The Effectiveness and Safety of 1470 nm Non-Ablative Laser Therapy for the Treatment of Striae Distensae: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Study Population
2.2. Assesment and Treatment Protocol
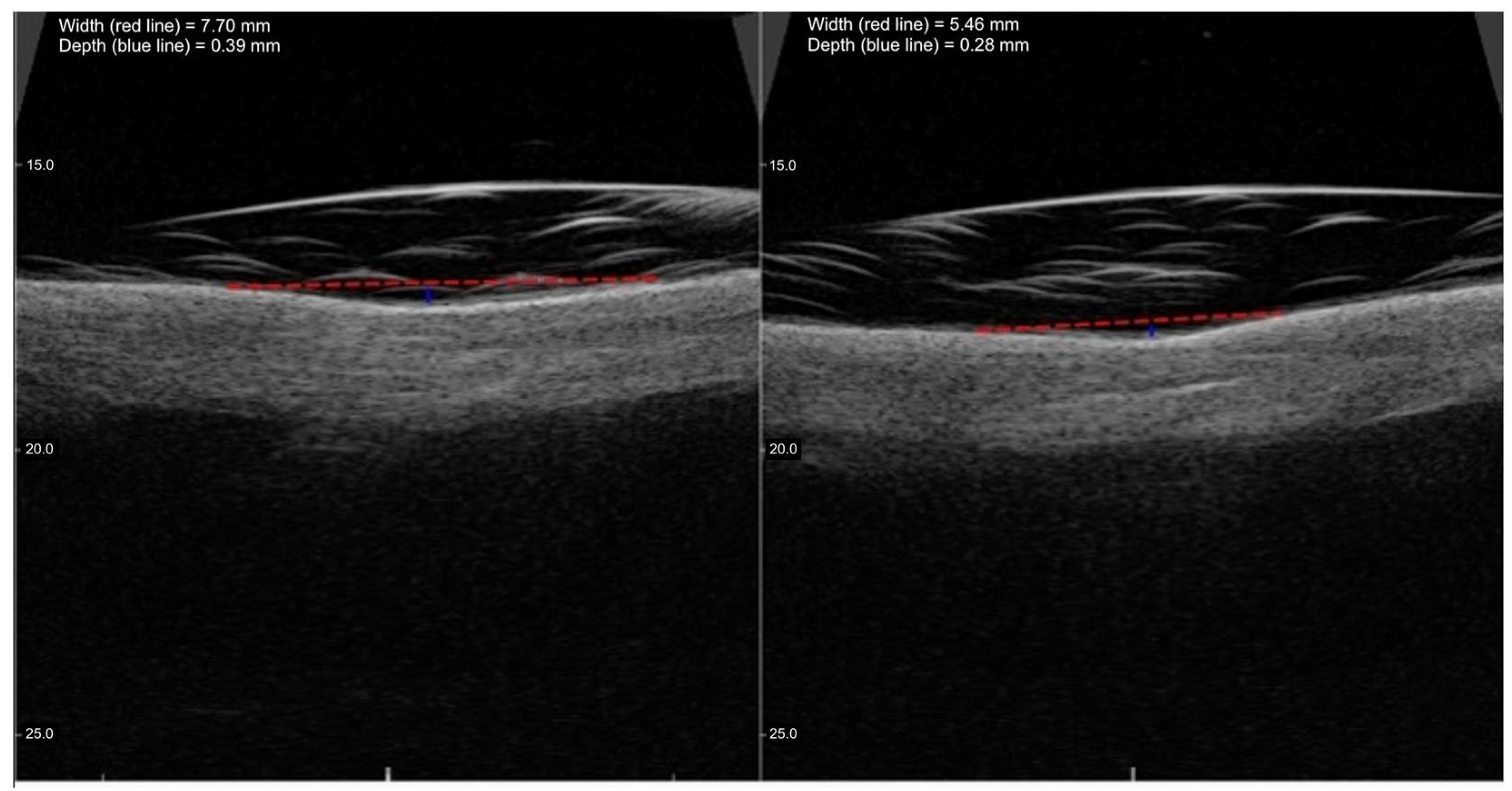
2.3. High-Frequency Ultrasound Evaluation
2.4. Statistical Analysis
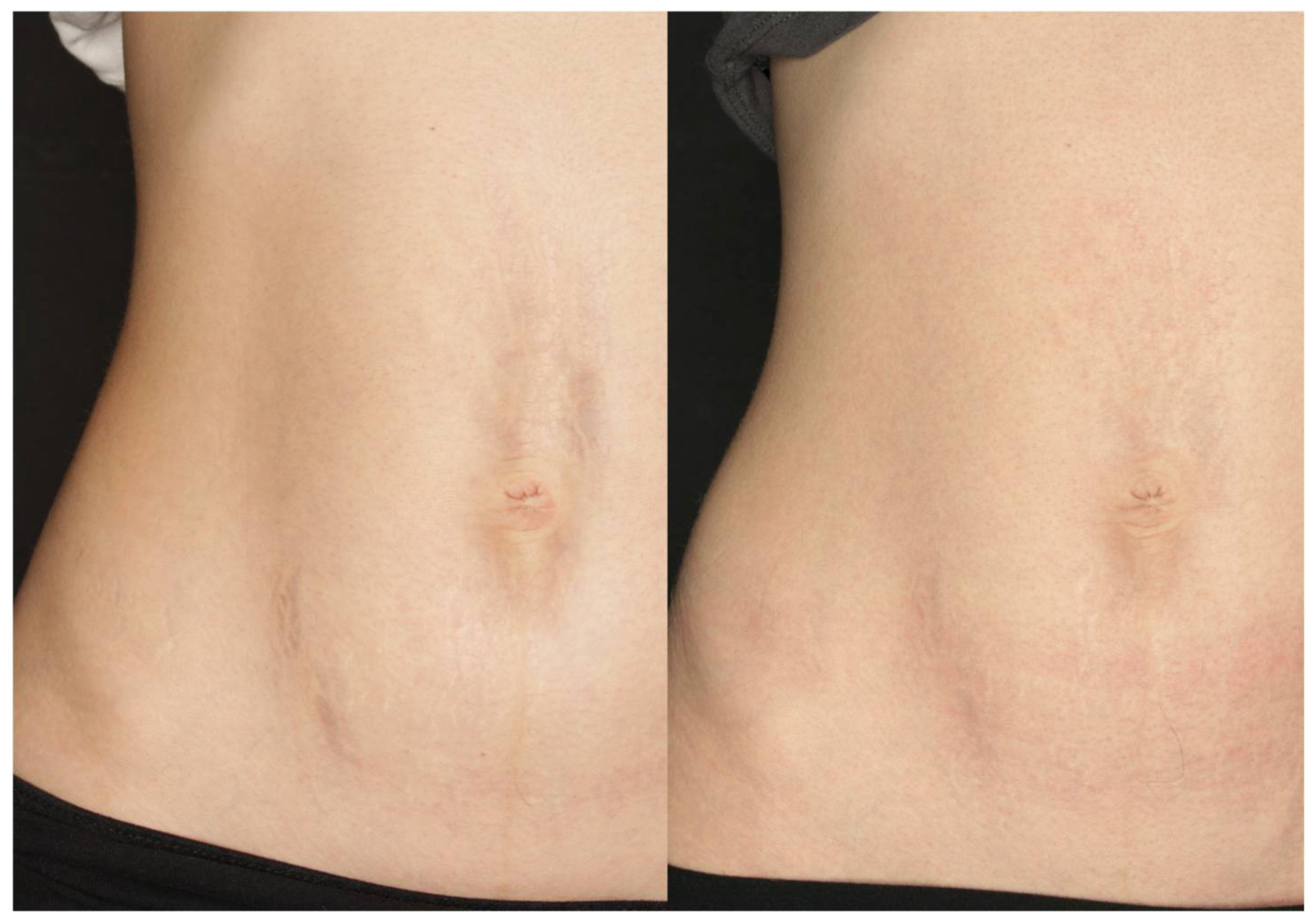
3. Results
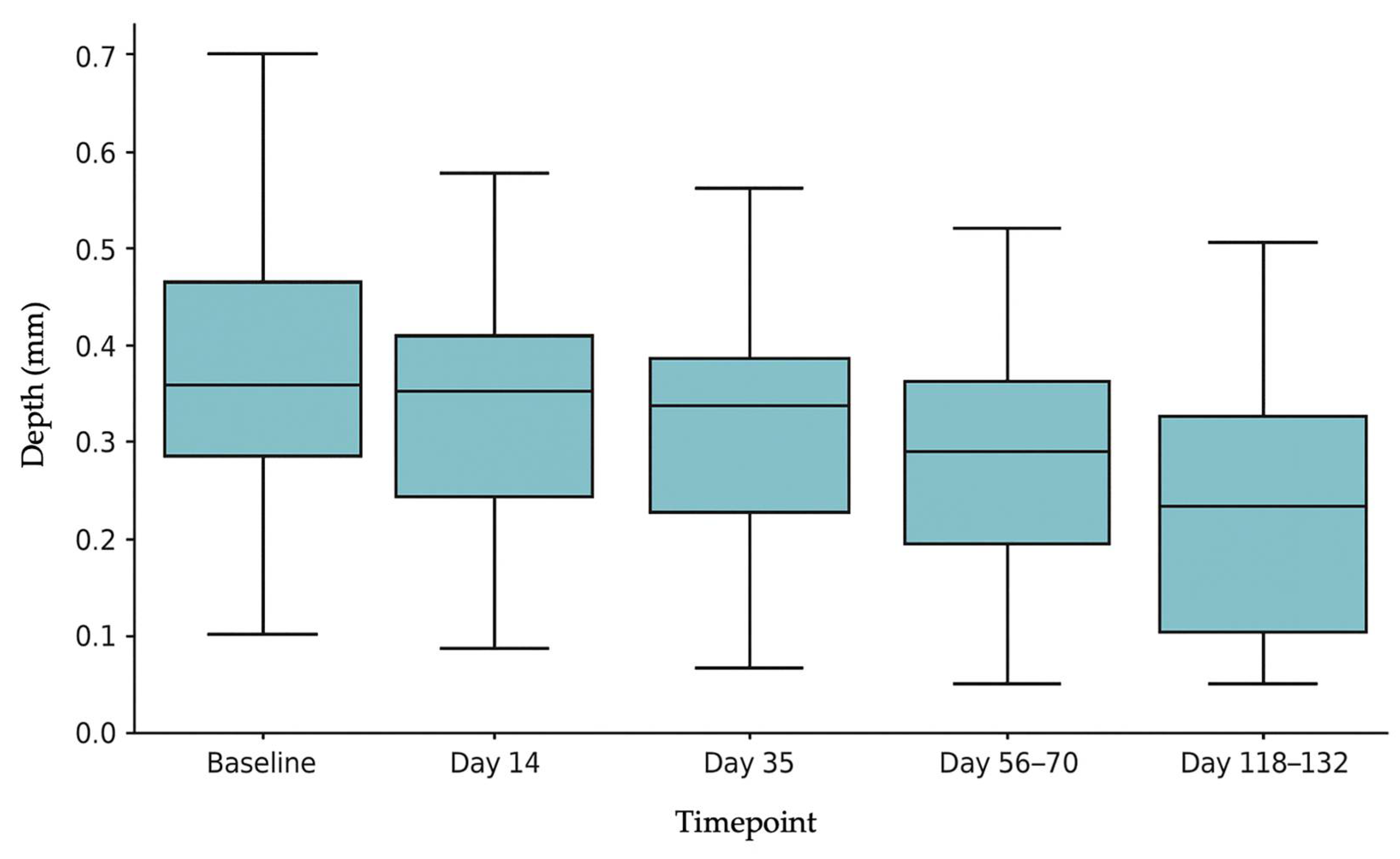
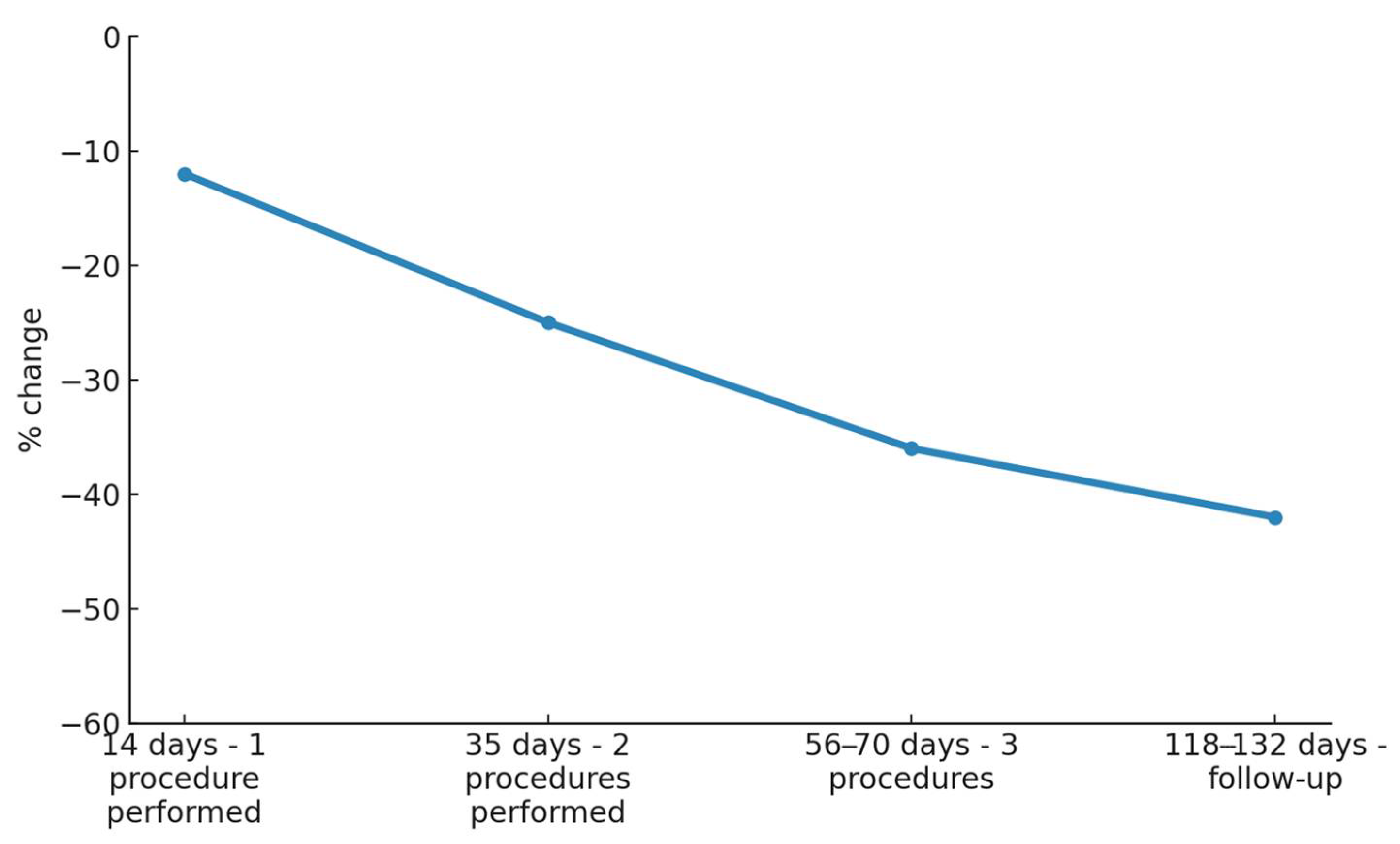
3.1. SD Depth
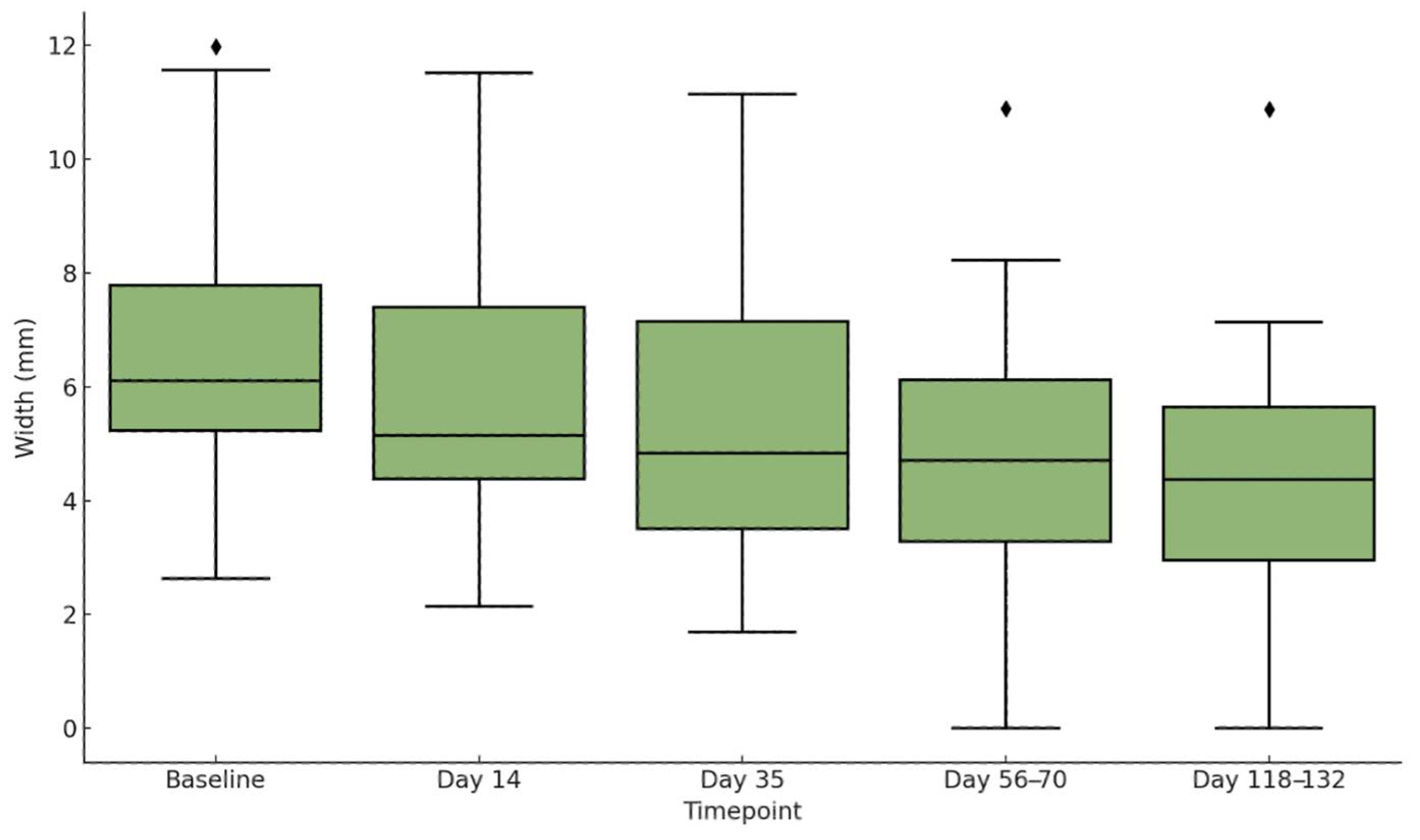
3.2. SD Width
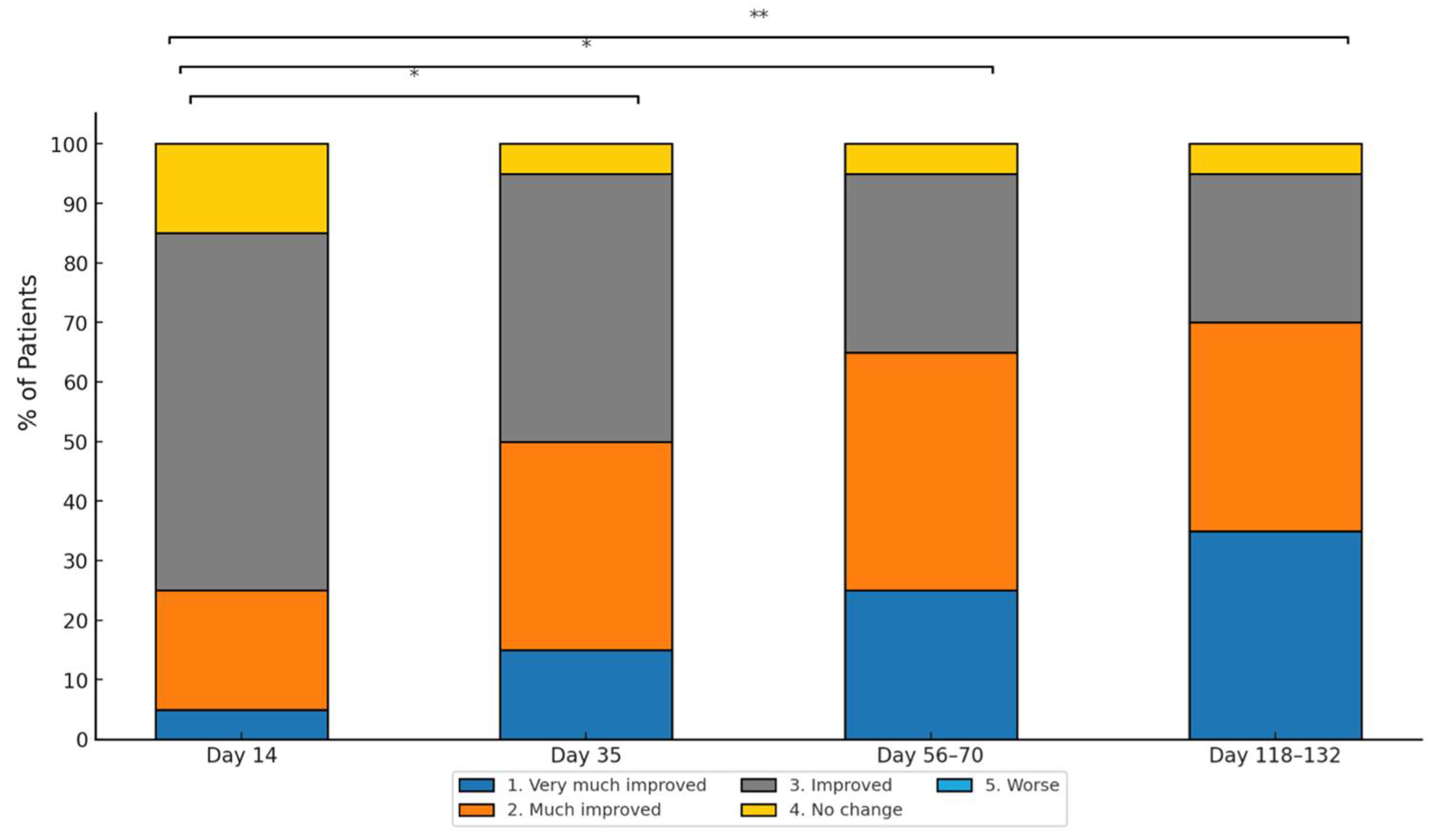
3.3. GAIS Score and Adverse Events
3.4. Safety and Tolerability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SD | Striae Distensae |
| ICD | International Classification of Diseases |
| HFUS | High-Frequency Ultrasound |
| SPSS | Statistical Package for the Social Sciences |
| ANOVA | Analysis of Variance |
| PRP | Platelet-Rich Plasma |
| SVF | Stromal Vascular Fraction |
| CO2 | Carbon Dioxide |
| Er:Glass | Erbium:Glass |
| Er:YAG | Erbium-doped Yttrium Aluminium Garnet |
| IPL | Intense Pulsed Light |
| PDL | Pulsed Dye Laser |
| GAIS | Global Aesthetic Improvement Scale |
| SD width | Width of Striae Distensae |
| SD depth | Depth of Striae Distensae |
References
- Yamaguchi, K.; Suganuma, N.; Ohashi, K. Quality of life evaluation in Japanese pregnant women with striae gravidarum: A cross-sectional study. BMC Res. Notes 2012, 5, 450. [Google Scholar] [CrossRef]
- Elsaie, M.L.; Baumann, L.S.; Elsaaiee, L.T. Striae distensae (stretch marks) and different modalities of therapy: An update. Dermatol. Surg. 2009, 35, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Al-Himdani, S.; Ud-Din, S.; Gilmore, S.; Bayat, A. Striae distensae: A comprehensive review and evidence-based evaluation of prophylaxis and treatment. Br. J. Dermatol. 2014, 170, 527–547. [Google Scholar] [CrossRef] [PubMed]
- Gauglitz, G.G.; Reinholz, M.; Kaudewitz, P.; Schauber, J.; Ruzicka, T. Treatment of striae distensae using an ablative Erbium: YAG fractional laser versus a 585-nm pulsed-dye laser. J. Cosmet. Laser. Ther. 2014, 16, 117–119. [Google Scholar] [CrossRef]
- Burrows, N.P.; Lovell, C.R. Disorders of connective tissue. In Rook’s Textbook of Dermatology, 7th ed.; Burns, T., Breathnach, S., Cox, N., Griffith, C., Eds.; Blackwell Science: Oxford, UK, 2004; pp. 46–47. [Google Scholar] [CrossRef]
- McDaniel, D.H. Laser therapy of stretch marks. Dermatol. Clin. 2002, 20, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.E.B.; Parry, E.J.; Humphries, J.D.; Jones, C.J.; Polson, D.W.; Kielty, C.M.; Griffiths, C.E.M. Fibrillin microfibrils are reduced in skin exhibiting striae distensae. Br. J. Dermatol. 1998, 138, 931–937. [Google Scholar] [CrossRef]
- Elson, M.L. Treatment of striae distensae with topical tretinoin. J. Dermatol. Surg. Oncol. 1990, 16, 267–270. [Google Scholar] [CrossRef]
- Kang, S.; Kim, K.J.; Griffiths, C.E.M.; Wong, T.-Y.; Talwar, H.S.; Fisher, G.J.; Gordon, D.; Hamilton, T.A.; Ellis, C.N.; Voorhees, J.J. Topical tretinoin (retinoic acid) improves early stretch marks. Arch. Dermatol. 1996, 132, 519–526. [Google Scholar] [CrossRef]
- Young, G.L.; Jewell, D. Creams for preventing stretch marks in pregnancy. Cochrane Database Syst. Rev. 2000, 2, CD000066. [Google Scholar] [CrossRef]
- Mallol, J.; Belda, M.; Costa, D.; Noval, A.; Sola, M. Prophylaxis of Striae gravidarum with a topical formulation. A double blind trial. Int. J. Cosmet. Sci. 1991, 13, 51–57. [Google Scholar] [CrossRef]
- Ash, K.; Lord, J.; Zukowskl, M.; McDANIEL, D.H. Comparison of topical therapy for striae alba (20% glycolic acid/0.05% tretinoin versus 20% glycolic acid/10% L-ascorbic acid). Dermatol. Surg. 1998, 24, 849–856. [Google Scholar] [CrossRef]
- Adatto, M.A.; Deprez, P. Striae treated by a novel combination treatment—Sand abrasion and a patent mixture containing 15% trichloracetic acid followed by 6–24 hrs of a patent cream under plastic occlusion. J. Cosmet. Dermatol. 2003, 2, 61–67. [Google Scholar] [CrossRef]
- Deprez, P. Easy Peel for the Treatment of Stretch Marks. Int. J. Cosmet. Surg. Aesthetic Dermatol. 2000, 2, 201–204. [Google Scholar] [CrossRef]
- Mazzarello, V.; Farace, F.; Ena, P.; Fenu, G.; Mulas, P.; Piu, L.; Rubino, C. A superficial texture analysis of 70% glycolic acid topical therapy and striae distensae. Plast. Reconstr. Surg. 2012, 129, 589e–590e. [Google Scholar] [CrossRef] [PubMed]
- Karimipour, D.J.; Kang, S.; Johnson, T.M.; Orringer, J.S.; Hamilton, T.; Hammerberg, C.; Voorhees, J.J.; Fisher, G. Microdermabrasion: A molecular analysis following a single treatment. J. Am. Acad. Dermatol. 2005, 52, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Lokhande, A.J.; Mysore, V. Striae Distensae Treatment Review and Update. Indian. Dermatol. Online J. 2019, 10, 380–395. [Google Scholar] [CrossRef] [PubMed]
- Park, K.Y.; Kim, H.K.; Kim, S.E.; Kim, B.J.; Kim, M.N. Treatment of striae distensae using needling therapy: A pilot study. Dermatol. Surg. 2012, 38, 1823–1828. [Google Scholar] [CrossRef]
- Aust, M.C.; Fernandes, D.; Kolokythas, P.; Kaplan, H.M.; Vogt, P.M. Percutaneous collagen induction therapy: An alternative treatment for scars, wrinkles, and skin laxity. Plast. Reconstr. Surg. 2008, 121, 1421–1429. [Google Scholar] [CrossRef]
- Kubik, P.; Gruszczyński, W.; Łukasik, B. Assessment of Safety and Mechanisms of Action of the 1470 nm LaserMe Device. Clin. Case Rep. Int. 2024, 8, 1683. [Google Scholar]
- Kubik, P.; Bighetti, S.; Bettolini, L.; Gruszczyński, W.; Łukasik, B.; Guida, S.; Stabile, G.; Herrera, E.M.M.; Carugno, A.; D’eSte, E.; et al. Effectiveness and safety of the use of 1470 nm laser therapy in patients suffering from acne scarring of the facial skin. Clin. Cosmet. Investig. Dermatol. 2025, 18, 543–551. [Google Scholar] [CrossRef]
- Oakley, A.M.; Patel, B.C. Stretch Marks. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Farahnik, B.; Park, K.; Kroumpouzos, G.; Murase, J. Striae gravidarum: Risk factors, prevention, and management. Int. J. Women’s Dermatol. 2016, 3, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Maione, V.; Bettolini, L.; Cozzi, C.; Bighetti, S.; Tomasi, C.; Calzavara-Pinton, P. Sexual quality of life in patients with pemphigus: A case-control study. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Bettolini, L.; Maione, V.; Arisi, M.; Rovaris, S.; Romanò, C.; Tomasi, C.; Calzavara-Pinton, P.; Zerbinati, N.; Bighetti, S. The influence of genital lichen sclerosus on sexual health and well-being: A tripartite comparative analysis. J. Low. Genit. Tract. Dis. 2024, 29, 81–87. [Google Scholar] [CrossRef]
- Forbat, E.; Al-Niaimi, F. Treatment of striae distensae: An evidence-based approach. J. Cosmet. Laser Ther. 2019, 21, 49–57. [Google Scholar] [CrossRef]
- Manstein, D.; Herron, G.S.; Sink, R.K.; Tanner, H.; Anderson, R.R. Fractional photothermolysis: A new concept for cutaneous remodeling using microscopic patterns of thermal injury. Lasers Surg. Med. 2004, 34, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Clementi, A.; Cannarozzo, G.; Guarino, L.; Zappia, E.; Cassalia, F.; Alma, A.; Sannino, M.; Longo, C.; Nisticò, S.P. Sequential Fractional CO2 and 1540/1570 nm Lasers: A Narrative Review of Preclinical and Clinical Evidence. J. Clin. Med. 2025, 14, 3867. [Google Scholar] [CrossRef]
- Karampinis, E.; Georgopoulou, K.-E.; Goudouras, G.; Lianou, V.; Kampra, E.; Schulze, A.V.R.; Zafiriou, E. Laser-Induced Koebner-Related Skin Reactions: A Clinical Overview. Medicina. 2024, 60, 1177. [Google Scholar] [CrossRef]

| Patient ID | Gender | Age | SD Location |
|---|---|---|---|
| 1 | F | 19 | breast |
| 2 | F | 50 | abdomen |
| 3 | F | 25 | abdomen |
| 4 | F | 18 | back |
| 5 | F | 56 | abdomen |
| 6 | F | 35 | abdomen |
| 7 | F | 34 | abdomen |
| 8 | F | 35 | buttocks |
| 9 | F | 32 | thighs |
| 10 | F | 35 | hips |
| 11 | F | 35 | thighs |
| 12 | F | 39 | thighs |
| 13 | F | 24 | thighs |
| 14 | F | 36 | abdomen |
| 15 | F | 35 | abdomen |
| 16 | F | 33 | buttocks |
| 17 | F | 36 | abdomen |
| 18 | F | 39 | buttocks |
| 19 | F | 23 | breast |
| 20 | F | 39 | abdomen |
| Timepoint | Mean Width (mm) | Standard Deviation (Width) | Change vs. Baseline (Width) | p-Value Width | Mean Depth (mm) | Standard Deviation (Depth) | Change vs. Baseline (Depth) | p-Value Depth |
|---|---|---|---|---|---|---|---|---|
| Before Treatments | 6.58 | 2.65 | - | - | 0.34 | 0.16 | - | - |
| Day 14 (14 days after 1st treatment) | 5.88 | 2.47 | −10.64% | <0.001 | 0.31 | 0.15 | −8.82% | <0.01 |
| Day 35 (21 days after 2nd treatment) | 5.20 | 2.33 | −20.97% | <0.001 | 0.27 | 0.13 | −20.59% | <0.001 |
| Day 56–70 (28–35 days after 3rd treatment) | 4.76 | 2.63 | −27.66% | <0.001 | 0.23 | 0.13 | −32.35% | <0.001 |
| Day 118–132 (90 days after end of treatments) | 4.40 | 2.52 | −33.13% | <0.001 | 0.18 | 0.15 | −47.06% | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubik, P.; Bighetti, S.; Bettolini, L.; Gruszczyński, W.; Łukasik, B.; Guida, S.; Stabile, G.; Paolino, G.; Murillo Herrera, E.M.; Carugno, A.; et al. The Effectiveness and Safety of 1470 nm Non-Ablative Laser Therapy for the Treatment of Striae Distensae: A Pilot Study. Cosmetics 2025, 12, 148. https://doi.org/10.3390/cosmetics12040148
Kubik P, Bighetti S, Bettolini L, Gruszczyński W, Łukasik B, Guida S, Stabile G, Paolino G, Murillo Herrera EM, Carugno A, et al. The Effectiveness and Safety of 1470 nm Non-Ablative Laser Therapy for the Treatment of Striae Distensae: A Pilot Study. Cosmetics. 2025; 12(4):148. https://doi.org/10.3390/cosmetics12040148
Chicago/Turabian StyleKubik, Paweł, Stefano Bighetti, Luca Bettolini, Wojciech Gruszczyński, Bartłomiej Łukasik, Stefania Guida, Giorgio Stabile, Giovanni Paolino, Elisa María Murillo Herrera, Andrea Carugno, and et al. 2025. "The Effectiveness and Safety of 1470 nm Non-Ablative Laser Therapy for the Treatment of Striae Distensae: A Pilot Study" Cosmetics 12, no. 4: 148. https://doi.org/10.3390/cosmetics12040148
APA StyleKubik, P., Bighetti, S., Bettolini, L., Gruszczyński, W., Łukasik, B., Guida, S., Stabile, G., Paolino, G., Murillo Herrera, E. M., Carugno, A., Valenti, M., Zane, C., Maione, V., D’Este, E., & Zerbinati, N. (2025). The Effectiveness and Safety of 1470 nm Non-Ablative Laser Therapy for the Treatment of Striae Distensae: A Pilot Study. Cosmetics, 12(4), 148. https://doi.org/10.3390/cosmetics12040148










