A 28-Day Pilot Study of the Effects on Facial Skin Hydration, Elasticity, and Texture of a Centella asiatica Extracellular Vesicle-Based Skin Care Formulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Description of Test Product
2.3. Assessment of Outcomes
- -
- Skin hydration: Assessed using the Corneometer® CM825 probe, which measures the dielectric constant related to epidermal moisture at a depth of 60–100 µm.
- -
- Skin melanin content: Measured with the Mexameter® MX18 probe, which detects melanin levels based on RGB light absorption at 568 nm, 660 nm, and 880 nm.
- -
- Skin elasticity index (R2 parameter): Evaluated using the Cutometer® Dual MPA580 probe, which analyzes the skin’s suction and stretching properties.
- -
- Wrinkle score: Measured under standard white light, which detects shadow variations to determine wrinkle distribution and count. A higher percentage indicates fewer wrinkles.
- -
- Redness score: Analyzed using RBX polarized light to identify vascular and inflammatory conditions. A higher percentage corresponds to fewer red zones.
- -
- Pore score: Measured under white light, detecting shadowed pore depressions and darker areas relative to the surrounding skin. A higher percentage reflects fewer visible pores.
- -
- Standardized capture protocol: All VISIA image captures and probe assessments followed strict SOPs (e.g., fixed lighting, angles, probe calibration), ensuring consistency and reducing subjective variability.
- -
- Automated software extraction: While images were reviewed by humans, primary endpoint data (e.g., lesion counts, texture scores) were derived via automated VISIA and probe software, limiting human interpretation.
- -
- Inter-rater reliability checks: A subset (~20%) of the images was independently analyzed by a second blinded assessor. Reliability metrics (e.g., intraclass correlation coefficients) were calculated to confirm consistency.
- -
- Periodic blinding checks: We periodically verified that the assessors remained blind to group assignments. Any deviations were flagged, reviewed, and resolved prior to unblinding.
2.4. Data Presentation and Statistical Analysis
3. Results
3.1. Participants
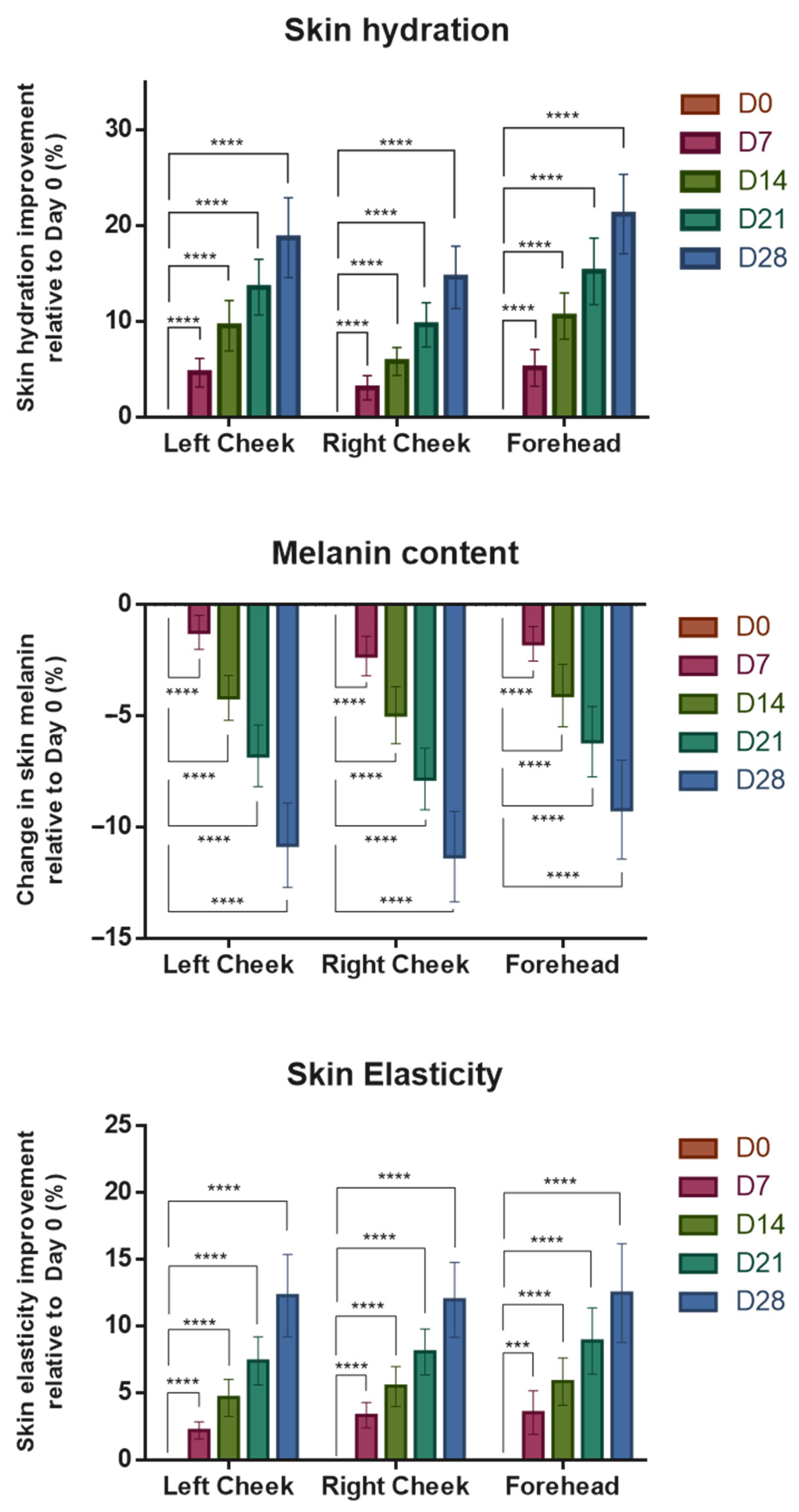
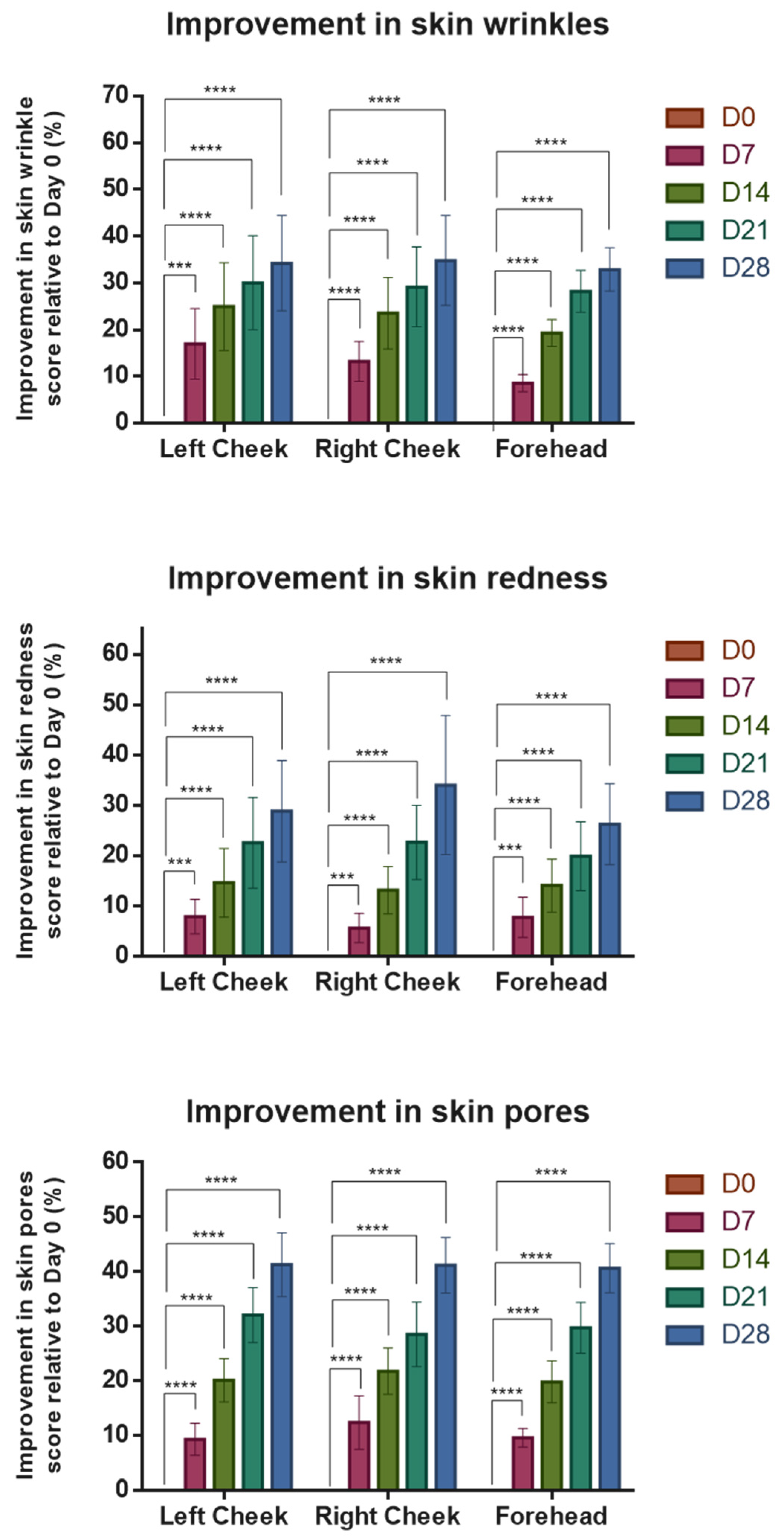
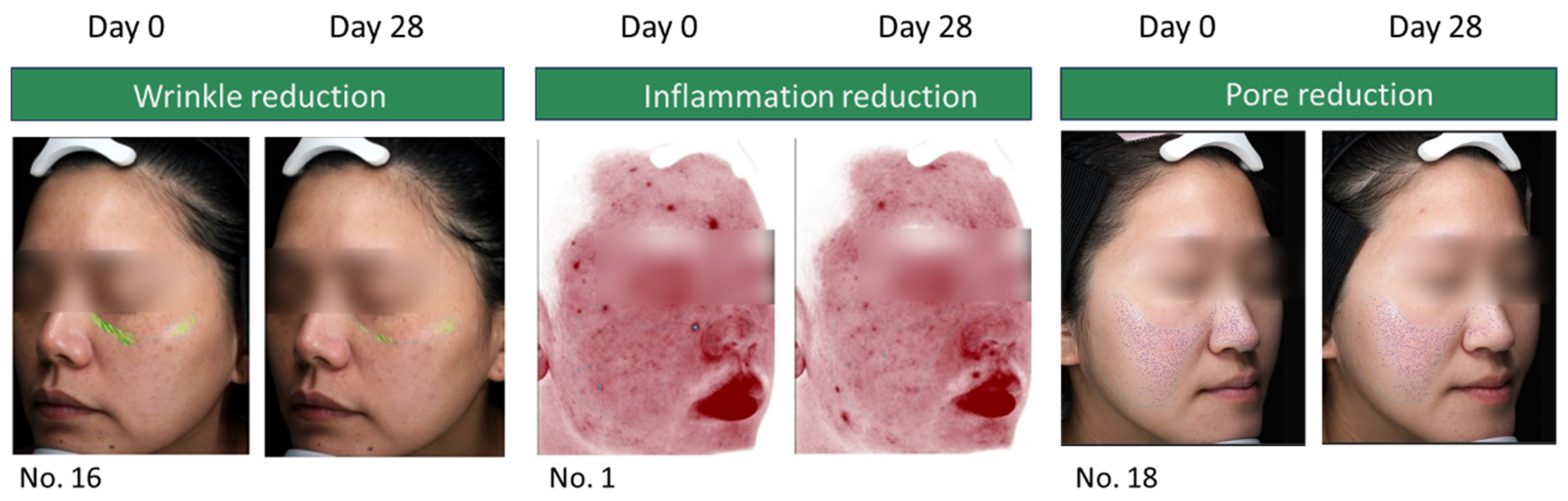
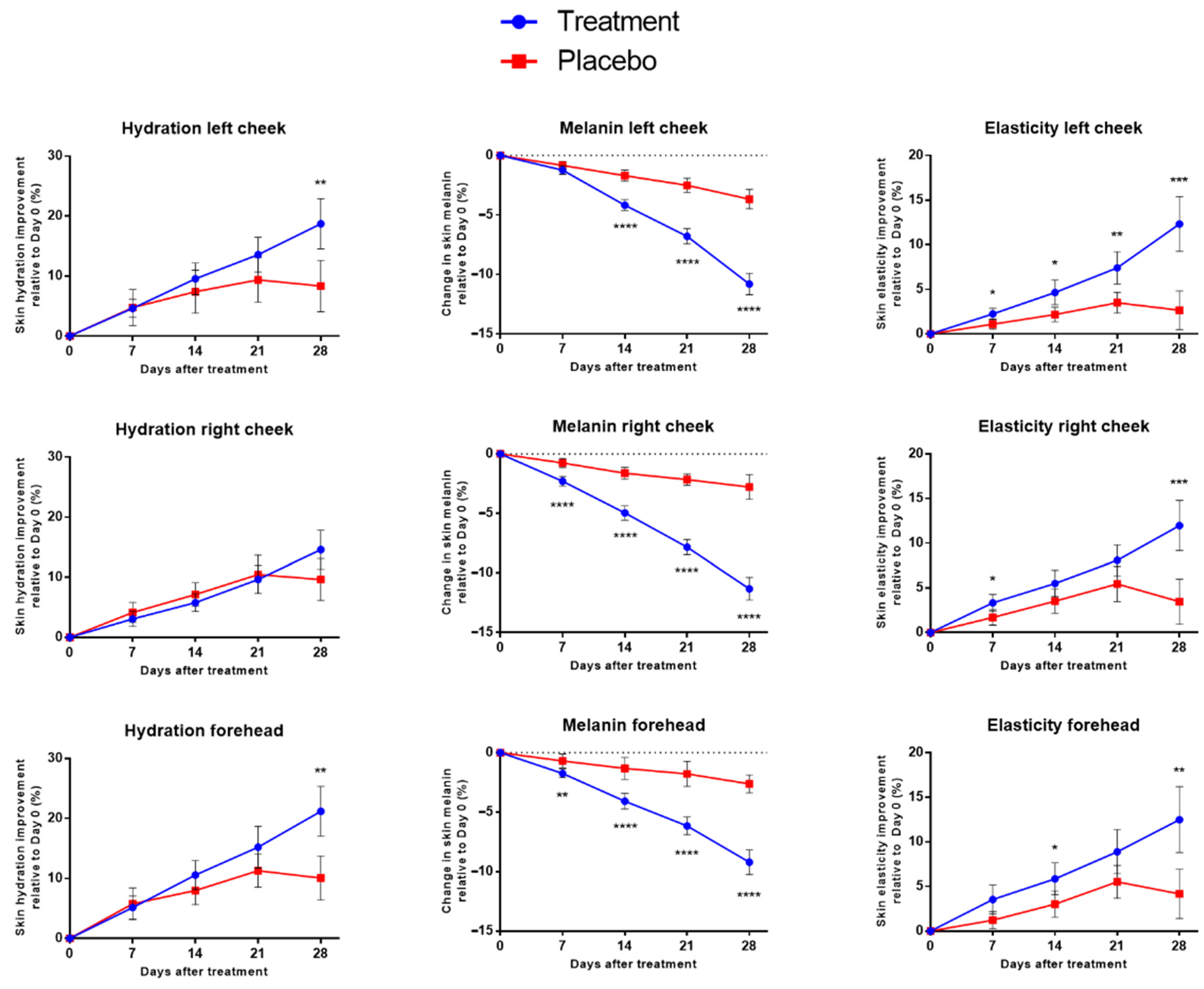
3.2. Assessments of Participants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajkumar, J.; Chandan, N.; Lio, P.; Shi, V. The Skin Barrier and Moisturization: Function, Disruption, and Mechanisms of Repair. Skin Pharmacol. Physiol. 2023, 36, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.C.; Aveleira, C.; Cavadas, C. Skin Senescence: Mechanisms and Impact on Whole-Body Aging. Trends Mol. Med. 2022, 28, 97–109. [Google Scholar] [CrossRef]
- The Beauty Boom and Beyond: Can the Industry Maintain Its Growth? Available online: https://www.mckinsey.com/industries/consumer-packaged-goods/our-insights/the-beauty-boom-and-beyond-can-the-industry-maintain-its-growth (accessed on 10 December 2024).
- Liu, J.K. Natural Products in Cosmetics. Nat. Prod. Bioprospect. 2022, 12, 40. [Google Scholar] [CrossRef]
- Biswas, D.; Mandal, S.; Chatterjee Saha, S.; Tudu, C.K.; Nandy, S.; Batiha, G.E.; Shekhaway, M.S.; Pandey, D.K.; Dey, A. Ethnobotany, Phytochemistry, Pharmacology, and Toxicity of Centella asiatica (L.) Urban: A Comprehensive Review. Phytother. Res. 2021, 35, 6624–6654. [Google Scholar] [CrossRef] [PubMed]
- Bandopadhyay, S.; Mandal, S.; Ghorai, M.; Jha, N.K.; Kumar, M.; Radha, N.; Ghosh, A.; Proćków, J.; Perez de la Lastra, J.M.; Dey, A. Therapeutic Properties and Pharmacological Activities of Asiaticoside and Madecassoside: A Review. J. Cell. Mol. Med. 2023, 27, 593–608. [Google Scholar] [CrossRef]
- Sun, B.; Wu, L.; Wu, Y.; Zhang, C.; Qin, L.; Hayashi, M.; Kudo, M.; Gao, M.; Liu, T. Therapeutic Potential of Centella asiatica and Its Triterpenes: A Review. Front. Pharmacol. 2020, 11, 568032. [Google Scholar] [CrossRef]
- Diniz, L.R.; Calado, L.L.; Duarte, A.B.; de Sousa, D.P. Centella asiatica and Its Metabolite Asiatic Acid: Wound Healing Effects and Therapeutic Potential. Metabolites 2023, 13, 276. [Google Scholar] [CrossRef]
- Farley, J.T.; Eldahshoury, M.K.; de Marcos Lousa, C. Unconventional secretion of plant extracellular vesicles and their benefits to human health: A mini review. Front. Cell Dev. Biol. 2022, 10, 883841. [Google Scholar] [CrossRef]
- Domaszewska-Szostek, A.; Krzyżanowska, M.; Polak, A.; Puzianowska-Kuźnicka, M. Effectiveness of extracellular vesicle application in skin aging treatment and regeneration: Do we have enough evidence from clinical trials? Int. J. Mol. Sci. 2025, 26, 2354. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Zhang, Q.; Franklin, J.L.; Coffey, R.J. Extracellular Vesicles and Nanoparticles: Emerging Complexities. Trends Cell Biol. 2023, 33, 667–681. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Hong, Y.D.; Kim, D.; Park, S.J.; Kim, J.S.; Kim, H.M.; Yoon, E.J.; Cho, J.S. Confirmation of Plant-Derived Exosomes as Bioactive Substances for Skin Application Through Comparative Analysis of Keratinocyte Transcriptome. Appl. Bio. Chem. 2022, 65, 8. [Google Scholar] [CrossRef]
- Kim, M.H.; Yoon, E.J.; Kim, J.S.; Park, S.J.; Lee, H. Comparative Analysis of the Transcriptome and Efficacy of Bioactive Centella asiatica Exosomes on Skin Cells. ResearchSquare 2023. Available online: https://www.researchsquare.com/article/rs-2787704/v1 (accessed on 11 November 2024).
- Li, A.; Li, D.; Gu, Y.; Liu, R.; Tang, X.; Zhao, Y.; Qi, F.; Wei, J.; Liu, J. Plant-Derived Nanovesicles: Further Exploration of Biomedical Function and Application Potential. Acta Pharm. Sin. 2023, 13, 3300–3320. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.M.; Wu, C.C.; Huang, H.C.; Wang, S.S.; Chuang, C.H.; Kao, P.L.; Tang, W.H.; Liu, L.T.C.; Qiu, W.Y.; Percec, I.; et al. Centella asiatica Tissue Culture-Derived Extracellular Vesicles: A Multifaceted Approach to Skincare Applications. bioRxiv 2024. [Google Scholar] [CrossRef]
- Taiwan Food and Drug Administration. Guidelines for Cosmetic Human Skin Patch Test (in Traditional Chinese). November 2019. Available online: https://www.fda.gov.tw/tc/includes/GetFile.ashx?id=f637108864795066124&type=2&cid=32306 (accessed on 7 April 2025).
- Hsu, T.F.; Su, Z.R.; Hsieh, Y.H.; Wang, M.F.; Oe, M.; Matsuoka, R.; Masuda, Y. Oral hyaluronan relieves wrinkles and improves dry skin: A 12-week double-blinded, placebo-controlled study. Nutrients 2021, 13, 2220. [Google Scholar] [CrossRef]
- Zang, S.; Chen, J.; Chevalier, C.; Zhang, J.; Li, S.; Wang, H.; Li, J.; Chen, Y.; Xu, H.; Sheng, L.; et al. Holistic investigation of the anti-wrinkle and repair efficacy of a facial cream enriched with C-xyloside. J. Cosmet. Dermatol. 2024, 23, 4017–4028. [Google Scholar] [CrossRef]
- Evans, M.; Lewis, E.D.; Zakaria, N.; Pelipyagina, T.; Guthrie, N. A randomized, triple-blind, placebo-controlled, parallel study to evaluate the efficacy of a freshwater marine collagen on skin wrinkles and elasticity. J. Cosmet. Dermatol. 2021, 20, 825–834. [Google Scholar] [CrossRef]
- Jung, E.; Lee, J.A.; Shin, S.; Roh, K.B.; Kim, J.H.; Park, D. Madecassoside Inhibits Melanin Synthesis by Blocking Ultraviolet-Induced Inflammation. Molecules 2013, 18, 15724–15736. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, J.; Ding, H.; Qiu, M.; Xue, L.; Ge, D.; Wen, G.; Ren, H.; Li, P.; Wang, J. Applications of Plant-Derived Extracellular Vesicles in Medicine. MedComm 2024, 5, e741. [Google Scholar] [CrossRef] [PubMed]
- The Inkey List. Exosome Hydro-Glow Complex. Available online: https://www.theinkeylist.com/products/exosome-hydro-glow-complex (accessed on 10 April 2025).
- Kim, Y.J.; Yoo, S.M.; Park, H.H.; Lim, H.J.; Kim, Y.L.; Lee, S.; Seo, K.W.; Kang, K.S. Exosomes Derived from Human Umbilical Cord Blood Mesenchymal Stem Cells Stimulates Rejuvenation of Human Skin. Biochem. Biophys. Res. Commun. 2017, 493, 1102–1108. [Google Scholar] [CrossRef]
- Johnson, J.; Law, S.Q.; Shojaee, M.; Hall, A.S.; Bhuiyan, S.; Lim, M.B.; Silva, A.; Kong, K.J.; Schoppet, M.; Blyth, C.; et al. First-in-Human Clinical Trial of Platelet-Derived Extracellular Vesicles for Wound Healing. J. Extracell. Vesicles 2023, 12, e12332. [Google Scholar] [CrossRef] [PubMed]
- Peredo, M.; Shivananjappa, S. Topical Human Mesenchymal Stem Cell-Derived Exosomes for Acceleration of Wound Healing Following Tissue Trauma and Aesthetic Procedures: A Case Series. J. Drugs. Dermatol. 2024, 23, 281–284. [Google Scholar] [CrossRef]
- Bai, G.; Truong, T.M.; Pathak, G.N.; Benoit, L.; Rao, B. Clinical applications of exosomes in cosmetic dermatology. Skin Health Dis. 2024, 4, e348. [Google Scholar] [CrossRef]
- Alzahrani, F.A.; Khan, M.I.; Kameli, N.; Alsahafi, E.; Riza, Y.M. Plant-derived extracellular vesicles and their exciting potential as the future of next-generation drug delivery. Biomolecules 2023, 13, 839. [Google Scholar] [CrossRef]
- Kim, H.S.; Hwang, H.J.; Seo, W.D.; Do, S.H. Oat (Avena sativa L.) Sprouts Restore Skin Barrier Function by Modulating the Expression of the Epidermal Differentiation Complex in Models of Skin Irritation. Int. J. Mol. Sci. 2023, 24, 17274. [Google Scholar] [CrossRef]
- Sah, A.; Naseef, P.P.; Kuruniyan, M.S.; Jain, G.K.; Zakir, F.; Aggarwal, G. A Comprehensive Study of Therapeutic Applications of Chamomile. Pharmaceuticals 2022, 15, 1284. [Google Scholar] [CrossRef]
- Huang, H.; Qian, J.; Zeng, L.; Zhang, J.; Peng, X.; Tang, X.; Tang, Y.; Fu, M.; Liu, Q.; Song, F.; et al. Full-Component Extract of Crithmum maritimum and its Effect on Epidermal Regeneration. RSC Adv. 2025, 15, 1713–1720. [Google Scholar] [CrossRef]
- Nowak, A.; Zielonka-Brzezicka, J.; Perużyńska, M.; Klimowicz, A. Epilobium angustifolium L. as a Potential Herbal Component of Topical Products for Skin Care and Treatment-A Review. Molecules 2022, 27, 3536. [Google Scholar] [CrossRef]
- Jesus, A.; Sousa, E.; Cidade, H.; Cruz, M.T.; Almeida, I.F. How to Fight Acute Sun Damage? Current Skin Care Strategies. Photochem. Photobiol. Sci. 2024, 23, 1915–1930. [Google Scholar] [CrossRef] [PubMed]
- Olsson, S.E.; Sreepad, B.; Lee, T.; Fasih, M.; Fijany, A. Public Interest in Acetyl Hexapeptide-8: Longitudinal Analysis. JMIR Dermatol. 2024, 7, e54217. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, Y.; Miyanishi, A.; Mizuno, H.; Kato, S.; Aoshima, H.; Kokubo, K.; Miwa, N. Super-Highly Hydroxylated Fullerene Derivative Protects Human Keratinocytes from UV-Induced Cell Injuries Together with the Decreases in Intracellular ROS Generation and DNA Damages. J. Photochem. Photobiol. B Biol. 2011, 102, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Yang, S.; Chen, J.; Li, C.; Zhou, C.; Hong, P.; Sun, S.; Qian, Z.J. Trehalose Against UVB-Induced Skin Photoaging by Suppressing MMP Expression and Enhancing Procollagen I Synthesis in HaCaT cells. J. Funct. Foods 2020, 74, 104198. [Google Scholar] [CrossRef]
- Garcia, C.; Valin, E.; Hernandez, E.; Kern, C.; Roso, A.; Garcia, C.; Valin, E.; Hernandez, E.; Kern, C.; Roso, A. Effect of a Simple Sugar-based Ingredient on Skin Moisturization: Biological Mode of Action and Clinical Effects. Asian J. Beauty Cosmetol. 2023, 21, 3–27. [Google Scholar] [CrossRef]

| Treatment | Placebo | |
|---|---|---|
| Number of participants | 20 | 10 |
| Gender | ||
| Female | 16 (80%) | 8 (80%) |
| Male | 4 (20%) | 2 (20%) |
| Age (mean ± SD in years) | 36.5 ± 9.2 | 40.1 ± 10.4 |
| Ethnicity | East Asian (100%) | East Asian (100%) |
| Fitzpatrick skin type | Type II (100%) | Type II (100%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, T.-M.; Wu, C.-C.; Huang, H.-C.; Lu, J.-Y.; Chuang, C.-H.; Kao, P.-L.; Tang, W.-H.; Liu, L.T.-C.; Qiu, W.-Y.; Percec, I.; et al. A 28-Day Pilot Study of the Effects on Facial Skin Hydration, Elasticity, and Texture of a Centella asiatica Extracellular Vesicle-Based Skin Care Formulation. Cosmetics 2025, 12, 186. https://doi.org/10.3390/cosmetics12050186
Chang T-M, Wu C-C, Huang H-C, Lu J-Y, Chuang C-H, Kao P-L, Tang W-H, Liu LT-C, Qiu W-Y, Percec I, et al. A 28-Day Pilot Study of the Effects on Facial Skin Hydration, Elasticity, and Texture of a Centella asiatica Extracellular Vesicle-Based Skin Care Formulation. Cosmetics. 2025; 12(5):186. https://doi.org/10.3390/cosmetics12050186
Chicago/Turabian StyleChang, Tsong-Min, Chung-Chin Wu, Huey-Chun Huang, Ji-Ying Lu, Ching-Hua Chuang, Pei-Lun Kao, Wei-Hsuan Tang, Luke Tzu-Chi Liu, Wei-Yin Qiu, Ivona Percec, and et al. 2025. "A 28-Day Pilot Study of the Effects on Facial Skin Hydration, Elasticity, and Texture of a Centella asiatica Extracellular Vesicle-Based Skin Care Formulation" Cosmetics 12, no. 5: 186. https://doi.org/10.3390/cosmetics12050186
APA StyleChang, T.-M., Wu, C.-C., Huang, H.-C., Lu, J.-Y., Chuang, C.-H., Kao, P.-L., Tang, W.-H., Liu, L. T.-C., Qiu, W.-Y., Percec, I., Chen, C., & Kuo, T.-Y. (2025). A 28-Day Pilot Study of the Effects on Facial Skin Hydration, Elasticity, and Texture of a Centella asiatica Extracellular Vesicle-Based Skin Care Formulation. Cosmetics, 12(5), 186. https://doi.org/10.3390/cosmetics12050186







