Cosmetic Upgrade of EGF: Genetically Modified Probiotic-Derived Cell-Free Supernatants Containing Human EGF Protein Exhibit Diverse Biological Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture
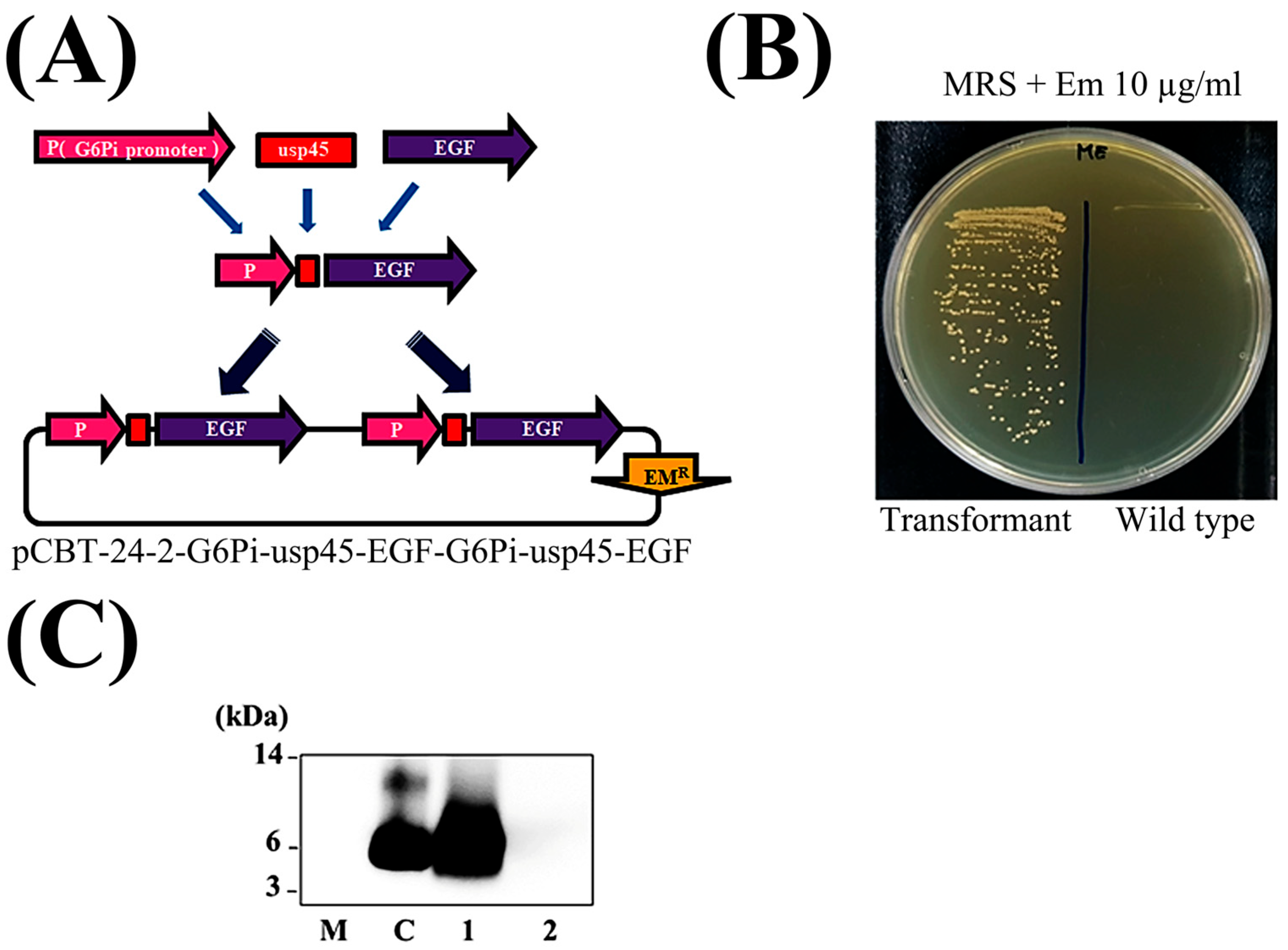
2.2. Construction of Plasmid Harboring DDS-EGF for Use in Pediococcus pentosaceus SL4 System
2.3. Transformation of Pediococcus pentosaceus SL4 (PP-EGF) and Detection of EGF Protein in Culture Supernatant
2.4. Cell Lines and Culture
2.5. Investigation of Bioactivities of PP-EGF Culture Supernatant
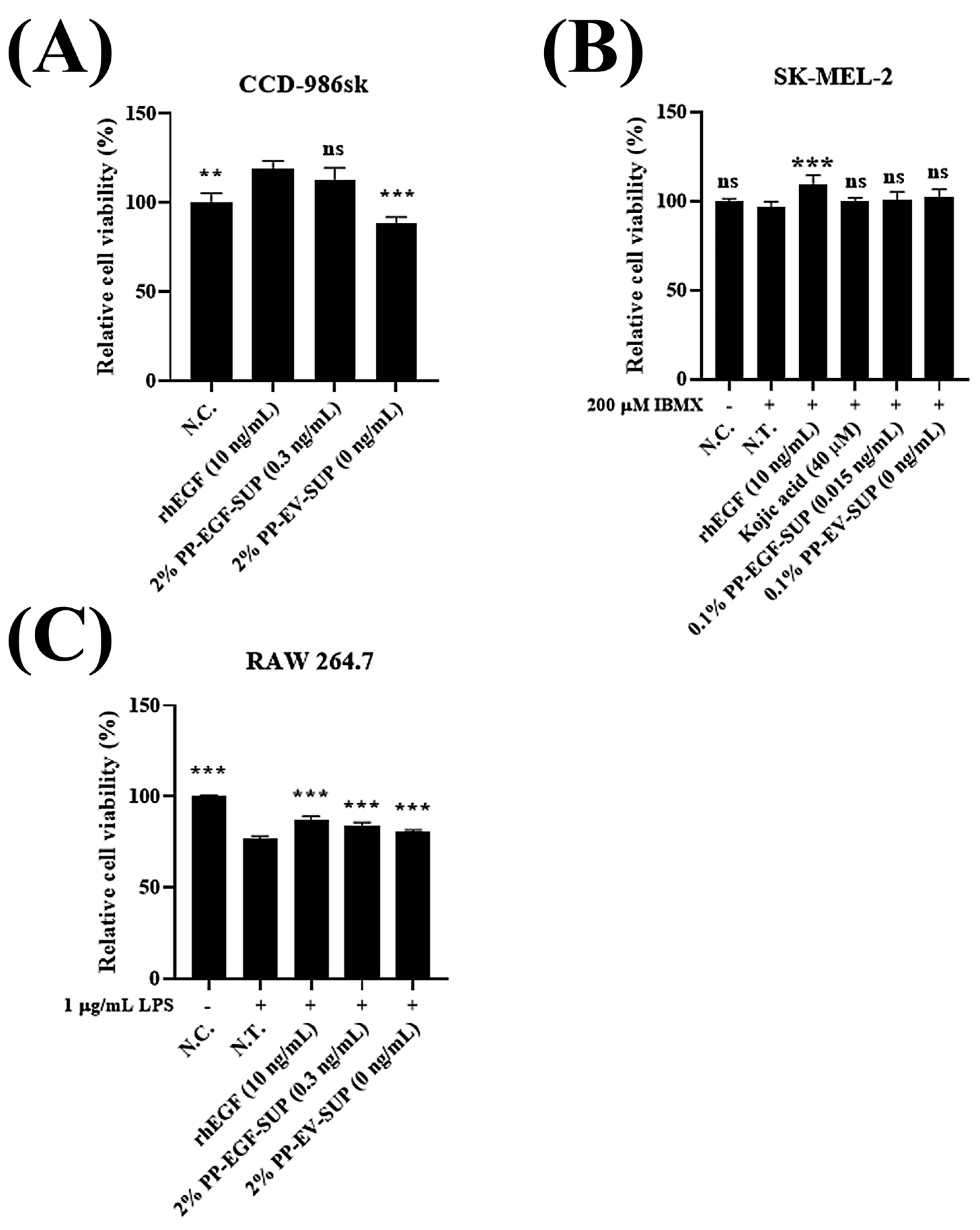
2.5.1. Cell Viability (Proliferation) Test
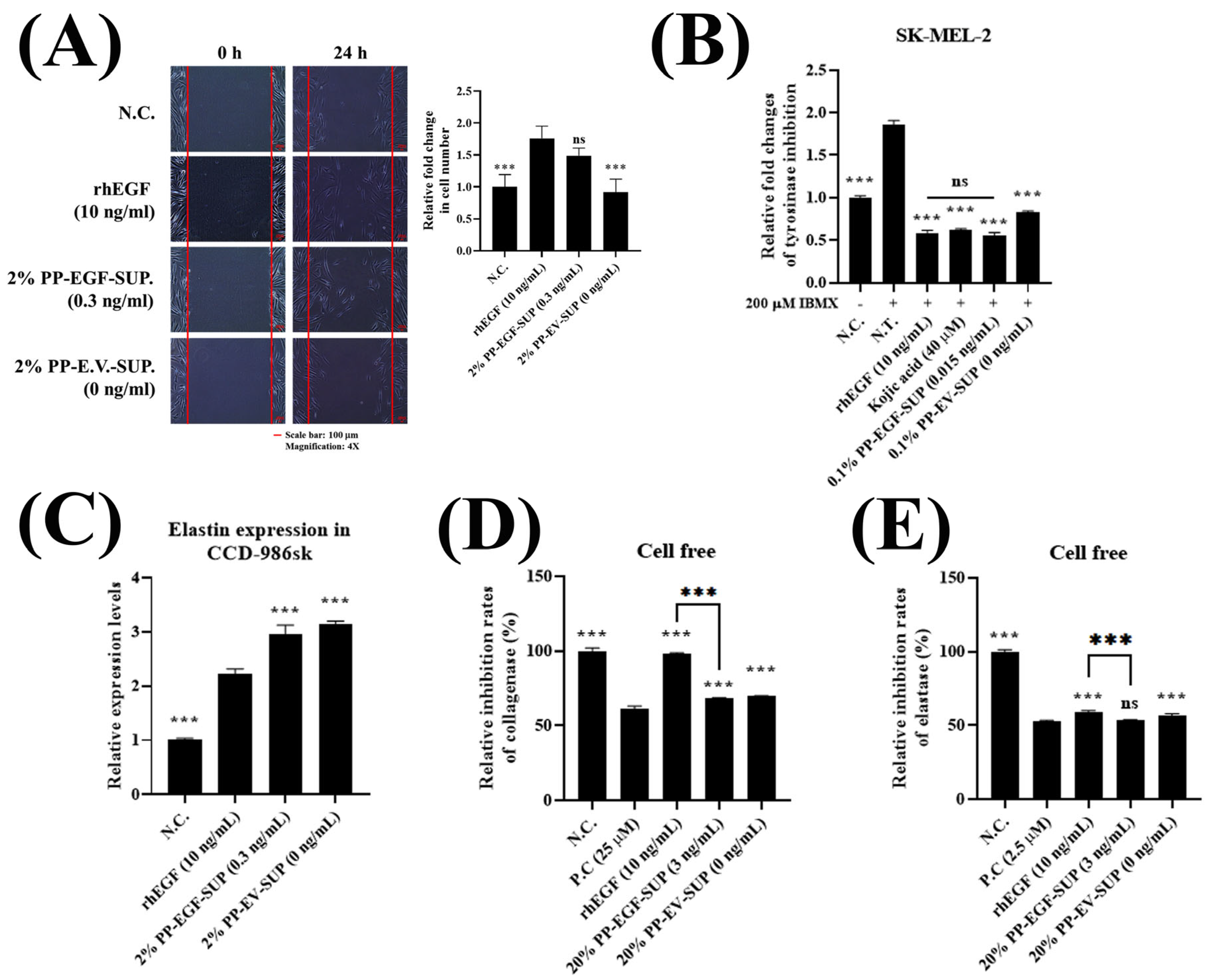
2.5.2. Cell Scratch Wound Healing Test
2.5.3. ELISA Analysis
2.5.4. Enzymatic Activity Test
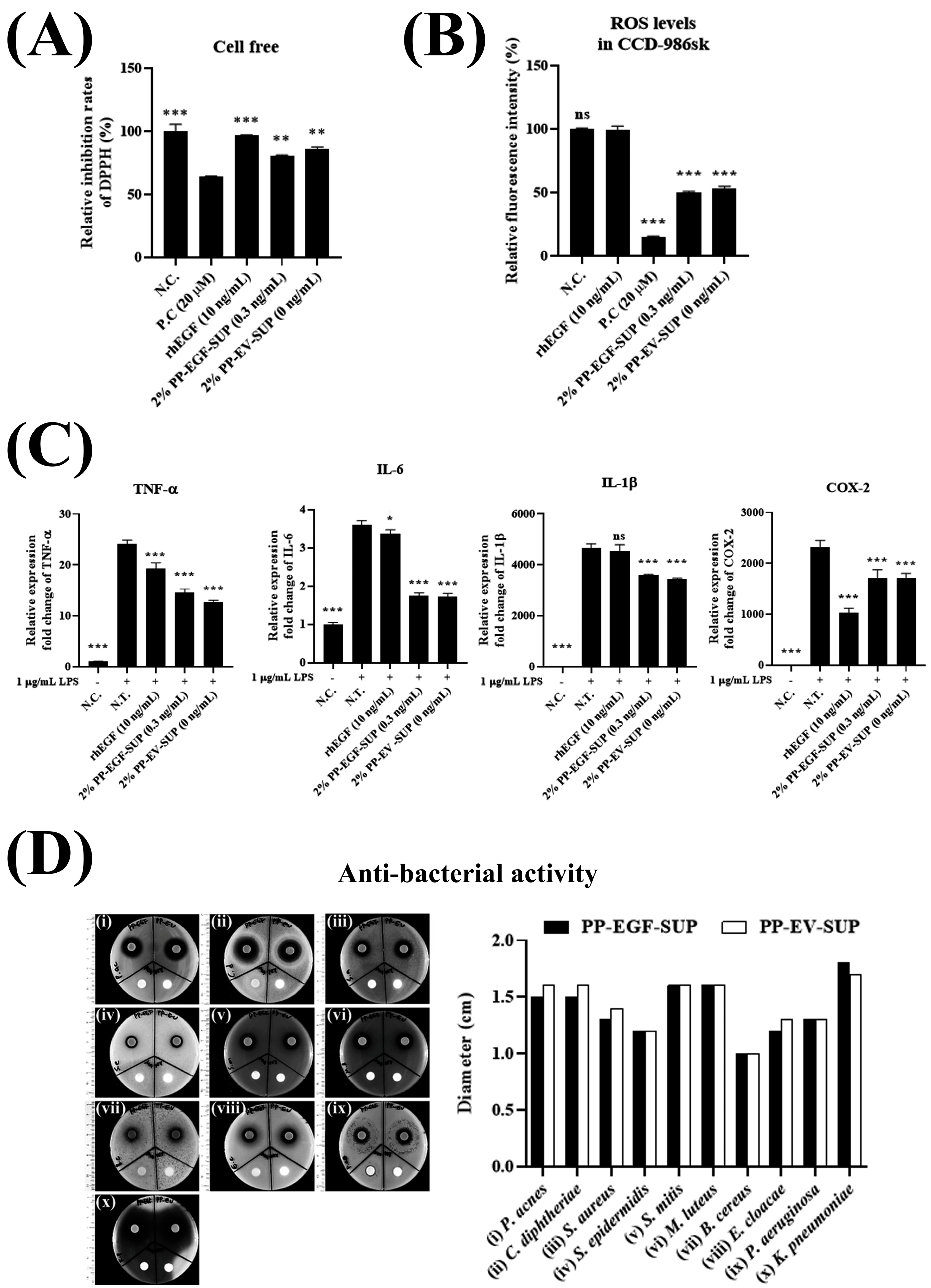
2.5.5. Antioxidant Activity Tests (DPPH and ROS Scavenging Assays)
2.5.6. Quantitative Real-Time PCR (Q-RT-PCR)
2.5.7. Evaluation of Anti-Bacterial Activity Using Disk Diffusion Method
2.6. Powdering of PP-EGF Culture Supernatant as a Cosmetic Ingredient
2.7. Stability Tests of PP-EGF
2.7.1. Thermal Stability
2.7.2. pH Stability
2.8. Statistical Analysis
2.9. Cosmetic Human Application Test
2.9.1. Applicants
2.9.2. Treatment Protocol
Manufacture of Ampoule Formulation
Ampoule Treatment
2.9.3. Evaluations
2.9.4. Statistical Methods
3. Results
3.1. Development of EGF (PP-EGF) Drug Delivery System Using Probiotics
3.2. Investigation of PP-EGF-SUP-Derived Bioactivities
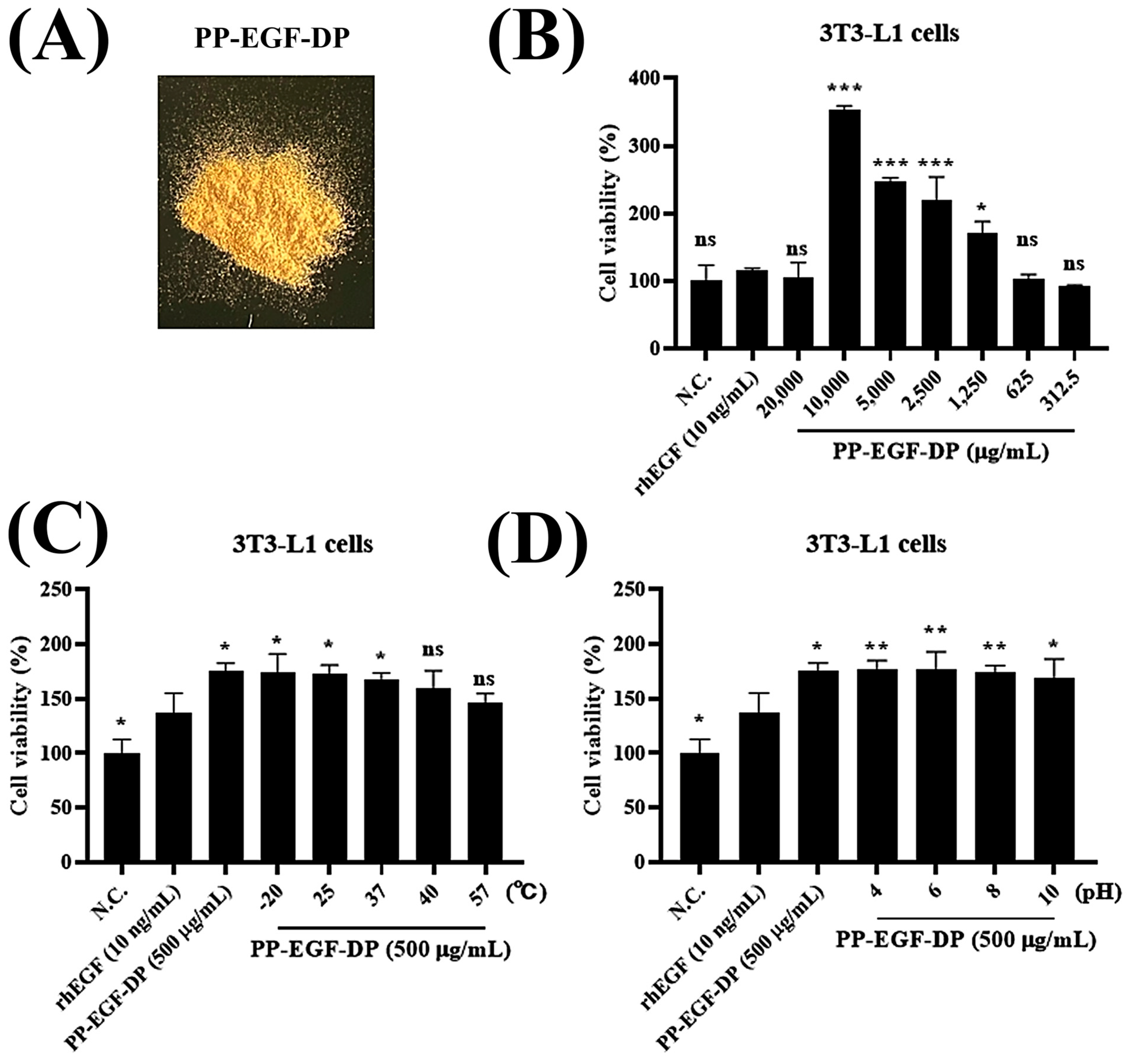
3.2.1. Toxicity Test of PP-EGF-SUP
3.2.2. EGF-Derived Bioactivities: Cell Scratch Wound Healing, Tyrosinase Inhibition, and Skin Barrier Improvement (Elastin Expression Stimulation and Collagenase and Elastase Suppression)
3.2.3. Probiotic-Derived Bioproperties: Antioxidant, Anti-Inflammatory, and Anti-Bacterial Activities
3.2.4. Evaluation of PP-EGF-DP as a Cosmetic Ingredient
3.2.5. Evaluation of Triple-Freckle (Light Freckles, Dark Freckles, and Freckle Area) Relief Using Antera 3D®CS
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Cohen, S. Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the new-born animal. J. Biol. Chem. 1962, 237, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Wenczak, B.A.; Lynch, J.B.; Nanney, L.B. Epidermal growth factor receptor distribution in burn wounds. Implications for growth factor-mediated repair. J. Clin. Investig. 1992, 90, 2392–2401. [Google Scholar] [CrossRef] [PubMed]
- Brake, A.J.; Merryweather, J.P.; Coit, D.G.; Heberlein, U.A.; Masiarz, F.R.; Mullenbach, G.T.; Urdea, M.S.; Valenzuela, P.; Barr, P.J. Alpha-Factor directed synthesis and secretion of mature foreign proteins in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 1984, 81, 4642–4646. [Google Scholar] [CrossRef]
- Hong, J.P.; Jung, H.D.; Kim, Y.W. Recombinant human epidermal growth factor to enhance healing for diabetic foot ulcers. Ann. Plast. Surg. 2006, 56, 394–398. [Google Scholar] [CrossRef]
- Tanaka, A.; Nagate, T.; Matsuda, H. Acceleration of wound healing by gelatin film dressings with epidermal growth factor. J. Vet. Med. Sci. 2005, 67, 909–913. [Google Scholar] [CrossRef]
- Yang, S.; Geng, Z.; Ma, K.; Sun, X.; Fu, X. Efficacy of topical recombinant human epidermal growth factor for treatment of diabetic foot ulcer: A systematic review and meta-analysis. Int. J. Low. Extrem. Wounds 2016, 15, 120–125. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.; Joo, K. Effects of recombinant human epidermal growth factor (rhEGF) on the recovery of skin barrier function after laser resurfacing. Dermatol. Surg. 2020, 46, 252–260. [Google Scholar]
- Noordam, R.; Gunn, D.A.; Tomlin, C.C.; Maier, A.B.; Griffiths, T.; Catt, S.D.; Ogden, S.; Slagboom, P.E.; Westendorp, R.G.; Griffiths, C.E.; et al. Leiden Longevity Study group. Serum insulin-like growth factor 1 and facial ageing: High levels associate with reduced skin wrinkling in a cross-sectional study. Br. J. Dermatol. 2013, 168, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.O.; Garman, K.A.; Lee, Y.G.; Zuo, C.; Beck, P.J.; Tan, M.; Agui-lar-Pimentel, J.A.; Ollert, M.; Schmidt-Weber, C.; Fuchs, H.; et al. The Role of Fibro-blast Growth Factor-Binding Protein 1 in Skin Carcinogenesis and Inflammation. J. Investig. Dermatol. 2018, 138, 179–188. [Google Scholar] [CrossRef]
- Dogan, S.; Demirer, S.; Kepenekci, I.; Erkek, B.; Kiziltay, A.; Hasirci, N.; Müftüoglu, S.; Nazikoglu, A.; Renda, N.; Dincer, U.D.; et al. Epidermal growth factor-containing wound closure enhances wound healing in non-diabetic and diabetic rats. Int. Wound J. 2009, 6, 107–115. [Google Scholar] [CrossRef]
- Krishnamurthy, R.; Manning, M.C. The stability factor: Importance in formulation development. Curr. Pharm. Biotechnol. 2002, 3, 361–371. [Google Scholar] [CrossRef]
- Ogiso, H.; Ishitani, R.; Nureki, O.; Fukai, S.; Yamanaka, M.; Kim, J.H.; Saito, K.; Sakamoto, A.; Inoue, M.; Shirouzu, M.; et al. Crystal structure of the complex of human epidermal growth factor and receptor extracellular domains. Cell 2002, 110, 775–787. [Google Scholar] [CrossRef]
- Choi, S.M.; Lee, K.M.; Kim, H.J.; Park, I.K.; Kang, H.J.; Shin, H.C.; Baek, D.; Choi, Y.; Park, K.H.; Lee, J.W. Effects of structurally stabilized EGF and bFGF on wound healing in type I and type II diabetic mice. Acta Biomater. 2018, 66, 325–334. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.; Huang, Z.; Kou, Y.; Hu, A. Efficacy and safety of nano-silver dressings combined with recombinant human epidermal growth factor for deep second-degree burns: A meta-analysis. Burns 2021, 47, 643–653. [Google Scholar] [CrossRef]
- Koppa Raghu, P.; Bansal, K.K.; Thakor, P.; Bhavana, V.; Madan, J.; Rosenholm, J.M.; Mehra, N.K. Evolution of nanotechnology in delivering drugs to eyes, skin and wounds via topical route. Pharmaceuticals 2020, 13, 167. [Google Scholar] [CrossRef]
- Choi, J.H.; Nam, S.H.; Song, Y.S.; Lee, H.W.; Lee, H.J.; Song, K.; Hong, J.W.; Kim, G.C. Treatment with low-temperature atmospheric pressure plasma enhances cutaneous delivery of epidermal growth factor by regulating E-cadherin-mediated cell junctions. Arch. Dermatol. Res. 2014, 306, 635–643. [Google Scholar] [CrossRef]
- Kim, J.; Jang, J.H.; Lee, J.H.; Choi, J.K.; Park, W.R.; Bae, I.H.; Bae, J.; Park, J.W. Enhanced topical delivery of small hydrophilic or lipophilic active agents and epidermal growth factor by fractional radiofrequency microporation. Pharm. Res. 2012, 29, 2017–2029. [Google Scholar] [CrossRef]
- Berlanga-Acosta, J.; Gavilondo-Cowley, J.; Barco-Herrera, D.G.; Martín-Machado, J.; Guillén-Nieto, G. Epidermal growth factor (EGF) and platelet-derived growth factor (PDGF) as tissue healing agents: Clarifying concerns about their possible role in malignant transformation and tumor progression. J. Carcinogene. Mutagene. 2011, 2, 100–115. [Google Scholar] [CrossRef]
- Shin, S.H.; Koh, Y.G.; Lee, W.G.; Seok, J.; Park, K.Y. The use of epidermal growth factor in dermatological practice. Int. Wound J. 2023, 20, 2414–2423. [Google Scholar] [CrossRef]
- Indira, M.; Venkateswarulu, T.C.; Abraham Peele, K.; Nazneen Bobby, M.; Krupanidhi, S. Bioactive molecules of probiotic bacteria and their mechanism of action: A review. 3 Biotech 2019, 9, 306. [Google Scholar] [CrossRef]
- Kodali, V.P.; Sen, R. Antioxidant and free radical scavenging activities of an exopolysaccharide from a probiotic bacterium. Biotechnol. J. 2008, 3, 245–251. [Google Scholar] [CrossRef]
- An, B.C.; Ryu, Y.; Yoon, Y.S.; Choi, O.; Park, H.J.; Kim, T.Y.; Kim, S.I.; Kim, B.K.; Chung, M.J. Colorectal Cancer Therapy Using a Pediococcus pentosaceus SL4 Drug Delivery System Secreting Lactic Acid Bacteria-Derived Protein p8. Mol. Cells 2019, 42, 755–762. [Google Scholar] [PubMed]
- Grinberg, I.; West, D.V.; Torres, M.; Gou, G.; Stein, D.M.; Wu, L.; Chen, G.; Gallo, E.M.; Akbashev, A.R.; Davies, P.K.; et al. Perovskite oxides for visible-light-absorbing ferroelectric and photovoltaic materials. Nature 2013, 503, 509–512. [Google Scholar] [CrossRef]
- Ngamsurach, P.; Praipipat, P. Antibacterial activities against Staphylococcus aureus and Escherichia coli of extracted Piper betle leaf materials by disc diffusion assay and batch experiments. RSC Adv. 2022, 12, 26435–26454. [Google Scholar] [CrossRef]
- Mamat, U.; Wilke, K.; Bramhill, D.; Schromm, A.B.; Lindner, B.; Kohl, T.A.; Corchero, J.L.; Villaverde, A.; Schaffer, L.; Head, S.R.; et al. Detoxifying Escherichia coli for endotoxin-free production of recombinant proteins. Microb. Cell Fact. 2015, 14, 57. [Google Scholar] [CrossRef]
- Gasaly, N.; de Vos, P.; Hermoso, M.A. Impact of bacterial metabolites on gut barrier function and host immunity: A focus on bacterial metabolism and its relevance for intestinal inflammation. Front. Immunol. 2021, 12, 658354. [Google Scholar] [CrossRef] [PubMed]
- Gillbro, J.M.; Olsson, M.J. The melanogenesis and mechanisms of skin-lightening agents--existing and new approaches. Int. J. Cosmet. Sci. 2011, 33, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-inflammatory and immunomodulatory effects of probiotics in gut inflammation: A door to the body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef]
- Fijan, S. Probiotics and Their Antimicrobial Effect. Microorganisms 2023, 11, 528. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Nanba, D.; Hata, M.; Higashiyama, S. The EGFR ligands and their signaling networks in epithelial cell biology and cancer: Roles in skin homeostasis and therapeutic implications. Cancer Sci. 2013, 104, 419–426. [Google Scholar]
- Sriwidodo, S.; Maksum, I.P.; Subroto, T.; Wathoni, N.; Subarnas, A.; Umar, A.K. Activity and effectiveness of recombinant rhEGF excreted by Escherichia coli BL21 on wound healing in induced diabetic mice. J. Exp. Pharmacol. 2020, 12, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Berlanga-Acosta, J.; Gavilondo-Cowley, J.; López-Saura, P.; González-López, T.; Castro-Santana, M.D.; López-Mola, E.; Guillén-Nieto, G.; Herrera-Martinez, L. Epidermal growth factor in clinical practice—A review of its biological actions, clinical indications and safety implications. Int. Wound J. 2009, 6, 331–346. [Google Scholar] [CrossRef]
- Indriyani, A.; Indrayati, N.; Sriwidodo, S.; Maksum, I. Optimization extracellular secretion of recombinant human epidermal growth factor (rhEGF) in Escherichia coli BL21 (DE3) pD881-OmpA-rhEGF by using response surface method (RSM). Int. J. Res. Pharm. Sci. 2019, 10, 1824–1831. [Google Scholar] [CrossRef]
- Shen, W.R.; Liew, M.W.O.; Ong, E. Production of recombinant human epidermal growth factor in Escherichia coli: Strategic upstream and downstream considerations for high protein yield. Process Biochem. 2024, 146, 81–96. [Google Scholar]
- de Groot, N.S.; Espargarö, A.; Morell, M.; Ventura, S. Studies on bacterial inclusion bodies. Future Microbiol. 2008, 3, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Wakelin, S.J.; Sabroe, I.; Gregory, C.D.; Poxton, I.R.; Forsythe, J.L.; Garden, O.J.; Howie, S.E. “Dirty little secrets”—Endotoxin contamination of recombinant proteins. Immunol. Lett. 2006, 106, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Eissazadeh, S.; Moeini, H.; Dezfouli, M.G.; Heidary, S.; Nelofer, R.; Abdullah, M.P. Production of recombinant human epidermal growth factor in Pichia pastoris. Braz. J. Microbiol. 2017, 48, 286–293. [Google Scholar] [CrossRef]
- George-Nascimento, C.; Gyenes, A.; Halloran, S.M.; Merryweather, J.; Valenzuela, P.; Steimer, K.S.; Masiarz, F.R.; Randolph, A. Characterization of recombinant human epidermal growth factor produced in yeast. Biochemistry 1988, 27, 797–802. [Google Scholar] [CrossRef]
- He, Y.; Schmidt, M.A.; Erwin, C.; Guo, J.; Sun, R.; Pendarvis, K.; Warner, B.W.; Herman, E.M. Transgenic soybean production of bioactive human epidermal growth factor (EGF). PLoS ONE 2016, 11, e0157034. [Google Scholar] [CrossRef]
- Negahdari, B.; Shahosseini, Z.; Baniasadi, V. Production of human epidermal growth factor using adenoviral based system. Res. Pharm. Sci. 2016, 11, 43–48. [Google Scholar] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Probiotic Cosmetic Products Market Forecast, Trend Analysis and Competition Tracking-Global Market Insights 2020 to 2030. Available online: https://www.factmr.com/report/4188/probiotic-cosmetic-products-market (accessed on 20 December 2020).
- Park, G.H.; do Rhee, Y.; Moon, H.R.; Won, C.H.; Lee, M.W.; Choi, J.H.; Moon, K.C.; Chang, S.E. Effect of an epidermal growth factor-containing cream on postinflammatory hyperpigmentation after Q-switched 532-nm neodymium-doped yttrium aluminum garnet laser treatment. Dermatol. Surg. 2015, 41, 131–135. [Google Scholar] [CrossRef]
- Yun, W.J.; Bang, S.H.; Min, K.H.; Kim, S.W.; Lee, M.W.; Chang, S.E. Epidermal growth factor and epidermal growth factor signaling attenuate laser-induced melanogenesis. Dermatol. Surg. 2013, 39, 1903–1911. [Google Scholar] [CrossRef]
- Tzaphlidou, M. The role of collagen and elastin in aged skin: An image processing approach. Micron 2004, 35, 173–177. [Google Scholar] [CrossRef] [PubMed]
- De Marco, S.; Sichetti, M.; Muradyan, D.; Piccioni, M.; Traina, G.; Pagiotti, R.; Pietrella, D. Probiotic cell-free supernatants exhibited anti-inflammatory and antioxidant activity on human gut epithelial cells and macrophages stimulated with LPS. Evid. Based Complement. Alternat. Med. 2018, 2018, 1756308. [Google Scholar] [CrossRef]
- Lescheid, D.W. Probiotics as regulators of inflammation: A review. Funct. Foods Health Dis. 2014, 4, 299–311. [Google Scholar] [CrossRef]
- Noordiana, N. Antibacterial agents produced by lactic acid bacteria isolated from Threadfin Salmon and Grass Shrimp. Int. Food Res. J. 2013, 20, 117–124. [Google Scholar]
- Drumond, M.M.; Tapia-Costa, A.P.; Neumann, E.; Nunes, Á.C.; Barbosa, J.W.; Kassuha, D.E.; Mancha-Agresti, P. Cell-free supernatant of probiotic bacteria exerted antibiofilm and antibacterial activities against Pseudomonas aeruginosa: A novel biotic therapy. Front. Pharmacol. 2023, 14, 1152588. [Google Scholar] [CrossRef]
- Shon, A.S.; Bajwa, R.P.S.; Russo, T.A. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: A new and dangerous breed. Virulence 2013, 4, 107–118. [Google Scholar] [CrossRef]
- Seo, J.G.; Park, J.C.; Ahn, H.D.; Lee, S.Y.; Kim, J.; Park, Y.S.; Cho, Y.K. Klebsiella pneumoniae multiorgan infection not accompanied by liver abscess: Report of 2 cases. Infect. Chemother. 2008, 40, 346–349. [Google Scholar] [CrossRef]
- Xu, J.; Chen, X.; Song, J.; Wang, C.; Xu, W.; Tan, H.; Suo, H. Antibacterial activity and mechanism of cell-free supernatants of Lacticaseibacillus paracasei against Propionibacterium acnes. Microb. Pathog. 2024, 189, 106598. [Google Scholar] [CrossRef] [PubMed]
- Hadfield, T.L.; McEvoy, P.; Polotsky, Y.; Tzinserling, V.A.; Yakovlev, A.A. The pathology of diphtheria. J. Infect. Dis. 2000, 181 (Suppl. 1), S116–S120. [Google Scholar] [CrossRef] [PubMed]
| Sample | EGF Concentration (μg/L) |
|---|---|
| PP-EV-SUP | 0 |
| PP-EGF-SUP | 14.99 ± 0.044 |
| Ampoules | Time Points | Light Freckles (%) | Change Rate (%) | Dark Freckles (%) | Change Rate (%) | Freckle Area (%) | Change Rate (%) | |
|---|---|---|---|---|---|---|---|---|
| 1% | Placebo | Before | 0.474 ± 0.042 | 0.84 | 0.777 ± 0.076 | 0.56 | 302.88 ± 78.61 | 2.79 |
| After 4 Wks | 0.478 ± 0.049 | 0.780 ± 0.07 | 286.05 ± 88.77 | |||||
| PP-EGF-DP | Before | 0.478 ± 0.037 | 3.38 | 0.779 ± 0.066 | 1.74 | 320.97 ± 77.8 | 21.21 | |
| After 4 Wks | 0.462 ± 0.04 | 0.76 ± 0.075 | 251.9 ± 99.06 | |||||
| 5% | Placebo | Before | 0.474 ± 0.053 | 0.08 | 0.731 ± 0.183 | 0.55 | 302.38 ± 87.33 | 5.93 |
| After 4 Wks | 0.474 ± 0.051 | 0.726 ± 0.179 | 274.68 ± 108.82 | |||||
| PP-EGF-DP | Before | 0.475 ± 0.057 | 3.79 | 0.745 ± 0.064 | 2.93 | 318.33 ± 97.68 | 29.10 | |
| After 4 Wks | 0.458 ± 0.059 | 0.723 ± 0.061 | 225.07 ± 94.77 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, J.Y.; Kim, S.; Ha, J.; Roh, Y.J.; Ryu, Y.; Chung, M.J.; Park, K.Y.; An, B.C. Cosmetic Upgrade of EGF: Genetically Modified Probiotic-Derived Cell-Free Supernatants Containing Human EGF Protein Exhibit Diverse Biological Activities. Cosmetics 2025, 12, 176. https://doi.org/10.3390/cosmetics12040176
Ahn JY, Kim S, Ha J, Roh YJ, Ryu Y, Chung MJ, Park KY, An BC. Cosmetic Upgrade of EGF: Genetically Modified Probiotic-Derived Cell-Free Supernatants Containing Human EGF Protein Exhibit Diverse Biological Activities. Cosmetics. 2025; 12(4):176. https://doi.org/10.3390/cosmetics12040176
Chicago/Turabian StyleAhn, Jun Young, Seungwoo Kim, Jaewon Ha, Yoon Jin Roh, Yongku Ryu, Myung Jun Chung, Kui Young Park, and Byung Chull An. 2025. "Cosmetic Upgrade of EGF: Genetically Modified Probiotic-Derived Cell-Free Supernatants Containing Human EGF Protein Exhibit Diverse Biological Activities" Cosmetics 12, no. 4: 176. https://doi.org/10.3390/cosmetics12040176
APA StyleAhn, J. Y., Kim, S., Ha, J., Roh, Y. J., Ryu, Y., Chung, M. J., Park, K. Y., & An, B. C. (2025). Cosmetic Upgrade of EGF: Genetically Modified Probiotic-Derived Cell-Free Supernatants Containing Human EGF Protein Exhibit Diverse Biological Activities. Cosmetics, 12(4), 176. https://doi.org/10.3390/cosmetics12040176






