Bioassay-Guided Isolation of Chemical Constituents from Lycopodiastrum casuarinoides and Targeted Evaluation of Their Potential Efficacy in Cosmetics
Abstract
1. Introduction
2. Materials and Methods
2.1. Instruments and Chemicals
2.2. Biomaterials
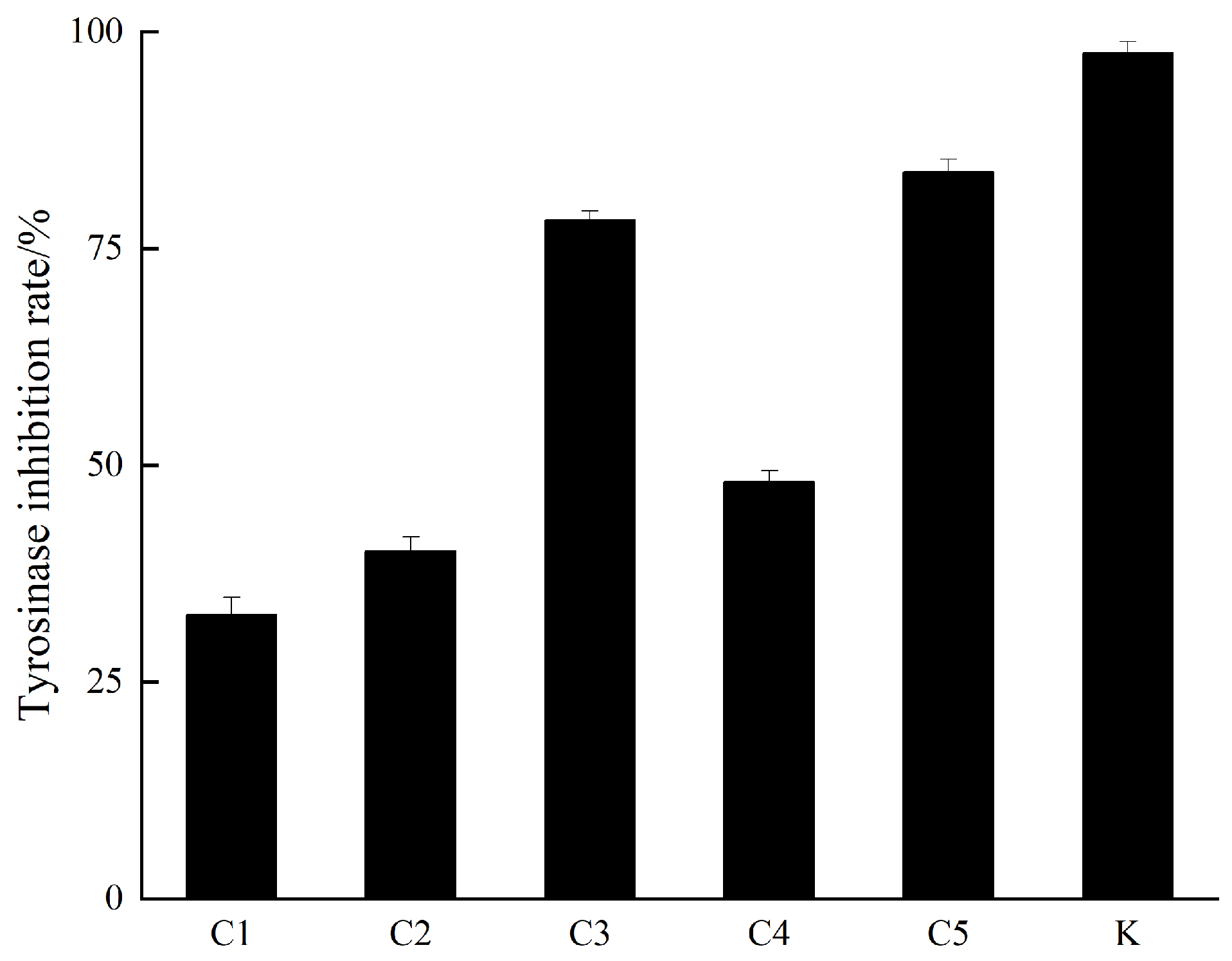
2.3. Extraction and Bioassay-Guided Isolation
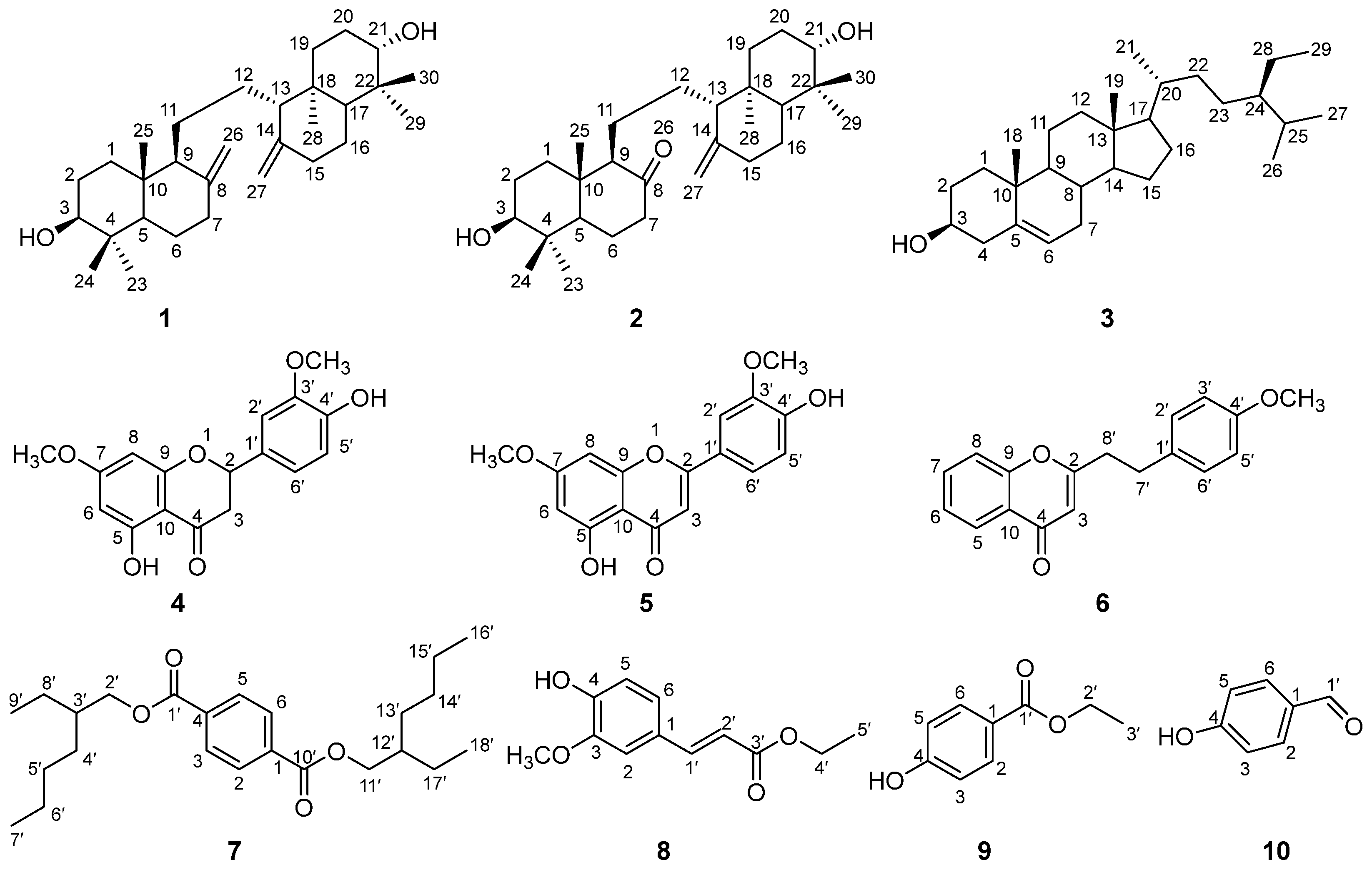
2.4. Characteristic 1H and 13C NMR and MS Spectral Data of Isolated Compounds
- Compound 1: 1H NMR (CDCl3, 600 MHz): δH 4.83 (2H, s, H-26α, H-27α), 4.56 (2H, s, H-26β, H-27β), 3.25 (2H, d, J = 11.7 Hz, H-3, H-21), 0.99 (6H, s, H-23, H-30), 0.76 (6H, s, H-24, H-29), 0.64 (6H, s, H-25, H-28); 13C NMR (CDCl3, 150 MHz): δC 37.2 (C-1, C-19), 28.1 (C-2, C-20), 79.1 (C-3, C-21), 39.4 (C-4, C-22), 54.8 (C-5, C-17), 24.2 (C-6, C-16), 38.4 (C-7, C-15), 148.6 (C-8, C-14), 57.7 (C-9, C-13), 39.3 (C-10, C-18), 22.8 (C-11, C-12), 28.5 (C-23, C-29), 15.5 (C-24, C-30), 14.7 (C-25, C-28), 106.9 (C-26, C-27). ESI-MS m/z: 465.1 [M + Na]+.
- Compound 2: 1H NMR (CDCl3, 400 MHz): δH 4.90 (2H, s, H-27), 3.33 (1H, d, J = 11.6 Hz, H-3), 3.23 (1H, d, J = 11.6 Hz, H-21), 1.08 (3H, s, H-25), 0.99 (3H, s, H-28), 0.81 (3H, s, H-24), 0.76 (3H, s, H-30), 0.69 (3H, s, H-23), 0.63 (3H, s, H-29); 13C NMR (CDCl3, 100 MHz): δC 37.1 (C-1), 28.5 (C-2), 78.8 (C-3), 39.3 (C-4), 54.9 (C-5), 27.7 (C-6), 42.5 (C-7), 212.0 (C-8), 64.9 (C-9), 42.5 (C-10), 21.2 (C-11), 23.7 (C-12), 57.4 (C-13), 147.5 (C-14), 37.3 (C-15), 23.7 (C-16), 53.7 (C-17), 38.4 (C-18), 37.1 (C-19), 28.1 (C-20), 79.1 (C-21), 39.4 (C-22), 27.7 (C-23), 15.5 (C-24), 14.6 (C-25), 108.0 (C-27), 14.9 (C-28), 28.5 (C-29), 15.5 (C-30). ESI-MS m/z: 467.2 [M + Na]+.
- Compound 3: 1H NMR (CDCl3, 600 MHz): δH 5.35 (1H, m, H-6), 3.52 (1H, tt, J = 11.1, 4.6 Hz, H-3), 1.01 (3H, s, H-19), 0.92 (3H, d, J = 6.6 Hz, H-21), 0.84 (3H, t, J = 7.7 Hz, H-29), 0.83 (3H, d, J = 6.7 Hz, H-26), 0.81 (3H, d, J = 6.8 Hz, H-27), 0.68 (3H, s, H-18); 13C NMR (CDCl3, 150 MHz): δC 37.4 (C-1), 32.1 (C-2), 72.0 (C-3), 42.4 (C-4), 140.9 (C-5), 121.9 (C-6), 32.1 (C-7), 31.8 (C-8), 50.3 (C-9), 36.7 (C-10), 21.2 (C-11), 39.9 (C-12), 42.5 (C-13), 56.9 (C-14), 24.5 (C-15), 28.4 (C-16), 56.2 (C-17), 12.0 (C-18), 19.2 (C-19), 36.3 (C-20), 18.9 (C-21), 34.1 (C-22), 26.2 (C-23), 46.0 (C-24), 29.3 (C-25), 19.5 (C-26), 20.0 (C-27), 23.2 (C-28), 12.1 (C-29). ESI-MS m/z: 415.2 [M + H]+.
- Compound 4: 1H NMR (CDCl3, 400 MHz): δH 12.03 (1H, s, 5-OH), 6.95 (3H, m, H-2′, H-5′, H-6′), 6.08 (1H, d, J = 2.4 Hz, H-6), 6.06 (1H, d, J = 2.3 Hz, H-8), 5.71 (1H, s, 4′-OH), 5.34 (1H, dd, J = 13.1, 3.0 Hz, H-2), 3.94 (3H, s, 3′-OCH3), 3.81 (3H, s, 7-OCH3), 3.10 (1H, dd, J = 17.2, 13.1 Hz, H-3α), 2.79 (1H, dd, J = 17.2, 3.0 Hz, H-3β); 13C NMR (CDCl3, 100 MHz): δC 79.5 (C-2), 43.6 (C-3), 196.1 (C-4), 164.3 (C-5), 95.3 (C-6), 168.1 (C-7), 94.4 (C-8), 163.0 (C-9), 103.3 (C-10), 130.4 (C-1′), 108.9 (C-2′), 146.9 (C-3′), 146.4 (C-4′), 114.7 (C-5′), 119.8 (C-6′), 56.2 (3′-OCH3), 55.8 (7-OCH3). ESI-MS m/z: 339.0 [M + Na]+.
- Compound 5: 1H NMR (CDCl3, 400 MHz): δH 12.79 (1H, s, 5-OH), 7.49 (1H, dd, J = 8.4, 2.0 Hz, H-6′), 7.33 (1H, d, J = 2.1 Hz, H-2′), 7.04 (1H, d, J = 8.4 Hz, H-5′), 6.57 (1H, s, H-3), 6.49 (1H, d, J = 2.2 Hz, H-8), 6.37 (1H, d, J = 2.2 Hz, H-6), 4.01 (3H, s, 3′-OCH3), 3.89 (3H, s, 7-OCH3); 13C NMR (CDCl3, 100 MHz): δC 164.2 (C-2), 104.7 (C-3), 182.6 (C-4), 162.4 (C-5), 98.2 (C-6), 165.7 (C-7), 92.8 (C-8), 157.9 (C-9), 105.7 (C-10), 123.6 (C-1′), 108.6 (C-2′), 149.4 (C-3′), 147.1 (C-4′), 115.2 (C-5′), 120.9 (C-6′), 56.3 (3′-OCH3), 56.0 (7-OCH3). ESI-MS m/z: 313.1 [M − H]−.
- Compound 6: 1H NMR (CDCl3, 400 MHz): δH 8.18 (1H, dd, J = 7.9, 1.6 Hz, H-5), 7.65 (1H, td, J = 7.8, 1.7 Hz, H-7), 7.44 (1H, d, J = 8.4 Hz, H-8), 7.39 (1H, t, J = 7.6 Hz, H-6), 7.12 (2H, d, J = 8.5 Hz, H-2′, H-6′), 6.83 (2H, d, J = 8.5 Hz, H-3′, H-5′), 6.14 (1H, s, H-3), 3.78 (3H, s, 4′-OCH3), 3.01 (2H, m, H-7′), 2.90 (2H, m, H-8′); 13C NMR (CDCl3, 100 MHz): δC 168.7 (C-2), 110.4 (C-3), 178.5 (C-4), 125.9 (C-5), 125.1 (C-6), 133.7 (C-7), 118.0 (C-8), 156.6 (C-9), 123.9 (C-10), 131.9 (C-1′), 129.4 (C-2′, C-6′), 114.2 (C-3′, C-5′), 158.4 (C-4′), 32.3 (C-7′), 36.6 (C-8′), 55.4 (4′-OCH3). ESI-MS m/z: 281.2 [M + H]+.
- Compound 7: 1H NMR (CDCl3, 600 MHz): δH 8.10 (4H, s, H-2, H-3, H-5, H-6), 4.26 (4H, m, H-2′, H-11′), 1.73 (2H, m, H-3′, H-12′), 1.46 (4H, m, H-4′, H-13′), 1.40 (4H, m, H-5′, H-14′), 1.34 (4H, m, H-6′, H-15′), 0.90 (6H, t, J = 7.0 Hz, H-7′, H-16′), 1.32 (4H, m, H-8′, H-17′), 0.95 (6H, t, J = 7.5 Hz, H-9′, H-18′); 13C NMR (CDCl3, 150 MHz): δC 134.4 (C-1, C-4), 129.6 (C-2, C-3, C-5, C-6), 166.1 (C-1′, C-10′), 67.9 (C-2′, C-11′), 39.1 (C-3′, C-12′), 30.7 (C-4′, C-13′), 29.1 (C-5′, C-14′), 23.1 (C-6′, C-15′), 14.2 (C-7′, C-16′), 24.1 (C-8′, C-17′), 11.2 (C-9′, C-18′). ESI-MS m/z: 391.3 [M + H]+.
- Compound 8: 1H NMR (CDCl3, 600 MHz): δH 7.61 (1H, d, J = 15.9 Hz, H-1′), 7.07 (1H, dd, J = 8.2, 1.9 Hz, H-6), 7.03 (1H, d, J = 1.9 Hz, H-2), 6.91 (1H, d, J = 8.2 Hz, H-5), 6.29 (1H, d, J = 15.9 Hz, H-2′), 5.86 (1H, s, 4-OH), 4.25 (2H, q, J = 7.1 Hz, H-4′), 3.93 (3H, s, 3-OCH3), 1.33 (3H, t, J = 7.1 Hz, H-5′); 13C NMR (CDCl3, 150 MHz): δC 127.2 (C-1), 109.4 (C-2), 148.0 (C-3), 146.9 (C-4), 114.8 (C-5), 123.2 (C-6), 144.8 (C-1′), 115.8 (C-2′), 167.4 (C-3′), 60.5 (C-4′), 14.5 (C-5′), 56.1 (3-OCH3). ESI-MS m/z: 245.1 [M + Na]+.
- Compound 9: 1H NMR (CDCl3, 400 MHz): δH 7.96 (2H, m, H-2, H-6), 6.87 (2H, m, H-3, H-5), 4.35 (2H, q, J = 7.1 Hz, H-2′), 1.38 (3H, t, J = 7.1 Hz, H-3′); 13C NMR (CDCl3, 100 MHz): δC 123.0 (C-1), 132.0 (C-2, C-6), 115.3 (C-3, C-5), 160.2 (C-4), 166.9 (C-1′), 61.0 (C-2′) 14.5 (C-3′). ESI-MS m/z: 189.0 [M + Na]+.
- Compound 10: 1H NMR (CDCl3, 600 MHz): δH 9.87 (1H, s, H-1′), 7.81 (2H, d, J = 8.7 Hz, H-2, H-6), 6.96 (2H, d, J = 8.6 Hz, H-3, H-5); 13C NMR (CDCl3, 150 MHz): δC 130.2 (C-1), 132.5, (C-2, C-6), 116.1 (C-3, C-5), 161.5 (C-4), 191.1 (C-1′). ESI-MS m/z: 123.0 [M + H]+.
2.5. In Vitro Mushroom Tyrosinase Inhibitory Activity Assay
2.6. In Silico Tyrosinase-Compound Binding Mechanism Analysis
3. Results and Discussion
3.1. Identification of Phytochemicals
3.2. Mushroom Tyrosinase Inhibitory Activity
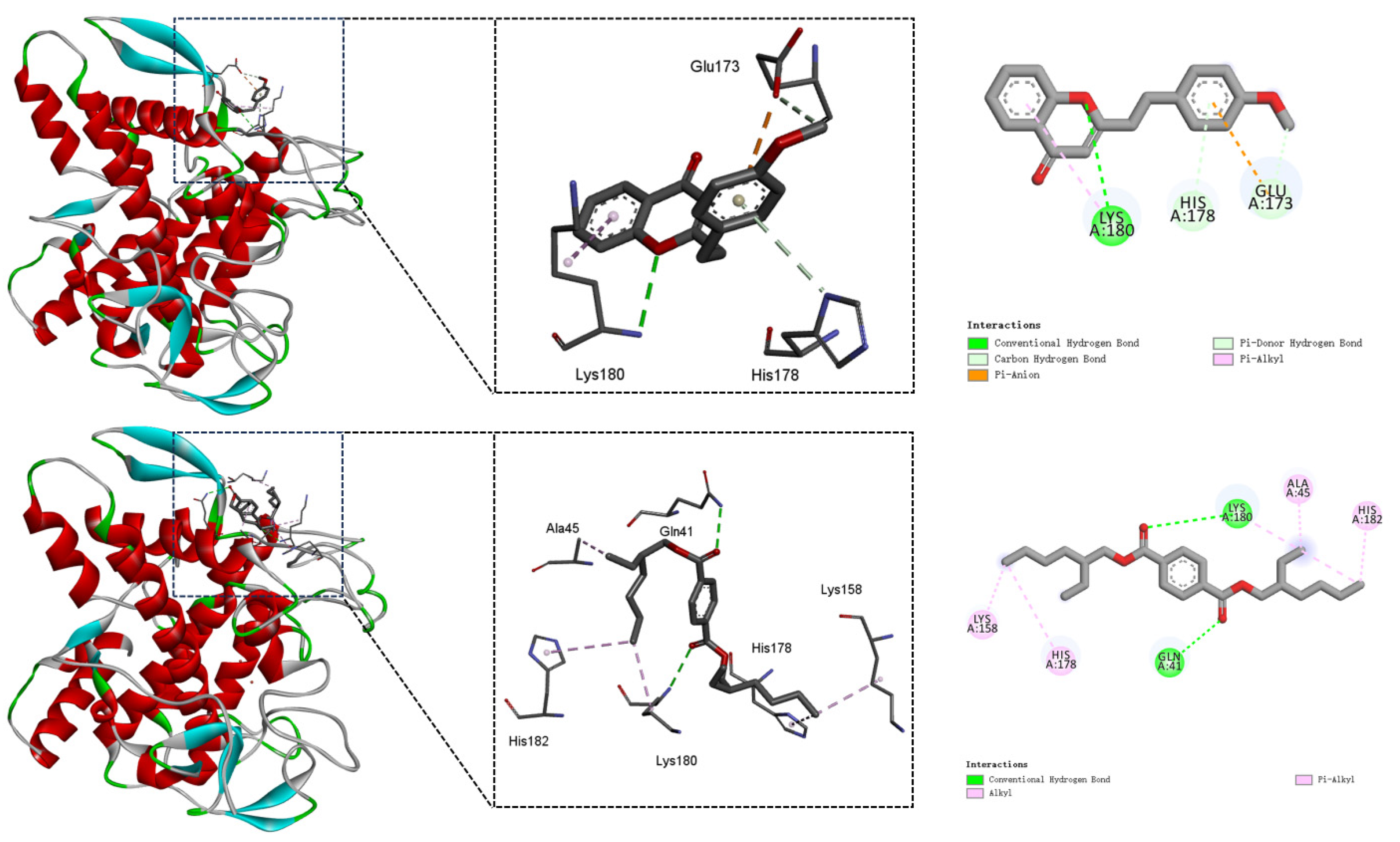
3.3. Mushroom Tyrosinase-Compound Binding Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| C | chloroform |
| CC | column chromatography |
| D | dichloromethane |
| E | ethyl acetate |
| K | kojic acid |
| M | methanol |
| P | petroleum ether |
References
- Chang, T.-S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef]
- Yang, S.; Jiang, J.; Zhou, A.; Zhou, Y.; Ye, W.; Cao, D.-S.; Yang, R. Substrate-photocaged enzymatic fluorogenic probe enabling sequential activation for light-controllable monitoring of intracellular tyrosinase activity. Anal. Chem. 2020, 92, 7194–7199. [Google Scholar] [CrossRef]
- Vanitha, V.; Soundhari, S. Isolation and characterisation of mushroom tyrosinase and screening of herbal extracts for anti-tyrosinase activity. Int. J. ChemTech Res. 2017, 10, 1156–1167. [Google Scholar]
- Arrowitz, C.; Schoelermann, A.M.; Mann, T.; Jiang, L.I.; Weber, T.; Kolbe, L. Effective tyrosinase inhibition by Thiamidol results in significant improvement of mild to moderate melasma. J. Investig. Dermatol. 2019, 139, 1691–1698. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Labay, C.; Hosta-Rigau, L. Tyrosinase-loaded multicompartment microreactor toward melanoma depletion. ACS Appl. Mater. Interfaces 2019, 11, 5862–5876. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]
- Bonesi, M.; Xiao, J.; Tundis, R.; Aiello, F.; Sicari, V.; Loizzo, R.M. Advances in the tyrosinase inhibitors from plant source. Curr. Med. Chem. 2019, 26, 3279–3299. [Google Scholar] [CrossRef] [PubMed]
- Parvez, S.; Kang, M.; Chung, H.-S.; Bae, H. Naturally occurring tyrosinase inhibitors: Mechanism and applications in skin health, cosmetics and agriculture industries. Phytother. Res. 2007, 21, 805–816. [Google Scholar] [CrossRef]
- Burlando, B.; Clericuzio, M.; Cornara, L. Moraceae plants with tyrosinase inhibitory activity: A review. Mini-Rev. Med. Chem. 2017, 17, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xu, X.; Liu, C.; Liu, L. Exploring the potential of lemon peel extracts in cosmetics: Chemical composition and bioactive properties. J. Microbiol. Biotechnol. 2025, 35, e2412042. [Google Scholar] [CrossRef] [PubMed]
- Qurat ul, A.; Saleem, M.; Nazir, M.; Riaz, N.; Tousif, M.I.; Tauseef, S.; Hassan, L.; Zengin, G.; Sharifi-Rad, M.; Shah, S.A.A. Secondary metabolite profiling, antioxidant capacity, enzyme inhibitory potential and in silico studies of Launaea intybacea (Jacq.) Beauverd: A multifunctional approach to probe into the new nutraceuticals. J. Mol. Struct. 2023, 1294, 136480. [Google Scholar] [CrossRef]
- Kuerban, G.; Turak, A.; Begmatov, N.B.; Zhao, J.; Aisa, H.A. Chemical composition of Artemisia scoparia and their bioactivities. Chem. Biodivers. 2024, 21, e202400414. [Google Scholar] [CrossRef]
- Gogoi, R.; Sarma, N.; Begum, T.; Chanda, S.K.; Lekhak, H.; Sastry, G.N.; Lal, M. Agarwood (Aquilaria malaccensis L.) a quality fragrant and medicinally significant plant based essential oil with pharmacological potentials and genotoxicity. Ind. Crops Prod. 2023, 197, 116535. [Google Scholar] [CrossRef]
- Al-Najjar, B.O.; Elshibani, F.; Sharkasi, M.A.; Shintiri, N.E.; Naili, E.E.; Abdulsayid, M.; Abozayed, R.S.; Mohammed, H.A. Phytochemical analysis, bioactivity, and molecular docking studies of Myrtus communis L. seeds and fruit peel extracts demonstrating antioxidant and anti-tyrosinase properties. Sci. Rep. 2025, 15, 5634. [Google Scholar] [CrossRef]
- Cakmak, U.; Tuncay, F.O.; Kolcuoglu, Y. Comparative analysis of LC-HRMS profiling, ATR FT-IR spectroscopy, antioxidant, anti-diabetic, and anti-tyrosinase activities of extracts from Cotoneaster frigidus Wall. Ex Lindl. “Cornubia” fruit. Food Res. Int. 2025, 201, 115535. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Song, W.; Jiang, Y.; Dai, Y.; Qin, Z.; Liu, L.; Wei, S.; Chen, H. Structural characterization of complex tannins from Euryale ferox fruit peels and their inhibitory mechanisms against tyrosinase activity and melanogenesis. Int. J. Biol. Macromol. 2025, 298, 139909. [Google Scholar] [CrossRef] [PubMed]
- Bu, Q.; Yang, Q.-B.; Ge, Z.-Y.; Shi, Z.-X.; Liang, L.-F. Phytochemicals and health-promoting evaluations of Ophiorrhiza puffii, a Chinese endemic plant. Nat. Prod. Res. 2025, in press. [CrossRef] [PubMed]
- Joo, Y.; Seo, Y.H.; Lee, S.; Shin, E.; Yeon, S.W.; Kim, S.B.; Lee, M.K. Antioxidant and tyrosinase-inhibitory activities and biological bioactivities of flavonoid derivatives from Quercus mongolica pollen. Molecules 2025, 30, 794. [Google Scholar] [CrossRef]
- Xu, Z.-Y.; Hu, Z.; La, C.-S.; Du, N.-N.; Bai, M.; Hao, J.; Lin, B.; Huang, X.-X.; Song, S.-J. Hydroxyl-amide alkaloids from pepper roots: Potential sources of natural antioxidants and tyrosinase inhibitors. J. Agric. Food Chem. 2024, 72, 19800–19811. [Google Scholar] [CrossRef]
- Bu, Q.; Ge, Z.-Y.; Liang, L.-F. Chemical and biological investigation on the potential ornamental plant Ophiorrhiza chinensis. Agronomy 2024, 14, 1872. [Google Scholar] [CrossRef]
- Watti, O.I.; Yalo, M.; Sharma, R.; Makhaba, M.; Hussein, A.A.; Mabusela, W.T. Phytochemistry, anti-tyrosinase, and anti-diabetes studies of extracts and chemical constituents of Dicerothamnus rhinocerotis leaves. Chemistry 2024, 6, 546–554. [Google Scholar] [CrossRef]
- Khan, M.T.H.; Khan, S.B.; Ather, A. Tyrosinase inhibitory cycloartane type triterpenoids from the methanol extract of the whole plant of Amberboa ramosa Jafri and their structure–activity relationship. Bioorg. Med. Chem. 2006, 14, 938–943. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.; Xie, Z.; Zheng, D.-K.; Li, J.; Tan, G.-S. Lycopodiastrum casuarinoides: An overview of their phytochemicals, biological activities, structure-activity relationship, biosynthetic pathway and 13C NMR data. Fitoterapia 2023, 165, 105425. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Li, W.-Y.; Gao, B.-B.; Zhao, Q.-S. Lycocasine A, a Lycopodium alkaloid from Lycopodiastrum casuarinoides and its acid-sensing ion channel 1a inhibitory activity. Molecules 2024, 29, 1581. [Google Scholar] [CrossRef]
- Jiang, S.; Li, W.-Y.; Gao, B.-B.; Ou, Y.-F.; Yuan, Z.-F.; Zhao, Q.-S. Casuattimines A–N, fourteen new Lycopodium alkaloids from Lycopodiastrum casuarinoides with Cav3.1 channel inhibitory activity. Bioorg. Chem. 2024, 142, 106962. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, J.; Li, D.; Li, X.-M.; Li, D.; Zhou, G.; Xu, K.-P.; Kang, F.-H.; Zou, Z.-X.; Xu, P.-S.; et al. Anti-cholinesterase activities of constituents isolated from Lycopodiastrum casuarinoides. Fitoterapia 2019, 139, 104366. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.N.; Thi Hong Nguyen, H.; Thuong Thi Trinh, D.; Hong Van Le, T.; Nguyen, T.D.; Vinh, D.; To, D.C.; Huynh, L.; Tran, M.H. Anti-inflammatory activity via NO production inhibition of compounds from Vietnamese Lycopodium casuarinoides Spring. Phytochem. Lett. 2023, 58, 42–48. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, X.-C.; Li, J.; Zou, Z.-X.; Xi, C.; Xu, K.-P.; Kang, F.-H.; Xu, P.-S.; Tan, G.-S. New unsaturated fatty acids from the aerial parts of Lycopodiastrum casuarinoides. Phytochem. Lett. 2021, 41, 55–60. [Google Scholar] [CrossRef]
- Jiang, M.-x.; Yao, X.-c.; Liu, Y.; Tan, G.-s.; Li, J. Chemical constituents from Lycopodiastrum casuarinoides. Chin. Tradit. Pat. Med. 2022, 44, 821–824. [Google Scholar] [CrossRef]
- Wang, L.-L.; Hao, L.-J.; Zhou, Z.-B.; Zhu, X.-L.; Shi, Z.-H.; Miyamoto, T.; Pan, K. Lycodine-type alkaloids and their glycosides from Lycopodiastrum casuarinoides. Phytochemistry 2018, 154, 63–72. [Google Scholar] [CrossRef]
- Zhang, H.-B.; Hu, J.; Li, J.-X.; Hao, S.-H. Cytotoxic lycodine alkaloids from the aerial parts of Lycopodiastrum casuarinoides. J. Asian Nat. Prod. Res. 2020, 22, 217–224. [Google Scholar] [CrossRef]
- Tang, Y.; Fu, Y.; Xiong, J.; Li, M.; Ma, G.-L.; Yang, G.-X.; Wei, B.-G.; Zhao, Y.; Zhang, H.-Y.; Hu, J.-F. Casuarinines A–J, lycodine-type alkaloids from Lycopodiastrum casuarinoides. J. Nat. Prod. 2013, 76, 1475–1484. [Google Scholar] [CrossRef]
- Ismaya, W.T.; Rozeboom, H.J.; Weijn, A.; Mes, J.J.; Fusetti, F.; Wichers, H.J.; Dijkstra, B.W. Crystal structure of Agaricus bisporus mushroom tyrosinase: Identity of the tetramer subunits and interaction with tropolone. Biochemistry 2011, 50, 5477–5486. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, W.-Y.; Chen, W.-J.; Wei, J.-H.; Liang, C.-Y.; Feng, X.; Lu, C.-S. Chemical constituents and antioxidant activity in vitro of Zhuang medicine Dalbergia rimosa. J. Chin. Med. Mater. 2024, 47, 1147–1152. [Google Scholar] [CrossRef]
- Teng, X.-F.; Liu, Y.-L.; Yang, F.; Wang, H.-J.; He, L. Chemical constituents from Lycopodium fargesii and their anti-osteoporotic activity. Nat. Prod. Res. Dev. 2022, 34, 226–231. [Google Scholar] [CrossRef]
- Vasconcelos, J.M.J.; Silva, A.M.S.; Cavaleiro, J.A.S. Chromones and flavanones from Artemisia campestris subsp. maritima. Phytochemistry 1998, 49, 1421–1424. [Google Scholar] [CrossRef]
- Peng, K.; Mei, W.-L.; Wu, J.; Dai, H.-F. Flavones from the stem of Aquilaria sinensis. J. Trop. Suptrop. Bot. 2010, 18, 97–100. Available online: http://jtsb.ijournals.cn/jtsb_en/article/abstract/2415 (accessed on 11 August 2025).
- Nakanishi, T.; Inada, A.; Nishi, M.; Yamagata, E.; Yoneda, K. A new and a known derivatives of 2-(2-phenylethyl) chromone from a kind of agarwood (“Kanankoh,” in Japanese) originating from Aquilaria agallocha. J. Nat. Prod. 1986, 49, 1106–1108. [Google Scholar] [CrossRef]
- Ma, Z.-Y.; Lu, Q.-X.; Nian, H.-F.; Teng, H.-D.; Yang, G.-Z.; Chen, Y. Chemical constituents from Garcinia multiflora fruits. Chin. Tradit. Herb. Drugs 2019, 50, 17–21. [Google Scholar] [CrossRef]
- Pal, P.P.; Begum, S.A.; Basha, A.S.; Araya, H.; Fujimoto, Y. A new lignan (polonilignan) and inhibitors of nitric oxide production from Penicillium polonicum, an endophytic fungi of Piper nigrum. Chem. Biodivers. 2023, 20, e202200840. [Google Scholar] [CrossRef]
- Wang, X.-N.; Du, J.-C.; Tan, R.-X.; Liu, J.-K. Chemical constituents of basidiomycete Hydnum repandum. Chin. Tradit. Herb. Drugs 2005, 36, 1126–1130. [Google Scholar] [CrossRef]
- Ashraf, Z.; Rafiq, M.; Nadeem, H.; Hassan, M.; Afzal, S.; Waseem, M.; Afzal, K.; Latip, J. Carvacrol derivatives as mushroom tyrosinase inhibitors; synthesis, kinetics mechanism and molecular docking studies. PLoS ONE 2017, 12, e0178069. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Z.; Li, Q.; Hu, Z.; Chen, Y.; Chen, H.; Cai, W.; Du, Q.; Zhang, P.; Xiong, D.; et al. Network pharmacology and molecular docking reveal the mechanisms of curcumin activity against esophageal squamous cell carcinoma. Front. Pharmacol. 2024, 15, 1282361. [Google Scholar] [CrossRef] [PubMed]
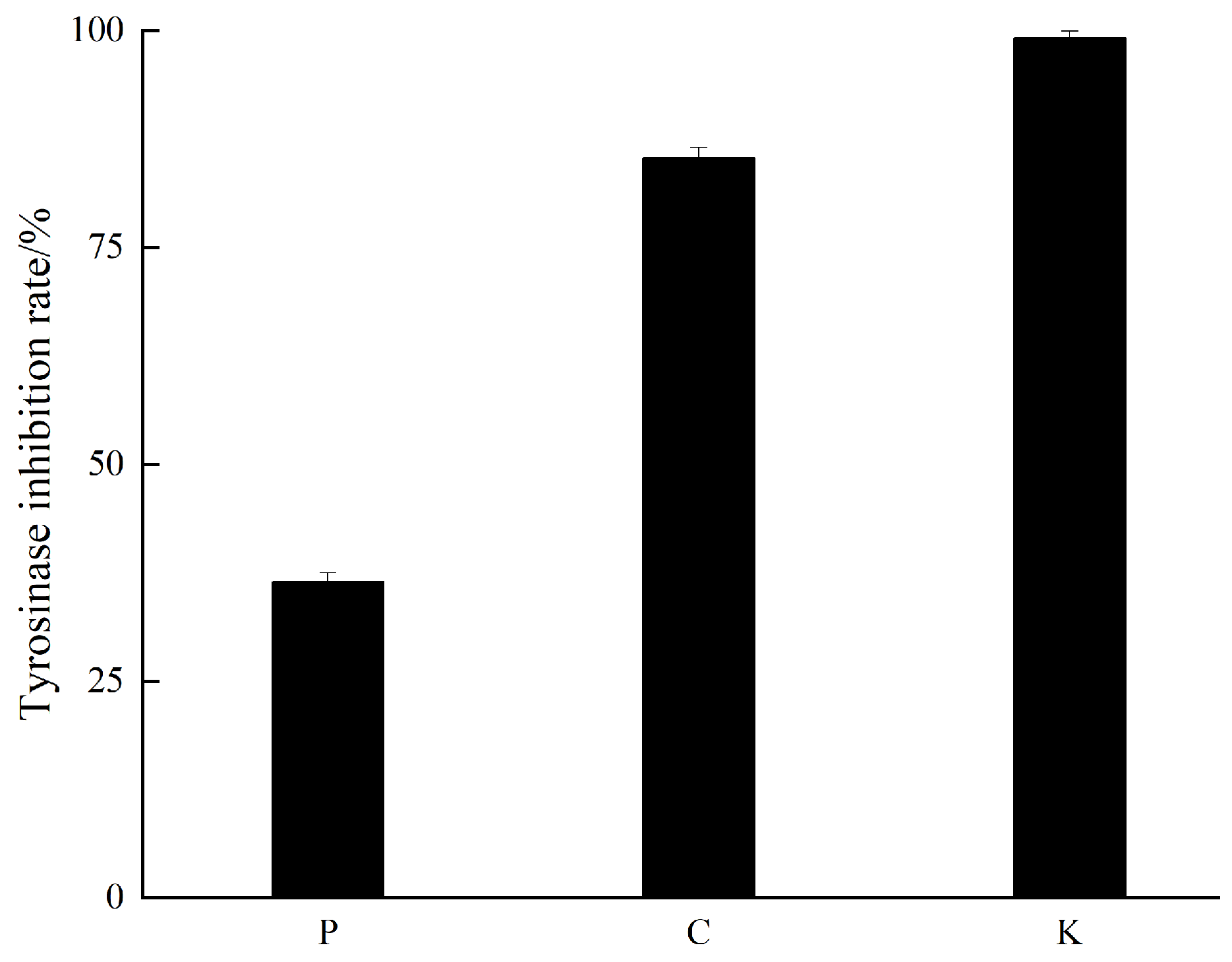

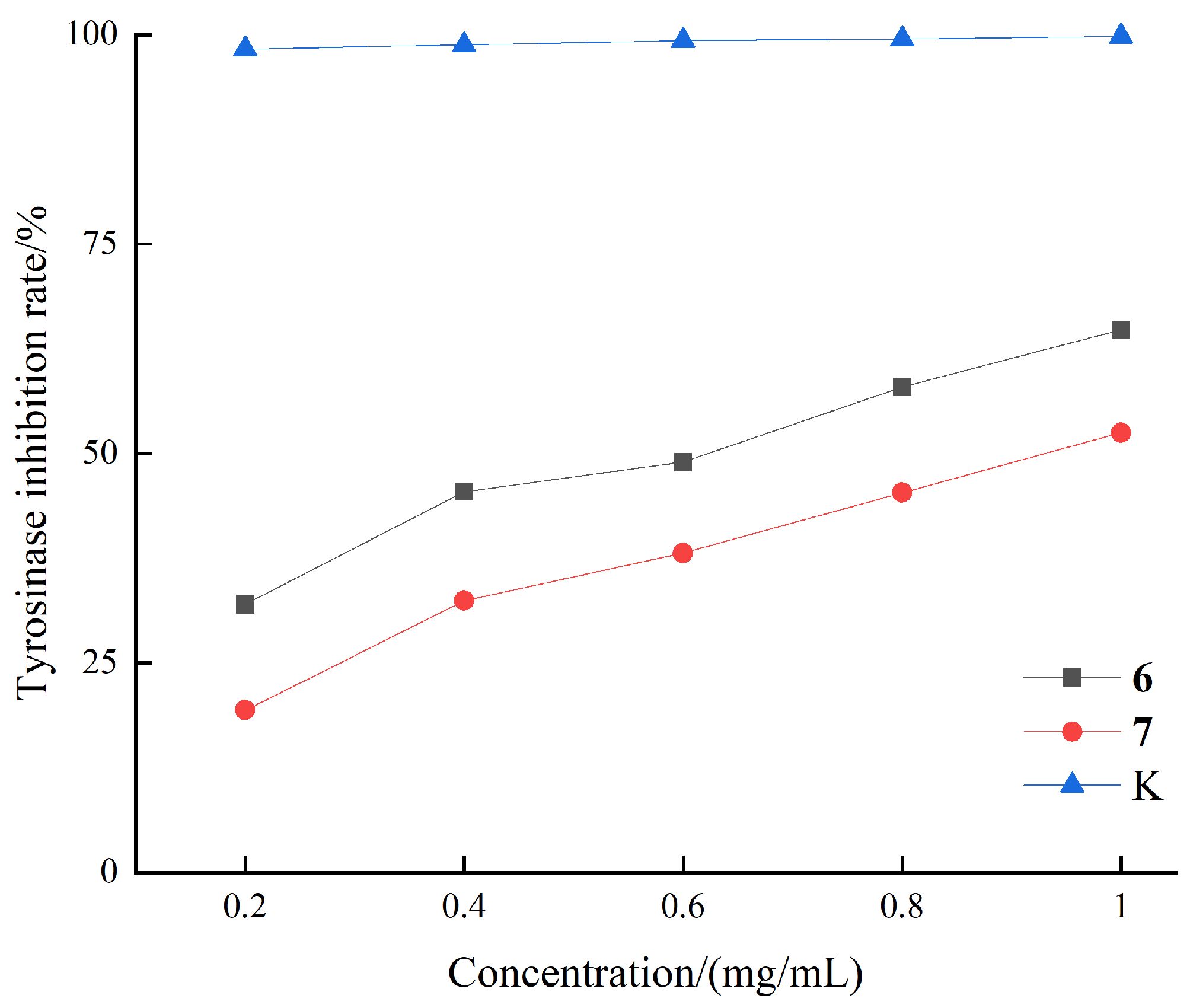
| Compounds | IC50 (mM) |
|---|---|
| 6 | 1.90 ± 0.03 |
| 7 | 2.43 ± 0.03 |
| Kojic acid 1 | 0.17 ± 0.03 |
| Compounds | Binding Energy (kcal/mol) |
|---|---|
| 6 | −6.60 |
| 7 | −5.75 |
| tropolone 1 | −6.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.-Y.; Ge, Z.-Y.; Yang, Q.-B.; Jiang, C.-F.; Wu, L.; Jiang, X.-Y.; Liang, L.-F. Bioassay-Guided Isolation of Chemical Constituents from Lycopodiastrum casuarinoides and Targeted Evaluation of Their Potential Efficacy in Cosmetics. Cosmetics 2025, 12, 174. https://doi.org/10.3390/cosmetics12040174
Zhu J-Y, Ge Z-Y, Yang Q-B, Jiang C-F, Wu L, Jiang X-Y, Liang L-F. Bioassay-Guided Isolation of Chemical Constituents from Lycopodiastrum casuarinoides and Targeted Evaluation of Their Potential Efficacy in Cosmetics. Cosmetics. 2025; 12(4):174. https://doi.org/10.3390/cosmetics12040174
Chicago/Turabian StyleZhu, Jian-Ye, Zeng-Yue Ge, Qi-Bin Yang, Cai-Fu Jiang, Lei Wu, Xin-Yuan Jiang, and Lin-Fu Liang. 2025. "Bioassay-Guided Isolation of Chemical Constituents from Lycopodiastrum casuarinoides and Targeted Evaluation of Their Potential Efficacy in Cosmetics" Cosmetics 12, no. 4: 174. https://doi.org/10.3390/cosmetics12040174
APA StyleZhu, J.-Y., Ge, Z.-Y., Yang, Q.-B., Jiang, C.-F., Wu, L., Jiang, X.-Y., & Liang, L.-F. (2025). Bioassay-Guided Isolation of Chemical Constituents from Lycopodiastrum casuarinoides and Targeted Evaluation of Their Potential Efficacy in Cosmetics. Cosmetics, 12(4), 174. https://doi.org/10.3390/cosmetics12040174








