1. Introduction
Dermal fillers and botulinum toxin injections are currently the most commonly performed esthetic procedures worldwide [
1,
2]. Among these, hyaluronic acid (HA) fillers have become the preferred choice due to their reversibility, safety profile, and ability to restore volume, improve hydration, and reduce facial wrinkles [
2,
3,
4].
Historically, permanent fillers such as silicone oil, methacrylates, and synthetic polymers were widely used [
5,
6,
7]. However, their tendency to migrate, induce inflammation, and cause irreversible esthetic damage has led to long-term complications and patient dissatisfaction. These risks, coupled with the difficulty of removal, continue to shape public perception and patient reluctance [
7,
8]. As a result, the field has shifted toward biocompatible and resorbable materials, although these too carry potential adverse effects [
9,
10,
11,
12,
13].
Early alternatives such as bovine and porcine collagen fillers were associated with high allergenic potential and limited duration, requiring pre-treatment allergy testing [
13]. Polylactic acid-based fillers, while offering a biostimulatory and semi-permanent volumizing effect, were initially plagued by granuloma formation [
11,
14]. Although improved in newer formulations, they remain used selectively. Calcium hydroxyapatite emerged with promising durability but was also linked to granulomas in early use [
15,
16,
17]. More recently, advancements in cross-linked HA fillers have enabled correction durability up to 12 months, rivaling other agents while offering superior biocompatibility [
3,
18,
19].
Several other materials—including carboxymethylcellulose and alginate—failed to gain widespread acceptance due to poor tissue integration, excessive stiffness, and inflammatory reactions [
11,
12,
14]. HA fillers, by contrast, are glycosaminoglycans naturally present in the extracellular matrix. They maintain hydration and elasticity, contribute to stromal resistance, and modulate inflammatory responses. Their degradation by endogenous hyaluronidases necessitated the development of cross-linking technologies to prolong tissue residence time [
1,
12,
13,
20].
Modern HA fillers are cross-linked using agents such as 1,4-butanediol diglycidyl ether (BDDE) or polyethylene glycol (PEG), forming chemical bridges between HA chains [
1,
21]. These products are then purified to minimize residual cross-linking agent (typically <12 ppm), resulting in a hydrogel whose rheological properties (elastic modulus G′ and viscosity G″) determine its clinical applications [
20,
22].
HA fillers are now produced in various formulations tailored for specific anatomical planes, from superficial dermal correction to deep periosteal augmentation. Beyond facial rejuvenation, body indications such as genital enhancement and gluteal volumization have gained traction. However, their use in breast augmentation remains contraindicated due to radiological opacity, which interferes with mammographic screening [
23,
24,
25,
26].
While the safety of HA fillers is generally well established—with most side effects being mild and transient (e.g., bruising, swelling, discomfort)—concerns persist regarding injection comfort, durability, and long-term tissue integration. One of the most frequent patient concerns is the perceived short duration of the effect, leading to repeat procedures and additional cost [
1,
3,
27].
Despite the availability of numerous high-quality HA fillers on the market, there remains a need for objective, reproducible methods to evaluate filler performance over time. Traditional assessments rely heavily on photography and subjective clinical grading scales, which are prone to inter-observer variability [
2,
28].
Recent imaging advances—such as VISIA
® CR Generation 5 and PRIMOS 3D optical profilometry—offer high-resolution, instrument-based evaluation of volumetric correction, skin texture, and surface irregularities. These technologies enable the precise tracking of outcomes and provide quantifiable data that complement subjective assessment [
29,
30].
Sofiderm® is a newer generation of cross-linked HA fillers based on medium cross-linking polyethylene ether (MCLPE) technology, designed for use at various tissue depths. Preliminary data suggest favorable rheological properties and versatility; however, independent, clinical studies using validated imaging modalities remain limited.
This prospective, single-arm, non-randomized pilot study aims to evaluate the corrective efficacy, durability, and tolerability of three Sofiderm® HA filler formulations in a small cohort of Caucasian patients with mild to moderate photoaging. In parallel, the study investigates the correlation between clinical aging severity, assessed using the Griffiths photoaging scale, and instrument-based outcomes derived from PRIMOS 3D and VISIA CR-5, including “color by distance” volumetric mapping.
By combining subjective clinical assessments with objective digital imaging, this study seeks to contribute to a more standardized, evidence-based approach to evaluating dermal filler performance.
2. Materials and Methods
2.1. Study Design and Population
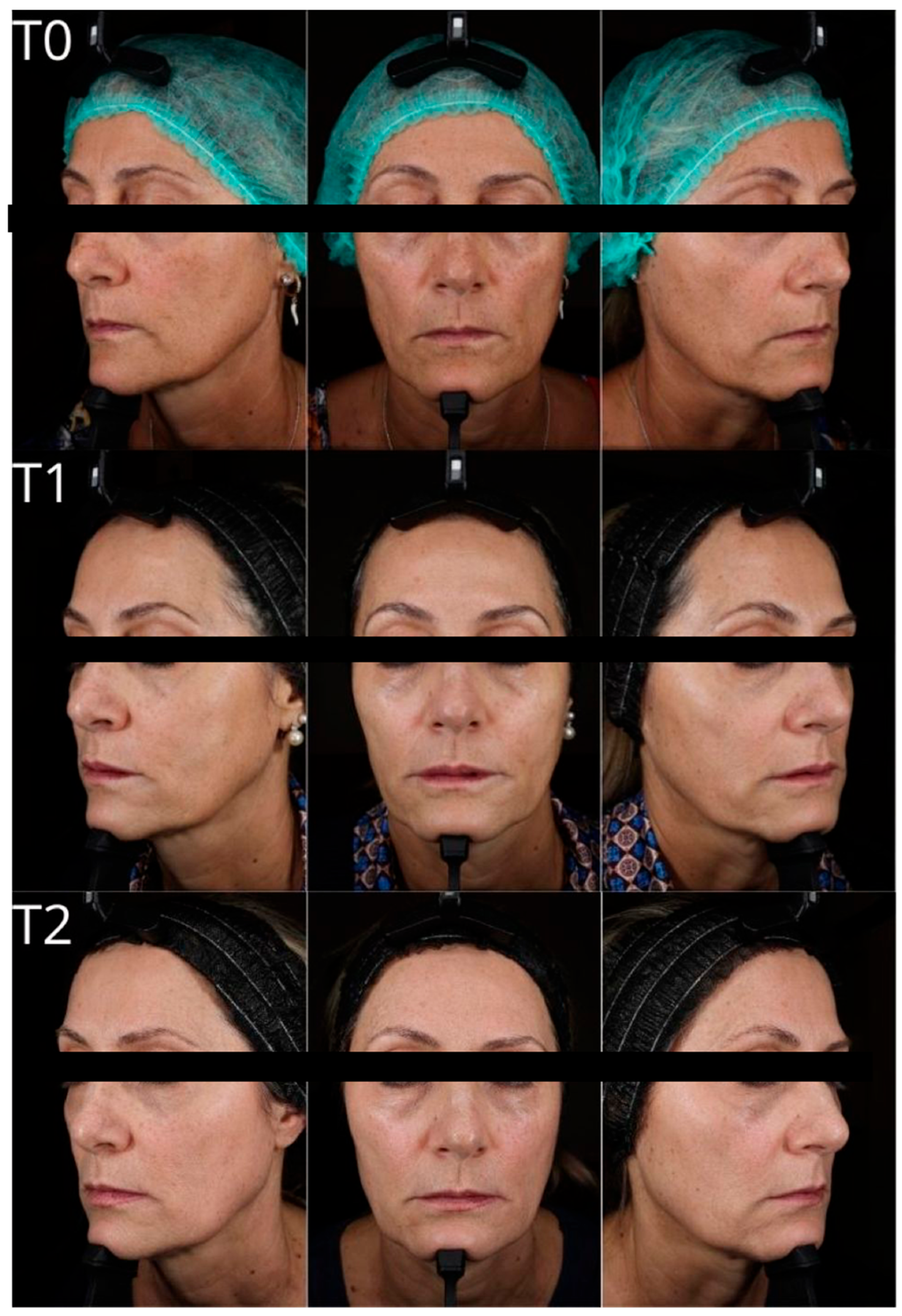
This prospective, single-arm, non-randomized pilot study enrolled five Caucasian patients (4 females and 1 male), aged between 46 and 73 years. Four patients were non-smokers or former smokers; one was an active smoker. At baseline, signs of facial photoaging were graded from 4 to 8 according to the Griffiths photoaging scale.
2.2. Ethical Considerations
All patients provided written informed consent for participation in the study and for the publication of anonymized images. The study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki. The study protocol was reviewed and approved by the Territorial Ethics Committee Lazio Area 1 (Comitato Etico Territoriale Lazio Area 1) on 12 March 2025 (Protocol No. 0224/2025).
2.3. Imaging and Assessment Tools
All patients underwent baseline and follow-up evaluations using two non-invasive imaging systems: VISIA® CR Generation 5 and PRIMOS 3D (Canfield Scientific Inc., Fairfield, NJ, USA. canfieldsci.com).
PRIMOS 3D was used to assess the following:
- ○
Surface Profile (SP): general skin texture;
- ○
Surface Roughness (SR): dilated pores and fine irregularities;
- ○
Surface Waviness (SW): deeper structural changes such as wrinkles and scars.
Color by distance mapping was used to quantify volume changes and symmetry over time.
VISIA CR images were analyzed with 2D Canfield Data Imager software (v.1.0.0) to evaluate pigmentation, UV damage, texture, and wrinkles.
Follow-up assessments were performed at 2 months (T1) and 9 months (T2) to evaluate correction efficacy and durability.
2.4. Product Characteristics and Injection Protocols
Patients were treated with varying volumes of Sofiderm
® hyaluronic acid fillers (Techderm Ltd., Hangzhou, China) concentration: 20 ± 3 mg/mL, chosen based on anatomical region and photoaging severity. The products, formulated using medium cross-linking polyethylene ether (MCLPE) technology, differ in particle size and rheological properties (
Table 1).
Injection techniques were tailored to target depth and region, employing a combination of needles (25 G × 16 mm, 27 G × 12 mm, 30 G × 12 mm) and cannulas (22 G × 90 mm, 27 G × 50 mm) using bolus, linear retrograde, or fan-shaped deposition methods.
2.5. Individual Treatment Protocol
2.5.1. Subject 1
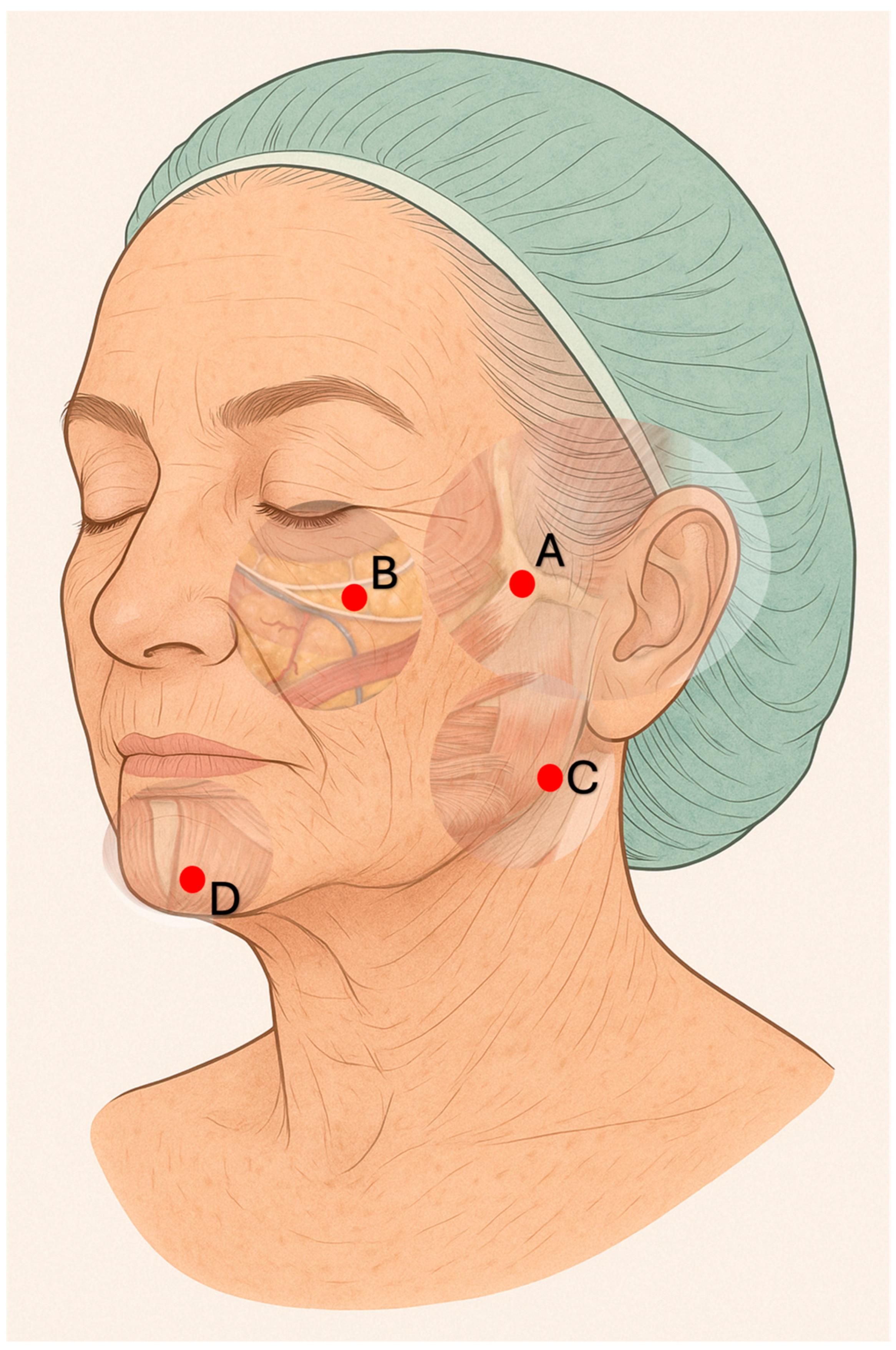
Subject 1 was a 73-year-old Caucasian female and active smoker, presenting with advanced signs of facial photoaging (Griffiths score: 8). Due to pronounced heliodermatitis and post-acne scarring, she first underwent preconditioning with plasma sublimation, followed by two sessions of intense pulsed light (IPL) therapy (550 nm, 17 J; Eterna Giovinezza, Quanta System), limited to the affected areas. The filler treatment included the injection of 2 mL of Sofiderm
® Derm Sub-Skin (1 mL per side) at the zygomatic-temporal junction using a 25 G × 16 mm needle in a periosteal bolus technique, and 4 mL (2 mL per side) in the deep malar fat pads, using the same needle and perpendicular approach. An additional 2 mL (1 mL per side) was injected into the gonion region through a mid-mandibular access using a 22 G × 90 mm cannula. (
Figure 1) From the same entry point, 1 mL per side was administered as a supraperiosteal bolus into the paramental area near the anterior mandibular foramen. In a subsequent session, 2 mL of Sofiderm
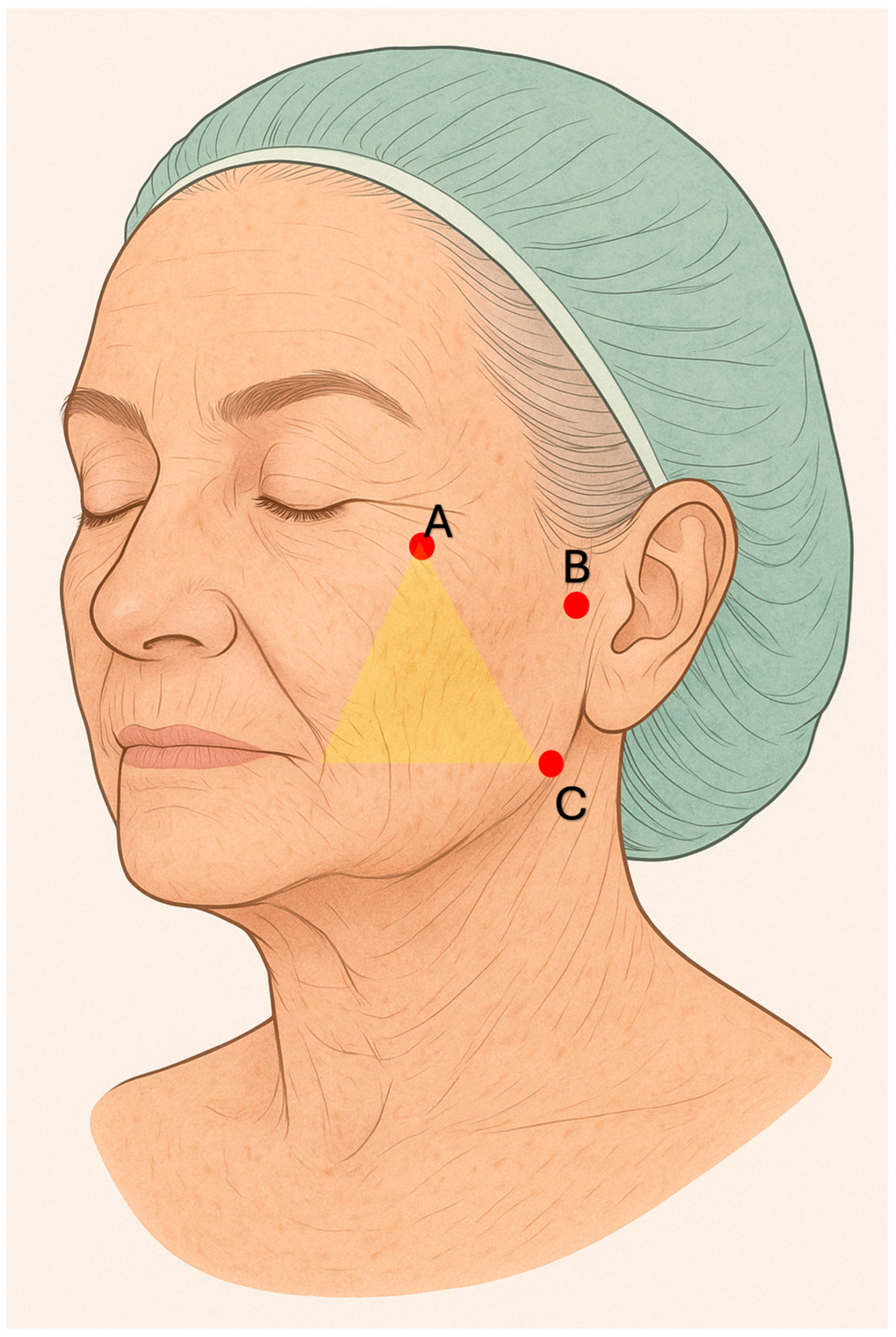
® Derm per side were injected subcutaneously in the region between the cheekbones, nasolabial folds, and mid-cheek using a fan-shaped technique with a 22 G × 90 mm cannula introduced at the zygomatic level. An additional 2 mL in total (1 mL per side) was delivered to the preauricular area, extending from the tragus to the zygomatic and lower cheek region, using the same cannula and access (
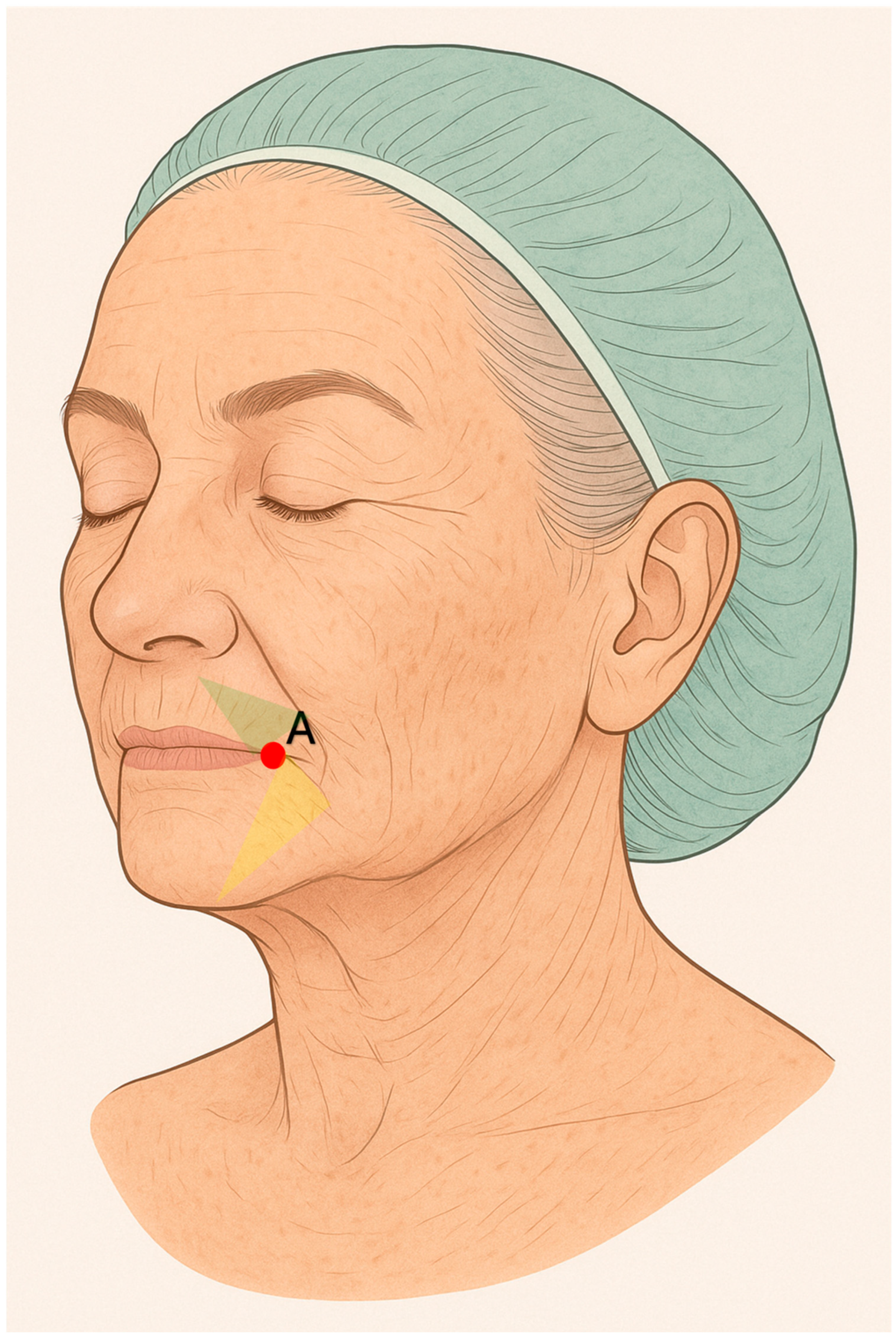
Figure 2). Through an entry point located approximately 3 cm lateral to each oral commissure, 1 mL of Sofiderm
® Derm per side was injected in a fan-shaped pattern between the chin and marionette lines. From the same access point, 1 mL of Sofiderm
® Fine Lines was administered to the upper lip (barcode lines) using a 27 G × 50 mm cannula, and 1 mL was injected into the lips with a 30 G × 12 mm needle to achieve lip rejuvenation (
Figure 3).
2.5.2. Subject 2
Subject 2 was a 63-year-old Caucasian female and heavy smoker, presenting with severe signs of facial aging (Griffiths score: 7). She received the same filler protocol as Subject 1. In addition, 2 mL of Sofiderm® Fine Lines (1 mL per side) were injected using a 30 G × 12 mm needle in a retrograde linear technique to treat fine lines in the periorbital region, nasal bridge, and mid-cheek areas.
2.5.3. Subject 3
Subject 3 was a 55-year-old Caucasian female smoker, with a Griffiths score of 6 and evidence of moderate photoaging. She presented with marked mandibular hypoplasia and chin retrusion, with the lower lip appearing disproportionately small and posteriorly positioned relative to the upper lip, and reduced vertical dimension of the lower third of the face. Treatment began with the injection of 1 mL per side of Sofiderm® Derm Sub-Skin using a 25 G × 16 mm needle with a perpendicular bolus technique: approximately 0.5 mL was placed on the periosteal plane of the zygomatic arch and 0.5 mL in the deep malar fat pad. A 22 G × 50 mm cannula was then used to deliver 1 mL per side of Sofiderm® Derm subcutaneously in a fan-shaped pattern in the mid-cheek region, accessing at the zygomatic level. Using the same cannula through a mid-mandibular entry point, 2 mL were placed on the supraperiosteal plane between the lower lip and pogonion, 2 mL were injected as bolus in the pogonion area, and another 2 mL were delivered to the region between the chin and marionette lines. Finally, an additional 0.5 mL of Sofiderm® Derm per side was injected subcutaneously medial to the marionette lines using the same access point and fan-shaped technique.
2.5.4. Subject 4
Subject 4 was a 52-year-old Caucasian female, non-smoker, with mild photoaging (Griffiths score: 4). Her primary concern was significant mid-cheek volume loss, which contributed to an overall aged appearance disproportionate to her chronological age. Treatment included the injection of 0.5 mL of Sofiderm® Derm Sub-Skin per side to the zygomatic and deep malar regions, delivered as supraperiosteal boluses with a 25 G × 16 mm needle. An additional 2 mL of Sofiderm® Derm per side were injected in a fan-shaped pattern on the subcutaneous plane of the mid-cheek using a 22 G × 90 mm cannula introduced approximately 4 cm medial to the tragus. Furthermore, 0.5 mL of Sofiderm® Derm per side was placed subcutaneously in a fan-shaped manner into the paramental region and marionette lines, using the same cannula through an access point located about 2 cm lateral to each oral commissure. Given the marked volume loss in the lateral canthal region, 0.5 mL of Sofiderm® Fine Lines was injected per side in a fan-shaped subcutaneous pattern using a 27 G × 50 mm cannula with a lateral canthal entry. Lip rejuvenation was also performed using Sofiderm® Fine Lines with a 30 G × 13 mm needle.
2.5.5. Subject 5
Subject 5 was a 55-year-old Caucasian male, non-smoker, with a Griffiths score of 5, and presented with intermediate signs of photoaging. Given his overall harmonious facial appearance, treatment was limited to softening the mental crease and enhancing zygomatic projection. To reduce the mental crease, 1 mL of Sofiderm® Derm was injected on the subcutaneous plane using a linear retrograde technique with a 27 G × 12 mm needle. Zygomatic enhancement was achieved with 0.5 mL per side of Sofiderm® Derm Sub-Skin, injected with a perpendicular technique on the supraperiosteal plane using a 25 G × 16 mm needle.
3. Results
3.1. Subject 1
Subject 1 reported the procedure as highly uncomfortable; however, no adverse effects were observed apart from mild, short-lived bruising. Notably, no post-procedure edema was reported.
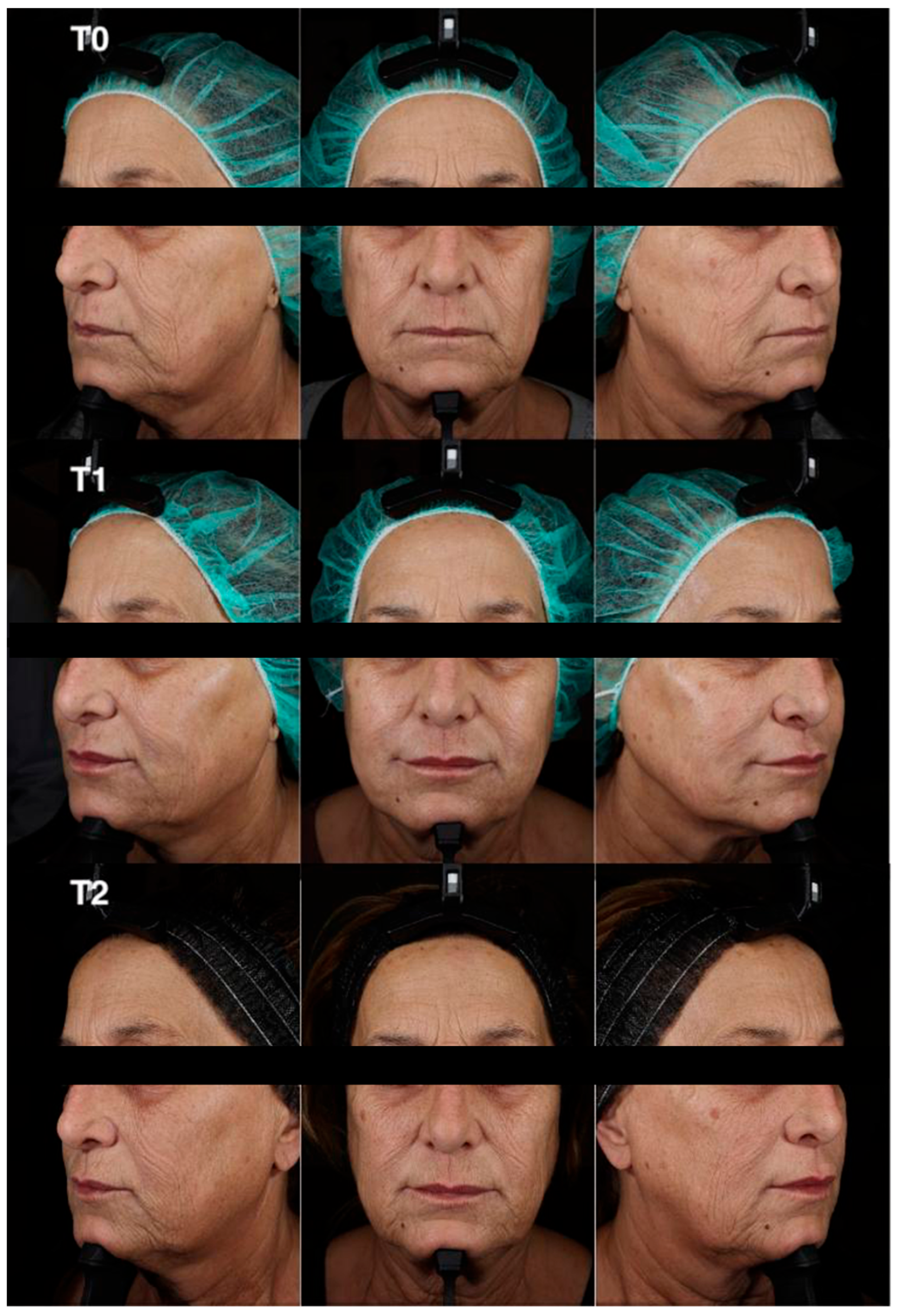
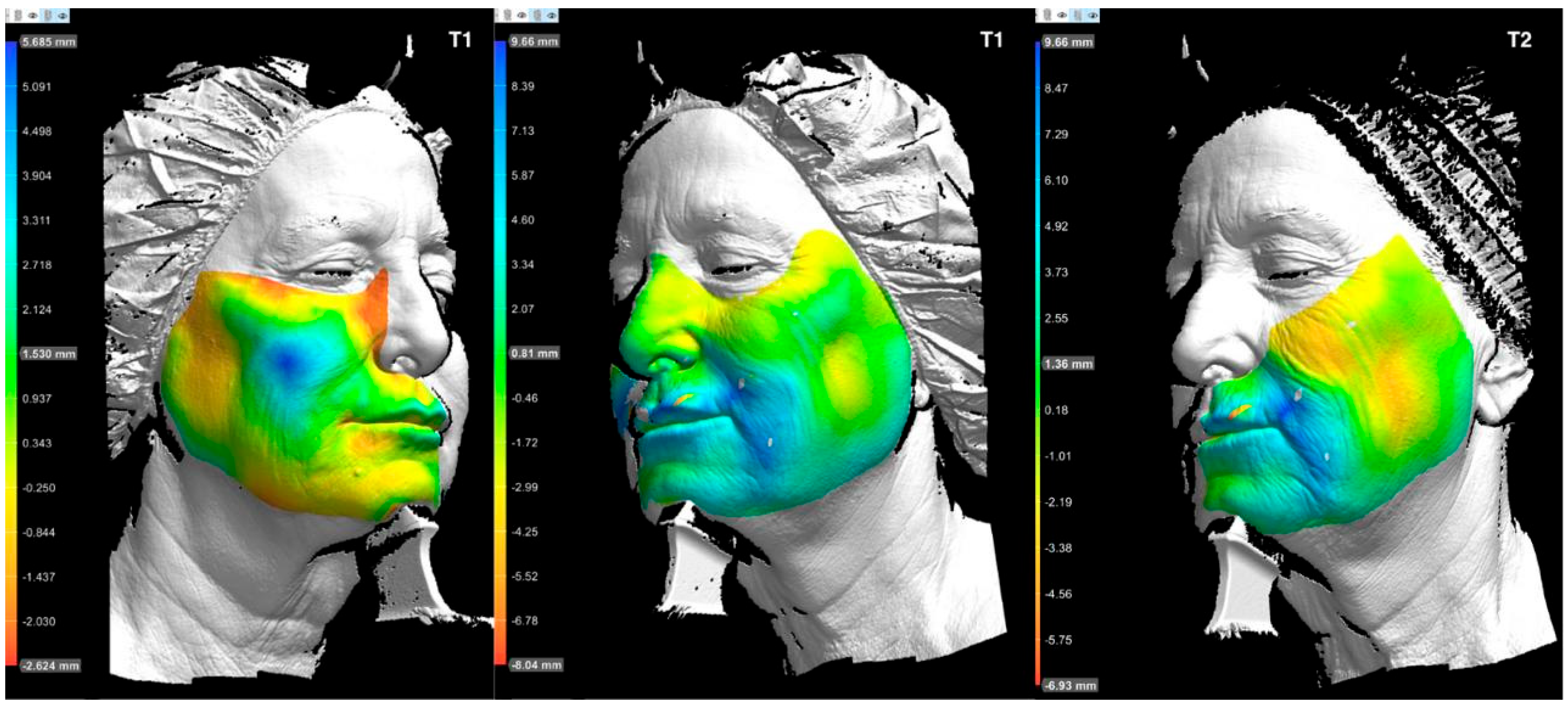
Clinical and instrumental assessments were performed at baseline (T0), 2 months post-treatment (T1), and 9 months post-treatment (T2) to evaluate filler effectiveness, duration of volumetric correction, and improvements in skin quality (
Scheme 1 and
Scheme 2). At T1, the subject’s Griffiths photoaging score improved from 8 to 3, corresponding to a 5-point reduction. This represents a substantial esthetic improvement in a short time frame.
Three-dimensional volumetric analysis at T1 (
Scheme 2) demonstrated a pronounced lifting and projection effect. Blue regions in the midface indicate increased projection, while the appearance of yellow and red tones in the lower third may reflect upward volumetric displacement, suggestive of an anti-gravitational “lifting” effect.
The psychological impact of the esthetic improvement was reportedly significant, with the patient attributing increased motivation for lifestyle changes to the treatment outcome. By the 9-month follow-up (T2), she had achieved a weight loss of 20 kg. Despite this reduction, the 3D volumetric analysis (
Scheme 2) still revealed persistent volume retention in the treated areas. The Griffiths score at T2 remained stable at 5, representing a sustained improvement from baseline.
Instrument-based assessments were consistent with clinical observations. Both 2D and 3D evaluations showed elevated values for all parameters at T0, marked improvement at T1, and a partial return toward baseline at T2, particularly in 2D skin texture and visible spots. These specific parameters worsened compared to baseline, potentially due to seasonal variation and changes in skin physiology associated with weight loss.
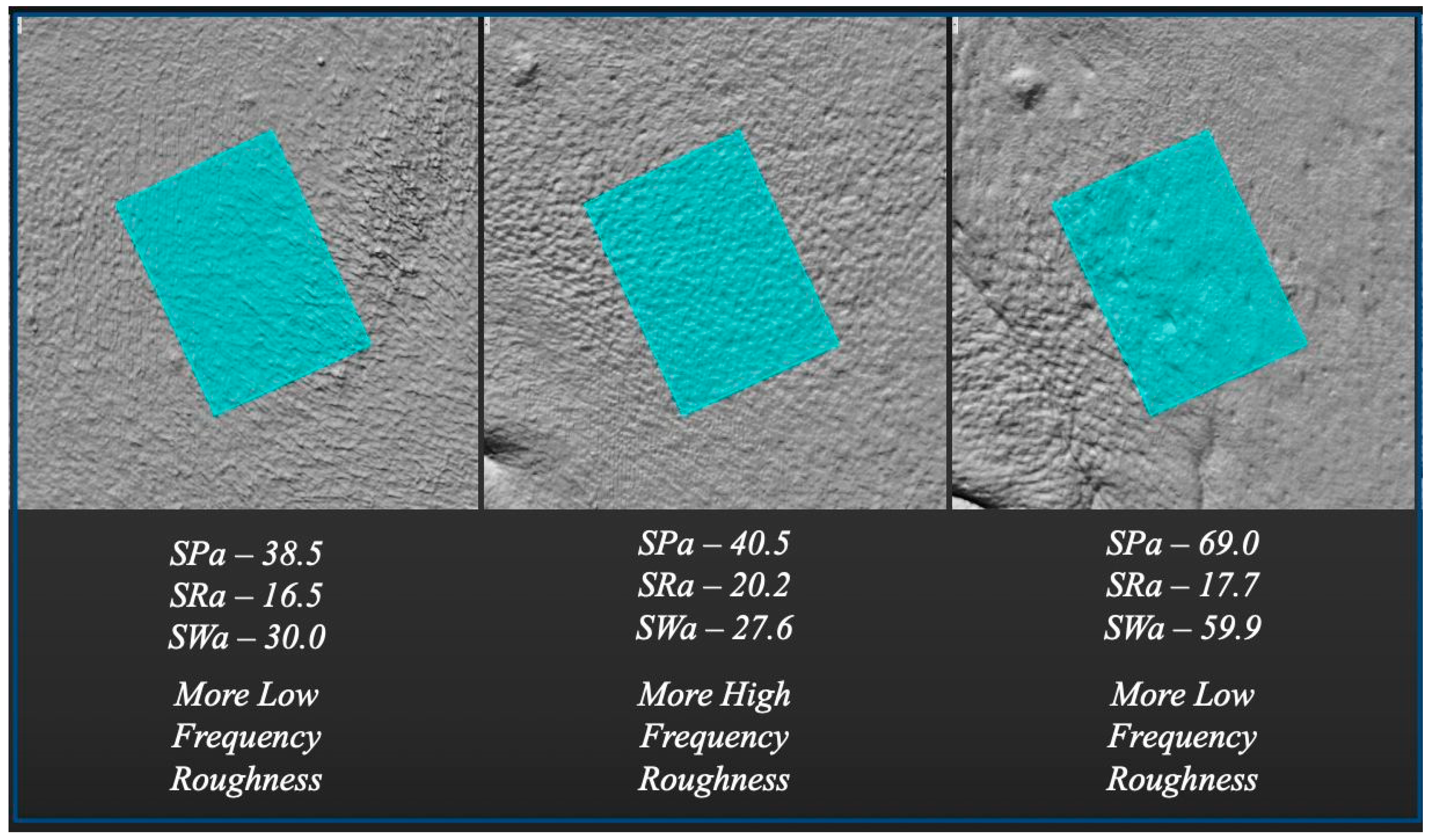
In contrast, 3D metrics such as Surface Profile (SP), Surface Roughness (SR), and Surface Waviness (SW) exhibited progressive improvement across all timepoints, with the exception of SPmax and SWmax. These two maximum values likely reflect isolated, deep skin defects (e.g., wrinkles or scars). It should be noted that these “max” metrics refer to single outlier values, while mean values for the same parameters continued to improve at T2, supporting a durable, widespread corrective effect.
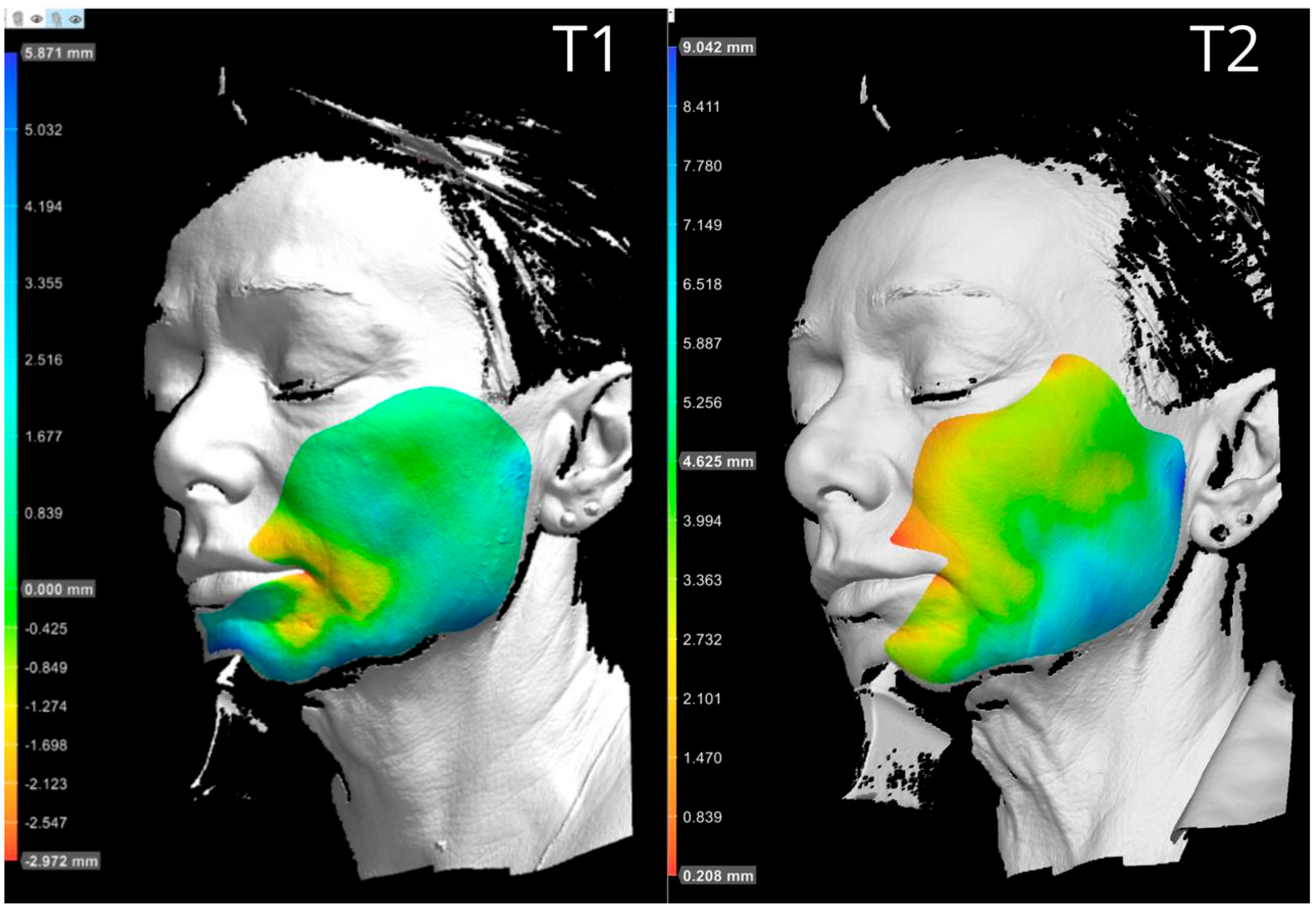
3.2. Subject 2
Subject 2, a 63-year-old female with a baseline Griffiths photoaging score of 7, described the procedure as moderately painful. She experienced moderate facial edema lasting approximately 24 h, while bruising was minimal and resolved quickly. Clinical evaluations were performed at baseline, 2 months, and 9 months after treatment to assess the effectiveness of the filler in terms of volumetric correction and skin quality improvement. At the 2-month follow-up, the patient’s Griffiths score had improved to 4, indicating a 3-point reduction and a noticeable esthetic enhancement. Volumetric analysis revealed widespread projection in the midface and lower facial third, with patterns suggestive of lifting effects. These results reflect upward redistribution of soft tissue, particularly in the malar and buccal compartments, likely due to deep-plane filler placement. At 9 months, a substantial portion of the volumetric correction remained visible, especially in the areas treated at the supraperiosteal level. Some degree of volume loss was observed in the malar and buccal regions, consistent with expected resorption over time. The Griffiths score at this timepoint was 5, reflecting partial regression from peak improvement but still improved compared to baseline. Instrumental analysis showed a consistent trend with clinical evaluation: an initial improvement in skin parameters at 2 months, followed by partial regression at 9 months. A mild worsening of skin texture was noted, which may be attributable to seasonal changes or physiological remodeling (
Scheme 3 and
Scheme 4). Based on the overall evaluation, a maintenance touch-up was considered appropriate to sustain the results achieved.
3.3. Subject 3
Subject 3, a 55-year-old female with moderate photoaging (Griffiths score: 6), reported the procedure as minimally painful. No bruising or edema was observed following treatment.
The improvement in facial harmony was notable, primarily due to enhanced volume and projection in deficient areas. However, the Griffiths score remained essentially unchanged throughout follow-up. This may be attributable to the patient’s significant sun exposure during the summer months, which likely influenced the stability of the photoaging scale.
Post-treatment assessment revealed increased chin projection, leading to improved lower facial contour. Although not yet fully proportional to the upper facial thirds, the projection was judged to be within an acceptable esthetic range. The vertical dimension between the gnathion and the lower lip appeared corrected, the mandibular line was more defined, and the midface showed a visible reduction in wrinkles. According to wrinkle-specific parameters, a 50% improvement was recorded at 2 months, followed by a partial regression of approximately 30% at 9 months. Overall facial projection patterns suggested upward repositioning of soft tissue, particularly involving the buccal fat compartments. This volumetric realignment, combined with anterior-inferior advancement of the chin, contributed to a visible improvement in the appearance of marionette lines. Instrument-based texture analysis showed improvement in 2D texture parameters (
Scheme 5 and
Scheme 6). However, PRIMOS 3D analysis indicated localized worsening in certain aspects of skin quality, likely reflecting environmental influences. In particular, photodamage indicators worsened at both follow-up visits, which may be related to increased sun exposure during the observation period.
3.4. Subject 4
Subject 4, a 52-year-old female with mild signs of photoaging (Griffiths score: 4), initially expressed high levels of anxiety related to anticipated pain. However, the procedure was well tolerated; she reported only mild discomfort and no pain. One localized ecchymosis measuring approximately 3 × 3 cm developed in the left lateral canthal region and resolved spontaneously within three weeks. No post-procedural edema was reported. At the first follow-up, the patient’s Griffiths score had improved to 2, reflecting a 2-point reduction compared to baseline. This score remained stable at the 9-month evaluation, suggesting a sustained effect of the treatment over time. Clinical assessment revealed a harmonious and homogeneous volumetric enhancement across the zygomatic, lateral canthal, and mid-cheek regions. The improvement contributed to a softening of facial contours and enhanced overall skin luminosity. Lip rejuvenation further supported a more balanced and youthful facial appearance. Quantitative skin analysis showed substantial improvements across multiple parameters. In particular, the severity of lateral canthal wrinkles improved by more than 60% at follow-up. Ultraviolet-induced photodamage was reduced by approximately 50%, while the extent of visible skin blemishes decreased by 20%. These results indicate a positive impact on both structural and superficial components of skin quality, aligning with the patient’s favorable clinical outcome (
Scheme 7 and
Scheme 8).
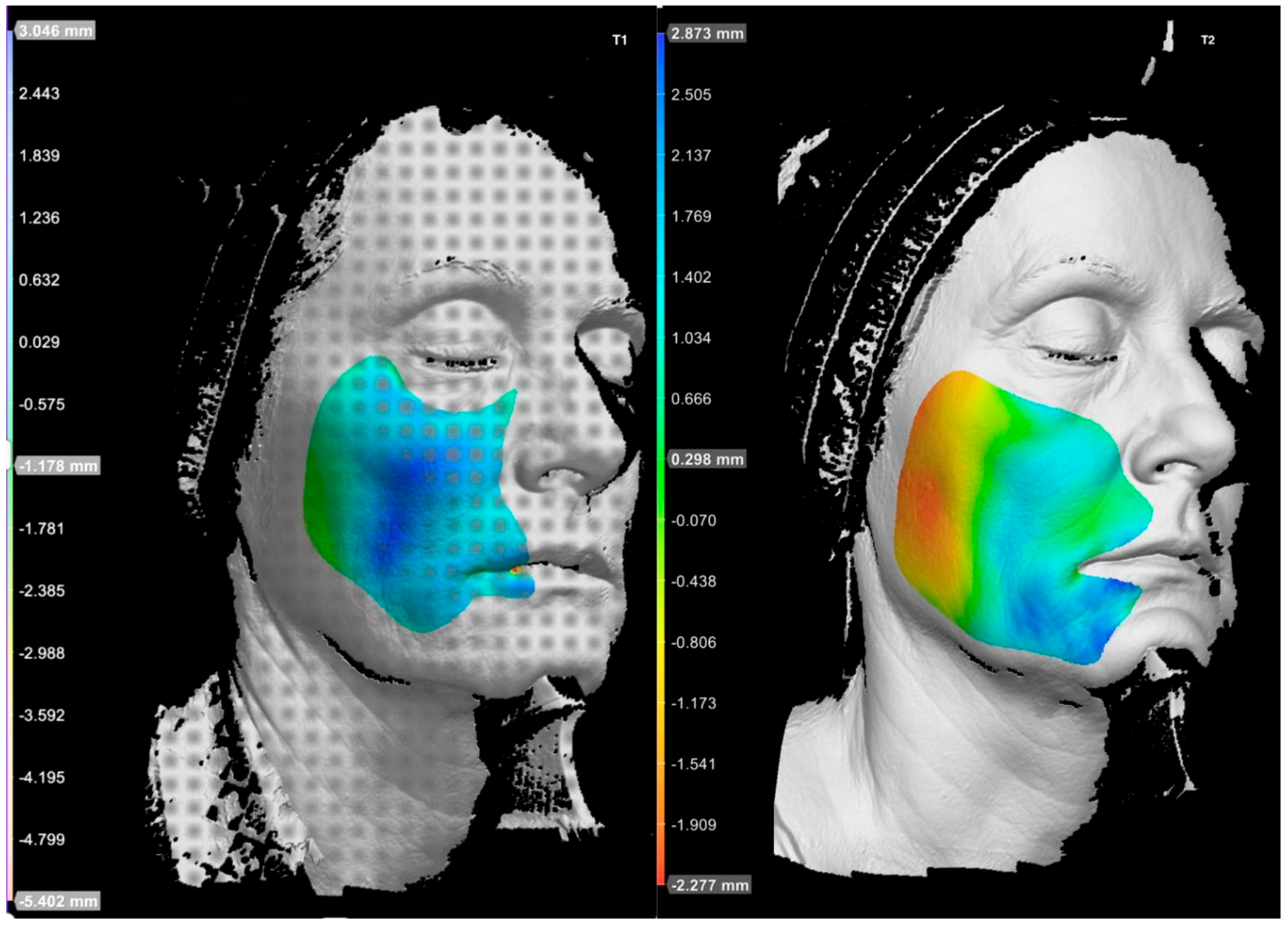
3.5. Subject 5
Subject 5, a 55-year-old male with intermediate signs of photoaging (Griffiths score: 5), reported no pain or bruising during the procedure. Only mild discomfort was experienced during correction of the mental crease. The correction of zygomatic projection was sufficient to restore midface convexity and counteract age-related flattening, a process partly attributable to physiological bone resorption, which also occurs in male patients. Aside from this targeted intervention, no modifications were necessary, as the patient’s facial proportions in terms of width, height, and projection were already balanced. The treatment of the mental crease resulted in moderate esthetic improvement, contributing to greater overall facial harmony. The product used in the zygomatic region was Sofiderm
® Derm Sub-Skin, a biphasic filler with a substantial free phase component that may exert biostimulatory effects. Improvements observed in skin quality parameters in the treated area are likely related to this biostimulatory action. However, parameters associated with deep structural irregularities—such as surface waviness (SW)—remained unchanged, as these features are not typically responsive to superficial biostimulation. This was the only subject to demonstrate a worsening in wrinkle severity (2D analysis) and UV-related skin damage at the 9-month follow-up. These findings may be attributed to frequent outdoor physical activity without consistent photoprotection (
Scheme 9 and
Scheme 10;
Figure 4,
Table 2).
4. Discussion
This exploratory pilot study offers preliminary insights into the clinical performance of Sofiderm
® hyaluronic acid fillers in facial rejuvenation. All five treated subjects showed measurable esthetic improvements, with an average reduction of 2 points on the Griffiths photoaging scale at 2 months (T1), which remained largely stable at 9 months (T2). The most notable results were observed in Subjects 1 and 2, with reductions of 5 and 3 points, respectively, reflecting potentially meaningful clinical benefits in select cases. These findings were supported by instrumental measurements using PRIMOS 3D and VISIA-CR, which demonstrated improvements in volumetric projection, skin texture, and surface smoothness. Although these outcomes are encouraging, they should be interpreted with caution given the study’s limited scale. The tolerability profile observed in this cohort was favorable, with minimal adverse effects such as mild bruising or transient edema, even in cases where relatively high filler volumes were used (up to 20 mL in a single subject). No delayed complications, such as granulomas or persistent swelling, were reported over a 15-month observation period. When compared to data on widely used hyaluronic acid fillers [
31,
32,
33,
34,
35,
36,
37,
38], the duration of Sofiderm
®’s effects in this cohort—particularly in the midface and mandibular regions—appears consistent with published expectations for crosslinked HA products, which typically range from 6 to 9 months. Although this study was not designed for direct comparison, the results suggest that the product’s performance may be in line with other formulations currently in use.
Recent literature highlights the relevance of rheological properties—such as elastic modulus (G′), viscous modulus (G″), cohesivity, and swelling behavior—in shaping filler performance [
36,
38].The favorable outcomes observed in this study may reflect the influence of Sofiderm
®’s MCLPE cross-linking technology and particle-size distribution. However, definitive conclusions on its comparative rheological profile cannot be drawn without standardized in vitro testing and head-to-head clinical trials.
The use of advanced imaging tools represents a key methodological strength of the present study. PRIMOS 3D enabled objective tracking of structural skin changes, while VISIA-CR provided complementary data on surface characteristics and pigmentation. The “Color by Distance” feature of PRIMOS added an intuitive, spatially precise means of visualizing projection changes. Although these technologies enhanced the granularity of outcome assessment, they should be considered as adjuncts to—but not replacements for—clinical judgment and patient-reported outcomes.
Several limitations must be acknowledged: the small sample size (n = 5), absence of a control group, and single-operator design restrict the generalizability of the findings. Confounding factors such as weight loss (Subject 1) and sun exposure (Subjects 3 and 5) may have influenced individual outcomes. These elements underline the exploratory nature of this study and highlight the need for more rigorous investigation.
5. Conclusions
This pilot study suggests that Sofiderm® hyaluronic acid fillers may offer clinically relevant improvements in facial volume restoration and skin quality, with a generally favorable safety and tolerability profile. Improvements observed both visually and instrumentally were sustained for up to 9 months in most cases, with some inter-individual variability. The average gain of 2 points per subject on the Griffiths photoaging scale at T1—confirmed by corresponding improvements in PRIMOS and VISIA-CR metrics—highlights the potential benefit of this treatment approach. A good correlation was observed between visual assessment and instrumental data, particularly in subjects with more pronounced clinical changes. Despite the use of relatively high filler volumes across the study population (60 mL in total), adverse events were minor and transient, and no delayed complications were reported during the extended follow-up. However, the study’s exploratory design and methodological constraints necessitate cautious interpretation. The observed rebound in 2D texture parameters at T2 across all subjects, potentially influenced by seasonal variation or photodamage, further illustrates the need for comprehensive and longitudinal evaluations. Importantly, the integration of advanced, non-invasive imaging systems allowed for the reproducible and quantitative assessment of treatment efficacy. These technologies may represent valuable tools in both research and clinical settings, contributing to more objective outcome measurement and helping inform product selection in a field where marketing pressure often exceeds the availability of comparative data.
Future research should aim to confirm these findings through larger, controlled studies that compare Sofiderm® directly with other HA fillers, investigate its rheologic profile in standardized conditions, and explore its suitability across different facial regions and skin types.