1. Introduction
Cellulite is a dermatological condition that primarily affects post-pubertal females [
1,
2,
3]. It is medically also referred to as Edematous Fibrosclerotic Panniculopathy (EFP), gynoid lipodystrophy, nodular liposclerosis, adiposis edematosa, dermopanniculosis deformans, and status protrusus cutis, each term reflecting different aspects of its pathophysiology [
3,
4]. Cellulite is a localised disorder of the subcutaneous tissue characterised by inflammatory, metabolic, and morphological alterations [
5,
6,
7,
8]. It manifests as visible skin textural alterations, characterised by cutaneous dimpling, surface irregularities, and dermal atrophy, producing the characteristic “orange peel” or “cottage cheese” appearance [
3,
9].
Cellulite severity is typically assessed using standardised clinical grading systems based on the visual and tactile appearance of the affected skin. Among these, the most widely adopted is the Nürnberger–Müller scale [
10], which classifies cellulite into four grades: Grade 0: Skin is smooth in both lying-down and standing positions; Grade I: Skin is smooth at rest but shows a mattress-like appearance on pinching; Grade II: Skin is smooth at rest but has a mattress-like appearance on standing; Grade III: Skin has a mattress-like appearance in both lying-down and standing positions. Grades II and III can be subdivided into mild, moderate, or severe. This scale provides a simple and reproducible framework for evaluating baseline severity and monitoring treatment-related improvements over time. Other scales (e.g.,: Curri Scale; DiBernardo Scoring System; Cellulite Severity Scale) have been used to evaluate cellulite, but Nürnberger–Müller remains the most used in the clinical practice.
1.1. Etiology and Pathophysiology
The exact etiopathogenesis of cellulite remains unclear, though it is recognised as a multifactorial condition involving anatomical, vascular, hormonal, inflammatory, and genetic factors, and may also be influenced by lifestyle and low levels of physical activity [
9,
11,
12,
13,
14,
15].
According to the architectural disorder hypothesis, cellulite results from imbalances in biomechanical forces within fibrous septa, adipose layers, and the dermis. Fibrous septa exert inward traction, while adipose tissue applies outward pressure, and the dermis provides containment. Short, unstable septa connecting the superficial fascia to the dermis fail to restrain superficial adipose lobules in cases of increased body mass index (BMI), leading to depressions where thickened septa anchor to the dermis [
3,
11,
16]. The enlargement of superficial adipose lobules and increased adipose layer thickness exacerbate these imbalances, resulting in pronounced dimpling and potential fat herniation through the long septa connecting the deep fascia to the dermis [
3,
11,
16]. Additionally, according to Piérard et al. [
17], genetic predisposition affects the extent of fascial bands and the vertical stretching of collagen septa contributes to connective tissue weakening and fat protrusion.
Sexually dimorphic skin architecture, influenced by reproductive hormones (e.g., estrogens), also plays a crucial role. Microimaging magnetic resonance imaging (MRI) and anatomical studies indicate that, in women, connective tissue septa run perpendicularly to the skin, facilitating fat herniation, whereas in men, septa are arranged at a 45° angle, forming a cross-linked pattern that inhibits fat protrusion [
2,
9,
18]. Male septa are also stronger and more stable, while women have fewer but larger and taller adipose lobules [
3,
19]. These differences, more pronounced in the superficial adipose layer, contribute to the lower prevalence of cellulite in men, even in cases of obesity [
3].
Vascular and inflammatory components have also been implicated. Vascular dysfunction may involve alterations in precapillary arterial sphincters, leading to increased capillary permeability, fluid retention, and fibrotic septal sclerosis, exacerbating cellulite’s characteristic irregularity [
3,
18,
20,
21]. Additionally, chronic low-grade inflammation may contribute to dermal atrophy and endothelial damage, further promoting fibrotic septa formation [
3,
13,
22]. Lifestyle factors, including diet, may also influence cellulite severity.
Cellulite primarily affects the buttocks, thighs, and hips [
18,
23,
24] but can also be found on the breasts, lower abdomen, arms, and nape [
18,
23]. It is classified into four severity stages, ranging from visible alterations upon skin pinching to persistent irregularities at rest, which may be associated with pain and tissue retraction [
16,
25].
1.2. Epidemiology
Although precise epidemiological data are lacking, cellulite affects up to 90% of postpubertal females [
4,
9,
26,
27,
28], with higher prevalence among Caucasian compared to Asian women [
15]. While independent of body weight [
8], cellulite severity correlates with increased body mass index (BMI; [
16,
22]). The typical onset between ages 20 and 30 suggests a hormonal etiology [
22]. Aging-associated adipose lobule hypertrophy may exacerbate biomechanical imbalances, increasing cellulite prevalence in older women, particularly those who are overweight. Male cases are rare and usually linked to androgen deficiency disorders such as Klinefelter syndrome or estrogen therapy for prostate cancer [
4,
9,
27].
1.3. Treatment Approaches
Cellulite is often perceived as an aesthetic concern, impacting self-esteem, psychosocial well-being, and quality of life [
3,
6,
29,
30]. Due to its complex etiology, no definitive treatment exists. Available therapies include topical agents, oral supplements, mechanical treatments, energy-based therapies, injectable treatments, and subcision [
3,
22].
1.4. Topical and Systemic Agents
Non-invasive treatments with biologically active substances involve topical formulations containing methylxanthines (e.g., caffeine, aminophylline, theophylline), retinoids [
11], and botanical extracts. Methylxanthines promote lipolysis and inhibit phosphodiesterase, reducing adipocyte volume and improving skin appearance [
11,
21]. Retinoids enhance cellular turnover, angiogenesis, and fibroblast activity while preventing preadipocyte differentiation [
11,
31]. Botanical extracts, such as forskolin, sacred lotus, carnitine, escin,
Ginkgo biloba,
Centella asiatica, and
Ruscus aculeatus, enhance skin elasticity, but their effects are temporary [
32].
Escin is often combined with hormone-based ingredients like L-thyroxine, a thyroid hormone. Escin enhances microvascular flow, while L-thyroxine stimulates localised lipolysis, improving blood and oxygen supply, skin elasticity, and tone. Studies suggest that topical L-thyroxine does not affect serum levels of T
3 and T
4, as it is converted into inactive rT3 in the dermis before entering circulation. This minimises systemic side effects and confines action to the application site, targeting cellulite reduction. However, rare cases of thyroid dysfunction symptoms have been reported. Excessive absorption of levothyroxine through the skin may trigger latent hyperthyroidism, leading to symptoms like insomnia, agitation, tachycardia, and anxiety (see [
33,
34,
35]). Due to its mechanism of action, this treatment is not recommended for individuals with thyroid disorders, cardiovascular issues, pregnancy or breastfeeding, hormonal and metabolic disorders, renal or hepatic insufficiency, or sensitive skin conditions such as dermatitis.
Overall, topical treatments provide only temporary aesthetic improvements with minimal impact on adipose and connective tissue structures [
11]. Given the lack of robust clinical data on efficacy and duration, no topical formulation for cellulite treatment has been approved by the U.S. Food and Drug Administration (FDA) [
3].
Various oral supplements with antioxidant properties are used for cellulite treatment [
26]. However, no solid studies confirm their efficacy, and they are not FDA-approved.
1.5. Mechanical Therapies and Energy Based Treatments
Mechanical therapies are among the oldest methods to treat cellulite. Massages can be manual or device assisted. These include manual massage, lymphatic drainage, Louis Paul Guitay (LPG) Endermologie, and pressotherapy. This approach stimulates local circulation and lymphatic drainage, enhancing tissue oxygenation and reducing fluid retention damage. These treatments are well tolerated and provide a temporary improvement in skin appearance. However, the lack of long-term effects makes this approach non-definitive [
21,
22,
36,
37].
Energy-based therapies, such as radiofrequency and lasers, aim to enhance circulation, collagen remodeling, neocollagenesis, and lipolysis. Radiofrequency (RF) devices deliver thermal energy, increasing the temperature in the targeted area to induce collagen denaturation, remodeling, and neocollagenesis, thereby improving skin appearance [
3,
9,
11]. Different RF devices exist, and their effects depend on their mode of action. Temperature-controlled devices reach deeper adipose tissue, enhancing lipolysis and improving treatment effectiveness and duration. Laser devices also deliver thermal energy with similar effects, with penetration depth depending on wavelength. These treatments are generally well tolerated and can reduce cellulite severity within 2 to 6 weeks [
38,
39]. Shockwave therapy is another option, using acoustic waves to penetrate the subcutaneous tissue, improving microcirculation, neocollagenesis, and lymphatic drainage.
Overall, energy-based therapies are well tolerated with minimal adverse effects, such as edema, but their long-term efficacy remains uncertain [
9,
11,
40,
41]. Furthermore, these treatments require specialised devices and must be performed in dedicated facilities. Additionally, multiple sessions are often necessary to achieve satisfactory results [
3], leading to costs that may not be affordable for all patients.
1.6. Injectable Treatments
Minimally invasive procedures, including dermal fillers and collagenase injections, have shown promising results. Collagenase enzymes hydrolyse collagen in fibrotic septa involved in cellulite formation [
42,
43]. This treatment is generally well tolerated, with bruising and injection site pain as the most common side effects, along with occasional prolonged skin discoloration [
44]. Injectable fillers provide volumising and biostimulatory effects. For instance, diluted calcium hydroxyapatite stimulates neocollagenesis and elastogenesis, enhancing dermal thickness, elasticity, and skin flexibility with good tolerance [
45]. Treatments with active substances include carboxytherapy, where CO
2 is injected subcutaneously to improve circulation and stimulate lipolysis. While beneficial for cellulite and localised fat deposits, it is contraindicated in pregnancy, chronic respiratory failure, and hepatic or renal insufficiency [
46,
47]. Mesotherapy involves injecting lipolytic substances (e.g., caffeine, aminophylline, theophylline) into the subcutaneous tissue [
15]. However, clinical studies are limited, and no FDA-approved treatments are available. Overall, large-scale studies are needed to assess long-term outcomes and potential adverse effects.
1.7. Subcision
Invasive treatments without active substances include manual subcision, a procedure that involves severing the fibrotic septa between the dermis and subcutaneous fibrous tissue. This facilitates the redistribution of biomechanical forces within the subcutaneous layer, reducing the protrusion of fat and thus limiting the signs of cellulite. Subcision can be performed not only manually but also with vacuum assistance, laser assistance, or through injections of collagenase (see injectable treatments), or acoustically using sound waves [
12,
41,
48,
49].
While effective, it carries risks such as oedema, pain, and bruising [
11,
50]. Moreover, the duration of the effects has not yet been fully clarified. Other options include ultrasonic liposculpture and autologous fat transplantation with Nd-YAG laser application [
51,
52].
1.8. Role of Natural Components and Cool-Warming Treatment for Cellulitis
Other than the invasive and non-invasive treatments reported above, natural components might have beneficial effects on the treatment of cellulitis, despite being less investigated. Specifically,
Curcuma longa (turmeric) offers anti-inflammatory and antioxidant properties, which may help mitigate local inflammatory processes implicated in the pathophysiology of cellulite [
53].
Eugenia caryophyllus (clove extract) and
Zingiber officinale (ginger) are both rich in phenolic compounds that support microcirculatory function and exhibit vasodilatory effects, enhancing blood flow in the affected dermal and subcutaneous tissues [
54,
55].
Spirulina platensis, a cyanobacterium rich in vitamins, minerals, and phycocyanins, contributes to antioxidative protection and may improve tissue oxygenation and metabolic activity [
56].
Lonicera japonica, traditionally used in Asian medicine, provides further anti-inflammatory action and supports the maintenance of extracellular matrix integrity [
57]. Genistein, a soy-derived isoflavone, has been shown to inhibit enzymes involved in collagen degradation and promote dermal remodelling by stimulating the synthesis of extracellular matrix components, thus potentially improving skin tone and elasticity. Caffeine, widely studied for its lipolytic properties, enhances the mobilisation of fatty acids by inhibiting phosphodiesterase and increasing intracellular cyclic AMP, leading to the breakdown of triglycerides in adipocytes [
58]. Carnitine acts as a cofactor in the mitochondrial β-oxidation of fatty acids, potentially improving local lipid metabolism and contributing to a reduction in adipose tissue volume. These active ingredients, if suspended in a gel matrix, might elicit a dual thermal sensation. This thermogenic response is designed to stimulate cutaneous microcirculation and promote lymphatic drainage, thereby facilitating the removal of metabolic waste and interstitial fluid. The resulting effects may contribute to a smoother skin appearance and a reduction in the cutaneous dimpling, surface irregularities, and dermal atrophy.
Despite the widespread availability of topical and device-based solutions, there is a lack of independent, in vivo clinical studies evaluating the real-world efficacy of over-the-counter thermogenic formulations enriched with natural bioactive compounds for the cosmetic improvement of moderate to severe cellulite. This study seeks to fill this gap by providing objective and patient-reported outcomes under controlled application protocols. The aesthetic and psychological burden of cellulite has led to a growing demand for effective and accessible treatment options. Existing therapies, however, present notable limitations: topical products frequently yield transient results with limited scientific validation; invasive procedures such as subcision or collagenase injections, though effective, require medical supervision and are associated with adverse effects including pain, bruising, and prolonged recovery time; and energy-based technologies (e.g., radiofrequency, laser) entail high costs, specialised equipment, and repeated sessions. In this regard, the present single-centre, prospective clinical study was designed to investigate the potential of a non-pharmacological, over-the-counter topical formulation, a safer, more convenient, and cost-effective alternative for the treatment of moderate to severe cellulite (Grades II–III on the Nürnberger–Müller scale). The product under evaluation is a thermogenic cream-gel enriched with botanical and bioactive compounds and designed to stimulate local microcirculation and lymphatic drainage through a dual thermal (cooling–warming) action. The primary aim of the study is to assess the efficacy of the topical formulation in reducing the clinical signs of cellulite. Secondary aims include evaluating improvements in skin texture and the reduction of visible surface irregularities such as the ‘orange peel’ appearance and localised hyperthermic zones. The study also seeks to determine whether this non-invasive approach could represent a viable and well-tolerated alternative to existing cellulite treatments with fewer systemic or procedural risks.
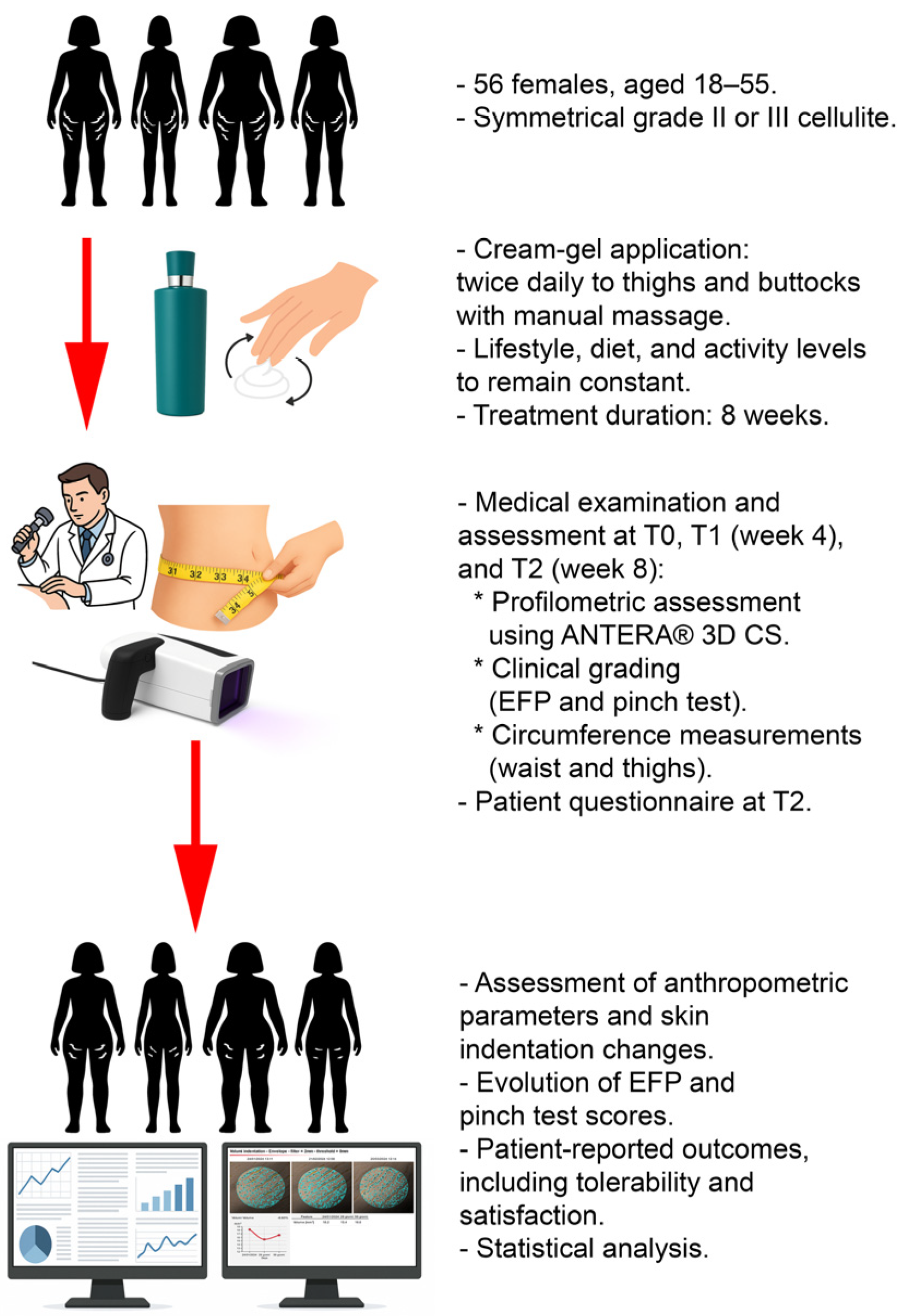
2. Materials and Methods
The study design was approved by the local Territorial Ethics Committee (Ethical approval: CET 99-2023) and registered with a recognised clinical trials platform (ClinicalTrials.gov ID: NCT06348615).
The study protocol was designed internally by the investigators, in accordance with current literature and standard clinical practice for cellulite evaluation. While no unified international guideline exists for testing the efficacy of non-pharmacological topical products in cellulite, validated tools were used, including the Nürnberger–Müller scale and instrumental profilometry (ANTERA® 3D CS imaging system, Miravex Limited, 11 St Stephen’s Green, Dublin 2, Dublin, Ireland), along with structured patient-reported outcomes.
2.1. Study Product Specifications and Application Protocol
The topical cream-gel used in this study, named ‘Defence My Body’ (BioNike, ICIM International, Milan, Italy), is a commercially available, over-the-counter medical device intended to improve the appearance of cellulite and skin surface irregularities. The formulation is intended to invoke a dual thermal action (cooling and warming), which purportedly stimulates capillary microcirculation and promotes the drainage of lymphatic fluids and metabolic wastes in subcutaneous tissue.
It is important to note that while no topical product for cellulite management has received FDA approval as a drug or medical treatment, in the European Union, such formulations may fall under the scope of Regulation (EU) 2017/745 on medical devices, provided their principal mode of action is physical rather than pharmacological. The formulation evaluated in this study is classified as a Class IIa medical device in the EU and is undergoing certification under Regulation (EU) 2017/745 (MDR).
According to the manufacturer’s specifications, the formulation includes the following key ingredients: Curcuma longa extract, Eugenia caryophyllus (Clove) extract, Zingiber officinale (Ginger) extract, Spirulina platensis extract, Lonicera japonica (Honeysuckle) extract, genistein, caffeine, and carnitine. These are incorporated into a cream-gel formulation system containing gel-forming polymers, glycols, glycerin, emollients, menthol, stabilising agents, and deionised and filtered water. The formulation is free of preservatives, gluten, and iodine, and contains trace levels of nickel (<0.00001%).
The formulation was stored at a controlled room temperature, not exceeding 30 °C, and protected from direct sunlight and extreme temperatures. Participants were provided with individual sachets to prevent contamination and ensure consistent dosage and application.
Participants were instructed to apply the topical cream-gel twice daily, in the morning and evening, specifically targeting areas commonly afflicted by cellulite, namely the thighs and buttocks, over a total duration of eight weeks. The application involved massaging the product into the skin using circular motions in a bottom-to-top direction to promote lymphatic drainage and enhance microcirculatory stimulation.
To maintain consistency in the study’s conditions, participants were required to keep their lifestyle, diet, and physical activity levels unchanged from the period prior to their recruitment.
Each application session was followed by thorough hand washing to prevent the product from coming into contact with sensitive areas, such as eyes and mucous membranes.
Medical check-ups were scheduled at baseline (T0), after 4 weeks (T1), and at the end of the 8-weeks clinical evaluation (T2). A preliminary skin sensitivity test was performed on a small area of skin before commencing the regular application to ensure compatibility and minimise the risk of adverse reactions.
2.2. Patient Recruitment and Assessment Methods
The study was designed as a single-arm trial due to the organoleptic properties of the topical product, which impart a distinctive scent, making it difficult to mask a placebo formulation for a potential control group.
The survey involved a panel of 56 female subjects with 2nd and 3rd degree cellulite on their lower limbs. Participants were selected based on specific inclusion and exclusion criteria essential for the integrity of the clinical outcomes. Women aged between 18 and 55 years with symmetrical grade II or III cellulite were included. Further details on the criteria for selection are summarised in
Table 1.
Recruitment was conducted through advertisements in clinics, ensuring diverse demographic representation. Each potential participant underwent a preliminary screening based on the above-mentioned criteria to confirm eligibility before enrolment in the study.
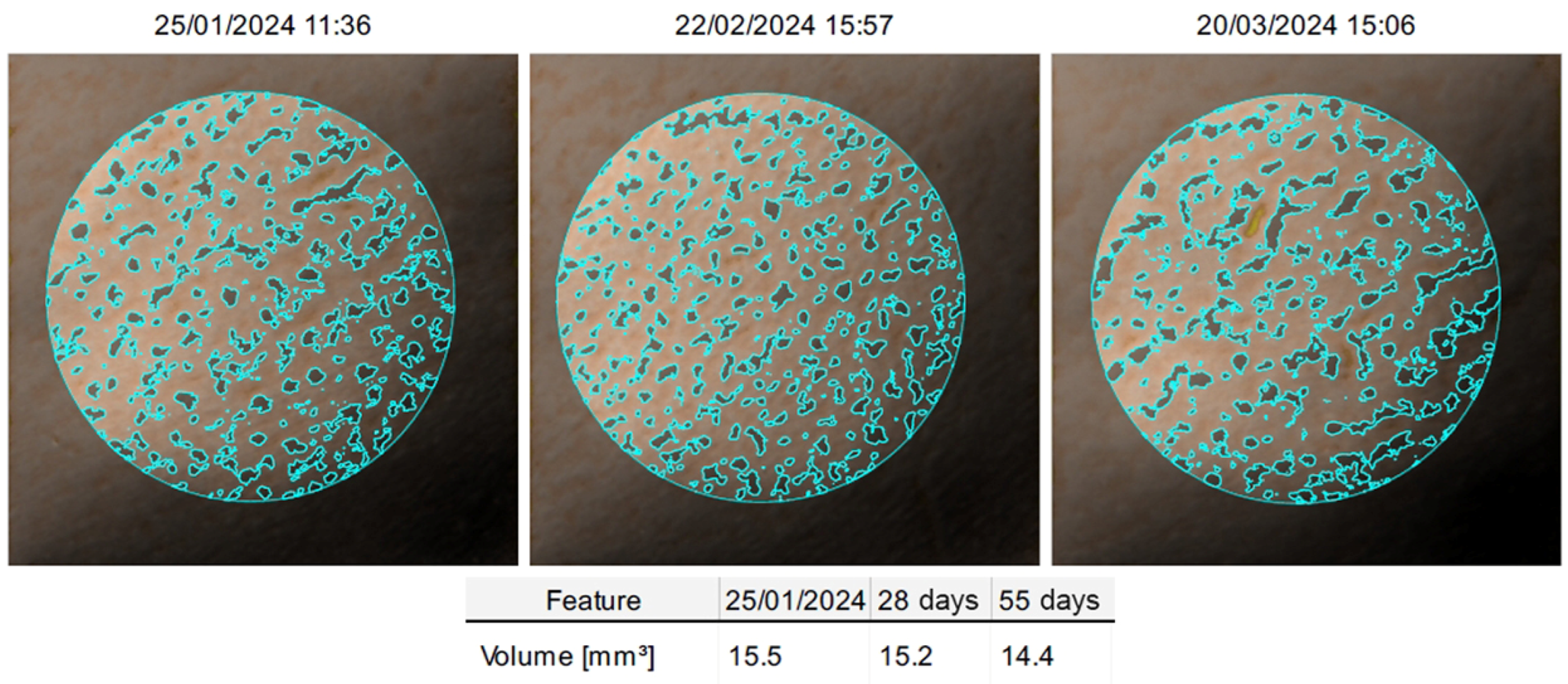
The primary variable was the change in skin profilometry measurements, assessed using the ANTERA
® 3D CS camera system (Miravex Ltd., Dublin, Ireland). This computerised analysis allowed for precise quantification of skin texture changes, including the reduction of cellulite appearance (see [
59,
60]).
Measurements were taken from four target anatomical sites: the left and right gluteus and the left and right thigh. Data were collected at baseline (T0), after four weeks (T1), and after eight weeks (T2). Skin indentation volumes were expressed in mm3. Complementary analyses were conducted by aggregating values from both gluteal areas (SMGL), both thigh areas (SMCO), and the sum of all four measurement areas (SM4).
Secondary variables included clinical evaluations of cellulite severity performed by a dermatologist using a categorical severity scale (EFP values ranging from 1 to 4), assessed at T0, T1, and T2. Additionally, severity assessments using the “pinch test” manoeuvre (PINCH values, ranging from 1 to 4) were conducted at the same time points. Circumference measurements of the thigh and waistline were recorded in centimetres. Thigh circumference was measured 1 cm below the gluteal crease, while waist circumference was measured at a consistent anatomical site.
At the final visit (T2), participants completed a structured questionnaire to assess subjective perceptions of the product’s efficacy. They rated improvements in the orange peel appearance, cellulite reduction, draining effect, skin elasticity, and smoothness on a 10-point scale (1 = no improvement, 10 = excellent improvement). Additional binary (yes/no) questions evaluated the product’s comfort upon skin contact, fragrance pleasantness, and ease of application. Overall satisfaction was rated on a five-point Likert scale (1 = very satisfied, 5 = very dissatisfied). Responses were recorded in patient diaries and analysed descriptively.
2.3. Statistical Analysis
To evaluate the efficacy of the product, we employed a comprehensive statistical approach under the guidance of ICIM International. The analysis encompassed both the Full Analysis Set (FAS), including all randomised participants who received at least one dose of the study product and had at least one post-baseline efficacy measurement, and the Per Protocol (PP) population, which included participants who completed the study without any major protocol deviations.
Primary efficacy analyses involved paired t-tests to compare ANTERA® values between T0 and T1, T0 and T2, and T1 and T2. Cumulative values (SMGL, SMCO, SM4) were also analysed using the same model. Descriptive statistics, including means, medians, standard deviations, minimum and maximum values, and quartiles, were calculated. Frequency distributions were summarised and represented using histograms.
For secondary outcomes involving ordinal data, such as EFP and PINCH values, the Friedman test was used to detect differences across multiple time points within the same subjects. Additionally, Cochran’s Q test was employed for binary transformations of these variables, assessing changes in the proportion of higher severity scores over time.
The Last Observation Carried Forward (LOCF) approach was applied for handling missing data, using the last observed value as the endpoint value when necessary. All adverse events (AEs) were categorised and tabulated, with severity and causality assessed according to MedDRA (Medical Dictionary for Regulatory Activities) standards. Both unsolicited and solicited AEs were considered.
All statistical tests were two-tailed, and a significance level of p < 0.05 was applied. Analyses were performed using Graph Pad Prism software (Version 9.0, Graph Pad Inc., San Diego, CA, USA).
The overall study design is summarised in
Figure 1.
3. Results
A total of 56 female participants were enrolled in the study between January and April 2024, with 53 of them receiving the study product. Among these, 51 participants constituted the FAS, while 45 participants completed the study without major protocol deviations, forming the PP population. Baseline characteristics (
Table 2) indicated a mean age of 42.47 years and a mean BMI of 22.41, with these values remaining relatively stable throughout the study period.
Significant reductions in skin indentation volumes were observed across the primary measurement sites. The left gluteus showed a significant reduction from a mean volume of 19.64 mm
3 at baseline to 17.18 mm
3 at eight weeks (
p < 0.001), while the right gluteus reduced from 19.54 mm
3 to 16.53 mm
3 over the same period (
p < 0.001;
Figure 2). Similar reductions were observed in the left and right thighs, with volumes decreasing from 17.58 mm
3 to 15.41 mm
3 (
p = 0.002) and from 17.75 mm
3 to 16.07 mm
3 (
p = 0.014), respectively. Cumulative measurements reflected these trends, with the sum of the four measurement areas (SM4) significantly decreasing from 74.50 mm
3 at baseline to 65.19 mm
3 at eight weeks (
p < 0.001) (
Table 3).
Clinical evaluations of cellulite severity, assessed through the EFP scale, demonstrated significant improvements over time. At baseline, 26 participants were classified as severity level 2 and 25 as level 3. By week 4, 23 participants remained in level 2 and 27 in level 3. At the end of the clinical evaluation (T2), 10 participants had improved to level 1, 18 remained at level 2, and 23 were still classified as level 3. No participants were classified as level 4 at any time point. These changes were confirmed by the Friedman test (Statistic = 20.93, p = 2.85 × 10−5) and further supported by the Cochran’s Q test (Statistic = 18.2, p = 1.12 × 10−4).
Similarly, the pinch test revealed progressive improvements. Initially, 26 participants were classified as grade 2 and 25 as grade 3. By week 4, 24 participants remained in grade 2, while 27 were in grade 3. At the end of the clinical evaluation, 10 participants achieved grade 1, 18 were in grade 2, and 23 in grade 3. The Friedman test confirmed significant changes over time (Statistic = 8.64, p = 0.013), while the Cochran’s Q test also indicated significant variation across the study period (Statistic = 19.86, p = 4.88 × 10−5).
Patient-reported outcomes at T2 indicated a generally positive perception of the product’s efficacy. Improvement in the orange peel appearance was rated between 7 and 10 by 53% of participants, while 57% reported similar improvements in overall cellulite appearance. Regarding the draining effect and reduction of swelling, 60% were assigned scores of 7 or higher. Skin elasticity improvement was noted by 70% of participants, and 68% reported enhanced skin smoothness, with most assigning scores of 7 to 10.
The product was well tolerated in terms of sensory experience, with 91% describing it as comfortable upon application, 74% appreciating its fragrance, and 100% finding it easy to apply. Overall satisfaction was high, with 83% of participants expressing satisfaction (36% very satisfied and 47% satisfied), and 17% reporting neutral opinions. No participants expressed dissatisfaction.
Adverse events were minimal, with only two participants discontinuing the study due to mild dermatological reactions, specifically urticaria and itching upon first application. No serious adverse events were reported, and the product was generally well-tolerated by the remaining participants.
4. Discussion
Cellulite represents a complex dermatological condition characterised by a spectrum of terms such as gynoid lipodystrophy and nodular liposclerosis, which reflect its multifactorial pathophysiology. This condition manifests visibly as skin textural alterations, notably the “orange peel” or “cottage cheese” appearance, primarily affecting post-pubertal females. The etiopathogenesis of cellulite is influenced by an array of factors including anatomical, vascular, hormonal, inflammatory, and genetic components, and may also be influenced by lifestyle, each contributing to the condition’s distinctive features.
Current management for cellulite varies in invasiveness and efficacy, encompassing a range from topical and systemic agents to more invasive mechanical and energy-based therapies. Non-invasive options, such as topical applications of methylxanthines and retinoids, target superficial symptoms but often provide only temporary relief without significant impact on the underlying adipose and connective tissues. On the other hand, energy-based therapies like radiofrequency and laser management offer a less invasive alternative that can achieve more durable results by targeting deeper tissue layers and stimulating collagen remodelling and lipolysis.
Despite the availability of various methods for cosmetic improvement, the challenge persists in finding an effective solution for patients who, by choice or necessity, wish to avoid pharmacological or invasive treatments.
Our single-centre prospective study aimed to assess the efficacy of a non-pharmacological cream-gel medical device intended for the management of cellulite, focusing on patients with moderate to severe manifestations (PEF severity scale 2 and 3). The statistical analysis, bolstered by objective skin profilometry and subjective clinical evaluations, supports significant improvements in cellulite severity over the 8-week clinical evaluation period.
The primary efficacy measures, captured through ANTERA® 3D skin profilometry, showed a marked reduction in skin indentation volumes at both gluteal and thigh measurement sites. At the end of the treatment period, mean values in all primary measurement sites significantly decreased, which reflects the cream-gel’s effectiveness in modulating skin morphology.
The clinical assessments align with these findings, with substantial improvements noted in both the dermatologist’s severity ratings and patient-reported outcomes. The use of the “pinch test”, a traditional method for assessing cellulite severity, further corroborated these results, indicating a reduction in the visibility of cellulite and a perceived improvement in skin texture and elasticity by the patients themselves.
The observed reduction in skin indentation volumes and improved surface texture are consistent with previously reported effects of topical formulations containing caffeine, carnitine, and botanical extracts, which are known to promote microcirculation, lipolysis, and dermal remodelling. For instance, Hexsel et al. [
61] demonstrated that caffeine-based formulations can significantly reduce thigh circumference and improve skin texture over 6–12 weeks, particularly when combined with mechanical massage. Similarly, studies evaluating cosmetic formulations enriched with genistein or
Centella asiatica [
62] have reported modest but measurable improvements in skin elasticity and cellulite appearance. However, many of these earlier studies lacked instrumental assessments or were conducted under less controlled application protocols. In contrast, our study employed 3D skin profilometry, allowing for objective, reproducible measurement of skin surface irregularities. The quantitative reduction in indentation volumes (mean reduction of 23.5%) aligns with or exceeds the improvements reported in comparable studies using energy-based technologies such as radiofrequency or shockwave therapy, which often require clinic-based equipment and multiple supervised sessions. In our study, while massage likely contributed to the observed effects, the consistent improvements across clinical, instrumental, and subjective parameters suggest a synergistic action between the mechanical stimulation and the bioactive components in the cream-gel, particularly given its thermogenic properties and complex formulation. In contrast to invasive modalities such as subcision or collagenase injections, the topical product evaluated here was well accepted and suitable for home use. This makes it an attractive option for patients seeking non-invasive, cost-effective alternatives with a favourable sensory profile and no systemic side effects. The positive patient-reported outcomes further reinforce the importance of acceptability in long-term management of aesthetic conditions like cellulite. Sensory characteristics such as ease of application, fragrance, and texture play a crucial role in compliance, as emphasised in recent consumer behaviour studies related to dermatocosmetic adherence.
Patient-reported outcomes highlight a positive reception to the regimen. A significant majority reported improvements in the appearance of cellulite and overall skin condition, with high scores for the product’s sensory attributes such as comfort on application and fragrance. These aspects are crucial for patient compliance and satisfaction, which in turn can influence the long-term success of cosmetic results.
The cream-gel was well-tolerated, with a low incidence of adverse events, which were mild in nature and limited to initial applications. The safety profile is particularly encouraging, considering the frequency of application required in the management of chronic conditions like cellulite. Indeed, participants did not report any local intolerance or discomfort during the study period.
This study was designed as a single-arm trial to evaluate a Class IIa medical device already on the market since 2013. The use of a control group with a placebo cream was not feasible due to the unique organoleptic characteristics of the tested product, including its distinct and recognisable fragrance, which would have complicated effective masking.
Beyond the challenges of trial design, it is also important to consider that the improvements observed may also be partially attributed to the action of massaging the cream onto the skin (see [
63]).
Future studies could benefit from incorporating a control group where the massage is performed with a placebo cream that has a fragrance similar to the active treatment, to distinctly quantify the effects of the massage versus the active ingredients.
While pharmaceutical products or surgical techniques may provide more consistent and noticeable results, the absence of contraindications and the minimal invasiveness of the tested product make it a valid option for those who cannot or choose not to undergo more invasive procedures.
This study has some limitations. The absence of a placebo-controlled group limits the ability to isolate the effects of the active ingredients from those of massage or other nonspecific factors. The single-centre design and relatively small sample size (n = 56) may reduce generalisability. The 8-week duration does not allow for assessment of long-term effects. No formal patch test was performed, although no adverse reactions were reported. Finally, the study relied on clinical and instrumental evaluations without histological or biochemical analysis, which could further elucidate the mechanisms of action.
5. Conclusions
This prospective, single-centre study demonstrated that an over-the-counter thermogenic cream-gel medical device, applied in association with manual massage, can lead to statistically and clinically significant improvements in the appearance of moderate to severe cellulite over an 8-week period. Objective skin profilometry showed a mean reduction of 23.5% in skin indentation volumes (p < 0.001), while clinical evaluations documented a shift from grade 2–3 to grade 1–2 in a substantial proportion of participants. Patient-reported outcomes confirmed perceived improvements in skin texture, elasticity, and appearance, with over 80% of participants expressing satisfaction with the product. The formulation was generally well tolerated, with only two cases of mild and transient dermatological reactions and no serious adverse events reported. These findings support the potential role of this product as a non-invasive, home-use option for individuals seeking alternatives to more aggressive or costly approaches. Nevertheless, further studies with placebo-controlled designs, longer follow-up periods, and histological analyses are warranted to better define the product’s long-term efficacy and underlying mechanisms of action.