | Baseline: 345.1 Post: 404.1 | 0.190 | Baseline: 292.3 Post: 334.6 | 0.224 |
| SPpm (µm) | Baseline: 232.0 Post: 233.1 | 0.916 | Baseline: 203.5 Post: 211.3 | 0.590 |
| SRa (µm) | Baseline: 21.5 Post: 21.4 | 0.987 | Baseline: 19.9 Post: 19.4 | 0.456 |
| SWp (µm) | Baseline: 166.2 Post: 173.0 | 0.667 | Baseline: 191.1 Post: 213.9 | 0.448 |
| Improvement Category | Protocol A—Standard (3 Sessions) | Protocol B—Simplified (2 Sessions) | Total (n = 17) |
|---|---|---|---|
| Complete | 6 | 5 | 11 |
| Partial | 2 | 3 | 5 |
| None | 0 | 1 | 1 |
| Total | 8 | 9 | 17 |
| Cluster | n | Complete | Partial | None |
|---|---|---|---|---|
| 1 | 7 | 6 | 1 | 0 |
| 2 | 6 | 3 | 3 | 0 |
| 3 | 4 | 2 | 1 | 1 |
| Total | 17 | 11 | 5 | 1 |
| Baseline (Mean ± SD) | Post-Treatment (Mean ± SD) | Δ (Post–Pre) (Mean ± SD) | p-Value (Within Group) | |
|---|---|---|---|---|
| SC thickness (µm) | 13.11 ± 1.17 | 13.92 ± 0.91 | 0.81 ± 1.43 | 0.032 |
| Epidermis thickness (µm) | 45.68 ± 5.30 | 48.31 ± 9.18 | 2.63 ± 9.19 | 0.255 |
| DEJ undulation (%) | 1.64 ± 3.92 | 3.36 ± 6.19 | 1.72 ± 3.77 | 0.079 |
| Keratinocyte density | 26,950 ± 4089 | 28,080 ± 3904 | 1130 ± 3511 | 0.203 |
| Average volume | 152.6 ± 8.3 | 154.8 ± 8.3 | 2.18 ± 9.82 | 0.374 |
| Compactness | 0.75 ± 0.02 | 0.75 ± 0.01 | −0.01 ± 0.02 | 0.157 |
| Cellular atypia | 0.20 ± 0.05 | 0.21 ± 0.04 | 0.00 ± 0.06 | 0.877 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Federica, T.; Giordano, V.; Di Guardo, A.; Simone, M.; Giovanni, P. Evaluating Efficacy and Tolerability of a New Intradermal Biorejuvenation with Free Hyaluronic Acid and Glycerol in Photoaging: A Retrospective Pilot Study. Cosmetics 2025, 12, 273. https://doi.org/10.3390/cosmetics12060273
Federica T, Giordano V, Di Guardo A, Simone M, Giovanni P. Evaluating Efficacy and Tolerability of a New Intradermal Biorejuvenation with Free Hyaluronic Acid and Glycerol in Photoaging: A Retrospective Pilot Study. Cosmetics. 2025; 12(6):273. https://doi.org/10.3390/cosmetics12060273
Chicago/Turabian StyleFederica, Trovato, Vespasiani Giordano, Antonio Di Guardo, Michelini Simone, and Pellacani Giovanni. 2025. "Evaluating Efficacy and Tolerability of a New Intradermal Biorejuvenation with Free Hyaluronic Acid and Glycerol in Photoaging: A Retrospective Pilot Study" Cosmetics 12, no. 6: 273. https://doi.org/10.3390/cosmetics12060273
APA StyleFederica, T., Giordano, V., Di Guardo, A., Simone, M., & Giovanni, P. (2025). Evaluating Efficacy and Tolerability of a New Intradermal Biorejuvenation with Free Hyaluronic Acid and Glycerol in Photoaging: A Retrospective Pilot Study. Cosmetics, 12(6), 273. https://doi.org/10.3390/cosmetics12060273








