Centella asiatica L. Urb. Extracellular Vesicle and Growth Factor Essence for Hair and Scalp Health: A 56-Day Exploratory Randomized Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
- Group A: Placebo control (Base formula without caffeine and panthenol, see below)
- Group B: Base formula (active ingredients: 0.1% caffeine and panthenol)
- Group C: Base formula + recombinant long-acting insulin growth factor-1 (rIGF-1) and recombinant long-acting fibroblast growth factor-7 (rFGF-7)
- Group D: Base formula + Centella asiatica extracellular vesicles (C. asiatica EV).
- Group E: Base formula + rIGF-1, rFGF-7, and C. asiatica EV.
- rIGF-1: sr-(Methionyl sh-Oligopeptide-2 Dipeptide-46 Oligopeptide-163 sh-Polypeptide-181), INCI ID:40196
- rFGF-7: sr-(Methionyl sh-Polypeptide-3 Dipeptide-46 Oligopeptide-163 sh-Polypeptide-181), INCI ID:40201
2.2. Randomization and Blinding
2.3. Assessment of Outcomes
- Scalp sebum levels were quantified using C+K Multi Probe Adaptor MPA580 system with Sebumeter® SM815 probe (Courage-Khazaka Electronic GmbH, Koln, Germany) via grease-spot photometry.
- Hair length, thickness, and density were assessed with the ScalpX Intelligent Scalp Diagnostic System (VAST Technologies Inc., Taipei City, Taiwan) with a 5-megapixel handheld USB digital microscope (DMC1213_USB 200X, Coresync Technology Corp., New Taipei City, Taiwan) to capture high-resolution scalp images and analyze using machine learning and artificial intelligence with an internal database.
2.4. Statistical Analysis
3. Results
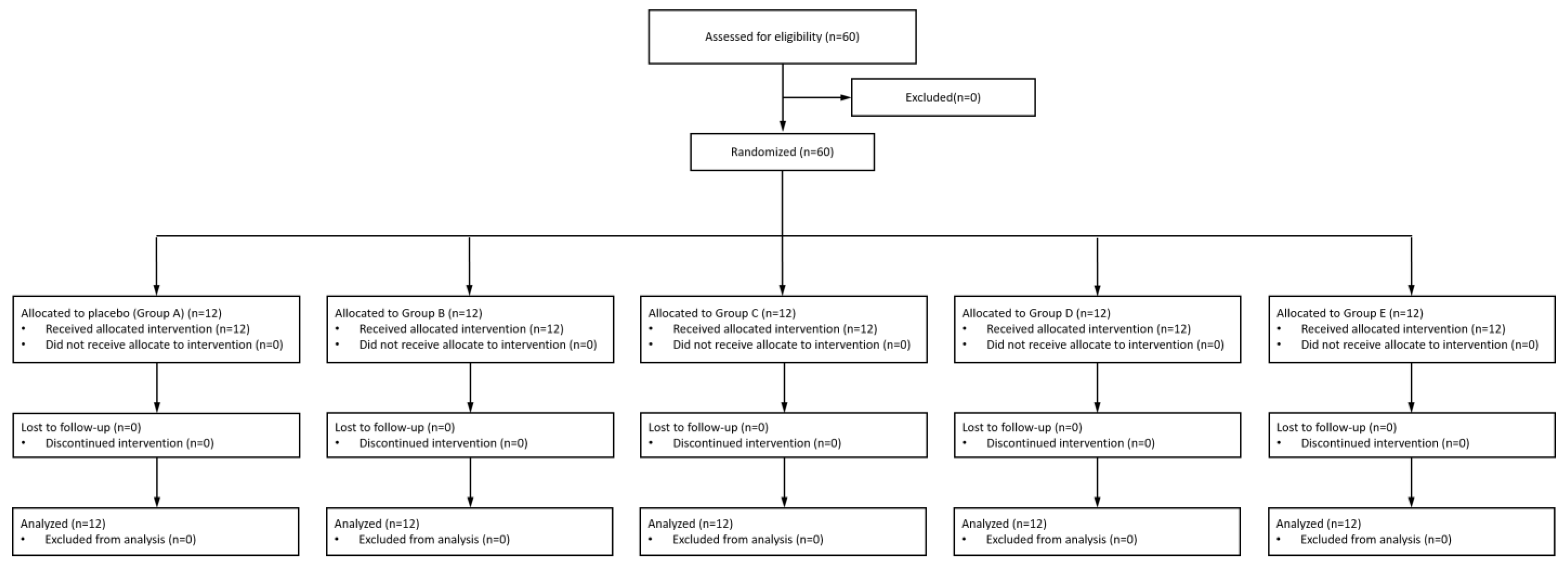
3.1. Participants
3.2. Assessments of Outcomes
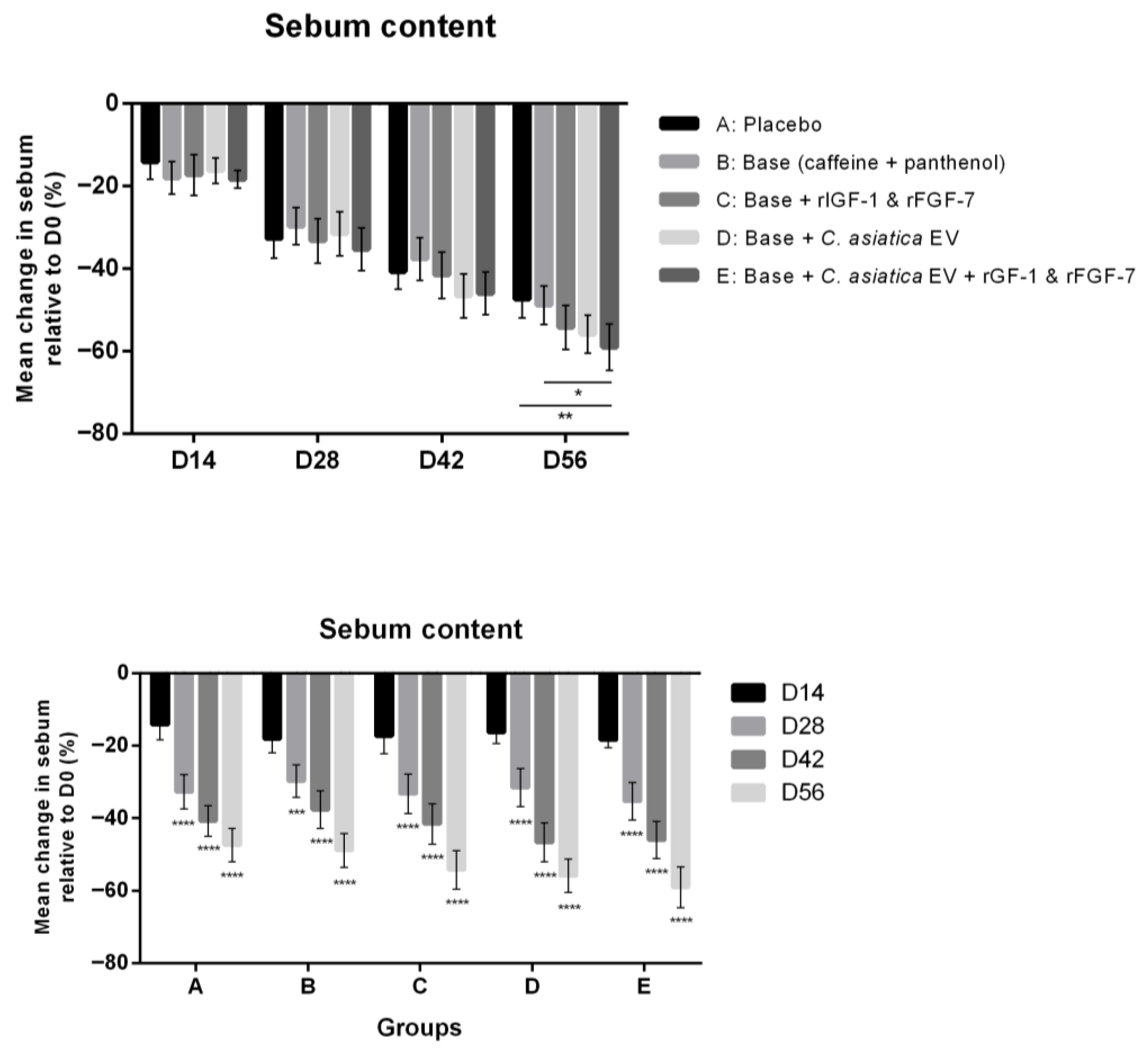
3.2.1. Sebum Content
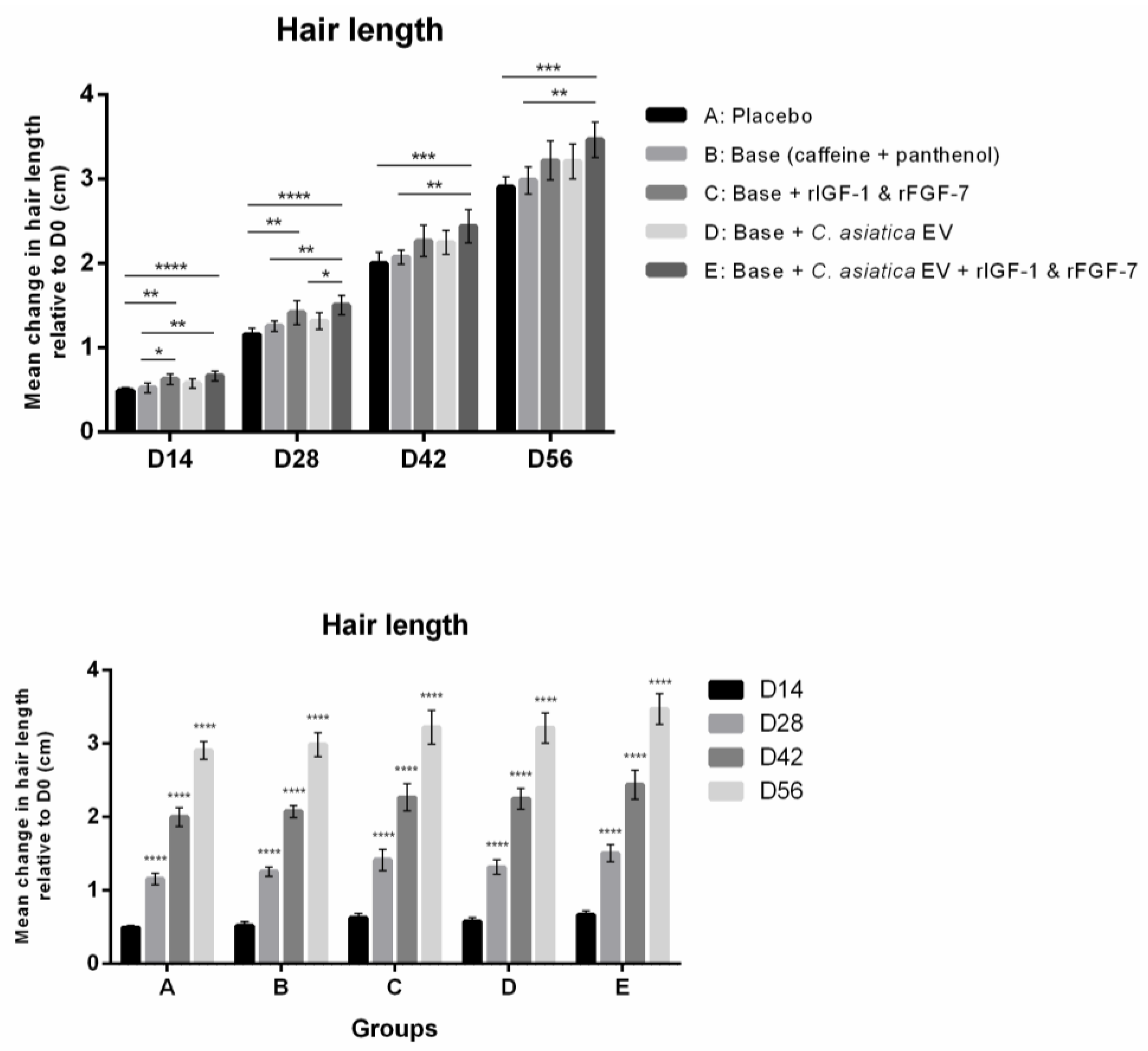
3.2.2. Hair Length
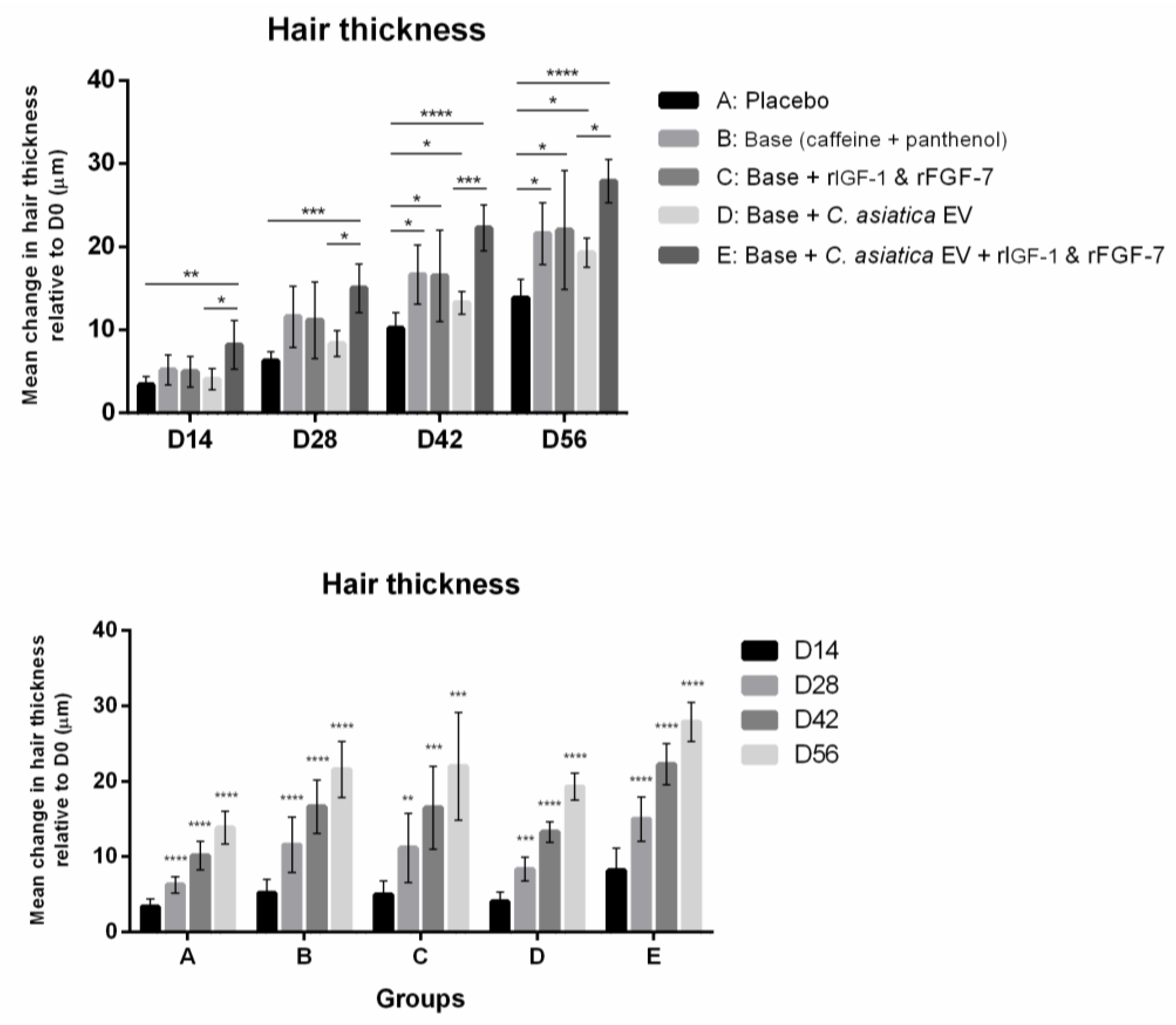
3.2.3. Hair Thickness
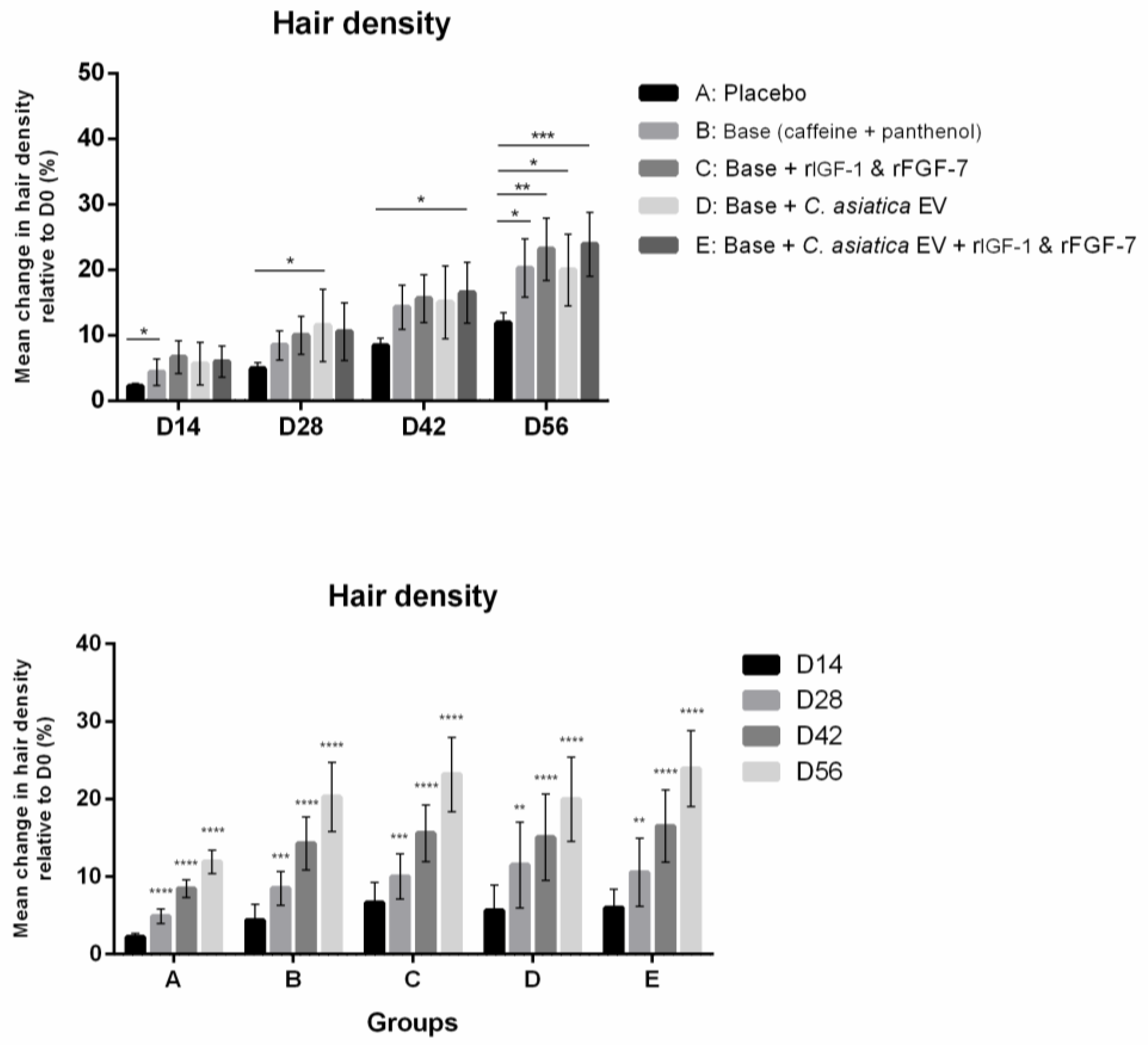
3.2.4. Hair Density
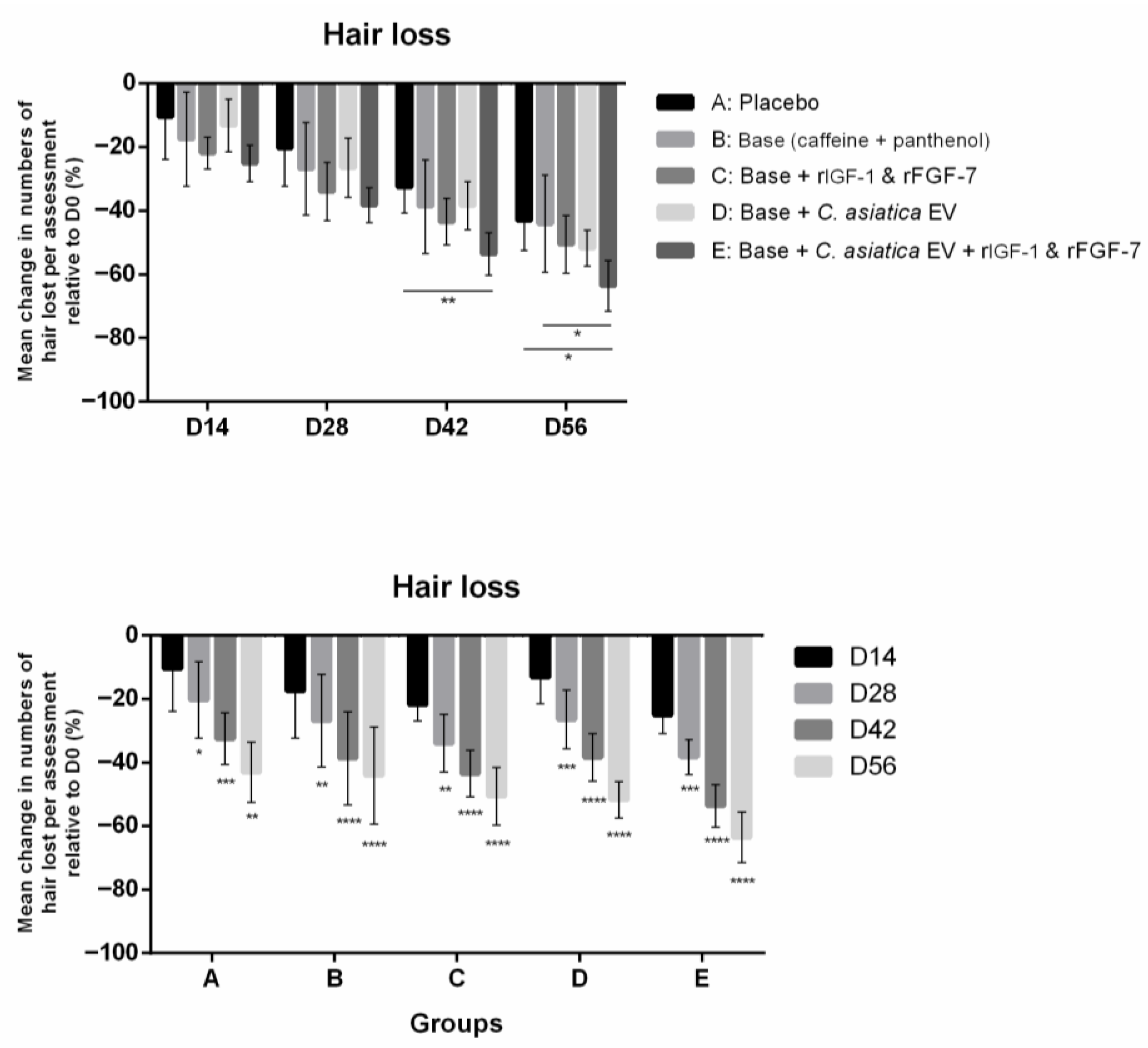
3.2.5. Hair Loss
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Norwood, O.T. Male pattern baldness: Classification and incidence. South Med. J. 1975, 68, 1359–1365. [Google Scholar] [CrossRef]
- Cash, T.F. The psychology of hair loss and its implications for patient care. Clin. Dermatol. 2001, 19, 161–166. [Google Scholar] [CrossRef]
- Alfonso, M.; Richter-Appelt, H.; Tosti, A.; Viera, M.S.; Garcia, M. The psychosocial impact of hair loss among men: A multinational European study. Curr. Med. Res. Opin. 2005, 21, 1829–1836. [Google Scholar] [CrossRef] [PubMed]
- Trueb, R.M. Molecular mechanisms of androgenetic alopecia. Exp. Gerontol. 2002, 37, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Randall, V.A. Androgens and hair growth. Dermatol. Ther. 2008, 21, 314–328. [Google Scholar] [CrossRef] [PubMed]
- Messenger, A.G.; Rundegren, J. Minoxidil: Mechanisms of action on hair growth. Br. J. Dermatol. 2004, 150, 186–194. [Google Scholar] [CrossRef]
- Kaufman, K.D.; Olsen, E.A.; Whiting, D.; Savin, R.; DeVillez, R.; Bergfeld, W.; Price, V.H.; Van Neste, D.; Roberts, J.L.; Hordinsky, M.; et al. Finasteride in the treatment of men with androgenetic alopecia. J. Am. Acad. Dermatol. 1998, 39, 578–589. [Google Scholar] [CrossRef]
- Irwig, M.S. Persistent sexual side effects of finasteride: Could they be permanent? J. Sex. Med. 2012, 9, 2927–2932. [Google Scholar] [CrossRef]
- Yuan, A.R.; Bian, Q.; Gao, J.Q. Current advances in stem cell-based therapies for hair regeneration. Eur. J. Pharmacol. 2020, 881, 173197. [Google Scholar] [CrossRef]
- Avci, P.; Gupta, G.K.; Clark, J.; Wikonkal, N.; Hamblin, M.R. Low-level laser (light) therapy (LLLT) for treatment of hair loss. Lasers Surg. Med. 2014, 46, 144–151. [Google Scholar] [CrossRef]
- Trueb, R.M. Further clinical evidence for the effect of IGF-1 on hair growth and alopecia. Skin Appendage Disord. 2018, 4, 90–95. [Google Scholar] [CrossRef]
- Danilenko, D.M.; Ring, B.D.; Yanagihara, D.; Benson, W.; Wiemann, B.; Starnes, C.O.; Pierce, G.F. Keratinocyte growth factor is an important endogenous mediator of hair follicle growth, development, and differentiation. Am. J. Pathol. 1995, 147, 145–154. [Google Scholar]
- Dhariwala, M.Y.; Ravikumar, P. An overview of herbal alternatives in androgenetic alopecia. J. Cosmet. Dermatol. 2019, 18, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Monthakantirat, O.; Tengamnuay, P.; De-Eknamkul, W. Identification of a new plant extract for androgenic alopecia treatment using a non-radioactive human hair dermal papilla cell-based assay. BMC Complement. Altern. Med. 2015, 16, 18. [Google Scholar] [CrossRef] [PubMed]
- Bandopadhyay, S.; Mandal, S.; Ghorai, M.; Jha, N.K.; Kumar, M.; Radha, N.; Ghosh, A.; Proćków, J.; Perez de la Lastra, J.M.; Dey, A. Therapeutic Properties and Pharmacological Activities of Asiaticoside and Madecassoside: A Review. J. Cell. Mol. Med. 2023, 27, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.M.; An, S.; Lee, J.; Lee, J.H.; Lee, J.N.; Kim, Y.S.; Ahn, K.J.; An, I.S.; Bae, S. Titrated extract of Centella asiatica increases hair inductive property through inhibition of STAT signaling pathway in three-dimensional spheroid cultured human dermal papilla cells. Biosci. Biotechnol. Biochem. 2017, 81, 2323–2329. [Google Scholar] [CrossRef]
- Togni, S.; Meneghin, M.; Eggenhoffner, R.; Giacomelli, L. Strengthening hair with Centella asiatica: A report of clinical and subjective efficacy of a local treatment with a 0.5% hair lotion. Esperienze Dermatol. 2018, 20 (Suppl. S2), 27–30. [Google Scholar] [CrossRef]
- Saansoomchai, P.; Surangkul, D.; Srikummool, T.C.M. Enhanced VEGF expression in hair follicle dermal papilla cells by Centella asiatica Linn. CMU J. Nat. Sci. 2018, 17, 25–37. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Zhang, Q.; Franklin, J.L.; Coffey, R.J. Extracellular vesicles and nanoparticles: Emerging complexities. Trends Cell Biol. 2023, 33, 667. [Google Scholar] [CrossRef]
- Moghassemi, S.; Dadashzadeh, A.; Sousa, M.J.; Vlieghe, H.; Yang, J.; León-Félix, C.M.; Amorim, C.A. Extracellular vesicles in nanomedicine and regenerative medicine: A re-view over the last decade. Bioact. Mater. 2024, 36, 126. [Google Scholar] [CrossRef]
- Yang, G.H.; Lee, Y.B.; Kang, D.; Choi, E.; Nam, Y.; Lee, K.H.; You, H.J.; Kang, H.J.; An, S.H.; Jeon, H. Overcome the barriers of the skin: Exosome therapy. Biomater. Res. 2023, 25, 22. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, F.; He, X.; Huang, K. Plant derived exosome-like nanoparticles and their therapeutic applications in glucolipid metabolism diseases. J. Agric. Food Chem. 2025, 73, 6385–6399. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.M.; Wu, C.C.; Huang, H.C.; Wang, S.S.; Chuang, C.H.; Kao, P.L.; Tang, W.H.; Liu, L.T.C.; Qiu, W.Y.; Percec, I.; et al. In Vitro Characterization of Centella asiatica Extracellular Vesicles and Their Skin Repair Effects in a UVB-Irradiated Mouse Model. Int. J. Mol. Sci. 2025, 26, 8982. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Li, D.; Gu, Y.; Liu, R.; Tang, X.; Zhao, Y.; Qi, F.; Wei, J.; Liu, J. Plant-Derived Nanovesicles: Further Exploration of Biomedical Function and Application Potential. Acta Pharm. Sin. 2023, 13, 3300–3320. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Yoon, E.J.; Kim, J.S.; Park, S.J.; Lee, H. Comparative analysis of the transcriptome and efficacy of bioactive Centella asiatica exosomes on skin cells. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Chang, T.-M.; Wu, C.-C.; Huang, H.-C.; Wang, S.-S.; Chuang, C.-H.; Kao, P.-L.; Tang, W.-H.; Liu, L.T.-C.; Qiu, W.-Y.; Percec, I.; et al. A 28-Day Pilot Study of the Effects on Facial Skin Hydration, Elasticity, and Texture of a Centella asiatica Extracellular Vesi-cle-Based Skin Care Formulation. Cosmetics 2025, 12, 186. [Google Scholar] [CrossRef]
- Su, H.Y.; Hickford, J.G.; Bickerstaffe, R.; Palmer, B.R. Insulin-like growth factor 1 and hair growth. Dermatol. Online J. 1999, 5, 1. [Google Scholar] [CrossRef]
- Li, J.; Yang, Z.; Li, Z.; Gu, L.; Wang, Y.; Sung, C. Exogenous IGF-1 promotes hair growth by stimulating cell proliferation and down regulating TGF-beta1 in C57BL/6 mice in vivo. Growth Horm. IGF Res. 2014, 24, 89–94. [Google Scholar] [CrossRef]
- Zhao, J.; Harada, N.; Okajima, K. Dihydrotestosterone inhibits hair growth in mice by inhibiting insulin-like growth factor-I production in dermal papillae. Growth Horm. IGF Res. 2011, 21, 260–267. [Google Scholar] [CrossRef]
- Philpott, M.P.; Sanders, D.A.; Kealey, T. Effects of insulin and insulin-like growth factors on cultured human hair follicles: IGF-I at physiologic concentrations is an important regulator of hair follicle growth in vitro. J. Investig. Dermatol. 1994, 102, 857–861. [Google Scholar] [CrossRef]
- Hayashi, Y.; Yamamoto, N.; Nakagawa, T.; Ito, J. Insulin-like growth factor 1 inhibits hair cell apoptosis and promotes the cell cycle of supporting cells by activating different downstream cascades after pharmacological hair cell injury in neonatal mice. Mol. Cell. Neurosci. 2013, 56, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shelat, H.; Geng, Y.J. IGF-1 prevents oxidative stress induced-apoptosis in induced pluripotent stem cells which is mediated by microRNA-1. Biochem. Biophys. Res. Commun. 2012, 426, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Chen, J.; Ding, J.; Zhang, J.; Chen, H. IGF1- and BM-MSC-incorporating collagen-chitosan scaffolds promote wound healing and hair follicle regeneration. Am. J. Transl. Res. 2020, 12, 6264–6276. [Google Scholar] [PubMed]
- Schlake, T. FGF signals specifically regulate the structure of hair shaft medulla via IGF-binding protein 5. Development 2005, 132, 2981–2990. [Google Scholar] [CrossRef]
- Perez-Mora, S.; Ocampo-Lopez, J.; Gomez-Garcia, M.D.C.; Perez-Ishiwara, D.G. BFNB enhances hair growth in C57BL/6 mice through the induction of EGF and FGF7 factors and the PI3K-AKT-beta-catenin pathway. Int. J. Mol. Sci. 2023, 24, 12110. [Google Scholar] [CrossRef]
- Kinoshita-Ise, M.; Tsukashima, A.; Kinoshita, T.; Yamazaki, Y.; Ohyama, M. Altered FGF expression profile in human scalp-derived fibroblasts upon WNT activation: Implication of their role to provide folliculogenetic microenvironment. Inflamm. Regen. 2020, 40, 35. [Google Scholar] [CrossRef]
- Iino, M.; Ehama, R.; Nakazawa, Y.; Iwabuchi, T.; Ogo, M.; Tajima, M.; Arase, S. Adenosine stimulates fibroblast growth factor-7 gene expression via adenosine A2b receptor signaling in dermal papilla cells. J. Investig. Dermatol. 2007, 127, 1318–1325. [Google Scholar] [CrossRef]
- Guo, L.; Degenstein, L.; Fuchs, E. Keratinocyte growth factor is required for hair development but not for wound healing. Genes Dev. 1996, 10, 165–175. [Google Scholar] [CrossRef]
- Lee, T.; Lee, D.; Jung, E.; Son, M.; Koo, K.; Choi, J. Engineering of long-acting human growth hormone-Fc fusion proteins: Effects of valency, fusion position, and linker design on pharmacokinetics and efficacy. PLoS ONE 2025, 20, e0323791. [Google Scholar] [CrossRef]
- Kim, S.J.; Kwak, H.H.; Cho, S.Y.; Sohn, Y.B.; Park, S.W.; Huh, R.; Kim, J.; Ko, A.R.; Jin, D.K. Pharmacokinetics, pharmacodynamics, and efficacy of a novel long-acting human growth hormone: Fc fusion protein. Mol. Pharm. 2015, 12, 3759–3765. [Google Scholar] [CrossRef]
- Fischer, T.W.; Hipler, U.C.; Elsner, P. Effect of caffeine and testosterone on the proliferation of human hair follicles in vitro. Int. J. Dermatol. 2007, 46, 27–35. [Google Scholar] [CrossRef]
- Fischer, T.W.; Herczeg-Lisztes, E.; Funk, W.; Zillikens, D.; Biro, T.; Paus, R. Differential effects of caffeine on hair shaft elongation, matrix and outer root sheath keratinocyte proliferation, and transforming growth factor-beta2/insulin-like growth factor-1-mediated regulation of the hair cycle in male and female human hair follicles in vitro. Br. J. Dermatol. 2014, 171, 1031–1043. [Google Scholar] [PubMed]
- Fischer, T.W.; Bergmann, A.; Kruse, N.; Kleszczynski, K.; Skobowiat, C.; Slominski, A.T.; Paus, R. New effects of caffeine on corticotropin-releasing hormone (CRH)-induced stress along the intrafollicular classical hypothalamic-pituitary-adrenal (HPA) axis (CRH-R1/2, IP3-R, ACTH, MC-R2) and the neurogenic non-HPA axis (substance P, p75NTR and TrkA) in ex vivo human male androgenetic scalp hair follicles. Br. J. Dermatol. 2021, 184, 96–110. [Google Scholar] [PubMed]
- Szendzielorz, E.; Spiewak, R. Caffeine as an active molecule in cosmetic products for hair loss: Its mechanisms of action in the context of hair physiology and pathology. Molecules 2025, 30, 167. [Google Scholar] [CrossRef] [PubMed]
- Sin, J.Y.; Kim, J.; Choi, Y.-H.; Kang, N.-G.; Lee, S. Dexpanthenol Promotes Cell Growth by Preventing Cell Senescence and Apoptosis in Cultured Human Hair Follicle Cells. Curr. Issues Mol. Biol. 2021, 43, 1361–1373. [Google Scholar] [CrossRef]
- Wang, Z.; Nan, W.; Si, H.; Wang, S.; Zhang, H.; Li, G. Pantothenic acid promotes dermal papilla cell proliferation in hair follicles of American minks via inhibitor of DNA Binding 3/Notch signaling pathway. Life Sci. 2020, 252, 117667. [Google Scholar] [CrossRef]
- Shieh, J.-S.; Chin, Y.-T.; Yeh, T.-T.; Guo, J.J.; Chang, F.-W.; Cheng, H.-R.; Hsu, H.-H.; Huang, W.-L.; Huang, H.-H.; Hsieh, Y.-Y.; et al. Topical Application of Bio-Pulsed Avian MSC-Derived Extracellular Vesicles Enhances Hair Regrowth and Skin Rejuvenation: Evidence from Clinical Evaluation and miRNA Profiling. Curr. Issues Mol. Biol. 2025, 47, 539. [Google Scholar] [CrossRef]
- Braunholtz, D.A.; Edwards, S.J.; Lilford, R.J. Are randomized clinical trials good for us (in the short term)? Evidence for a “trial effect”. J. Clin. Epidemiol. 2001, 54, 217–224. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Darlenski, R.; Surber, C. Glycerol and the skin: Holistic approach to its origin and functions. Br. J. Dermatol. 2008, 159, 23–34. [Google Scholar] [CrossRef]
- Craighead, D.H.; Alexander, L.M. Topical menthol increases cutaneous blood flow. Microvasc. Res. 2016, 107, 39–45. [Google Scholar] [CrossRef]

| Contained in Formulation | ||||||
|---|---|---|---|---|---|---|
| Component | % or Concentration | A | B | C | D | E |
| C. asiatica EV | 1011 particles/mL | − | − | − | + | + |
| rIGF-1 | 25 μg/mL | − | − | + | - | + |
| rFGF-7 | 15 μg/mL | − | − | + | - | + |
| Glycerin | 7% | + | + | + | + | + |
| Capryloyl Glycine | 0.6% | + | + | + | + | + |
| Xylitylglucoside | 0.075% | + | + | + | + | + |
| Hexylene Glycol | 0.75% | + | + | + | + | + |
| Propanediol | 1.17% | + | + | + | + | + |
| Caprylhydroxamic acid | 0.09% | + | + | + | + | + |
| 1,2-Hexanediol | 0.54% | + | + | + | + | + |
| Panthenol | 0.5% | − | + | + | + | + |
| Xanthan Gum | 0.25% | + | + | + | + | + |
| Menthol | 0.6% | + | + | + | + | + |
| Menthyl Lactate | 0.4% | + | + | + | + | + |
| Caffeine | 0.1% | − | + | + | + | + |
| PPG-10 Methyl Glucose Ether | 0.05% | + | + | + | + | + |
| Aqua | To 100% | + | + | + | + | + |
| Allocated Group | A | B | C | D | E | p-Value * |
|---|---|---|---|---|---|---|
| n | 12 | 12 | 12 | 12 | 12 | ns |
| Gender | ns | |||||
| Female | 11 (91.7%) | 10 (83.3%) | 10 (83.3%) | 10 (83.3%) | 10 (83.3%) | |
| Male | 1 (8.3%) | 2 (16.7%) | 2 (16.7%) | 2 (16.7%) | 2 (16.7%) | |
| Baseline values (mean ± SD) | ||||||
| Age (years) | 36.3 ± 8.5 | 34.7 ± 10.5 | 38.3 ± 10.3 | 36.2 ± 11.1 | 36.3 ± 8.7 | ns |
| Sebum content (a.u.) | 58.0 (24.6) | 49.9 (20.2) | 62.8 (23.3) | 56.2 (13.5) | 47.8 (11.0) | ns |
| Hair length (cm) | 36.6 (17.3) | 33.0 (14.6) | 35.1 (16.8) | 38.5 (14.3) | 33.0 (15.1) | ns |
| Hair thickness (µm) | 75.8 (6.3) | 73.4 (8.5) | 70.3 (9.8) | 76.3 (5.8) | 71.0 (5.9) | ns |
| Hair density (no. of hairs/cm2) | 146.1 (11.3) | 148.5 (16.9) | 133.7 (14.5) | 146.6 (21.7) | 153.5 (14.2) | ns |
| Hair loss (no. of hairs lost/assessment) | 13.3 (5.4) | 15.6 (5.6) | 15.0 (4.1) | 16.4 (6.2) | 16.1 (5.1) | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, T.-M.; Wu, C.-C.; Huang, H.-C.; Lu, J.-Y.; Chuang, C.-H.; Kao, P.-L.; Tang, W.-H.; Hsieh, W.-J.; Liu, L.T.-C.; Qiu, W.-Y.; et al. Centella asiatica L. Urb. Extracellular Vesicle and Growth Factor Essence for Hair and Scalp Health: A 56-Day Exploratory Randomized Trial. Cosmetics 2025, 12, 253. https://doi.org/10.3390/cosmetics12060253
Chang T-M, Wu C-C, Huang H-C, Lu J-Y, Chuang C-H, Kao P-L, Tang W-H, Hsieh W-J, Liu LT-C, Qiu W-Y, et al. Centella asiatica L. Urb. Extracellular Vesicle and Growth Factor Essence for Hair and Scalp Health: A 56-Day Exploratory Randomized Trial. Cosmetics. 2025; 12(6):253. https://doi.org/10.3390/cosmetics12060253
Chicago/Turabian StyleChang, Tsong-Min, Chung-Chin Wu, Huey-Chun Huang, Ji-Ying Lu, Ching-Hua Chuang, Pei-Lun Kao, Wei-Hsuan Tang, Wang-Ju Hsieh, Luke Tzu-Chi Liu, Wei-Yin Qiu, and et al. 2025. "Centella asiatica L. Urb. Extracellular Vesicle and Growth Factor Essence for Hair and Scalp Health: A 56-Day Exploratory Randomized Trial" Cosmetics 12, no. 6: 253. https://doi.org/10.3390/cosmetics12060253
APA StyleChang, T.-M., Wu, C.-C., Huang, H.-C., Lu, J.-Y., Chuang, C.-H., Kao, P.-L., Tang, W.-H., Hsieh, W.-J., Liu, L. T.-C., Qiu, W.-Y., Percec, I., Chen, C., & Kuo, T.-Y. (2025). Centella asiatica L. Urb. Extracellular Vesicle and Growth Factor Essence for Hair and Scalp Health: A 56-Day Exploratory Randomized Trial. Cosmetics, 12(6), 253. https://doi.org/10.3390/cosmetics12060253







