Safety Validation of Plant-Derived Materials for Skin Application
Abstract
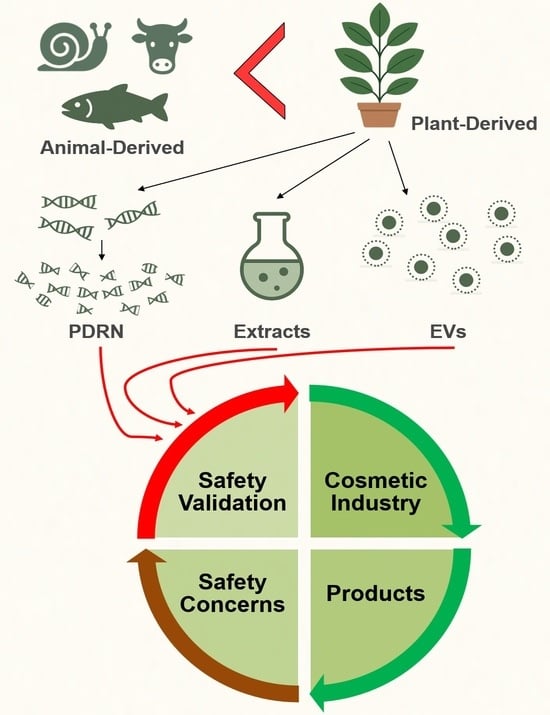
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Callus Induction, Culture, and Extraction
2.3. Production of Polydeoxyribonucleotide
2.4. Exosome Isolation
2.5. Assessment of Cell Viability
2.6. Wound-Healing Assay
2.7. Immunocytochemistry
2.8. Primary Irritant Patch Test
2.8.1. Test Subjects
Inclusion Criteria for Study Participants
- (1)
- Healthy adult men and women between the ages of 20 and 59, with no acute or chronic physical illnesses, including skin diseases;
- (2)
- Individuals who have received sufficient explanation from the principal investigator or a designated researcher regarding the study and have voluntarily signed the informed consent form;
- (3)
- Individuals who are able to undergo follow-up observation throughout the study period.
Exclusion Criteria for Study Participants
- (1)
- Pregnant or lactating women, or women who are likely to become pregnant;
- (2)
- Individuals who have used topical steroids for the treatment of skin diseases for more than one month;
- (3)
- Individuals who have participated in a similar study within the past four weeks;
- (4)
- Individuals with sensitivity or allergic reactions to cosmetics, pharmaceuticals, or daily sun exposure;
- (5)
- Individuals with irritation or severe allergic reactions to adhesive tapes;
- (6)
- Individuals with sensitive or hypersensitive skin;
- (7)
- Individuals with moles, acne, tattoos, scars, erythema, telangiectasia, or burn marks on the test site that could interfere with the study.
2.8.2. General Method
2.9. Statistical Analysis
3. Results
3.1. Samples
3.2. In Vitro Assessments of Plant-Derived Materials
3.3. Clinical Tests
3.3.1. Study Participants
3.3.2. Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CCK | Cell counting kit |
| EV | Extracellular vesicle |
| FLG | Filaggrin |
| ICDRG | International Contact Dermatitis Research Group |
| INCI | International nomenclature of cosmetic ingredients |
| NTA | Nanoparticle tracking analysis |
| PDM | Plant-derived material |
| PDRN | Polydeoxyribonucleotide |
| TEM | Transmission electron microscopy |
References
- Yapar, E.A. Intellectual property and patent in cosmetics. Marmara Pharm. J. 2017, 21, 419–424. [Google Scholar]
- Nohynek, G.J.; Antignac, E.; Re, T.; Toutain, H. Safety assessment of personal care products/cosmetics and their ingredients. Toxicol. Appl. Pharmacol. 2010, 243, 239–259. [Google Scholar] [CrossRef] [PubMed]
- César, F.C.; Carnevale, F.; Porto, G.S.; Campos, P.M.M. Patent analysis: A look at the innovative nature of plant-based cosmetics. Química Nova 2017, 40, 840–847. [Google Scholar] [CrossRef]
- Liu, J.-K. Natural products in cosmetics. Nat. Prod. Bioprospect. 2022, 12, 40. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Wang, S.-L. Recent advances on polydeoxyribonucleotide extraction and its novel application in cosmeceuticals. Int. J. Biol. Macromol. 2024, 282, 137051. [Google Scholar] [CrossRef]
- Squadrito, F.; Bitto, A.; Irrera, N.; Pizzino, G.; Pallio, G.; Minutoli, L.; Altavilla, D. Pharmacological activity and clinical use of PDRN. Front. Pharmacol. 2017, 8, 224. [Google Scholar] [CrossRef]
- Galeano, M.; Pallio, G.; Irrera, N.; Mannino, F.; Bitto, A.; Altavilla, D.; Vaccaro, M.; Squadrito, G.; Arcoraci, V.; Colonna, M.R. Polydeoxyribonucleotide: A promising biological platform to accelerate impaired skin wound healing. Pharmaceuticals 2021, 14, 1103. [Google Scholar] [CrossRef]
- Colangelo, M.T.; Galli, C.; Guizzardi, S. The effects of polydeoxyribonucleotide on wound healing and tissue regeneration: A systematic review of the literature. Regen. Med. 2020, 15, 1801–1821. [Google Scholar] [CrossRef]
- Lee, J.H.; Han, J.W.; Byun, J.H.; Lee, W.M.; Kim, M.H.; Wu, W.H. Comparison of wound healing effects between Oncorhynchus keta-derived polydeoxyribonucleotide (PDRN) and Oncorhynchus mykiss-derived PDRN. Arch. Craniofac. Surg. 2018, 19, 20–34. [Google Scholar] [CrossRef]
- Negi, P.S. Plant extracts for the control of bacterial growth: Efficacy, stability and safety issues for food application. Int. J. Food Microbiol. 2012, 156, 7–17. [Google Scholar] [CrossRef]
- Bäurle, P.; Suter, A.; Wormstall, H. Safety and effectiveness of a traditional ginkgo fresh plant extract—Results from a clinical trial. Forsch. Komplementmed. 2009, 16, 156–161. [Google Scholar]
- Kazemi, M.; Mohammadifar, M.; Aghadavoud, E.; Vakili, Z.; Aarabi, M.H.; Talaei, S.A. Deep skin wound healing potential of lavender essential oil and licorice extract in a nanoemulsion form: Biochemical, histopathological and gene expression evidences. J. Tissue Viability 2020, 29, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Tollenaere, M.D.; Sennelier, B.; Lambert, C.; Durduret, A.; Kim, S.-Y.; Seo, H.-H.; Lee, J.-H.; Scandolera, A.; Reynaud, R. Analysis of Active Components and Transcriptome of Freesia refracta Callus Extract and Its Effects against Oxidative Stress and Wrinkles in Skin. Int. J. Mol. Sci. 2024, 25, 8150. [Google Scholar] [CrossRef] [PubMed]
- Saba, E.; Kim, S.-H.; Lee, Y.Y.; Park, C.-K.; Oh, J.-W.; Kim, T.-H.; Kim, H.-K.; Roh, S.-S.; Rhee, M.H. Korean Red Ginseng extract ameliorates melanogenesis in humans and induces antiphotoaging effects in ultraviolet B-irradiated hairless mice. J. Ginseng Res. 2020, 44, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Hong, Y.D.; Kim, D.; Park, S.J.; Kim, J.S.; Kim, H.-M.; Yoon, E.J.; Cho, J.-S. Confirmation of plant-derived exosomes as bioactive substances for skin application through comparative analysis of keratinocyte transcriptome. Appl. Biol. Chem. 2022, 65, 8. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, Y.; Liao, H.; Fu, H.; Yang, X.; Xiang, Q.; Zhang, S. Plant exosome-like nanoparticles as biological shuttles for transdermal drug delivery. Bioengineering 2023, 10, 104. [Google Scholar] [CrossRef]
- Choi, H.Y.; Lee, Y.J.; Kim, C.M.; Lee, Y.-M. Revolutionizing cosmetic ingredients: Harnessing the power of antioxidants, probiotics, plant extracts, and peptides in personal and skin care products. Cosmetics 2024, 11, 157. [Google Scholar] [CrossRef]
- Aroman, M.S.; Guillot, P.; Dahan, S.; Coustou, D.; Mortazawi, K.; Zourabichvili, O.; Aardewijn, T. Efficacy of a repair cream containing Rhealba oat plantlets extract l-ALA–l-GLU dipeptide, and hyaluronic acid in wound healing following dermatological acts: A meta-analysis of > 2000 patients in eight countries corroborated by a dermatopediatric clinical case. Clin. Cosmet. Investig. Dermatol. 2018, 11, 579–589. [Google Scholar]
- Tangwattanachuleeporn, M.; Muanwien, P.; Teethaisong, Y.; Somparn, P. Optimizing concentration of polyethelene glycol for exosome isolation from plasma for downstream application. Medicina 2022, 58, 1600. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Li, Y.-J.; Hu, X.-B.; Huang, S.; Xiang, D.-X. Preservation of small extracellular vesicles for functional analysis and therapeutic applications: A comparative evaluation of storage conditions. Drug Deliv. 2021, 28, 162–170. [Google Scholar] [CrossRef]
- Kim, E.; Kim, S.-Y.; Song, J.; Jang, J.; Seo, H.H.; Lee, J.H.; Moh, S.H. Skin Recovery by Lavandula angustifolia Leaf Callus Extract: Redox Control of Nrf2 Signaling. Free Radic. Biol. Med. 2025, 235, 390–404. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Choi, S.; Kim, S.Y.; Jang, S.J.; Lee, S.; Kim, H.; Jang, J.H.; Seo, H.H.; Lee, J.H.; Choi, S.S.; et al. Wound healing effect of polydeoxyribonucleotide derived from Hibiscus sabdariffa callus via Nrf2 signaling in human keratinocytes. Biochem. Biophys. Res. Commun. 2024, 728, 150335. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Jang, J.; Lim, M.-J.; Kim, S.-Y.; Yun, S.K.; Song, J.; Seo, H.H.; Lee, J.H.; Moh, S.H. Effective release of Eryngium maritimum L. callus extract via encapsulation in multilayered liposomes for skin delivery. Ther. Deliv. 2025, 16, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Murthy, H.N.; Georgiev, M.I.; Park, S.-Y.; Dandin, V.S.; Paek, K.-Y. The safety assessment of food ingredients derived from plant cell, tissue and organ cultures: A review. Food Chem. 2015, 176, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Lim, S.I. Plant extract-based synthesis of metallic nanomaterials, their applications, and safety concerns. Biotechnol. Bioeng. 2022, 119, 2273–2304. [Google Scholar] [CrossRef]
- Boncler, M.; Różalski, M.; Krajewska, U.; Podsędek, A.; Watala, C. Comparison of PrestoBlue and MTT assays of cellular viability in the assessment of anti-proliferative effects of plant extracts on human endothelial cells. J. Pharmacol. Toxicol. Methods 2014, 69, 9–16. [Google Scholar] [CrossRef]
- Chan, S.M.; Khoo, K.S.; Sit, N.W. Interactions between plant extracts and cell viability indicators during cytotoxicity testing: Implications for ethnopharmacological studies. Trop. J. Pharm. Res. 2015, 14, 1991–1998. [Google Scholar] [CrossRef]
- Şahin, F.; Koçak, P.; Güneş, M.Y.; Özkan, İ.; Yıldırım, E.; Kala, E.Y. In vitro wound healing activity of wheat-derived nanovesicles. Appl. Biochem. Biotechnol. 2019, 188, 381–394. [Google Scholar] [CrossRef]
- Filippi, A.; Petrussa, E.; Peresson, C.; Bertolini, A.; Vianello, A.; Braidot, E. In Vivo assay to monitor flavonoid uptake across plant cell membranes. FEBS Open Bio 2015, 5, 748–752. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Apone, F.; Tito, A.; Carola, A.; Arciello, S.; Tortora, A.; Filippini, L.; Monoli, I.; Cucchiara, M.; Gibertoni, S.; Chrispeels, M.J. A mixture of peptides and sugars derived from plant cell walls increases plant defense responses to stress and attenuates ageing-associated molecular changes in cultured skin cells. J. Biotechnol. 2010, 145, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Koufan, M.; Belkoura, I.; Mazri, M.A.; Amarraque, A.; Essatte, A.; Elhorri, H.; Zaddoug, F.; Alaoui, T. Determination of antioxidant activity, total phenolics and fatty acids in essential oils and other extracts from callus culture, seeds and leaves of Argania spinosa (L.) Skeels. Plant Cell Tissue Organ Cult. 2020, 141, 217–227. [Google Scholar] [CrossRef]
- Chalageri, G.; Dhananjaya, S.; Raghavendra, P.; Kumar, L.S.; Babu, U.; Varma, S.R. Substituting plant vegetative parts with callus cell extracts: Case study with Woodfordia fruticosa Kurz.—A potent ingredient in skin care formulations. S. Afr. J. Bot. 2019, 123, 351–360. [Google Scholar] [CrossRef]
- Marques, C.; Porcello, A.; Cerrano, M.; Hadjab, F.; Chemali, M.; Lourenço, K.; Hadjab, B.; Raffoul, W.; Applegate, L.A.; Laurent, A.E. From Polydeoxyribonucleotides (PDRNs) to Polynucleotides (PNs): Bridging the Gap Between Scientific Definitions, Molecular Insights, and Clinical Applications of Multifunctional Biomolecules. Biomolecules 2025, 15, 148. [Google Scholar] [CrossRef]
- Parra, A.L.; Yhebra, R.S.; Sardiñas, I.G.; Buela, L.I. Comparative study of the assay of Artemia salina L. and the estimate of the medium lethal dose (LD50 value) in mice, to determine oral acute toxicity of plant extracts. Phytomed. Plus 2001, 8, 395–400. [Google Scholar]
- Teshome, K.; Gebre-Mariam, T.; Asres, K.; Perry, F.; Engidawork, E. Toxicity studies on dermal application of plant extract of Plumbago zeylanica used in Ethiopian traditional medicine. J. Ethnopharmacol. 2008, 117, 236–248. [Google Scholar] [CrossRef]
- Seremet, O.C.; Olaru, O.T.; Gutu, C.M.; Nitulescu, G.M.; Ilie, M.; Negres, S.; Zbarcea, C.E.; Purdel, C.N.; Spandidos, D.A.; Tsatsakis, A.M. Toxicity of plant extracts containing pyrrolizidine alkaloids using alternative invertebrate models. Mol. Med. Rep. 2018, 17, 7757–7763. [Google Scholar] [CrossRef]
- Watanabe-Takahashi, M.; Yamasaki, S.; Murata, M.; Kano, F.; Motoyama, J.; Yamate, J.; Omi, J.; Sato, W.; Ukai, H.; Shimasaki, K. Exosome-associated Shiga toxin 2 is released from cells and causes severe toxicity in mice. Sci. Rep. 2018, 8, 10776. [Google Scholar] [CrossRef] [PubMed]
- Irrera, N.; Arcoraci, V.; Mannino, F.; Vermiglio, G.; Pallio, G.; Minutoli, L.; Bagnato, G.; Anastasi, G.P.; Mazzon, E.; Bramanti, P.; et al. Activation of A2A receptor by PDRN reduces neuronal damage and stimulates WNT/β-CATENIN driven neurogenesis in spinal cord injury. Front. Pharmacol. 2018, 9, 506. [Google Scholar] [CrossRef]
- Kim, E.; Choi, S.; Jang, J.; Shin, H.Y.; Ahn, K.; Kim, S.J.; Seo, H.H.; Lee, J.H.; Moh, S.H. Effects of polydeoxyribonucleotide derived from Gynostemma pentaphyllum callus on skin barrier function. Biochem. Biophys. Res. Commun. 2025, 776, 152180. [Google Scholar] [CrossRef]




| Skin Irritation Index | Criterion |
|---|---|
| 0.00–0.75 | No irritation |
| 0.76–1.50 | Low irritation |
| 1.51–2.50 | Slight irritation |
| 2.51–4.00 | Moderate irritation |
| 4.01–5.00 | Severe irritation |
| Grade | Symbol | Criteria | |
|---|---|---|---|
| 0 | - | No reaction | - |
| 1 | + | Uncertain positive reaction | Slight erythema |
| 2 | ++ | Mild positive reaction | Erythema, infiltration, or papules |
| 3 | +++ | Strong positive reaction | Erythema, infiltration, papules, and small vesicles |
| 4 | ++++ | Very strong positive reaction | Severe erythema, infiltration, papules, and irregular vesicles |
| IR | IR (irritant reaction) | Irritant reaction | Single erythema or papule without edema or infiltration |
| Sample No. | Sample Type | Name of Material | International Nomenclature of Cosmetic Ingredients (INCI) |
|---|---|---|---|
| 1 | Callus | Aloe vera phytoplacenta extract | Aloe Barbadensis Phytoplacenta Extract |
| 2 | Callus | Aloe vera callus extract | Aloe Vera Callus Extract |
| 3 | Callus | Angelica keiskei callus extract | Angelica Keiskei Callus Extract |
| 4 | Callus | Artemisia princeps callus extract | Artemisia Princeps Callus Extract |
| 5 | Callus | Aster spathulifolius callus extract | Aster Spathulifolius Callus Extract |
| 6 | Callus | Camellia japonica callus extract | Camellia Japonica Callus Extract |
| 7 | Callus | Camellia japonica phytoplacenta extract | Camellia Japonica Phytoplacenta Extract |
| 8 | Callus | Camellia sinensis callus culture extract | Camellia Sinensis Callus Culture Extract |
| 9 | Callus | Campanula punctata callus extract | Campanula Punctata Callus Extract |
| 10 | Callus | Centella asiatica callus extract | Centella Asiatica Callus Extract |
| 11 | Callus | Dryas octopetala callus cultureextract | Dryas Octopetala Callus Culture Extract |
| 12 | Callus | Glycine max callus culture extract | Glycine Max (Soybean) Callus Culture Extract |
| 13 | Callus | Gynostemma pentaphyllum callus extract | Gynostemma Pentaphyllum Callus Extract |
| 14 | Callus | Leontopodium alpinum callus culture extract | Leontopodium Alpinum Callus Culture Extract |
| 15 | Callus | Lilium candidum callus culture extract | Lilium Candidum Callus Culture Extract |
| 16 | Callus | Myrothamnus flabellifolia callus culture extract | Myrothamnus Flabellifolia Callus Culture Extract |
| 17 | Callus | Neofinetia falcata callus culture extract | Neofinetia Falcata Callus Culture Extract |
| 18 | Callus | Opuntia ficus-indica callus culture extract | Opuntia Ficus-Indica Callus Culture Extract |
| 19 | Callus | Oryza sativa callus culture extract | Oryza Sativa (Rice) Callus Culture Extract |
| 20 | Callus | Orostachys japonica callus extract | Orostachys Japonica Callus Extract |
| 21 | Callus | Panax ginseng callus culture extract | Panax Ginseng Callus Culture Extract |
| 22 | Callus | Rhizophora mangle callus culture extract | Rhizophora Mangle Callus Culture Extract |
| 23 | Callus | Rosa damascena callus culture extract | Rosa Damascena Callus Culture Extract |
| 24 | Callus | Salicornia herbacea callus culture extract | Salicornia Herbacea Callus Culture Extract |
| 25 | Callus | Solanum lycopersicum callus culture extract | Solanum Lycopersicum (Tomato) Callus Culture Extract |
| 26 | EV | Camellia sinensis callus extracellular vesicles | Camellia Sinensis Callus Extracellular Vesicles |
| 27 | EV | Centella asiatica callus extracellular vesicles | Centella Asiatica Callus Extracellular Vesicles |
| 28 | EV | Glycine max callus extracellular vesicles | Glycine Max Callus Extracellular Vesicles |
| 29 | EV | Panax ginseng adventitious root extracellular vesicles | Panax Ginseng Adventitious Root Extracellular Vesicles |
| 30 | Filtrate | Eryngium maritimum callus culture filtrate | Eryngium Maritimum Callus Culture Filtrate |
| 31 | Filtrate | Eryngium maritimum callus Culture Filtrate Liposome | Eryngium Maritimum Callus Culture Filtrate |
| 32 | PDRN (Callus) | Adenium obesum Sodium DNA | Sodium DNA |
| 33 | PDRN (Plant) | Brassica oleracea Sodium DNA | Sodium DNA |
| 34 | PDRN (Plant) | Camellia japonica Sodium DNA | Sodium DNA |
| 35 | PDRN (Plant) | Centella asiatica Sodium DNA | Sodium DNA |
| 36 | PDRN (Callus) | Glycine max Sodium DNA | Sodium DNA |
| 37 | PDRN (Callus) | Gynostemma pentaphyllum Sodium DNA | Sodium DNA |
| 38 | PDRN (Plant) | Houttuynia cordata Sodium DNA | Sodium DNA |
| 39 | PDRN (Callus) | Lavandula angustifolia Sodium DNA | Sodium DNA |
| 40 | PDRN (Callus) | Leontopodium alpinum Sodium DNA | Sodium DNA |
| 41 | PDRN (Plant) | Melaleuca alternifolia Sodium DNA | Sodium DNA |
| 42 | PDRN (Callus) | Morinda citrifolia Sodium DNA | Sodium DNA |
| 43 | PDRN (Plant) | Narcissus tazetta Sodium DNA | Sodium DNA |
| 44 | PDRN (Plant) | Oryza sativa Sodium DNA | Sodium DNA |
| 45 | PDRN (Plant) | Panax ginseng Sodium DNA | Sodium DNA |
| 46 | PDRN (Plant) | Pinus densiflora Sodium DNA | Sodium DNA |
| 47 | PDRN (Plant) | Rosa damascena Sodium DNA | Sodium DNA |
| 48 | PDRN (Callus) | Rosa damascena (callus) Sodium DNA | Sodium DNA |
| 49 | PDRN (Plant) | Solanum lycopersicum Sodium DNA | Sodium DNA |
| 50 | PDRN (Callus) | Vitis vinifera (callus) Sodium DNA | Sodium DNA |
| Sample No. | Name of Materials | No. of Participants | Index | Irritation |
|---|---|---|---|---|
| 1 | Aloe vera phytoplacenta extract | 32 | 0.00 | None |
| 2 | Aloe vera callus extract | 31 | 0.00 | None |
| 3 | Angelica keiskei callus extract | 31 | 0.00 | None |
| 4 | Artemisia princeps callus extract | 31 | 0.36 | None |
| 5 | Aster spathulifolius callus extract | 32 | 0.00 | None |
| 6 | Camellia japonica callus extract | 31 | 0.9 | None |
| 7 | Camellia japonica phytoplacenta extract | 31 | 0.00 | None |
| 8 | Camellia sinensis callus culture extract | 31 | 0.36 | None |
| 9 | Campanula punctata callus extract | 32 | 0.00 | None |
| 10 | Centella asiatica callus extract | 32 | 0.00 | None |
| 11 | Dryas octopetala callus cultureextract | 33 | 0.36 | None |
| 12 | Glycine max callus culture extract | 32 | 0.00 | None |
| 13 | Gynostemma pentaphyllum callus extract | 32 | 0.00 | None |
| 14 | Leontopodium alpinum callus culture extract | 31 | 0.00 | None |
| 15 | Lilium candidum callus culture extract | 31 | 0.00 | None |
| 16 | Myrothamnus flabellifolia callus culture extract | 31 | 0.00 | None |
| 17 | Neofinetia falcata callus culture extract | 32 | 0.00 | None |
| 18 | Opuntia ficus-indica callus culture extract | 31 | 0.36 | None |
| 19 | Oryza sativa callus culture extract | 31 | 0.00 | None |
| 20 | Orostachys japonica callus extract | 31 | 0.36 | None |
| 21 | Panax ginseng callus culture extract | 32 | 0.00 | None |
| 22 | Rhizophora mangle callus culture extract | 31 | 0.72 | None |
| 23 | Rosa damascena callus culture extract | 30 | 0.00 | None |
| 24 | Salicornia herbacea callus culture extract | 32 | 0.00 | None |
| 25 | Solanum lycopersicum callus culture extract | 32 | 0.00 | None |
| 26 | Camellia sinensis callus extracellular vesicles | 32 | 0.00 | None |
| 27 | Centella asiatica callus extracellular vesicles | 32 | 0.00 | None |
| 28 | Glycine max callus extracellular vesicles | 31 | 0.00 | None |
| 29 | Panax ginseng adventitious root extracellular vesicles | 32 | 0.00 | None |
| 30 | Eryngium maritimum callus culture filtrate | 32 | 0.00 | None |
| 31 | Eryngium maritimum callus Culture Filtrate Liposome | 32 | 0.00 | None |
| 32 | Adenium obesum Sodium DNA | 33 | 0.00 | None |
| 33 | Brassica oleracea Sodium DNA | 33 | 0.00 | None |
| 34 | Camellia japonica Sodium DNA | 33 | 0.00 | None |
| 35 | Centella asiatica Sodium DNA | 33 | 0.00 | None |
| 36 | Glycine max Sodium DNA | 33 | 0.00 | None |
| 37 | Gynostemma pentaphyllum Sodium DNA | 33 | 0.00 | None |
| 38 | Houttuynia cordata Sodium DNA | 33 | 0.00 | None |
| 39 | Lavandula angustifolia Sodium DNA | 33 | 0.00 | None |
| 40 | Leontopodium alpinum Sodium DNA | 33 | 0.00 | None |
| 41 | Melaleuca alternifolia Sodium DNA | 33 | 0.00 | None |
| 42 | Morinda citrifolia Sodium DNA | 33 | 0.00 | None |
| 43 | Narcissus tazetta Sodium DNA | 33 | 0.00 | None |
| 44 | Oryza sativa Sodium DNA | 33 | 0.00 | None |
| 45 | Panax ginseng Sodium DNA | 33 | 0.00 | None |
| 46 | Pinus densiflora Sodium DNA | 33 | 0.00 | None |
| 47 | Rosa damascena Sodium DNA | 33 | 0.00 | None |
| 48 | Rosa damascena (callus) Sodium DNA | 33 | 0.00 | None |
| 49 | Solanum lycopersicum Sodium DNA | 33 | 0.00 | None |
| 50 | Vitis vinifera (callus) Sodium DNA | 33 | 0.00 | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.; Seo, H.H.; Shin, D.S.; Song, J.; Yun, S.K.; Lee, J.H.; Moh, S.H. Safety Validation of Plant-Derived Materials for Skin Application. Cosmetics 2025, 12, 153. https://doi.org/10.3390/cosmetics12040153
Kim E, Seo HH, Shin DS, Song J, Yun SK, Lee JH, Moh SH. Safety Validation of Plant-Derived Materials for Skin Application. Cosmetics. 2025; 12(4):153. https://doi.org/10.3390/cosmetics12040153
Chicago/Turabian StyleKim, Euihyun, Hyo Hyun Seo, Dong Sun Shin, Jihyeok Song, Seon Kyu Yun, Jeong Hun Lee, and Sang Hyun Moh. 2025. "Safety Validation of Plant-Derived Materials for Skin Application" Cosmetics 12, no. 4: 153. https://doi.org/10.3390/cosmetics12040153
APA StyleKim, E., Seo, H. H., Shin, D. S., Song, J., Yun, S. K., Lee, J. H., & Moh, S. H. (2025). Safety Validation of Plant-Derived Materials for Skin Application. Cosmetics, 12(4), 153. https://doi.org/10.3390/cosmetics12040153