Injectable Poly-L-Lactic Acid (PLLA-SCA™) as a Versatile Treatment in Current Aesthetic Medicine: Expert Recommendations Based on Italian Clinical Experience
Abstract
1. Introduction
2. Methods
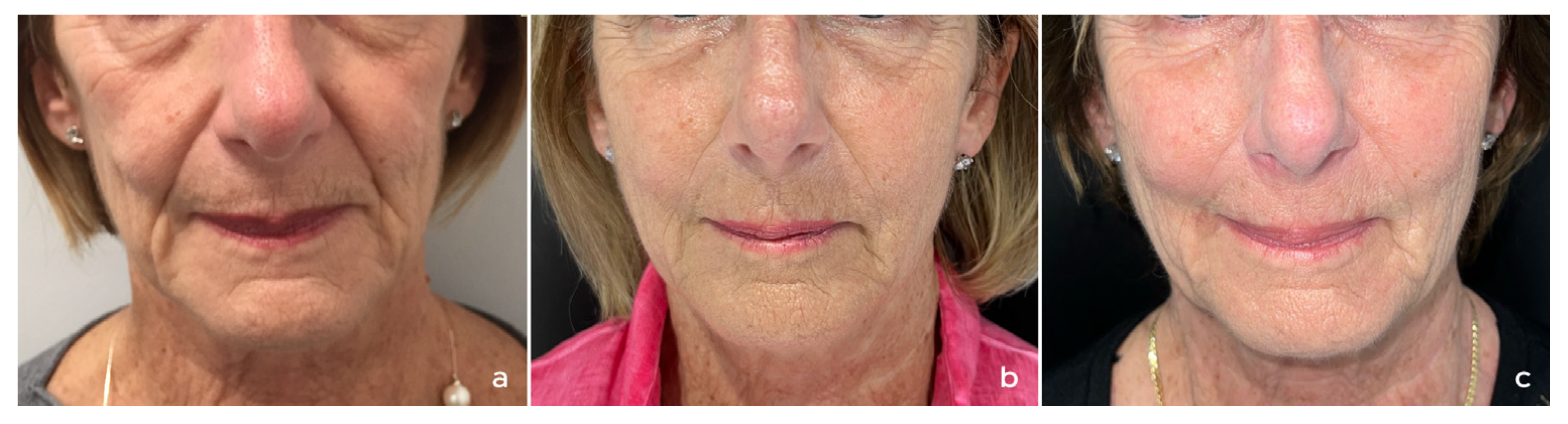
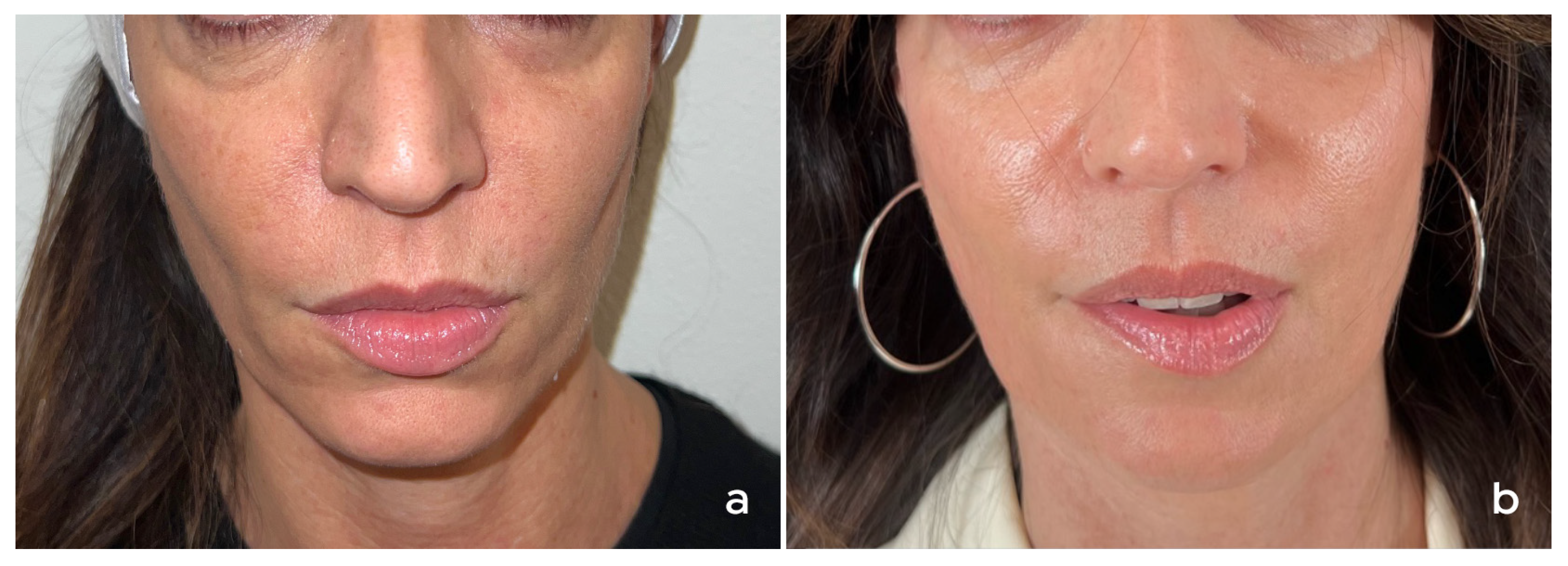
3. Treatment of the Face
3.1. Authors’ Recommendations for Treatment of the Face
3.1.1. Patient Selection
3.1.2. Product Preparation and Treatment Protocol
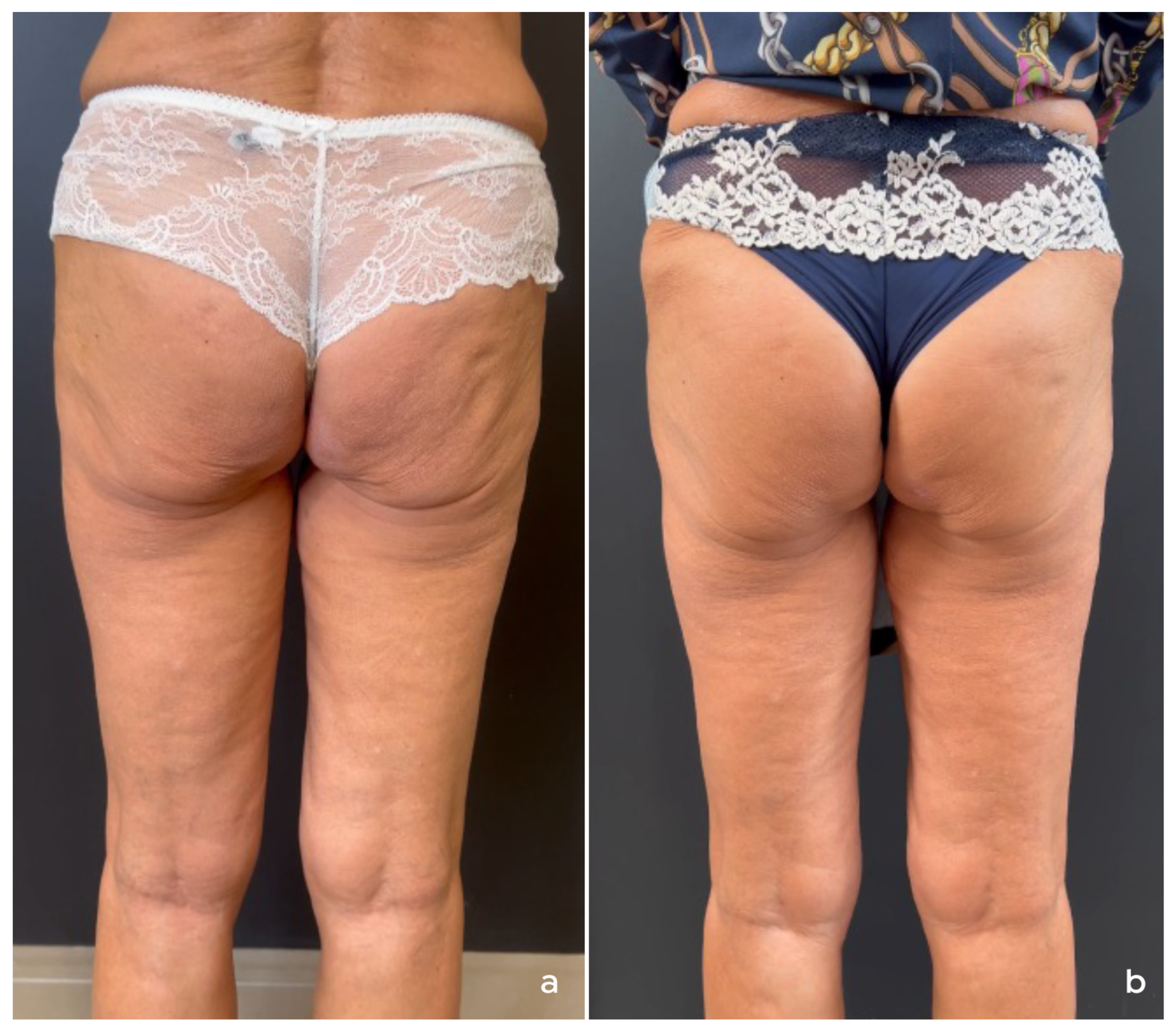
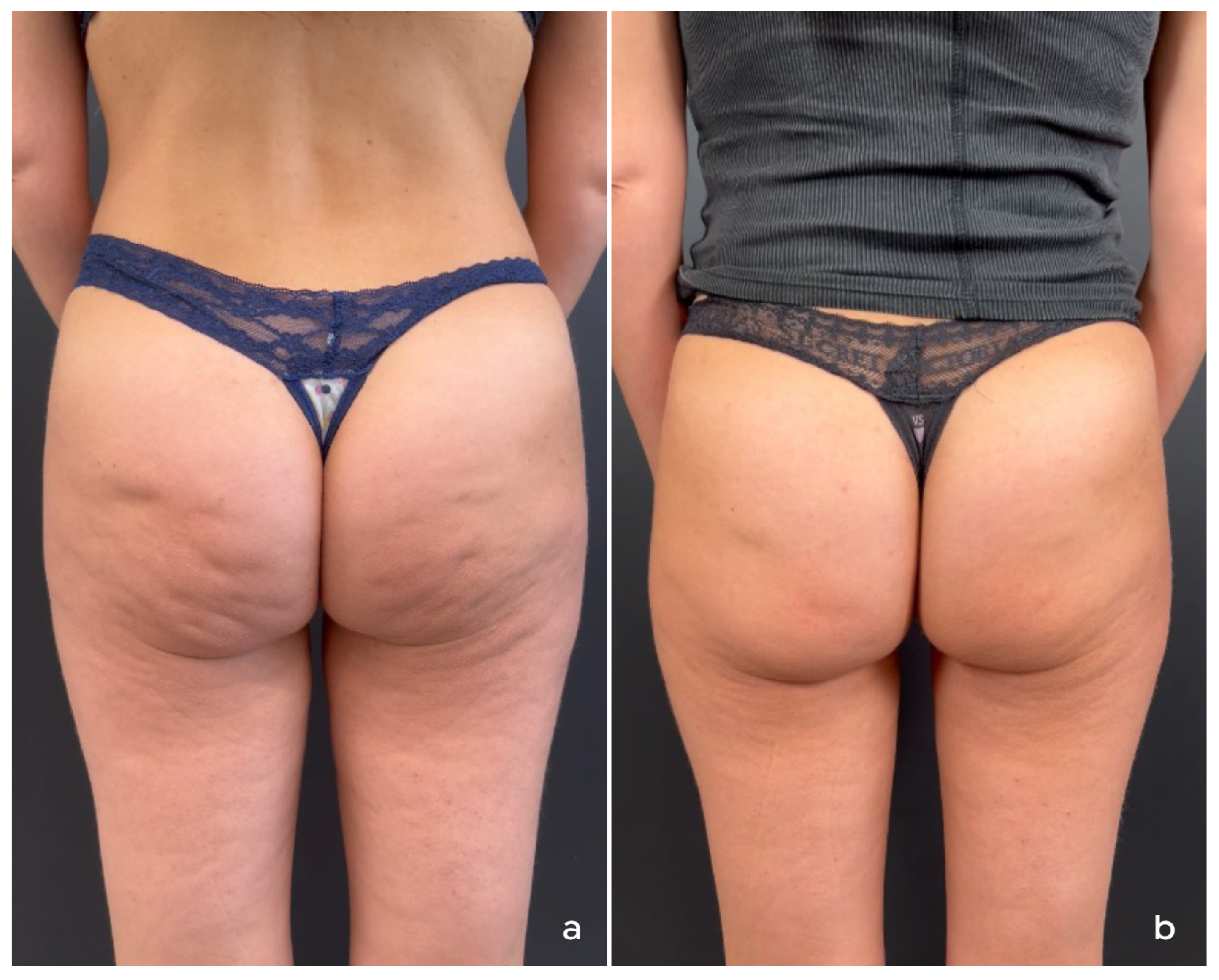
4. Treatment of Non-Facial Body Areas
4.1. Authors’ Recommendations for Treatment of Non-Facial Body Areas
4.1.1. Patient Selection
4.1.2. Product Preparation and Treatment Protocol
5. Combination of PLLA-SCA™ with Other Aesthetic Treatments
Authors’ Recommendations for Treatment Combinations
6. Safety of PLLA-SCA™
Authors’ Recommendations for Safety
7. Limitations
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, X.; Gao, X.; Xie, W. Research Progress in Skin Aging, Metabolism, and Related Products. Int. J. Mol. Sci. 2023, 24, 15930. [Google Scholar] [CrossRef] [PubMed]
- Wang, H. A Review of the Effects of Collagen Treatment in Clinical Studies. Polymers 2021, 13, 3868. [Google Scholar] [CrossRef]
- Kwan, K.R.; Kolansky, Z.; Abittan, B.J.; Farberg, A.S.; Goldenberg, G. Skin tightening. Cutis 2020, 106, 134–137, 139, E1. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Fang, W.; Wang, F. Injectable fillers: Current status, physicochemical properties, function mechanism, and perspectives. RSC Adv. 2023, 13, 23841–23858. [Google Scholar] [CrossRef] [PubMed]
- Go, B.C.; Frost, A.S.; Friedman, O. Using injectable fillers for chin and jawline rejuvenation. World J. Otorhinolaryngol.-Head Neck Surg. 2023, 9, 131–137. [Google Scholar] [CrossRef]
- Avelar, L.E.; Nabhani, S.; Wüst, S. Unveiling the Mechanism: Injectable Poly-L-Lactic Acid’s Evolving Role-Insights From Recent Studies. J. Cosmet. Dermatol. 2024, 24, e16635. [Google Scholar] [CrossRef]
- Zhu, W.; Dong, C. Poly-L-Lactic acid increases collagen gene expression and synthesis in cultured dermal fibroblast (Hs68) through the TGF-β/Smad pathway. J. Cosmet. Dermatol. 2023, 22, 1213–1219. [Google Scholar] [CrossRef]
- Waibel, J.; Ziegler, M.; Nguyen, T.Q.; Le, J.H.; Qureshi, A.; Widgerow, A.; Meckfessel, M. Comparative Bulk RNA-Seq Analysis of Poly-l-Lactic Acid Versus Calcium Hydroxylapatite Reveals a Novel, Adipocyte-Mediated Regenerative Mechanism of Action Unique to PLLA. Dermatol. Surg. 2024, 50, S166–S171. [Google Scholar] [CrossRef]
- Vleggaar, D.; Fitzgerald, R.; Lorenc, Z.P. The history behind the use of injectable poly-L-lactic acid for facial and nonfacial volumization: The positive impact of evolving methodology. J. Drugs Dermatol. 2014, 13 (Suppl. 4), s32–s34. [Google Scholar]
- Sculptra™ Instructions for Use [Package Insert], Europe; Galderma Laboratories: Dallas, TX, USA, 2020.
- Simamora, P.; Chern, W. Poly-L-lactic acid: An overview. J. Drugs Dermatol. 2006, 5, 436–440. [Google Scholar]
- Eason, C.; Snyder, A.; Favre, C.; Schlesinger, T. Sculptra®—History and how it is best used today. Dermatol. Rev. 2023, 4, 115–120. [Google Scholar] [CrossRef]
- Goldberg, D.; Guana, A.; Volk, A.; Daro-Kaftan, E. Single-arm study for the characterization of human tissue response to injectable poly-L-lactic acid. Dermatol. Surg. 2013, 39, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Stein, P.; Vitavska, O.; Kind, P.; Hoppe, W.; Wieczorek, H.; Schürer, N.Y. The biological basis for poly-L-lactic acid-induced augmentation. J. Dermatol. Sci. 2015, 78, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Vleggaar, D.; Bauer, U. Facial enhancement and the European experience with Sculptra (poly-l-lactic acid). J. Drugs Dermatol. 2004, 3, 542–547. [Google Scholar] [PubMed]
- Huth, S.; Huth, L.; Marquardt, Y.; Jansen, M.; Lin, C.; Bartneck, M.; Baron, J.M. Molecular Insights Into the Effects of PLLA-SCA™ on Gene Expression and Collagen Synthesis in Human 3D Skin Models Containing Macrophages. J. Drugs Dermatol. 2004, 23, 285–288. [Google Scholar] [CrossRef]
- Instructions for Use Sculptra Aesthetic US. Galderma Laboratories, 2023. Available online: https://www.galderma.com/us/sites/default/files/2023-04/Sculptra_USA_eIFU.pdf (accessed on 30 July 2024).
- Baumann, K.; Alm, J.; Norberg, M.; Ejehorn, M. Immediate Use After Reconstitution of a Biostimulatory Poly-L-Lactic Acid Injectable Implant. J. Drugs Dermatol. 2020, 19, 1199–1203. [Google Scholar] [CrossRef]
- Palm, M.; Weinkle, S.; Cho, Y.; LaTowsky, B.; Prather, H. A Randomized Study on PLLA Using Higher Dilution Volume and Immediate Use Following Reconstitution. J. Drugs Dermatol. 2021, 20, 760–766. [Google Scholar] [PubMed]
- Vleggaar, D.; Fitzgerald, R.; Lorenc, Z.P.; Andrews, J.T.; Butterwick, K.; Comstock, J.; Hanke, C.W.; O’Daniel, T.G.; Palm, M.D.; Roberts, W.E.; et al. Consensus recommendations on the use of injectable poly-L-lactic acid for facial and nonfacial volumization. J. Drugs Dermatol. 2014, 13 (Suppl. 4), s44–s51. [Google Scholar]
- Redaelli, A.; Rzany, B.; Eve, L.; Grangier, Y.; Herranz, P.; Olivier-Masveyraud, F.; Vleggaar, D. European expert recommendations on the use of injectable poly-L-lactic acid for facial rejuvenation. J. Drugs Dermatol. 2014, 13, 1057–1066. [Google Scholar]
- Haddad, A.; Menezes, A.; Guarnieri, C.; Coimbra, D.; Ribeiro, E.; Sarubi, J.; Avelar, L.E.; Del Nero, M.P.; da Cunha, M.G.; Mazzuco, R.; et al. Recommendations on the Use of Injectable Poly-L-Lactic Acid for Skin Laxity in Off-Face Areas. J. Drugs Dermatol. 2019, 18, 929–935. [Google Scholar]
- Avelar, L.; Ong, A.; Ong, D.; Wai, A.C.S.; Wai, A.Y.T.; Sungkyu, J.; Seok, L.H.; Tam, E.; Leng, S.E.; Huang, J.; et al. Consensus recommendations on the use of injectable poly-l-lactic acid in Asian patients. J. Cosmet. Dermatol. 2023, 22, 3223–3231. [Google Scholar] [CrossRef]
- Haddad, A.; Avelar, L.; Fabi, S.G.; Sarubi, J.; Somenek, M.; Coimbra, D.D.; Palm, M.; Durairaj, K.K.; Somji, M.; Vasconcelos-Berg, R.; et al. Injectable Poly-L-Lactic Acid for Body Aesthetic Treatments: An International Consensus on Evidence Assessment and Practical Recommendations. Aesthetic Plast. Surg. 2025, 49, 1507–1517. [Google Scholar] [CrossRef]
- Narins, R.S.; Baumann, L.; Brandt, F.S.; Fagien, S.; Glazer, S.; Lowe, N.J.; Monheit, G.D.; Rendon, M.I.; Rohrich, R.J.; Werschler, W.P. A randomized study of the efficacy and safety of injectable poly-L-lactic acid versus human-based collagen implant in the treatment of nasolabial fold wrinkles. J. Am. Acad. Dermatol. 2010, 62, 448–462. [Google Scholar] [CrossRef]
- Bohnert, K.; Dorizas, A.; Lorenc, P.; Sadick, N.S. Randomized, Controlled, Multicentered, Double-Blind Investigation of Injectable Poly-L-Lactic Acid for Improving Skin Quality. Dermatol. Surg. 2019, 45, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Alcotzer, I.; Liassidou, A.; Hexsel, D.; Shenhav, L.T.; Artzi, O. Optimal Changes Seen in Patients After Treatment with Poly-L-Lactic Acid: A Retrospective Descriptive Study. Dermatol. Surg. 2024, 50, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Palm, M.; Mayoral, F.; Rajani, A.; Goldman, M.P.; Fabi, S.; Espinoza, L.; Andriopoulos, B.; Harper, J. Chart Review Presenting Safety of Injectable PLLA Used with Alternative Reconstitution Volume for Facial Treatments. J. Drugs Dermatol. 2021, 20, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Fabi, S.; Hamilton, T.; LaTowsky, B.; Kazin, R.; Marcus, K.; Mayoral, F.; Joseph, J.; Hooper, D.; Shridharani, S.; Hicks, J.; et al. Effectiveness and Safety of Sculptra Poly-L-Lactic Acid Injectable Implant in the Correction of Cheek Wrinkles. J. Drugs Dermatol. 2024, 23, 1297–1305. [Google Scholar] [CrossRef]
- Mu, X.; Luo, S.; Zhao, H. 12-Month effectiveness and safety of PLLA treatment of midface in Chinese subjects: A multicenter, randomized, no-treatment controlled study. In Proceedings of the AMWC Congress, Monte Carlo, Monaco, 30 March–1 April 2023. [Google Scholar]
- LaTowsky, B.; Fabi, S.; Hamilton, T. Long-term effect of biostimulatory Sculptra Poly-L-Lactic Acid injectable (PLLA-SCA™) implant on correction of cheek wrinkles and nasolabial fold contour deficiencies. In Proceedings of the AMWC World Congress, Monte Carlo, Monaco, 27–29 March 2024. [Google Scholar]
- Avelar, L.E.T.; Haddad, A. Facial assessment for poly-l-lactic acid application—One product, different outcomes. J. Cosmet. Dermatol. 2023, 7, 75–77. [Google Scholar] [CrossRef]
- Christen, M.O. Collagen Stimulators in Body Applications: A Review Focused on Poly-L-Lactic Acid (PLLA). Clin. Cosmet. Investig. Dermatol. 2022, 15, 997–1019. [Google Scholar] [CrossRef]
- Almukhtar, R.M.; Wood, E.S.; Loyal, J.; Hartman, N.; Fabi, S.G. A Randomized, Single-Center, Double-Blinded, Split-Body Clinical Trial of Poly-L-Lactic Acid for the Treatment of Cellulite of the Buttocks and Thighs. Dermatol. Surg. 2023, 49, 378–382. [Google Scholar] [CrossRef]
- Durairaj, K.K.; Devgan, L.; Lee, A.; Khachatourian, N.; Nguyen, V.; Issa, T.; Baker, O. Poly-L-Lactic Acid for Gluteal Augmentation found to be Safe and Effective in Retrospective Clinical Review of 60 Patients. Dermatol. Surg. 2020, 46 (Suppl. 1), S46–S53. [Google Scholar] [CrossRef]
- Nikolis, A.; Enright, K.; Avelar, L.; Rice, S.; Sinno, H.; Rizis, D.; Cotofana, S. A Prospective, Multicenter Trial on the Efficacy and Safety of Poly-L-Lactic Acid for the Treatment of Contour Deformities of the Buttock Regions. J. Drugs Dermatol. 2022, 21, 304–308. [Google Scholar]
- Harper, J.; Avelar, L.; Haddad, A.; Novello, J.; Mest, D.; Guarnieri-Munia, C.; Rice, S.; Vega, J.; Andriopolous, B.; Nogueria, A.; et al. Expert Recommendations on the Use of Injectable Poly-L-lactic Acid for Contour Deficiencies of the Buttocks. J. Drugs Dermatol. 2022, 21, 21–26. [Google Scholar] [CrossRef]
- Swearingen, A.; Medrano, K.; Ferzli, G.; Sadick, N.; Arruda, S. Randomized, Double-Blind, Placebo-Controlled Study of Poly-L-Lactic acid for Treatment of Cellulite in the Lower Extremities. J. Drugs Dermatol. 2021, 20, 529–533. [Google Scholar]
- Zubair, R.; Ishii, L.; Loyal, J.; Hartman, N.; Fabi, S.G. SPLASH: Split-Body Randomized Clinical Trial of Poly-L-Lactic Acid for Adipogenesis and Volumization of the Hip Dell. Dermatol. Surg. 2024, 50, 1155–1162. [Google Scholar] [CrossRef]
- Joseph, J.; Palm, M.; Bråsäter, D.; Weinberg, F.; Prygova, I. Evaluation of the safety and effectiveness of higher reconstitution volumes of Poly-L-Lactic Acid injectable (PLLA-SCA™) for correction of wrinkles in the décolletage area. In Proceedings of the AMWC World Congress, Monte Carlo, Monaco, 27–29 March 2024. [Google Scholar]
- Sadick, N.S.; Arruda, S. The Use of Poly-L-Lactic Acid in the Abdominal Area. Dermatol. Surg. 2017, 43, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Sarubi, J.; Guarnieri, C.; Del Nero, M.P.; Kamamoto, C.; Honda, M.; Saito, F.; Haddad, A. Targeted and Individualized Gluteal Poly-L-Lactic Acid Injection for Optimal Aesthetic Results in the Gluteal Region. J. Clin. Aesthetic Dermatol. 2023, 16, 30–36. [Google Scholar]
- Sadick, N.S.; Manhas-Bhutani, S.; Krueger, N. A novel approach to structural facial volume replacement. Aesthetic Plast. Surg. 2013, 37, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri Munia, C.; Morais, M.; Sato, M.; Parada, M.; Avelar, L.E. Optimizing Skin Quality with Injectable Poly-L-Lactic Acid and Hyaluronic Acid. J. Clin. Aesthetic Dermatol. 2023, 16, 26–29. [Google Scholar]
- Sarubi, J.; Avelar, L.E.T.; Nero, M.P.D.; Kamamoto, C.; Morais, M. Facial rejuvenation on the use of injectable poly-L-lactic acid and hyaluronic acid: Combined technique. J. Cosmet. Dermatol. 2022, 21, 5261–5263. [Google Scholar] [CrossRef]
- Duracinsky, M.; Leclercq, P.; Herrmann, S.; Christen, M.O.; Dolivo, M.; Goujard, C.; Chassany, O. Safety of poly-L-lactic acid (New-Fill®) in the treatment of facial lipoatrophy: A large observational study among HIV-positive patients. BMC Infect. Dis. 2014, 14, 474. [Google Scholar] [CrossRef] [PubMed]
- Lafaille, P.; Benedetto, A. Fillers: Contraindications, side effects and precautions. J. Cutan. Aesthetic Surg. 2010, 3, 16–19. [Google Scholar] [CrossRef] [PubMed]
- King, M.; Bassett, S.; Davies, E.; King, S. Management of Delayed Onset Nodules. J. Clin. Aesthetic Dermatol. 2016, 9, E1–E5. [Google Scholar]
- De Boulle, K.; Heydenrych, I. Patient factors influencing dermal filler complications: Prevention, assessment, and treatment. Clin. Cosmet. Investig. Dermatol. 2015, 8, 205–214. [Google Scholar] [CrossRef] [PubMed]
| Topic | Recommendations |
|---|---|
| TREATMENT OF THE FACE | PATIENT SELECTION
|
PRODUCT PREPARATION/TREATMENT PROTOCOL
| |
| TREATMENT OF NON-FACIAL BODY AREAS | PATIENT SELECTION
|
PRODUCT PREPARATION/TREATMENT PROTOCOL
| |
| TREATMENT COMBINATIONS |
|
| SAFETY |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Innocenti, A.; Battistella, T.; Gregorio, C.D.; Leporati, M.; Luni, M.; Rossati, L. Injectable Poly-L-Lactic Acid (PLLA-SCA™) as a Versatile Treatment in Current Aesthetic Medicine: Expert Recommendations Based on Italian Clinical Experience. Cosmetics 2025, 12, 264. https://doi.org/10.3390/cosmetics12060264
Innocenti A, Battistella T, Gregorio CD, Leporati M, Luni M, Rossati L. Injectable Poly-L-Lactic Acid (PLLA-SCA™) as a Versatile Treatment in Current Aesthetic Medicine: Expert Recommendations Based on Italian Clinical Experience. Cosmetics. 2025; 12(6):264. https://doi.org/10.3390/cosmetics12060264
Chicago/Turabian StyleInnocenti, Alessandro, Tommaso Battistella, Carlo Di Gregorio, Massimiliano Leporati, Massimo Luni, and Leonardo Rossati. 2025. "Injectable Poly-L-Lactic Acid (PLLA-SCA™) as a Versatile Treatment in Current Aesthetic Medicine: Expert Recommendations Based on Italian Clinical Experience" Cosmetics 12, no. 6: 264. https://doi.org/10.3390/cosmetics12060264
APA StyleInnocenti, A., Battistella, T., Gregorio, C. D., Leporati, M., Luni, M., & Rossati, L. (2025). Injectable Poly-L-Lactic Acid (PLLA-SCA™) as a Versatile Treatment in Current Aesthetic Medicine: Expert Recommendations Based on Italian Clinical Experience. Cosmetics, 12(6), 264. https://doi.org/10.3390/cosmetics12060264





