Design and Synthesis of a Novel in Chemico Reactivity Probe N,N-dimethyl N-(2-(1-naphthyl)acetyl)-l-cysteine (NNDNAC) for Rapid Skin Sensitization Assessment of Cosmetic Ingredients
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
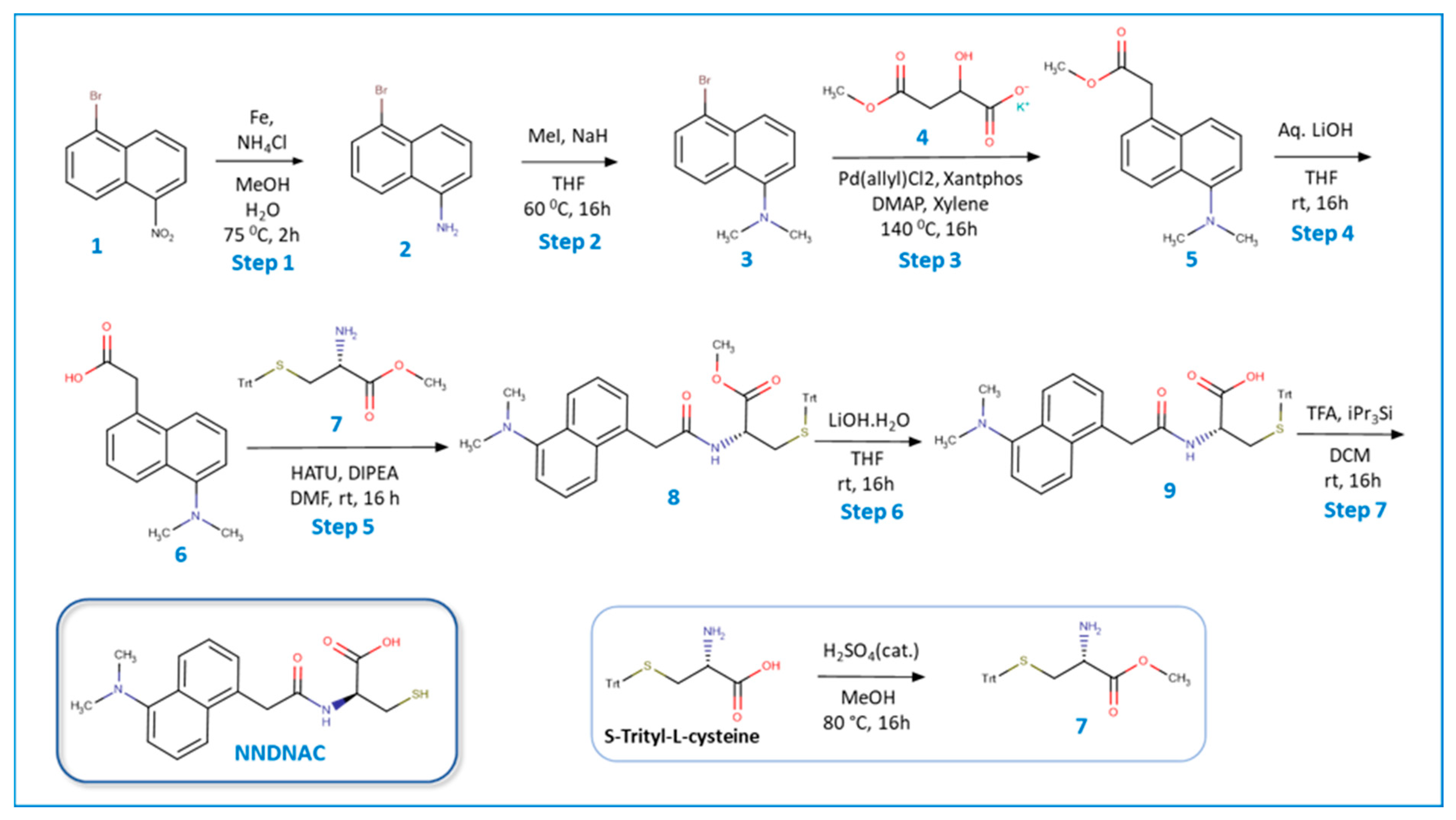
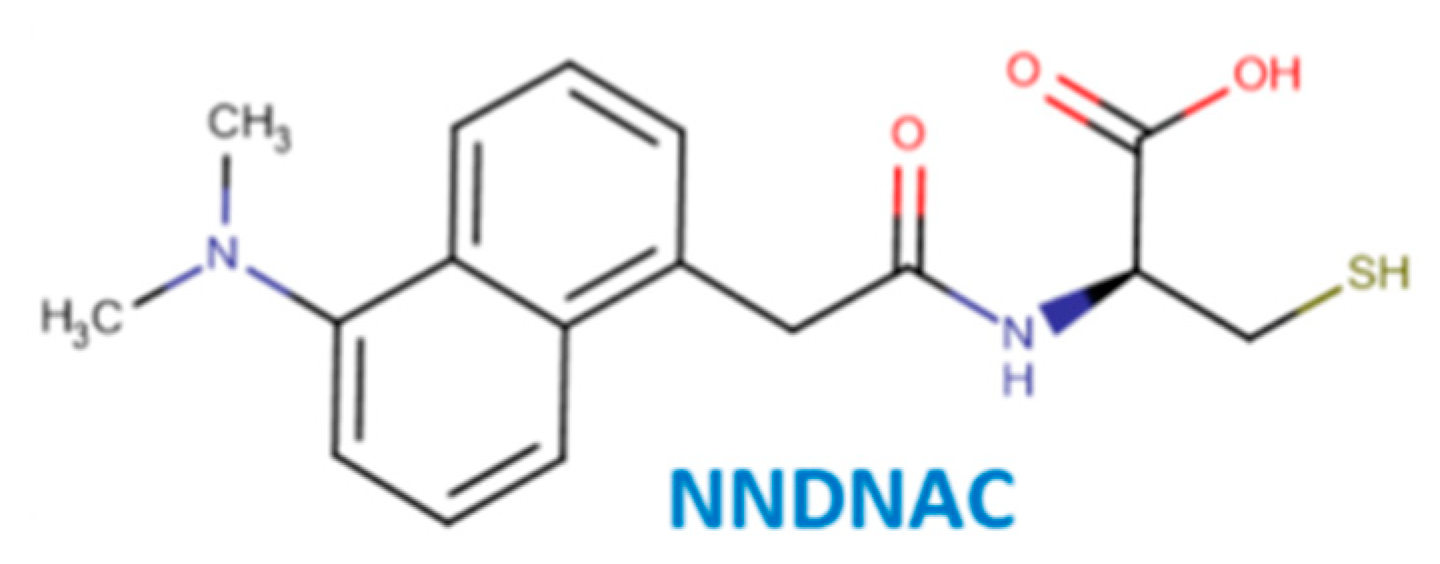
2.2. Synthesis of the Probe NNDNAC
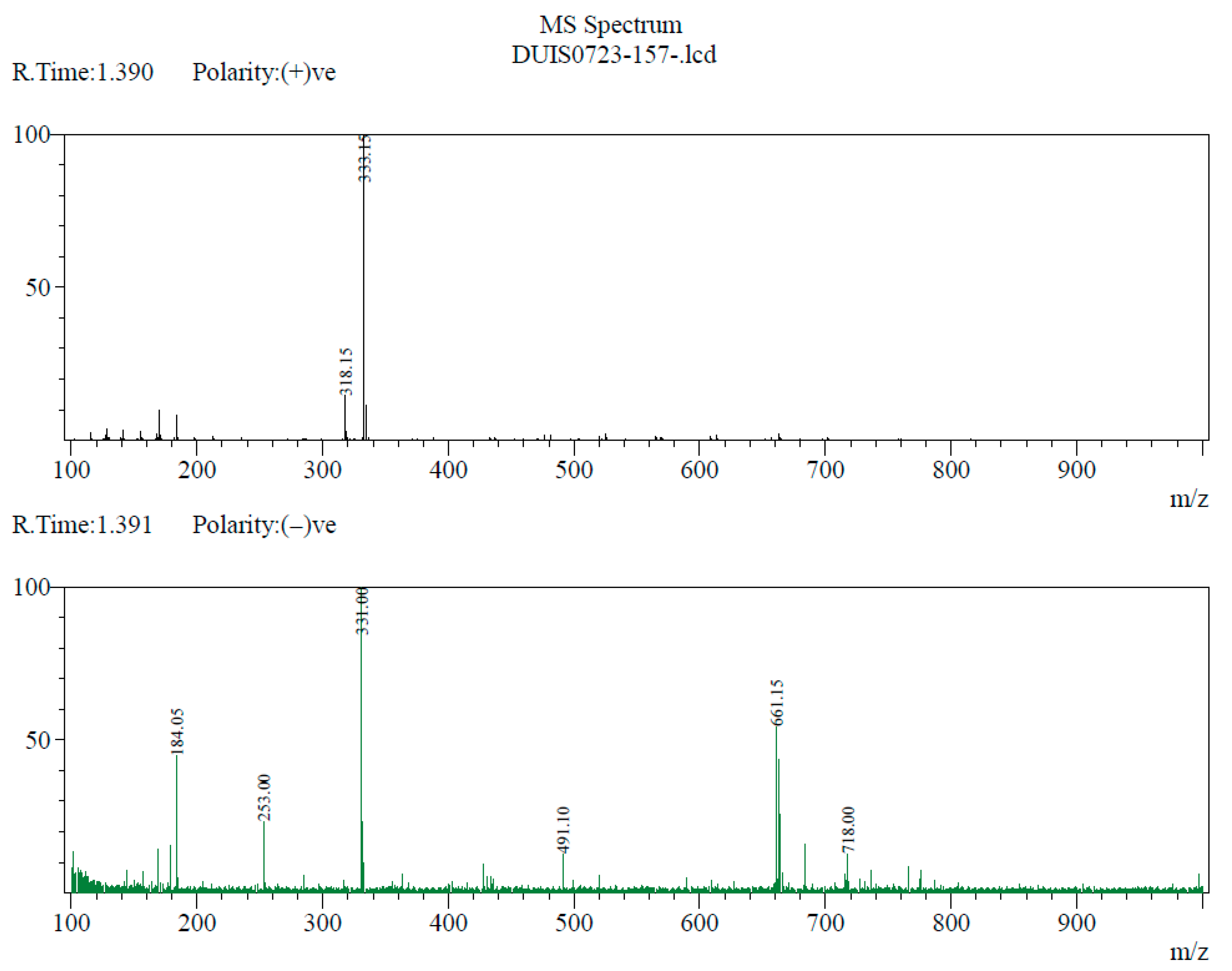
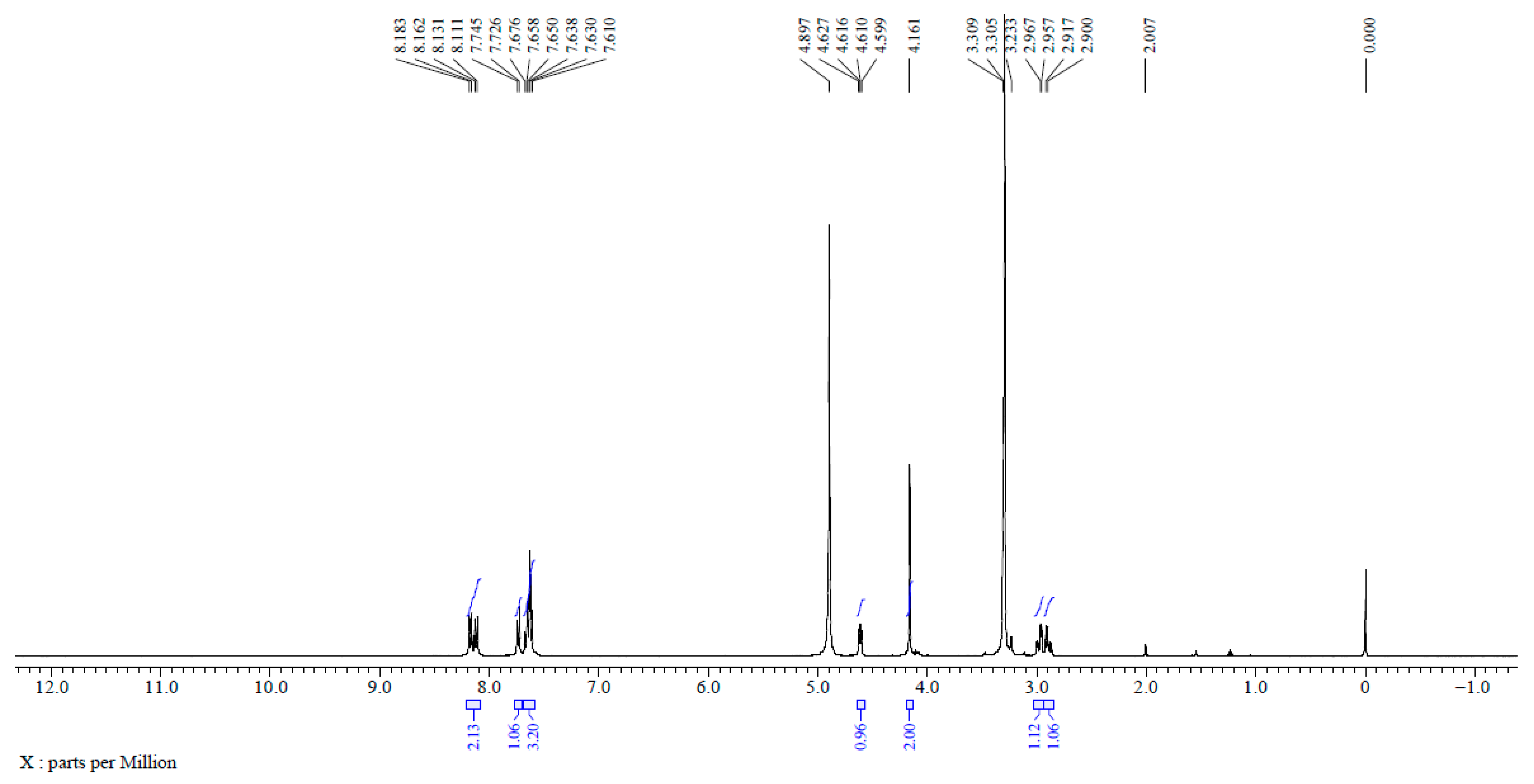
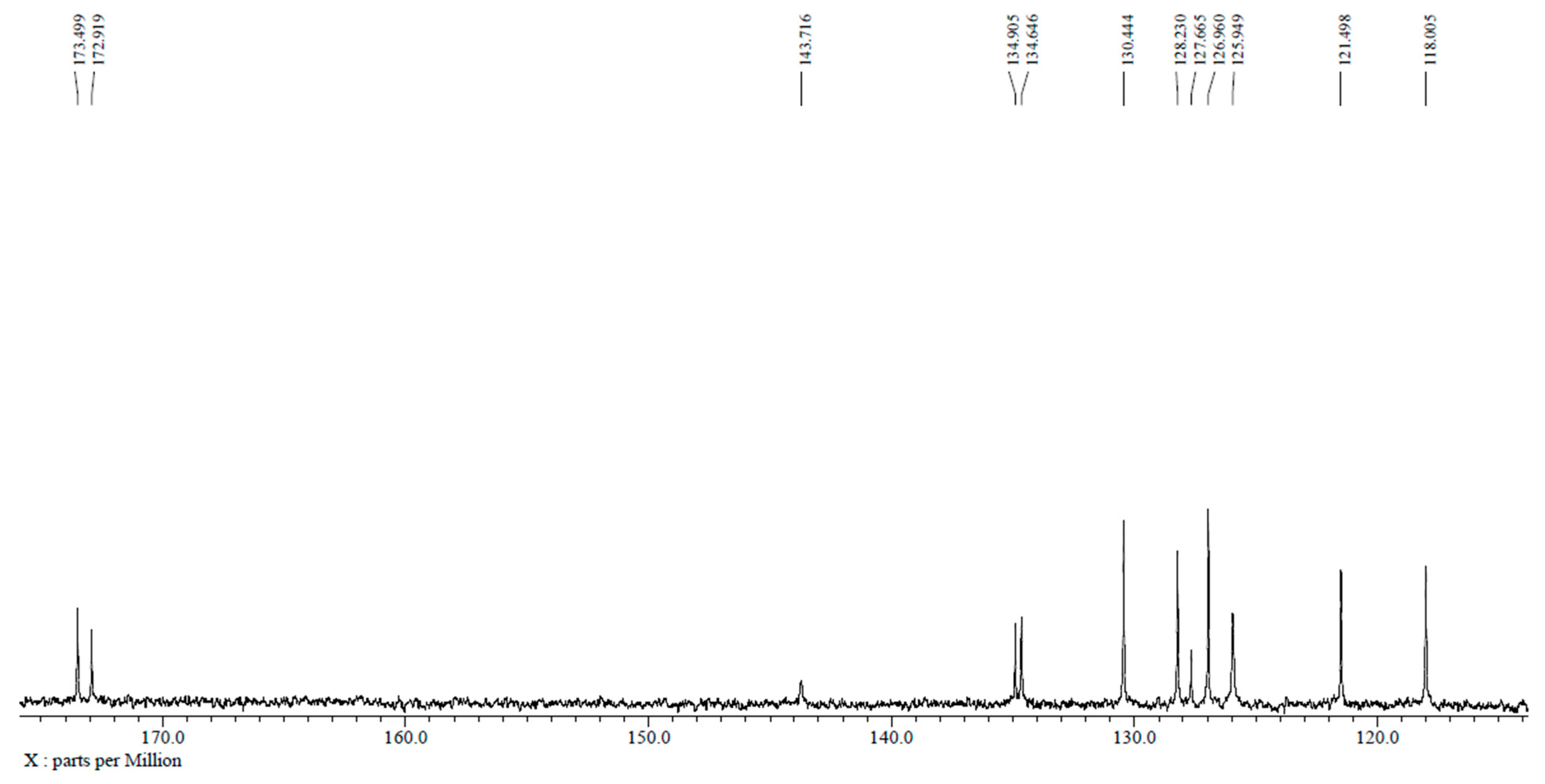
2.3. Structural Confirmation of NNDNAC
2.4. Reactivity Assay for Skin Sensitization Using NNDNAC
2.4.1. Preparation of the NNDNAC Stock Solution and Standard Curve
2.4.2. Preparation of Test Chemical Solution
2.4.3. Preparation of Reaction Mixture
2.4.4. LC Method Optimization for Quantification of NNDNAC
2.5. Comparative Skin Sensitization Assay
- Direct Peptide Reactivity Assay (DPRA) with cysteine (Cys-DPRA);
- Amino Acid Derivative Reactivity Assay (ADRA) with N-acetylcysteine (NAC-ADRA).
3. Results
3.1. Synthesis and Structural Characterization of NNDNAC
3.2. NNDNAC Reactivity and Comparative Skin Sensitization Assessment
3.2.1. Reactivity of NNDNAC with Test Compounds
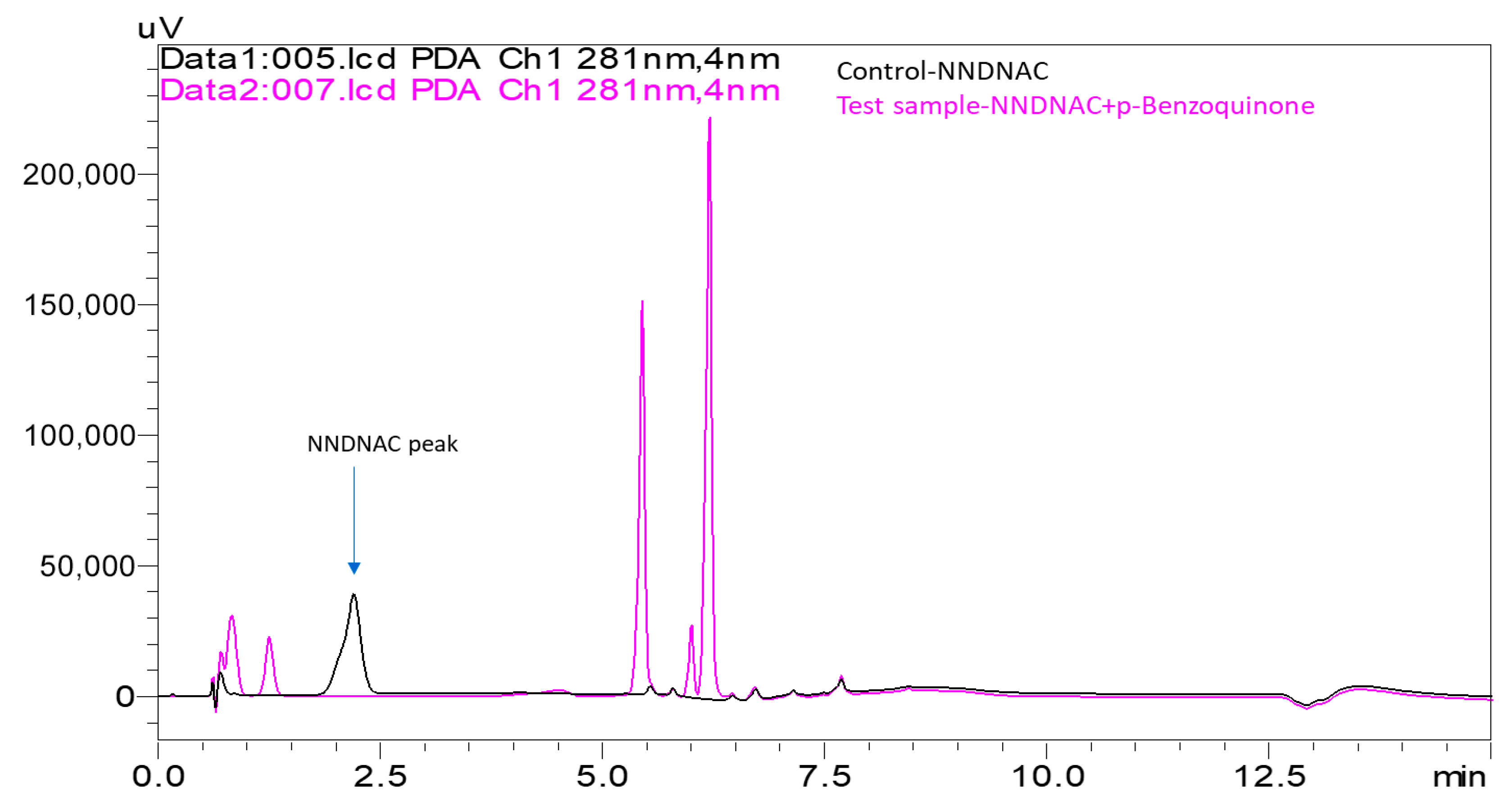
- Strong Electrophilic Sensitizers: Both p-benzoquinone and 2-methyl-4-isothiazolin-3-one showed very high depletion rates with NNDNAC at 100% and 98%, respectively. These compounds were consistently classified as strong sensitizers across all assays, with KeratinoSens™ EC3 values of 32.77 mM and 29.56 mM, and LLNA EC3 values of 0.01% and 1.9%, respectively.
- Pro-electrophiles: For p-phenylenediamine and 4-aminophenol, NNDNAC showed 0% depletion. This indicates that these compounds do not directly react with NNDNAC under the assay condition (1 h incubation). Both are known sensitizers, with p-phenylenediamine having an LLNA EC3 of 0.16%.
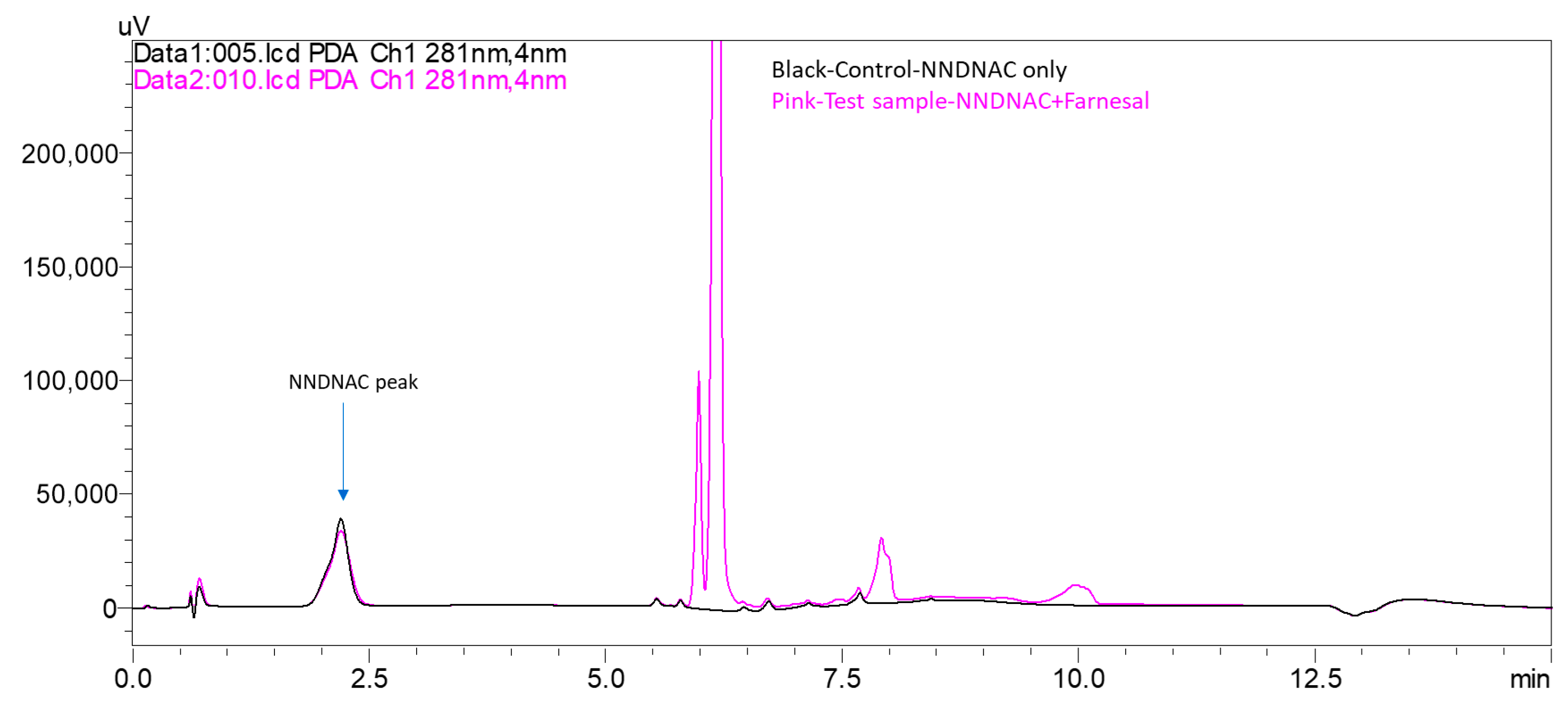
- Weak/Non-sensitizers: Farnesal exhibited a low NNDNAC depletion of 3%. This aligns well with its classification as a weak sensitizer based on KeratinoSens™ (EC3 > 2000 mM) and LLNA (EC3 12%). Similarly, cinnamyl alcohol and lactic acid showed minimal or no depletion with NNDNAC (1% and 0%, respectively), which is consistent with their non-sensitizing or very weak sensitizing classification across the other assays (KeratinoSens™ EC3 > 2000 mM, LLNA EC3 21% for cinnamyl alcohol, and ND for lactic acid).
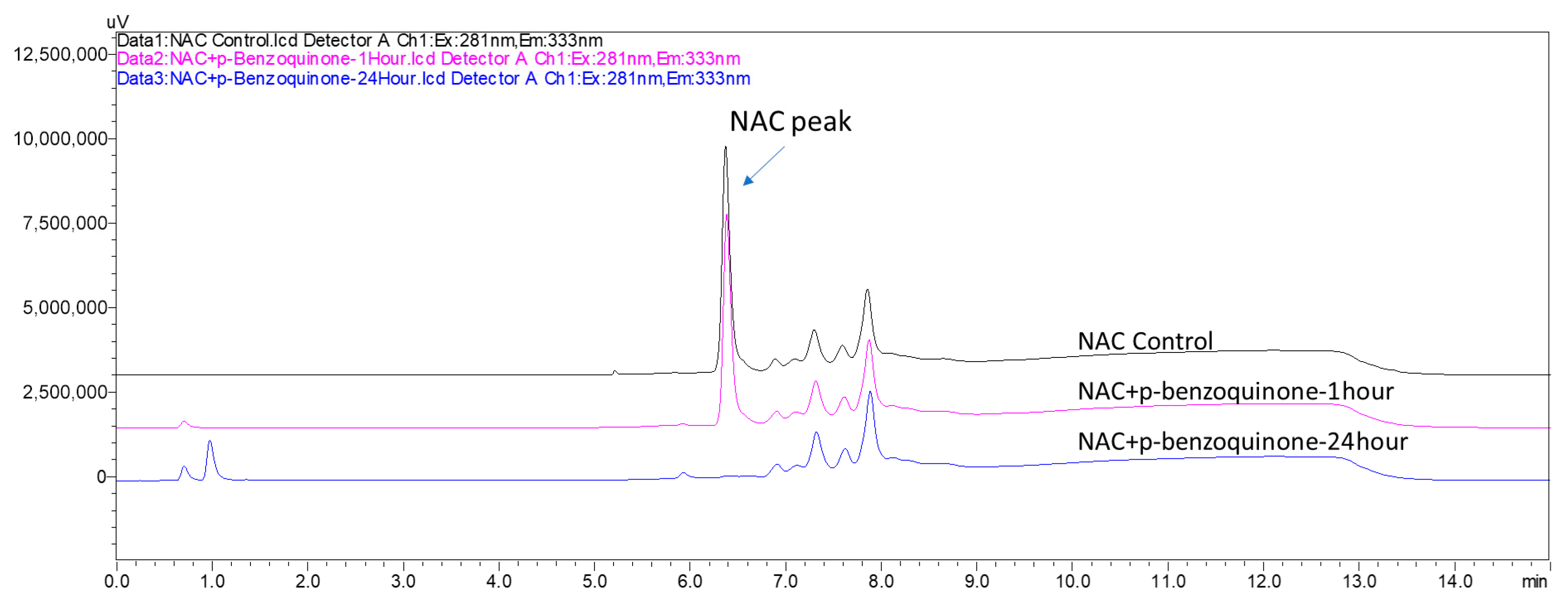
3.2.2. Comparison with Cys-DPRA and NAC-ADRA
- p-Benzoquinone and 2-methyl-4-isothiazolin-3-one resulted in 100% depletion in both Cys-DPRA and NAC-ADRA, consistent with their strong sensitizing potential.
- p-Phenylenediamine and 4-aminophenol, identified as pro-electrophiles, also showed 98.6% and 100% depletion in Cys-DPRA and 100% in NAC-ADRA, respectively. The high depletion rates in Cys-DPRA and NAC-ADRA for these pro-electrophiles (in contrast to NNDNAC’s 0% depletion under short incubation) highlight a key differentiation capability of the NNDNAC assay, which can distinguish direct electrophiles from pro-electrophiles based on incubation time.
- Farnesal showed moderate depletion (15–55% in Cys-DPRA and 20–40% in NAC-ADRA), which is a broader range compared to NNDNAC’s tighter 3% depletion, and less aligned with KeratinoSens™ and LLNA data.
- Cinnamyl alcohol and lactic acid showed 0% depletion in both Cys-DPRA and NAC-ADRA, consistent with NNDNAC’s results and their non-sensitizing nature.
3.3. Comparative Analysis of NNDNAC and NAC-ADRA Assay Performance
3.3.1. Performance with Direct Electrophilic Sensitizers
3.3.2. Performance with Pro-Electrophilic Sensitizers
3.3.3. Performance with Non-Reactive Compounds
4. Discussion
5. Conclusions
6. Patents
| Patent Title | Official Filing Number | Filing Date |
| A COMPOUND, A PROBE, AND METHODS THEREOF | 202341086958 | 19 December 2023 |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choudhury, R.P.; Singh, A.; Mathai, E.; Sudhakar, D.; Tourneix, F.; Alépée, N.; Gautier, F. The dimer effect: A refinement approach towards skin sensitization assessment in-chemico using Amino acid Derivative Reactivity Assay. J. Appl. Toxicol. 2024, 44, 1804–1815. [Google Scholar] [CrossRef] [PubMed]
- Bialas, I.; Zelent-Kraciuk, S.; Jurowski, K. The skin sensitisation of cosmetic ingredients: Review of actual regulatory status. Toxics 2023, 11, 392. [Google Scholar] [CrossRef] [PubMed]
- OECD. The Adverse Outcome Pathway for Skin Sensitisation Initiated by Covalent Binding to Proteins; OECD Series on Testing and Assessment; OECD Publishing: Paris, France, 2014; No. 168. [Google Scholar]
- Hardonnière, K.; Szely, N.; El Ali, Z.; Pallardy, M.; Kerdine-Römer, S. Models of dendritic cells to assess skin sensitization. Front. Toxicol. 2022, 4, 851017. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.S.; Jindal, R.; Mitra, B.; Lee, S.; Li, L.; Maguire, T.J.; Schloss, R.; Yarmush, M.L. Perspectives on non-animal alternatives for assessing sensitization potential in allergic contact dermatitis. Cell. Mol. Bioeng. 2012, 5, 52–72. [Google Scholar] [CrossRef] [PubMed]
- Gądarowska, D.; Kalka, J.; Daniel-Wójcik, A.; Mrzyk, I. Alternative methods for skin-sensitization assessment. Toxics 2022, 10, 740. [Google Scholar] [CrossRef] [PubMed]
- Avonto, C.; Chittiboyina, A.G.; Rua, D.; Khan, I.A. A fluorescence high throughput screening method for the detection of reactive electrophiles as potential skin sensitizers. Toxicol. Appl. Pharmacol. 2015, 289, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Alépée, N.; Tourneix, F.; Singh, A.; Ade, N.; Grégoire, S. Off to a good start? Review of the predictivity of reactivity methods modelling the molecular initiating event of skin sensitization. ALTEX 2023, 40, 606–618. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 442C: In Chemico Skin Sensitisation: Assays Addressing the Adverse Outcome Pathway Key Event on Covalent Binding to Proteins; OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2025. [Google Scholar]
- Fujita, M.; Yamamoto, Y.; Wanibuchi, S.; Katsuoka, Y.; Kasahara, T. A newly developed means of HPLC-fluorescence analysis for predicting the skin sensitization potential of multi-constituent substances using ADRA. Toxicol. Vitr. 2019, 59, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Yamamoto, Y.; Watanabe, S.; Sugawara, T.; Wakabayashi, K.; Tahara, Y.; Horie, N.; Fujimoto, K.; Takeuchi, K.; Kamiya, K.; et al. The within- and between-laboratory reproducibility and predictive capacity of the in-chemico amino acid derivative reactivity assay: Results of validation study implemented in four participating laboratories. J. Appl. Toxicol. 2019, 39, 1492–1505. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Yamamoto, Y.; Watanabe, S.; Sugawara, T.; Wakabayashi, K.; Tahara, Y.; Horie, N.; Fujimoto, K.; Kusakari, K.; Kurokawa, Y.; et al. Cause of and countermeasures for oxidation of the cysteine-derived reagent used in the amino acid derivative reactivity assay. J. Appl. Toxicol. 2019, 39, 191–208. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 442D: In Vitro Skin Sensitisation: ARE-Nrf2 Luciferase Test Method; OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2022. [Google Scholar]
- OECD. Test No. 442E: In Vitro Skin Sensitisation: In Vitro Skin Sensitisation Assays Addressing the Key Event on Activation of Dendritic Cells on the Adverse Outcome Pathway for Skin Sensitisation; OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2023. [Google Scholar]
- Grabowski, Z.R.; Rotkiewicz, K.; Rettig, W. Structural changes accompanying intramolecular electron transfer: Focus on twisted intramolecular charge-transfer states and structures. Chem. Rev. 2003, 103, 3899–4032. [Google Scholar] [CrossRef] [PubMed]
- Klymchenko, A.S. Solvatochromic and fluorogenic dyes as environment-sensitive probes: Design and biological applications. Acc. Chem. Res. 2017, 50, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Mayr, H.; Kempf, B.; Ofial, A.R. π-Nucleophilicity in carbon-carbon bond-forming reactions. Acc. Chem. Res. 2003, 36, 66–77. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, I.; Balasubramanian, C.; Chernyshev, V.; Vitali, M. Role of polycyclic aromatic hydrocarbons and their metabolites in inducing developmental toxicity and cancer. Environ. Sci. Pollut. Res. 2017, 24, 8121–8132. [Google Scholar]
- Loving, G.S.; Sainlos, M.; Imperiali, B. Monitoring protein interactions and dynamics with solvatochromic fluorophores. Trends Biotechnol. 2010, 28, 73–83. [Google Scholar] [CrossRef] [PubMed]
- De Ávila, R.I.; Lindstedt, M.; Valadares, M.C. The 21st century movement within the area of skin sensitization assessment: From the animal context towards current human-relevant in vitro solutions. Regul. Toxicol. Pharmacol. 2019, 108, 104445. [Google Scholar] [CrossRef] [PubMed]
- Clouet, E.; Kerdine-Römer, S.; Ferret, P.-J. Comparison and validation of an in vitro skin sensitization strategy using a data set of 33 chemical references. Toxicol. Vitr. 2017, 45, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Caloni, F.; De Angelis, I.; Hartung, T. Replacement of animal testing by integrated approaches to testing and assessment (IATA): A call for in vivitrosi. Arch. Toxicol. 2022, 96, 1935–1950. [Google Scholar] [CrossRef] [PubMed]
- Maertens, A.; Golden, E.; Luechtefeld, T.H.; Hoffmann, S.; Tsaioun, K.; Hartung, T. Probabilistic risk assessment—The keystone for the future of toxicology. ALTEX 2022, 39, 3–29. [Google Scholar] [CrossRef] [PubMed]
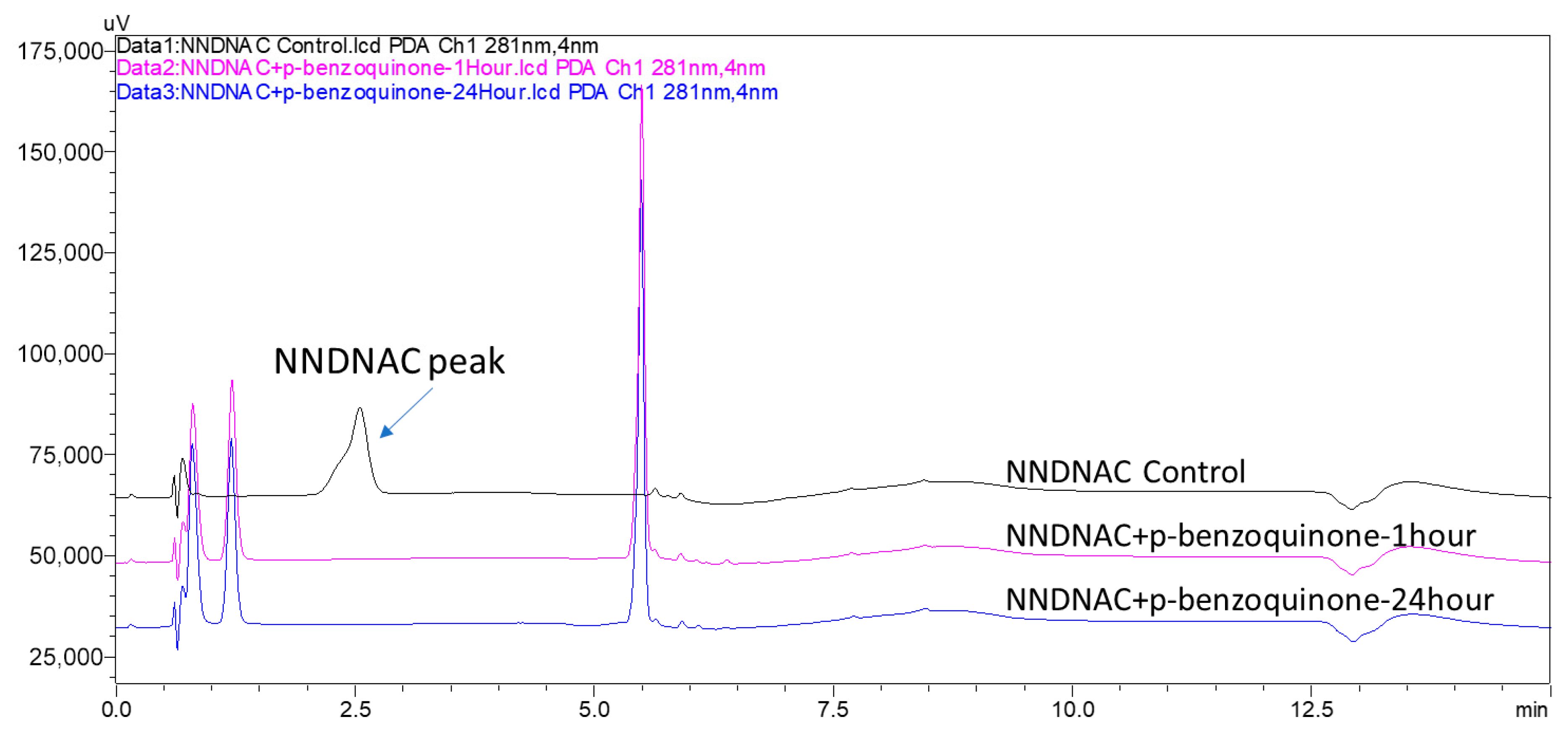
| CAS NO. | % Depletion of Probe Compound/Peptide | KeratinoSens EC3 (mM) | LLNA EC3 (%) | |||
|---|---|---|---|---|---|---|
| NNDNAC | Cys-DPRA | NAC-ADRA | ||||
| p-Benzoquinone | 106-51-4 | 100 | 100 | 100 | 32.77 | 0.01 |
| 2-Methyl-4 isothiazolin 3-one | 2682-20-4 | 98 | 100 | 100 | 29.56 | 1.9 |
| Farnesal | 502-67-0 | 3 | 15–55 | 20–40 | >2000 | 12 |
| Cinnamyl alcohol | 104-54-1 | 1 | 0 | 0 | >2000 | 21 |
| Lactic acid | 50-21-5 | 0 | 0 | 0 | >2000 | ND |
| p-Phenylenediamine | 106-50-3 | 0 | 98.6 | 100 | ND | 0.16 |
| 4-Aminophenol | 123-30-8 | 0 | 100 | 100 | ND | ND |
| Test Compound | % Depletion of Probe Compound/Peptide | |||
|---|---|---|---|---|
| NNDNAC | NAC-ADRA | |||
| Incubation Time | ||||
| 1 h | 24 h | 1 h | 24 h | |
| p-Benzoquinone | 100 | 100 | 4 | 100 |
| 2-Methyl 4-isothiazolin 3-one | 98 | 100 | 4.8 | 100 |
| Cinnamyl alcohol | 1 | 2 | 0 | 0 |
| Lactic acid | 0 | 0 | 0 | 0 |
| p-Phenylenediamine | 0 | 100 | 0 | 100 |
| 4-Aminophenol | 0 | 100 | 0 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, A.; Sudhakar, D.G.S.; Choudhury, R.P. Design and Synthesis of a Novel in Chemico Reactivity Probe N,N-dimethyl N-(2-(1-naphthyl)acetyl)-l-cysteine (NNDNAC) for Rapid Skin Sensitization Assessment of Cosmetic Ingredients. Cosmetics 2025, 12, 268. https://doi.org/10.3390/cosmetics12060268
Singh A, Sudhakar DGS, Choudhury RP. Design and Synthesis of a Novel in Chemico Reactivity Probe N,N-dimethyl N-(2-(1-naphthyl)acetyl)-l-cysteine (NNDNAC) for Rapid Skin Sensitization Assessment of Cosmetic Ingredients. Cosmetics. 2025; 12(6):268. https://doi.org/10.3390/cosmetics12060268
Chicago/Turabian StyleSingh, Akanksha, D. G. S. Sudhakar, and Ratnadeep Paul Choudhury. 2025. "Design and Synthesis of a Novel in Chemico Reactivity Probe N,N-dimethyl N-(2-(1-naphthyl)acetyl)-l-cysteine (NNDNAC) for Rapid Skin Sensitization Assessment of Cosmetic Ingredients" Cosmetics 12, no. 6: 268. https://doi.org/10.3390/cosmetics12060268
APA StyleSingh, A., Sudhakar, D. G. S., & Choudhury, R. P. (2025). Design and Synthesis of a Novel in Chemico Reactivity Probe N,N-dimethyl N-(2-(1-naphthyl)acetyl)-l-cysteine (NNDNAC) for Rapid Skin Sensitization Assessment of Cosmetic Ingredients. Cosmetics, 12(6), 268. https://doi.org/10.3390/cosmetics12060268






