Green Synthesis of Iron Oxide Nanoparticles for Use in Pickering Emulsions: In Vitro UV-Absorbing and Antimicrobial Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Green Tea Extract
2.3. Green Synthesis of Iron Oxide Nanoparticles
2.4. Preparation of Pickering Emulsion
2.5. Characterization of Iron Oxide Nanoparticles
2.6. Characterization of Pickering Emulsion
2.7. Rheological Analyses
2.8. Determination of Sun Protection Factor (SPF)
2.9. Antimicrobial Study of Formulations
2.10. Cell Viability Assay
- −
- FeCl2 in PBS: 1, 10, and 100 µg/mL.
- −
- IONP PE in PBS: 1, 10, and 100 µg/mL.
2.11. Statistical Analysis
3. Results and Discussion
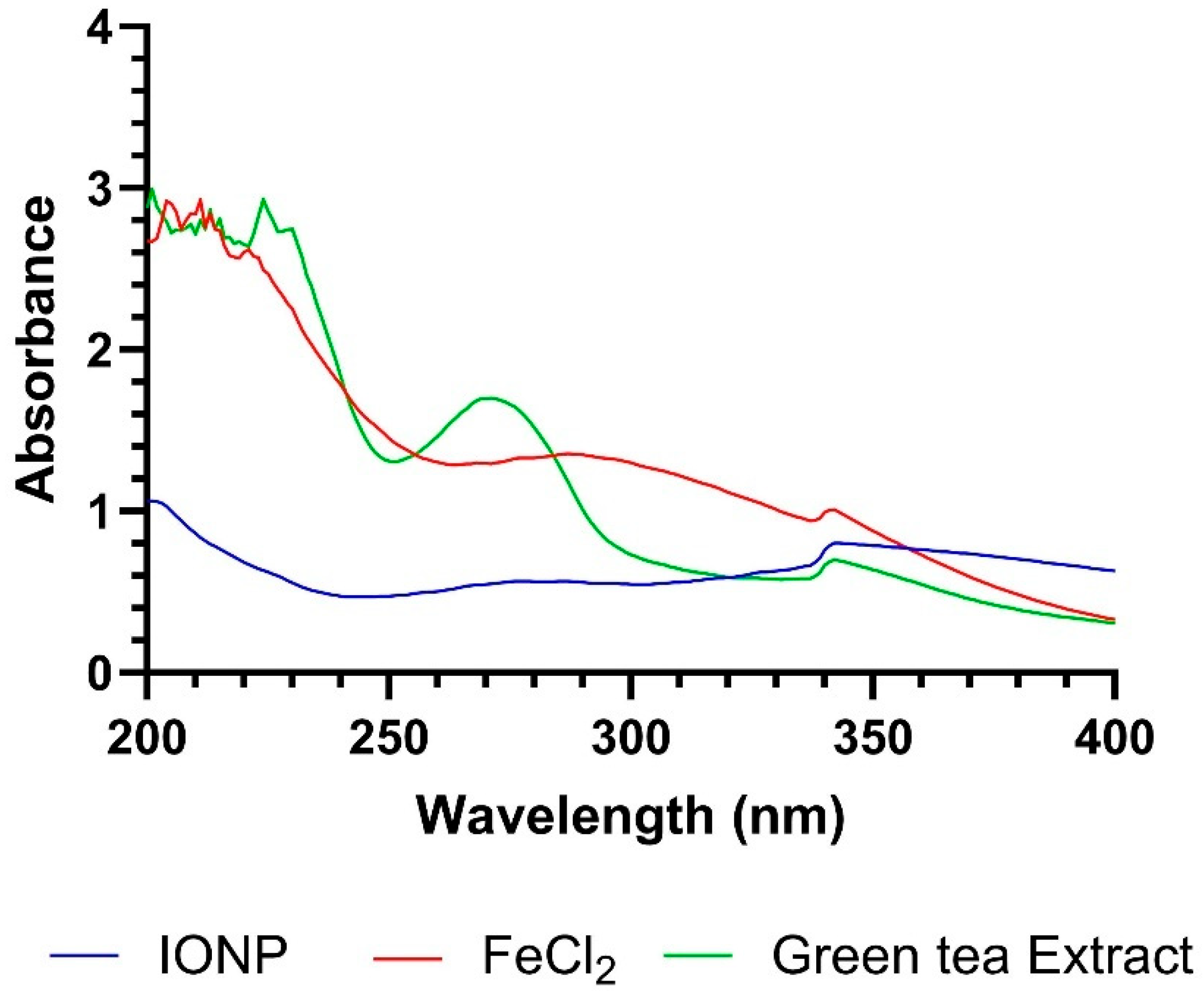
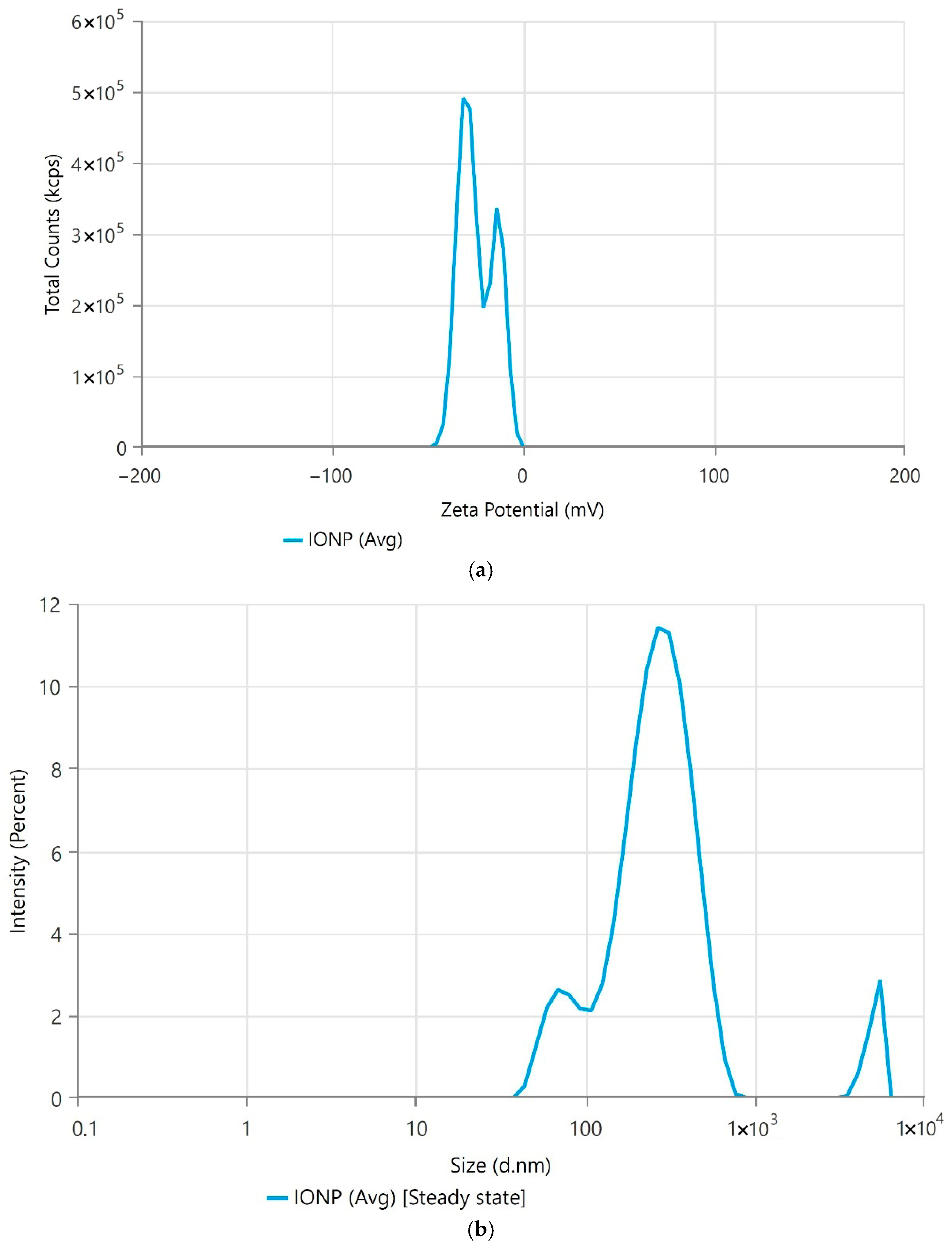
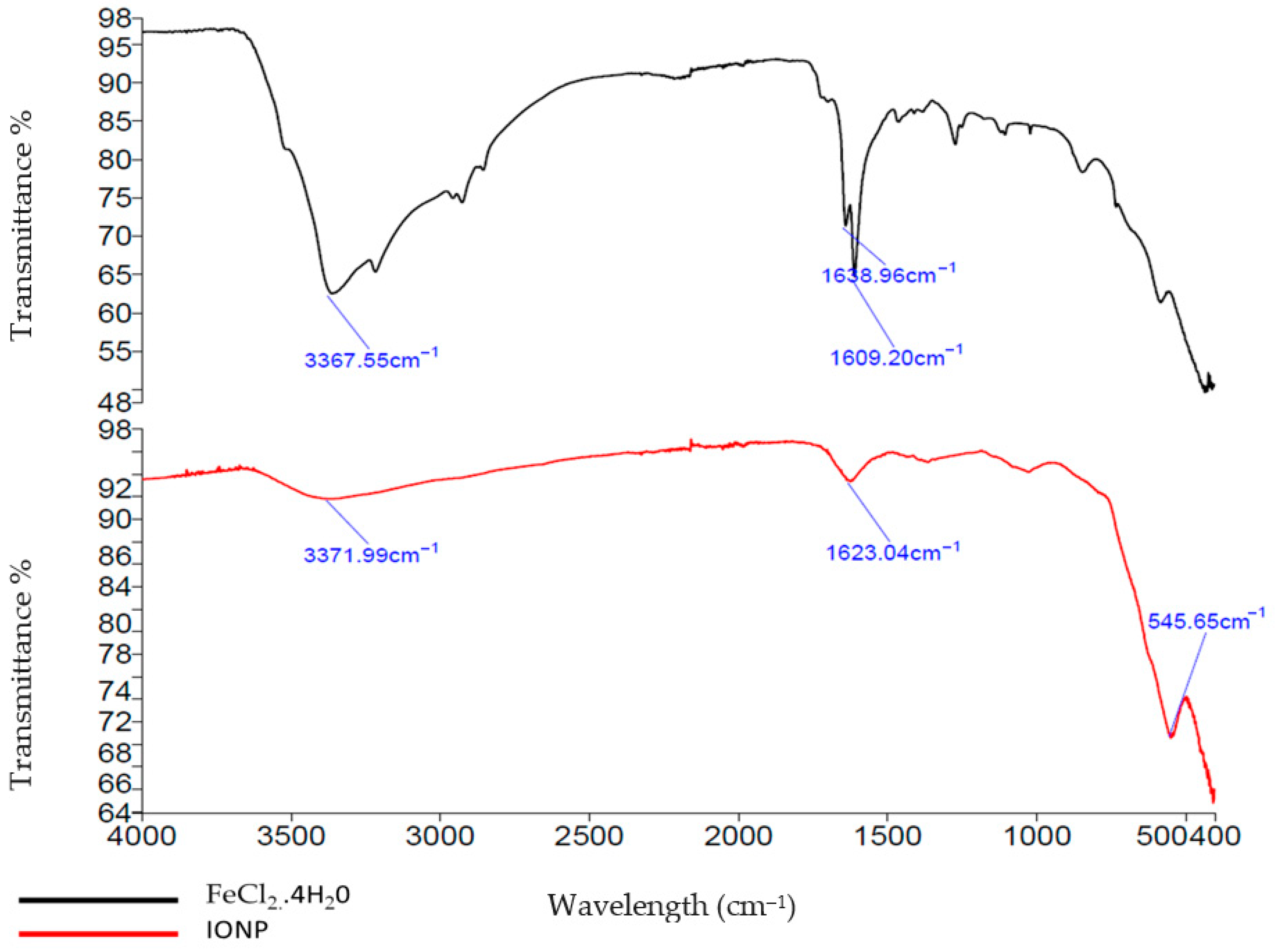
3.1. Iron-Oxide Nanoparticle Preparation
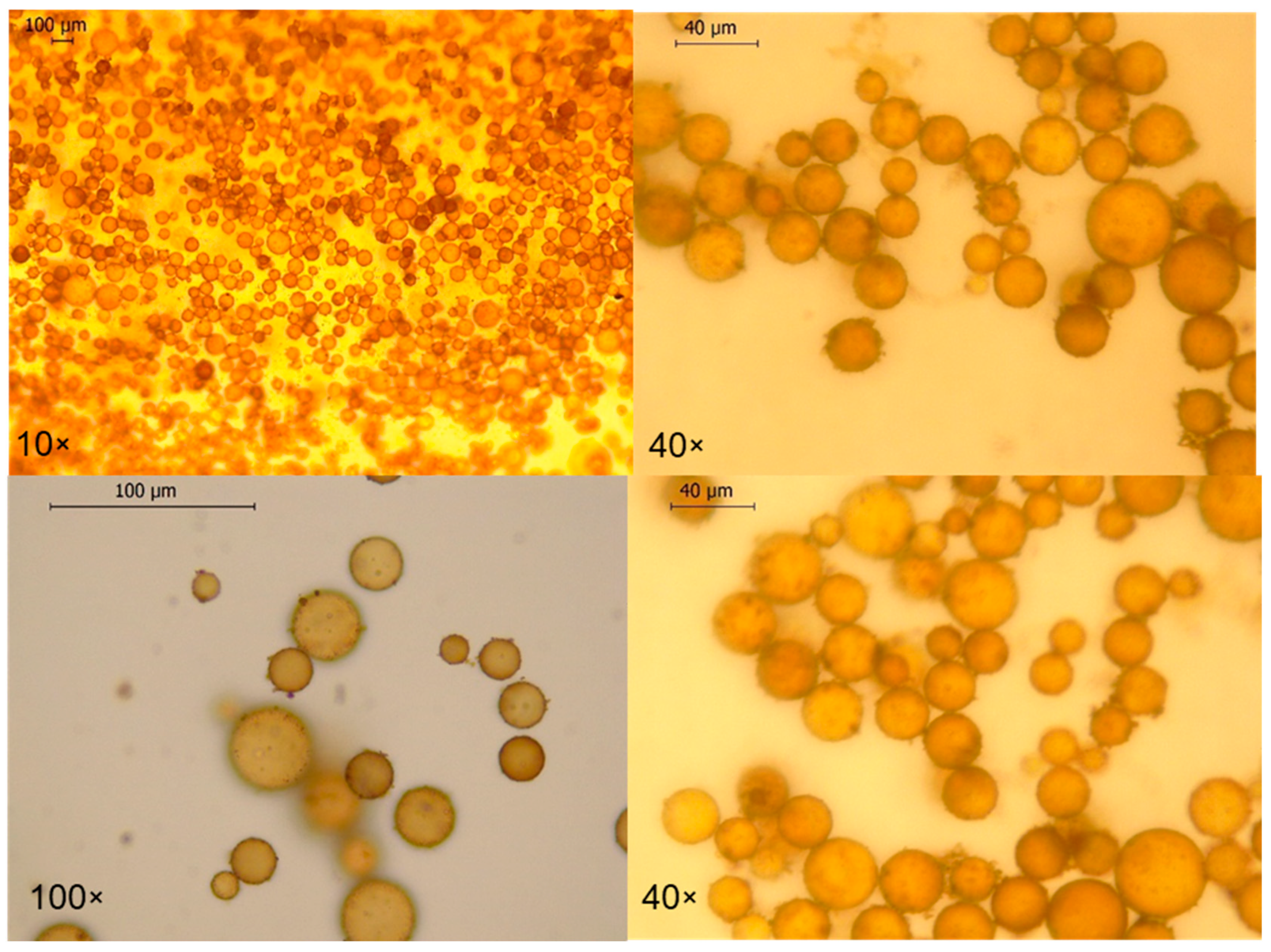
3.2. Preparation and Evaluation of Pickering Emulsion
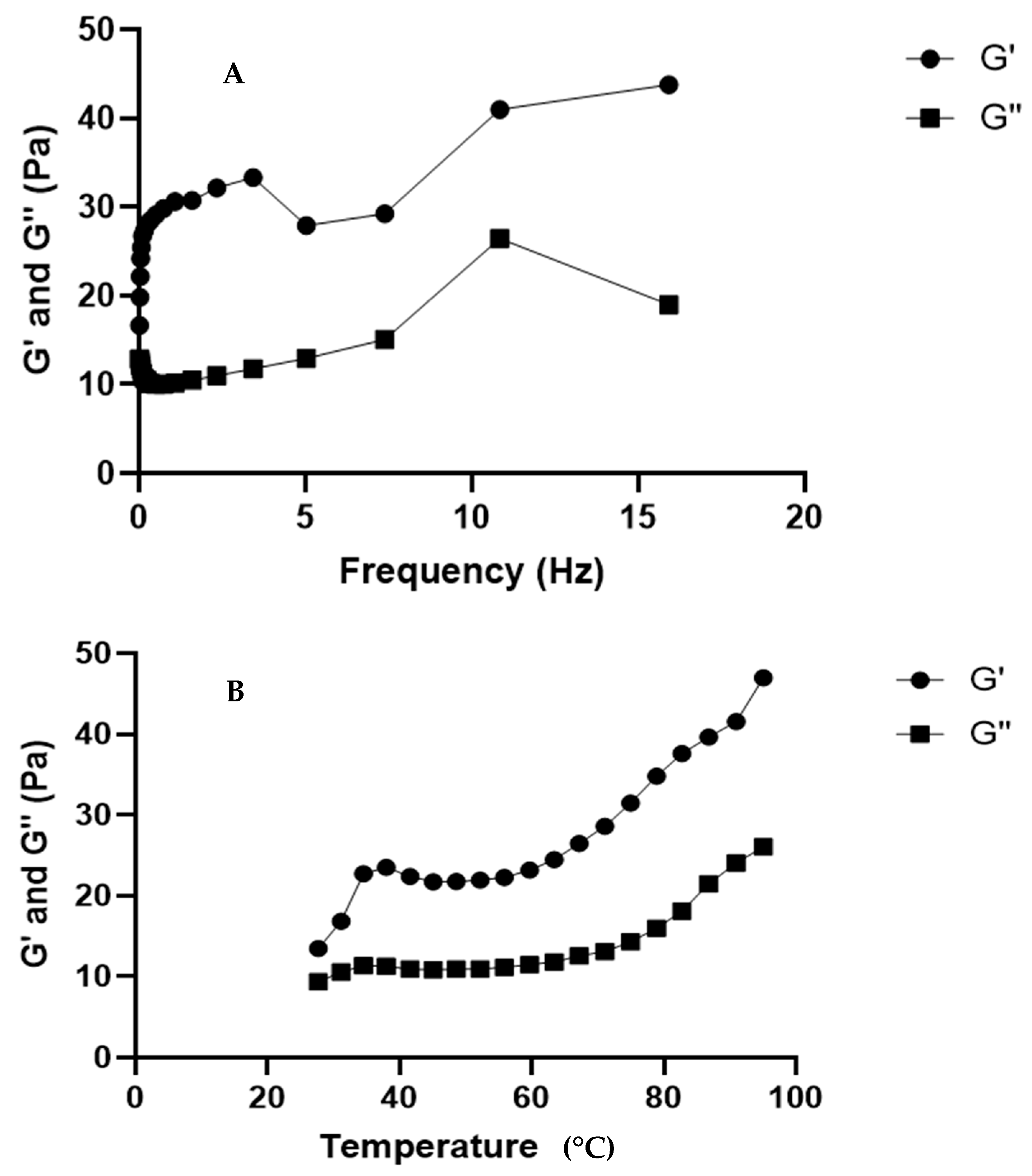
Rheological Properties
3.3. Antimicrobial Study
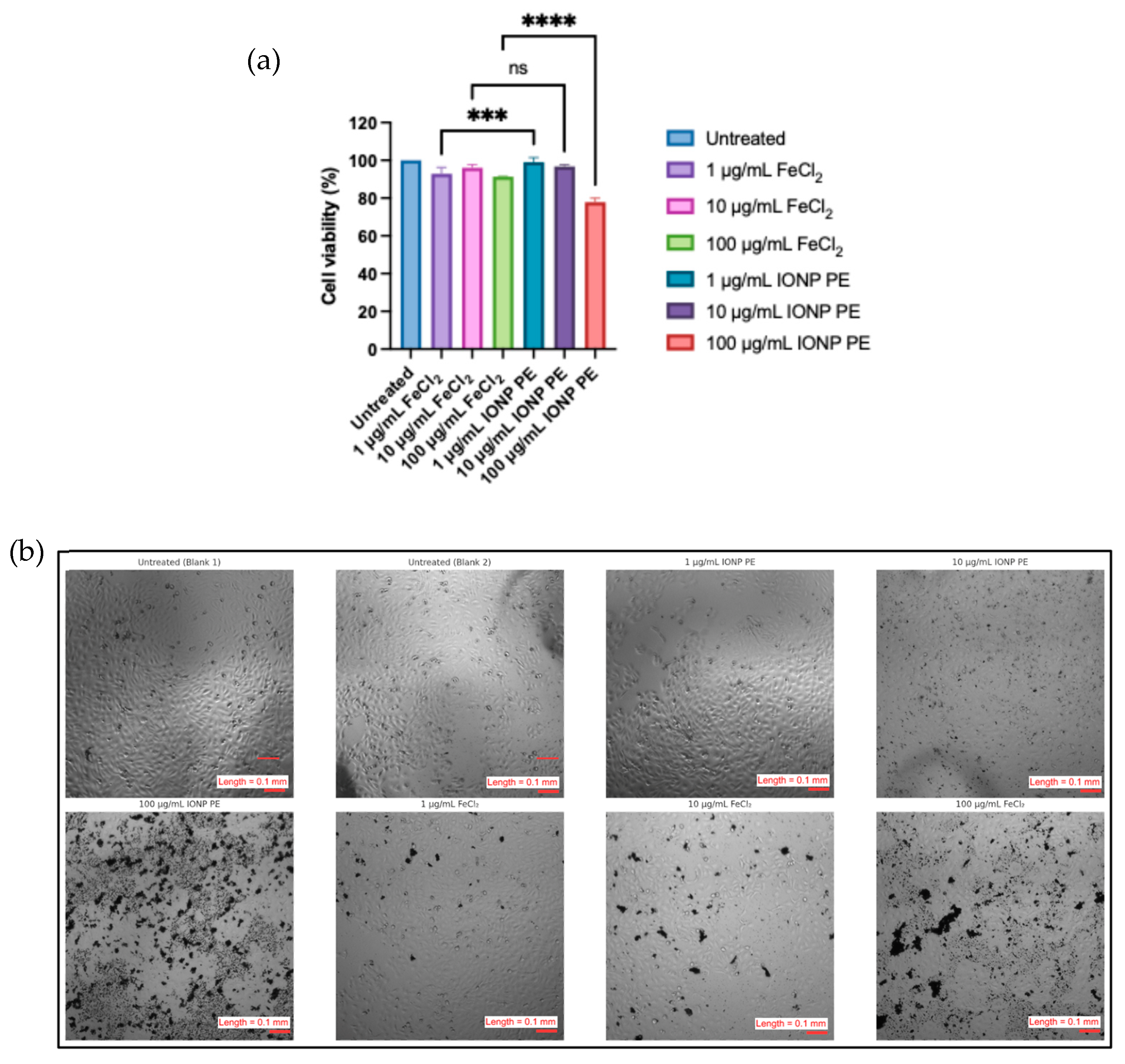
3.4. Human Keratinocyte (HaCAT) Cell Viability Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Academy of Dermatology. Sunscreen FAQs. Available online: https://www.aad.org/media/stats-sunscreen (accessed on 2 May 2023).
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef]
- Schneider, S.L.; Lim, H.W. Review of environmental effects of oxybenzone and other sunscreen active ingredients. J. Am. Acad. Dermatol. 2019, 80, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Altay Benetti, A.; Tarbox, T.; Benetti, C. Current Insights into the Formulation and Delivery of Therapeutic and Cosmeceutical Agents for Aging Skin. Cosmetics 2023, 10, 54. [Google Scholar] [CrossRef]
- Petrazzuoli, M. Advances in Sunscreens. Curr. Probl. Dermatol. 2000, 12, 287–290. [Google Scholar] [CrossRef]
- Singh, P.; Nanda, A. Enhanced sun protection of nano-sized metal oxide particles over conventional metal oxide particles: An in vitro comparative study. Int. J. Cosmet. Sci. 2014, 36, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Krasnikov, I.V.; Seteikin, A.Y.; Popov, A.P. Measurement of sun and heat protecting properties of human skin through addition of titanium dioxide nanoparticles. Opt. Spectrosc. 2010, 109, 298–303. [Google Scholar] [CrossRef]
- Afsar, O.; Oltulu, C. Evaluation of the cytotoxic effect of titanium dioxide nanoparticles in human embryonic lung cells. Turk. J. Med. Sci. 2023, 53, 1648–1657. [Google Scholar] [CrossRef]
- Zhou, C.; Lee, C.; Salas, J.; Luke, J. Guide to tinted sunscreens in skin of color. Int. J. Dermatol. 2024, 63, 272–276. [Google Scholar] [CrossRef]
- Cole, Y.; Ilyas, A.M.; Ilyas, E.N. Availability of Adequate Photoprotection for Skin of Color. Cureus 2023, 15, e42794. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, E.F.; Sarkas, H.W.; Boland, P. Iron Oxides in Novel Skin Care Formulations Attenuate Blue Light for Enhanced Protection Against Skin Damage. J. Cosmet. Dermatol. 2020, 20, 532–537. [Google Scholar] [CrossRef]
- Castanedo-Cazares, J.P.; Hernandez-Blanco, D.; Carlos-Ortega, B.; Fuentes-Ahumada, C.; Torres-Alvarez, B. Near-visible light and UV photoprotection in the treatment of melasma: A double-blind randomized trial. Photodermatol. Photoimmunol. Photomed. 2014, 30, 35–42. [Google Scholar] [CrossRef]
- Dumbuya, H.; Grimes, P.E.; Lynch, S.; Ji, K.; Brahmachary, M.; Zheng, Q.; Bouez, C.; Wangari-Talbot, J. Impact of Iron-Oxide Containing Formulations Against Visible Light-Induced Skin Pigmentation in Skin of Color Individuals. J. Drugs Dermatol. 2020, 19, 712–717. [Google Scholar] [CrossRef]
- Tanaka, Y.; Parker, R.; Aganahi, A. Photoprotective Ability of Colored Iron Oxides in Tinted Sunscreens against Ultraviolet, Visible Light and Near-Infrared Radiation. Opt. Photonics J. 2023, 13, 199–208. [Google Scholar] [CrossRef]
- Dewi, D.A.R. Sunscreen Protection Against Visible Light: Is It Needed? Malahayati Nurs. J. 2022, 4, 2527–2536. [Google Scholar] [CrossRef]
- Shahwan, T.; Sirriah, S.A.; Nairat, M.; Boyacı, E.; Eroğlu, A.E.; Scott, T.B.; Hallam, K. Green Synthesis of Iron Nanoparticles and Their Application as a Fenton-Like Catalyst for the Degradation of Aqueous Cationic and Anionic Dyes. Chem. Eng. J. 2011, 172, 258–266. [Google Scholar] [CrossRef]
- Win, T.T.; Khan, S.; Bo, B.; Zada, S.; Fu, P. Green Synthesis and Characterization of Fe3O4 Nanoparticles Using Chlorella-K01 Extract for Potential Enhancement of Plant Growth Stimulating and Antifungal Activity. Sci. Rep. 2021, 11, 21996. [Google Scholar] [CrossRef] [PubMed]
- Kürsteiner, R.; Ding, Y.; Ritter, M.; Panzarasa, G. Formation of Iron (Hydr)Oxide Nanoparticles With a pH-Clock. Nanomaterials 2022, 12, 3743. [Google Scholar] [CrossRef] [PubMed]
- Sangode, C.M.; Mahant, S.A.; Tidke, P.C.; Umekar, M.J.; Lohiya, R.T. Green Synthesized of Novel Iron Nanoparticles as Promising Antimicrobial Agent: A Review. GSC Biol. Pharm. Sci. 2021, 015, 117–127. [Google Scholar] [CrossRef]
- Kuang, Y.; Wang, Q.; Chen, Z.; Megharaj, M.; Naidu, R. Heterogeneous Fenton-Like Oxidation of Monochlorobenzene Using Green Synthesis of Iron Nanoparticles. J. Colloid Interface Sci. 2013, 410, 67–73. [Google Scholar] [CrossRef]
- Makarov, V.V.; Makarova, S.S.; Love, A.J.; Синицына, О.В.; Dudnik, A.O.; Яминский, И.B.; Taliansky, M.; Kaлинина, H.B. Biosynthesis of Stable Iron Oxide Nanoparticles in Aqueous Extracts of Hordeum vulgare and Rumex acetosa Plants. Langmuir 2014, 30, 5982–5988. [Google Scholar] [CrossRef]
- Kaur, H.; Kumar, S.; Saini, R.; Singh, P.P.; Pugazhendhi, A. One-pot biogenic synthesis of C. limon/TiO2 with dual applications as an advance photocatalyst and antimicrobial agent. Chemosphere 2023, 335, 139106. [Google Scholar] [CrossRef]
- Kaur, H.; Kumar, S.; Kaur, G.; Kaur, N.; Badru, R.; Saini, R. An emerging expanse: Novel and eco-friendly-biogenic synthesis of E. cardamomum-wrapped TiO2 nanoparticles for environmental and biological applications. Environ. Res. 2023, 234, 116599. [Google Scholar] [CrossRef]
- Yusuf, N.; Irby, C.; Katiyar, S.K.; Elmets, C.A. Photoprotective Effects of Green Tea Polyphenols. Photodermatol. Photoimmunol. Photomed. 2007, 23, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Hajihossein, R.; Eslamirad, Z.; Rafiei, F.; Naderi, G.; Assadi, M.K. Anti-Acanthamoeba Effect of Camellia sinensis Extract (Black and Green Tea) in Vitro. J. Med. Plants 2020, 1, 163–169. [Google Scholar] [CrossRef]
- Bernegossi, J.; Barbosa, R.M.C.; Rustice, P.M.; Chorilli, M. Green Tea Glycolic Extract-Loaded Liquid Crystal Systems: Development, Characterization and Microbiological Control. Braz. J. Pharm. Sci. 2016, 52, 383–390. [Google Scholar] [CrossRef]
- Amrutha, G.S.; Kalpashree, M.M.; Krishna, K. Green Synthesis of Nanoparticles Using Plumbago Auriculata Lam. Floral Extract and Study of Its Antimicrobial Effects. Int. J. Res. Appl. Sci. Eng. Technol. 2022, 10, 1208–1216. [Google Scholar] [CrossRef]
- Udoetok, I.A.; Wilson, L.D.; Headley, J.V. Stabilization of Pickering Emulsions by Iron oxide Nano-particles (Fe3O4 NPs). Adv. Mater. Sci. 2016, 1, 24–33. [Google Scholar] [CrossRef]
- Bordes, C.; Bolzinger, M.A.; El Achak, M.; Pirot, F.; Arquier, D.; Agusti, G.; Chevalier, Y. Formulation of Pickering emulsions for the development of surfactant-free sunscreen creams. Int. J. Cosmet. Sci. 2021, 43, 432–445. [Google Scholar] [CrossRef]
- Kanellis, V.G. Sharing recipes and creating potentially dangerous homemade sunscreens. Australas. J. Dermatol. 2020, 61, 161. [Google Scholar] [CrossRef]
- Tiwari, R.; Indu, S.; Gupta, M.; Singh, L.P.; Tiwari, G. Formulation and Evaluation of Herbal Sunscreens: An Assessment Towards Skin Protection From Ultraviolet Radiation. Pharmacophore 2022, 13, 41–49. [Google Scholar] [CrossRef]
- Kaur, C.D.; Saraf, S. In vitro sun protection factor determination of herbal oils used in cosmetics. Pharmacogn. Res. 2010, 2, 22–25. [Google Scholar] [CrossRef]
- Nareswari, T.L.; Syafitri, E.; Nurjannah, O. Sunscreen lip balm stick formulation containing a combination of virgin coconut oil and crude palm oil. Pharm. Rep. 2022, 2, 22–25. [Google Scholar] [CrossRef]
- Banswal, L.K.; Mane, D.S.; Khan, M.S. A Review on Formulation and Evaluation of Herbal Sunscreen Cream. Int. J. Res. Publ. Rev. 2023, 4, 3740–3751. [Google Scholar] [CrossRef]
- Bildir, B.; Demirkan, Z.; Kaya, B. Biosynthesis, Characterization and Determination of Sun Protection Factor (Spf) of Iron Nanoparticles with Bee Bread. Türk Doğa Ve Fen Derg. 2022, 11, 110–117. [Google Scholar] [CrossRef]
- Sayre, R.M.; Agin, P.P.; LeVee, G.J.; Marlowe, E. A comparison of in vivo and in vitro testing of sunscreening formulas. Photochem. Photobiol. 1979, 29, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Mansur, J.S.; Breder, M.N.R.; Mansur, M.C.A.; Azulay, R.D. Determination of Sun Protection Factor by Spectrophotometry. An. Bras. Dermatol. 1986, 61, 121–124. [Google Scholar]
- EUCAST—European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters, version 14.0; EUCAST: Växjö, Sweden, 2024. [Google Scholar]
- Kaynak Onurdağ, F.; Yavaş, Ç.; Ökten, S. Are The Disinfectants Used In Hospitals Also Effective On Bacteria That Cause Nosocomial Infections?: A University Hospital Investigation. J. Fac. Pharm. Ank. Univ. 2022, 46, 129–143. [Google Scholar] [CrossRef]
- Hussain, A.; Yasar, M.; Ahmad, G.; Ijaz, M.; Aziz, A.; Nawaz, M.G.; Khan, F.A.; Iqbal, H.; Shakeel, W.; Momand, H.; et al. Synthesis, characterization, and applications of iron oxide nanoparticles. Int. J. Health Sci. 2023, 17, 3–10. [Google Scholar] [PubMed]
- Katiyar, S.K.; Matsui, M.S.; Elmets, C.A.; Mukhtar, H. Polyphenolic antioxidant (−)-epigallocatechin-3-gallate from green tea reduces UVB-induced inflammatory responses and infiltration of leukocytes in human skin. Photochem. Photobiol. 1999, 69, 148–153. [Google Scholar] [CrossRef]
- Alves, T.; Arranca, D.; Martins, A.; Ribeiro, H.; Raposo, S.; Marto, J. Complying with the Guideline for Quality and Equivalence for Topical Semisolid Products: The Case of Clotrimazole Cream. Pharmaceutics 2021, 13, 555. [Google Scholar] [CrossRef]
- Ansari, S.; Rashid, M.A.I.; Waghmare, P.R.; Nobes, D.S. Measurement of the flow behavior index of Newtonian and shear-thinning fluids via analysis of the flow velocity characteristics in a mini-channel. SN Appl. Sci. 2020, 2, 1787. [Google Scholar] [CrossRef]
- Asadi, A.; Alarifi, I.M.; Nguyen, H.M.; Moayedi, H. Feasibility of least-square support vector machine in predicting the effects of shear rate on the rheological properties and pumping power of MWCNT–MgO/oil hybrid nanofluid based on experimental data. J. Therm. Anal. Calorim. 2021, 143, 1439–1454. [Google Scholar] [CrossRef]
- Owen, D.H.; Peters, J.J.; Kieweg, S.L.; Geonnotti, A.R.; Schnaare, R.L.; Katz, D.F. Biophysical analysis of prototype microbicidal gels. J. Pharm. Sci. 2007, 96, 661–669. [Google Scholar] [CrossRef]
- Gokce, E.H.; Korkmaz, E.; Tuncay-Tanrıverdi, S.; Dellera, E.; Sandri, G.; Bonferoni, M.C.; Ozer, O. A comparative evaluation of coenzyme Q10-loaded liposomes and solid lipid nanoparticles as dermal antioxidant carriers. Int. J. Nanomed. 2012, 7, 5109–5117. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, H.; Jiang, Y.; Zhang, Y.; Jiang, L. A review of constitutive models for non-Newtonian fluids. Fract. Calc. Appl. Anal. 2024, 27, 1483–1526. [Google Scholar] [CrossRef]
- Benetti, A.A.; Modh, H.; Panczyk, T.; Badruddoza, A.Z.; Shah, J.; Wacker, M.G.; Pastorin, G. Understanding the Structure-Release Relationships of Sepineo™: Molecular dynamics, physico-chemical characterization, in vitro and ex vivo permeation. J. Drug Deliv. Sci. Technol. 2025, 109, 106957. [Google Scholar] [CrossRef]
- Van Kempen, S.E.H.J.; Schols, H.A.; van der Linden, E.; Sagis, L.M.C. Non-linear surface dilatational rheology as a tool for understanding microstructures of air/water interfaces stabilized by oligofructose fatty acid esters. Soft. Matter. 2013, 9, 9579–9592. [Google Scholar] [CrossRef] [PubMed]
- Moch, K.; Böhmer, R.; Gainaru, C. Temperature oscillations provide access to high-order physical aging harmonics of a glass forming melt. J. Chem. Phys. 2023, 159, 221102. [Google Scholar] [CrossRef]
- Migliozzi, S.; Angeli, P.; Mazzei, L. Gelation kinetics of non-aqueous Carbopol dispersions. Colloids Surf. A Physicochem. Eng. Asp. 2019, 577, 84–95. [Google Scholar] [CrossRef]
- Xu, J.; Wang, P.; Zhou, Z.; Yuan, B.; Zhang, H. Nonlinear oscillatory rheology of aqueous suspensions of cellulose nanocrystals and nanofibrils. J. Rheol. 2024, 68, 491–508. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. Do Iron Oxide Nanoparticles Have Significant Antibacterial Properties? Antibiotics 2021, 10, 884. [Google Scholar] [CrossRef] [PubMed]
- Fatih, H.J.; Ashengroph, M.; Sharifi, A.; Zorab, M.M. Green-synthesized alpha-Fe(2)O(3)-nanoparticles as potent antibacterial, anti-biofilm and anti-virulence agent against pathogenic bacteria. BMC Microbiol. 2024, 24, 535. [Google Scholar] [CrossRef]
- Flieger, J.; Pasieczna-Patkowska, S.; Zuk, N.; Panek, R.; Korona-Glowniak, I.; Susniak, K.; Pizon, M.; Franus, W. Characteristics and Antimicrobial Activities of Iron Oxide Nanoparticles Obtained via Mixed-Mode Chemical/Biogenic Synthesis Using Spent Hop (Humulus lupulus L.) Extracts. Antibiotics 2024, 13, 111. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.; Mir, A.; Mallik, D.; Sinha, A.; Nayar, S.; Webster, T.J. Bactericidal effect of iron oxide nanoparticles on Staphylococcus aureus. Int. J. Nanomed. 2010, 5, 277–283. [Google Scholar] [CrossRef] [PubMed]



| Formulation | IONP (w/w)(%) | Coconut Oil (w/w) (%) | Green Tea Extract (w/w) (%) |
|---|---|---|---|
| PE-1 | 10 | 30 | 60 |
| PE-2 | 10 | 20 | 70 |
| PE-3 | 10 | 10 | 80 |
| PE-4 | 7.5 | 30 | 62.5 |
| PE-5 | 5 | 30 | 65 |
| PE-6 | 2.5 | 30 | 67.5 |
| Formulation | SPF ± SD |
|---|---|
| 0.1 M IONP | 0.447 ± 0.002 |
| 0.5 M IONP | 5.185 ± 0.015 |
| 1.0 M IONP | 6.202 ± 0.013 |
| TiO2 NP * | 3.982 ± 0.006 |
| Cutibacterium acnes ATCC 6919 | S. aureus ATCC 29213 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| S1 (1 M) (mg/mL) | S2 (0.5 M) (mg/mL) | S3 (0.25 M) (mg/mL) | CLR (µg/mL) | S1 (1 M) (mg/mL) | S2 (0.5 M) (mg/mL) | S3 (0.25 M) (mg/mL) | GN (µg/mL) | CIP (µg/mL) | CZ (µg/mL) |
| 0.5 | 0.5 | 0.5 | 8 | 0.5 | 0.5 | 0.5 | 8 | 8 | 8 |
| 0.25 | 0.25 | 0.25 | 4 | 0.25 | 0.25 | 0.25 | 4 | 4 | 4 |
| 0.125 | 0.125 | 0.125 | 2 | 0.125 | 0.125 | 0.125 | 2 | 2 | 2 |
| 0.0625 | 0.0625 | 0.0625 | 1 | 0.0625 | 0.0625 | 0.0625 | 1 | 1 | 1 |
| 0.03125 | 0.03125 | 0.03125 | 0.5 | 0.03125 | 0.03125 | 0.03125 | 0.5 | 0.5 | 0.5 |
| 0.015625 | 0.015625 | 0.015625 | 0.25 | 0.015625 | 0.015625 | 0.015625 | 0.25 | 0.25 | 0.25 |
| 0.0078125 | 0.0078125 | 0.0078125 | 0.125 | 0.0078125 | 0.0078125 | 0.0078125 | 0.125 | 0.125 | 0.125 |
| 0.00390625 | 0.00390625 | 0.00390625 | 0.0625 | 0.00390625 | 0.00390625 | 0.00390625 | 0.0625 | 0.0625 | 0.0625 |
| 0.00195312 | 0.00195312 | 0.00195312 | 0.0313 | 0.00195312 | 0.00195312 | 0.00195312 | 0.0313 | 0.0313 | 0.0313 |
| 0.00097656 | 0.00097656 | 0.00097656 | 0.0156 | 0.00097656 | 0.00097656 | 0.00097656 | 0.0156 | 0.0156 | 0.0156 |
| 0.00048828 | 0.00048828 | 0.00048828 | 0.00048828 | 0.00048828 | 0.00048828 | ||||
| 0.00024414 | 0.00024414 | 0.00024414 | 0.00024414 | 0.00024414 | 0.00024414 | ||||
| Application Times | t = 0 (CFU/mL) | 2nd Hour (CFU/mL) | 4th Hour (CFU/mL) | 6th Hour (CFU/mL) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1/2 | 1/4 | 1 | 1/2 | 1/4 | 1 | 1/2 | 1/4 | |||
| C.acnes | Formulation | 0.4 × 107 | 0.1 × 106 | 0.1 × 106 | 0.1 × 106 | tntc | tntc | tntc | tntc | tntc | tntc |
| Coconut oil | 0.4 × 107 | tntc | 0.4 × 107 | 0.3 × 107 | 0.1 × 106 | tntc | tntc | 0.1 × 107 | tntc | tntc | |
| S. aureus | Formulation | 0.5 × 107 | 0.7 × 105 | 0.5 × 105 | 0.1 × 106 | 0.9 × 105 | 0.6 × 106 | tntc | tntc | tntc | tntc |
| Coconut oil | 0.5 × 107 | tntc | 0.7 × 106 | 0.7 × 106 | 0.1 × 105 | tntc | tntc | 0.1 × 106 | tntc | tntc | |
| S. epidermidis | Formulation | 0.4 × 107 | tntc | 0.8 × 107 | 0.8 × 108 | sçk | 0.4 × 105 | 0.1 × 106 | 0.1 × 105 | 0.2 × 107 | 0.3 × 107 |
| Coconut oil | 0.4 × 107 | tntc | tntc | tntc | 0.3 × 106 | 0.2 × 105 | 0.2 × 106 | tntc | tntc | tntc | |
| Cutibacterium acnes ATCC 6919 | S. aureus ATCC 29213 | S. epidermidis NCTC 11047 | |||||
|---|---|---|---|---|---|---|---|
| Formulation | Coconut Oil | Formulation | Coconut Oil | Formulation | Coconut Oil | ||
| 1/1 | 2nd hour | 97.5 | 0.00 | 98.6 | 0.00 | 0.00 | 0.00 |
| 4th hour | 0.00 | 97.5 | 98.2 | 99.8 | 0.00 | 92.5 | |
| 6th hour | 0.00 | 75.0 | 0.00 | 98.0 | 99.9 | 0.00 | |
| 1/2 | 2nd hour | 97.5 | 0.00 | 99.0 | 86.0 | 0.00 | 0.00 |
| 4th hour | 0.00 | 0.00 | 88.0 | 0.00 | 99.0 | 99.5 | |
| 6th hour | 0.00 | 0.00 | 0.00 | 0.00 | 50.0 | 0.00 | |
| 1/4 | 2nd hour | 9.50 | 25.0 | 98.0 | 86.0 | 0.00 | 0.00 |
| 4th hour | 0.00 | 0.00 | 0.00 | 0.00 | 97.5 | 95.0 | |
| 6th hour | 0.00 | 0.00 | 0.00 | 0.00 | 25.0 | 0.00 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ergin, A.D.; Erbay, Z.B.; Karaca, M.; Ökten, S.; Kuyucuklu, G.; Benetti, C.; Benetti, A.A. Green Synthesis of Iron Oxide Nanoparticles for Use in Pickering Emulsions: In Vitro UV-Absorbing and Antimicrobial Properties. Cosmetics 2025, 12, 208. https://doi.org/10.3390/cosmetics12050208
Ergin AD, Erbay ZB, Karaca M, Ökten S, Kuyucuklu G, Benetti C, Benetti AA. Green Synthesis of Iron Oxide Nanoparticles for Use in Pickering Emulsions: In Vitro UV-Absorbing and Antimicrobial Properties. Cosmetics. 2025; 12(5):208. https://doi.org/10.3390/cosmetics12050208
Chicago/Turabian StyleErgin, Ahmet Doğan, Zeynep Betül Erbay, Müberra Karaca, Suzan Ökten, Gülcan Kuyucuklu, Camillo Benetti, and Ayça Altay Benetti. 2025. "Green Synthesis of Iron Oxide Nanoparticles for Use in Pickering Emulsions: In Vitro UV-Absorbing and Antimicrobial Properties" Cosmetics 12, no. 5: 208. https://doi.org/10.3390/cosmetics12050208
APA StyleErgin, A. D., Erbay, Z. B., Karaca, M., Ökten, S., Kuyucuklu, G., Benetti, C., & Benetti, A. A. (2025). Green Synthesis of Iron Oxide Nanoparticles for Use in Pickering Emulsions: In Vitro UV-Absorbing and Antimicrobial Properties. Cosmetics, 12(5), 208. https://doi.org/10.3390/cosmetics12050208






