Stability, Bioactivity, and Skin Penetration of Prunus Leaf Extracts in Cream Formulations: A Clinical Study on Skin Irritation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Collection and Identification of Plant Materials
2.3. Preparation of Prunus Species Leaf Extracts
2.4. Determination of the Total Phenolic Content (TPC)
2.5. Determination of the Total Condensed Tannin Content (TTC)
2.6. Determination of Antioxidant Activity Using DPPH Assay
2.7. Determination of Lipid Peroxidation Inhibitory Activity Using theThiobarbituric Acid Reactive Substances (TBARS) Assay
2.8. Stability Test of the Prunus Species Leaf Extracts
2.9. Developed Cream Formulation and Its Stability Evaluation
2.10. In Vitro Skin Permeation Study of Developed Cream Formulation Using Franz Diffusion Cells
2.11. Clinical Evaluation
2.11.1. Study Design and Methods
- Male or female participants aged 30–50 years.
- Availability for the entire study duration.
- No use of medication or medical care for at least one month prior to and during the study.
- Free from any dermatological or systemic disorder that could interfere with test results or increase the risk of adverse reactions. Examples of systemic disorders include autoimmune diseases, diabetes, or severe cardiovascular conditions.
- Visible skin diseases at the study site (e.g., eczema, psoriasis) that could interfere with the evaluation.
- Systemic disorders such as autoimmune diseases, diabetes, or chronic inflammatory conditions that could affect skin response.
- Use of systemic or topical drugs or medication that could mask or affect study results.
- History of serious diseases, such as cardiovascular disease or malignancies, or known sensitivity to cosmetics.
- Diagnosis of chronic skin allergies.
- Known or suspected intolerance or hypersensitivity to the investigational products or any of their ingredients.
- Pregnancy or lactation during the study period.
2.11.2. Skin Safety Study in Human Volunteers
2.12. Statistical Analysis
3. Results and Discussion
3.1. Extraction of Prunus Species Leaf
3.2. Total Phenolic and Total Condensed Tannin Contents of Prunus Species Leaf Extracts (EL, JL, and CL)
3.3. Antioxidant Activity of Prunus Species Leaf Extracts (EL, JL, and CL) Using DPPH Assay
3.4. Lipid Peroxidation Inhibitory Activity of Prunus Species Leaf Extracts (EL, JL, and CL) Using the TBARS Assay
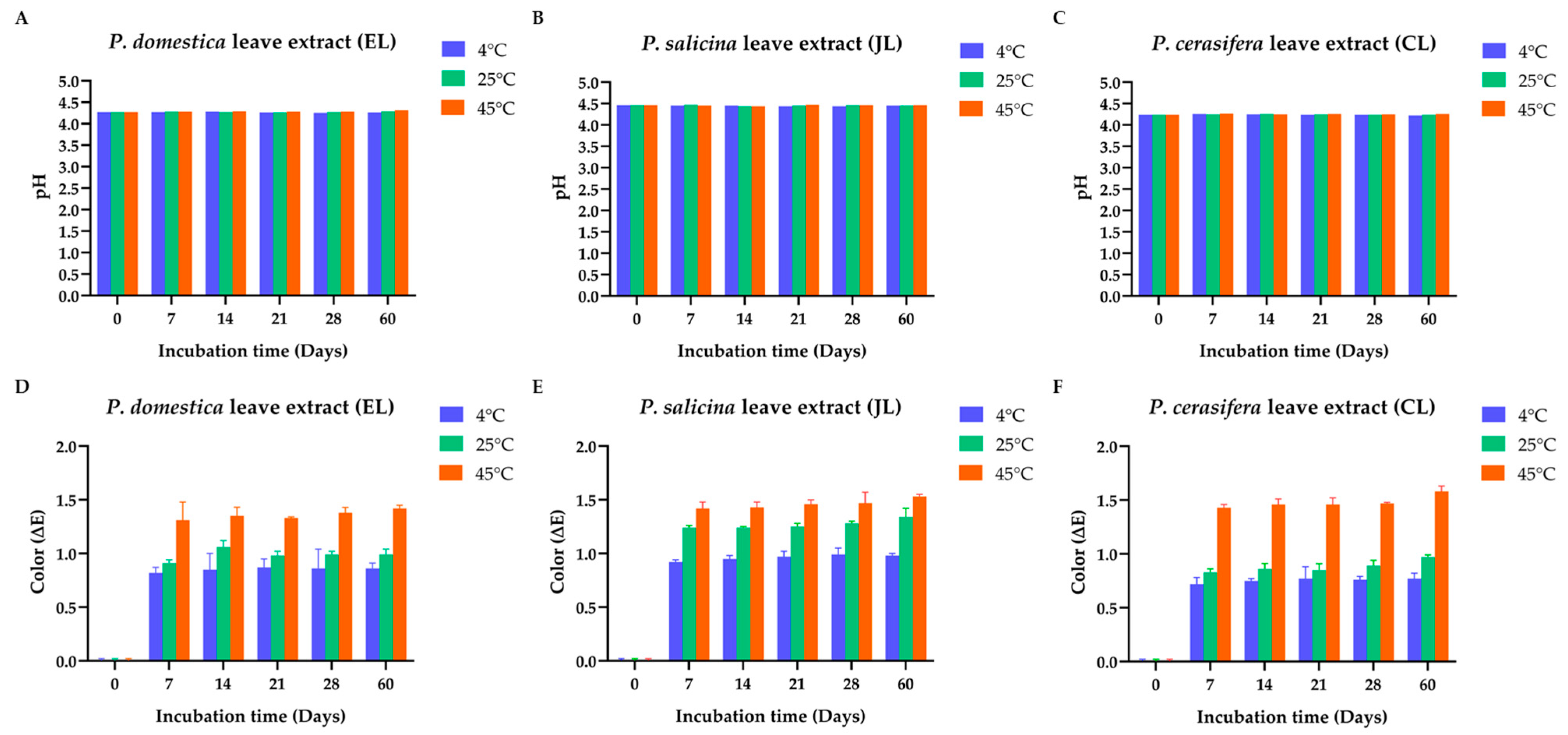
3.5. Stability Test of the Prunus Species Leaf Extracts (EL, JL, and CL)
3.5.1. Physicochemical Characteristics of the Prunus Species Leaf Extracts
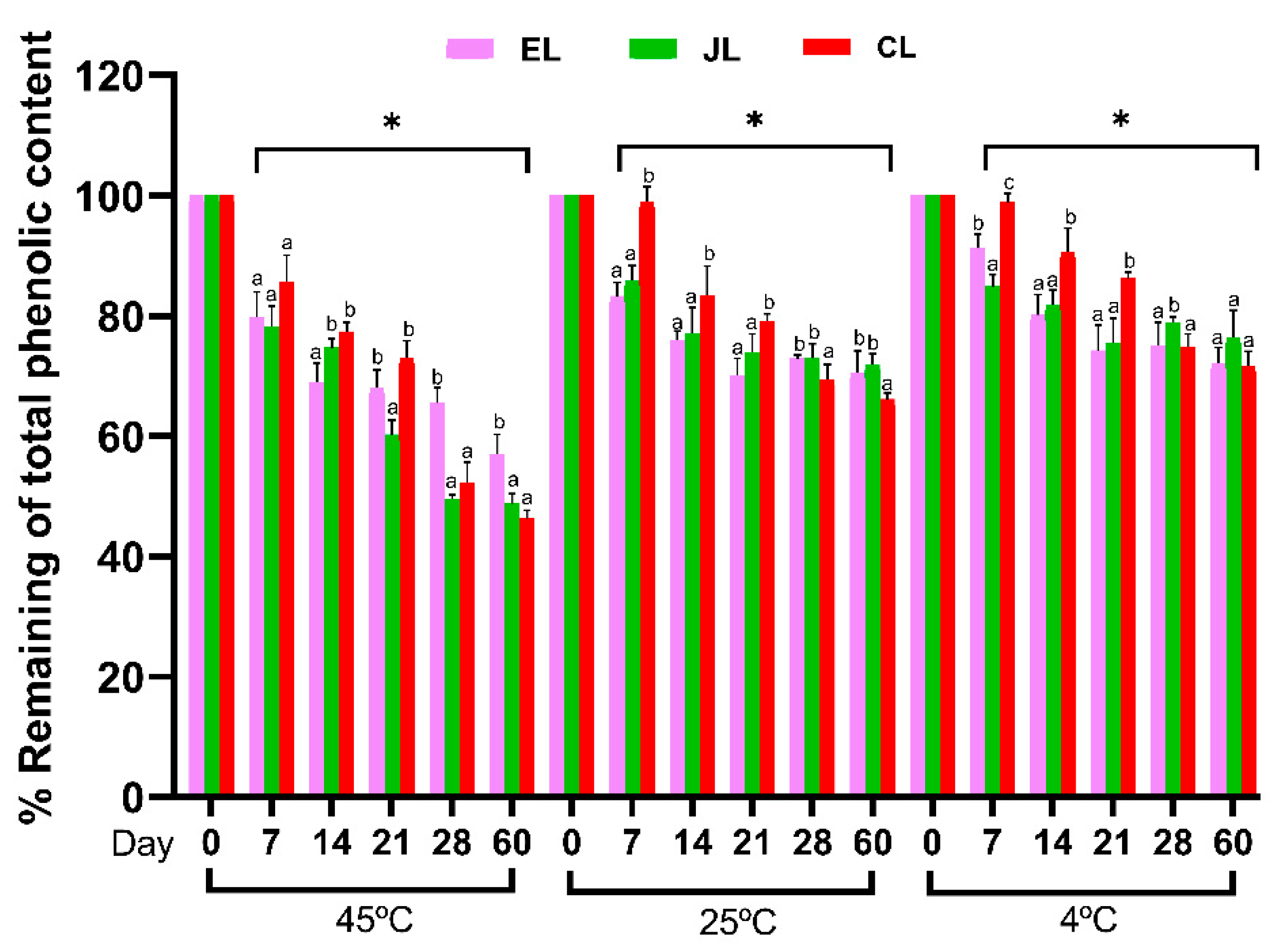
3.5.2. TPC of the Prunus Species Leaf Extracts (EL, JL, and CL) in Stability Tests
3.5.3. TTC of the Prunus Species Leaf Extracts (EL, JL, and CL) in Stability Tests
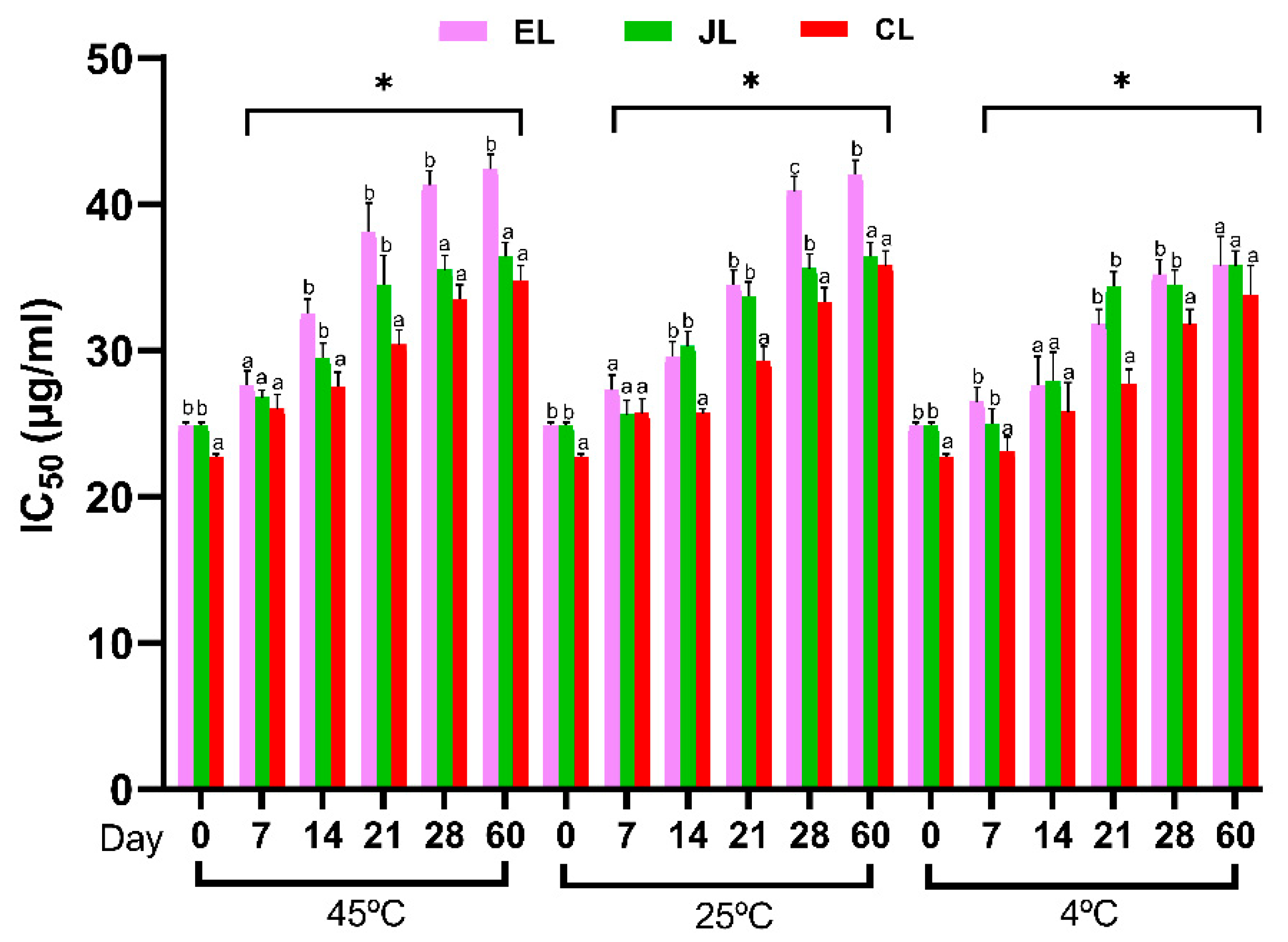
3.5.4. Antioxidant Activity Using DPPH Assay of the Prunus Species Leaf Extracts (EL, JL, and CL) in Stability Tests
3.6. Stability Test of Cream Formulation
3.6.1. Physicochemical Stability of the Cream Formulation Containing CL (CCL)
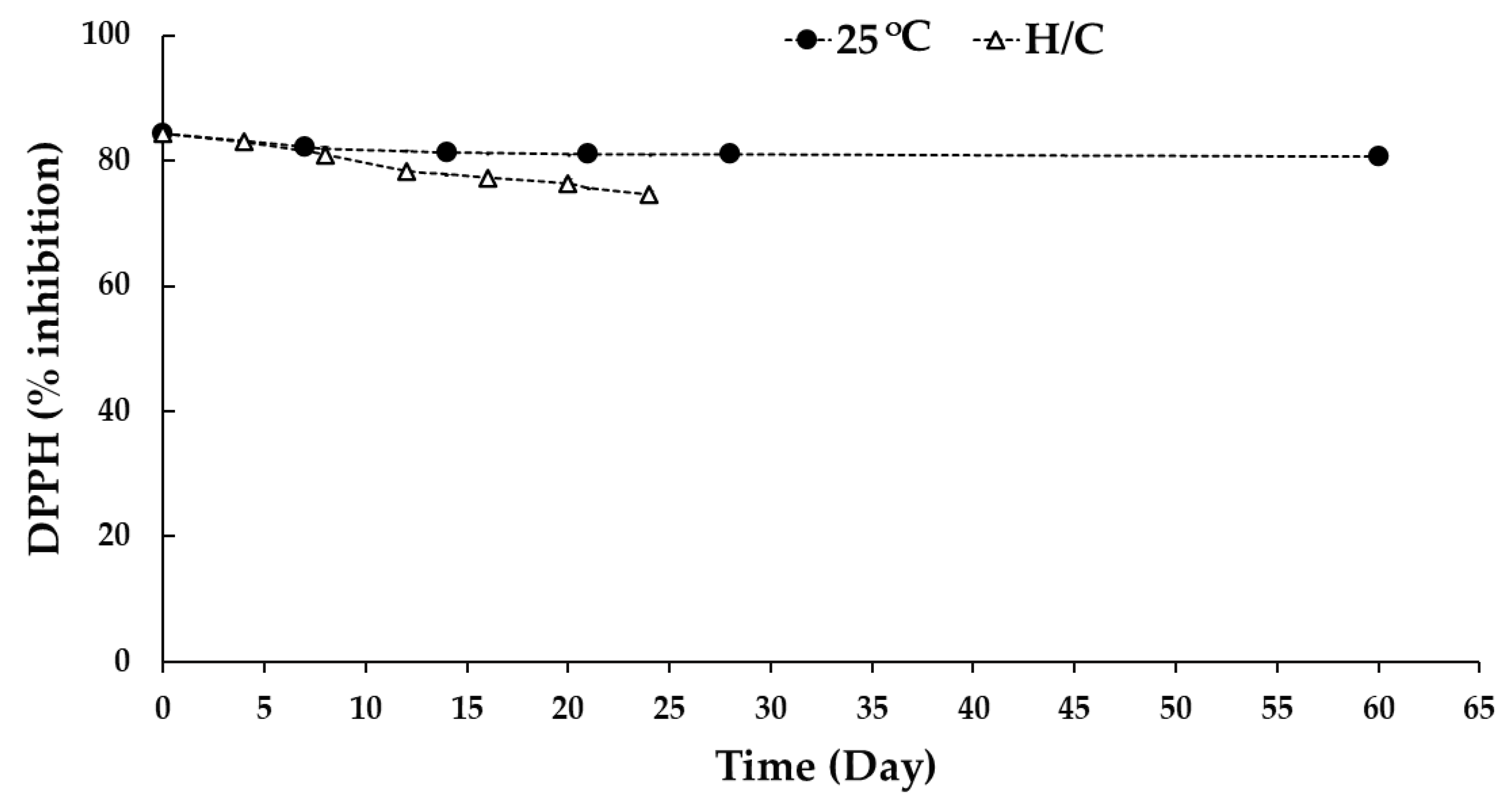
3.6.2. DPPH Free Radical Scavenging Activity Determination of CCL
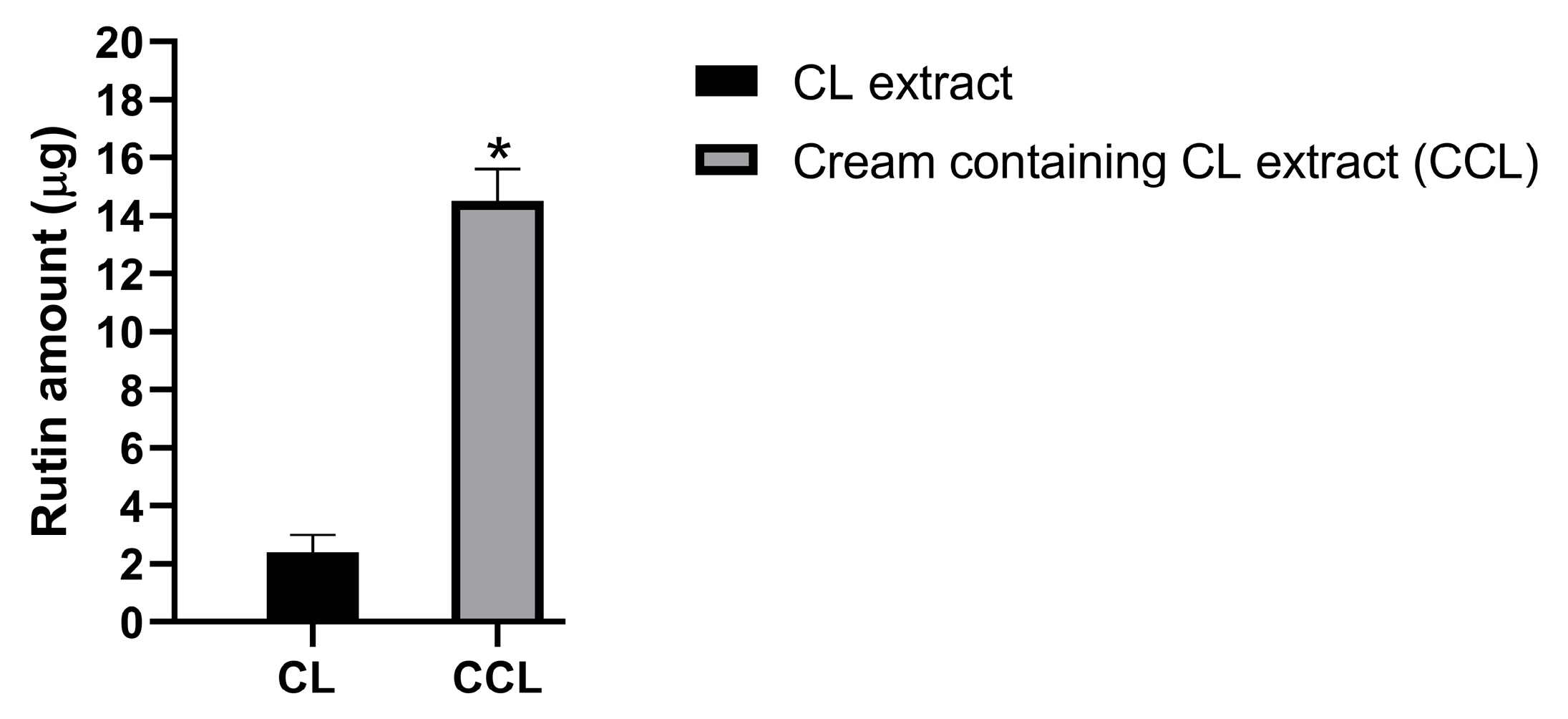
3.7. Skin Permeation Study of CCL
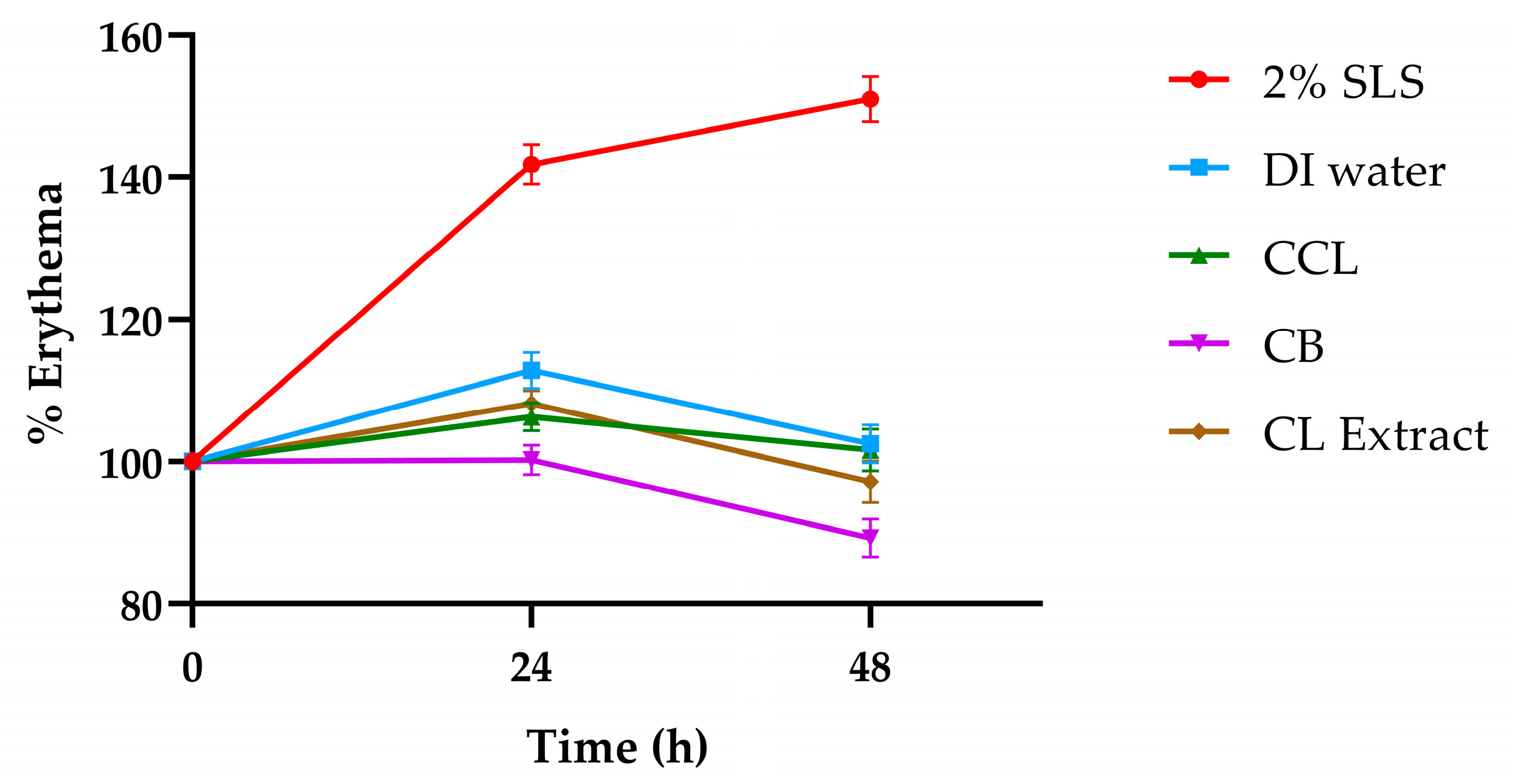
3.8. Skin Safety Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Pauzi, N.A.M.; Cheema, M.S.; Ismail, A.; Ghazali, A.R.; Abdullah, R. Safety assessment of natural products in Malaysia: Current practices, challenges, and new strategies. Rev. Environ. Health 2022, 37, 169–179. [Google Scholar] [CrossRef]
- Lee, J.; Hyun, C.-G. Natural Products for Cosmetic Applications. Molecules 2023, 28, 534. [Google Scholar] [CrossRef] [PubMed]
- Brar, H.S.; Kaur, P.; Subramanian, J.; Nair, G.R.; Singh, A. Effect of Chemical Pretreatment on Drying Kinetics and Physio-chemical Characteristics of Yellow European Plums. Int. J. Fruit Sci. 2020, 20, S252–S279. [Google Scholar] [CrossRef]
- Bioforsk Vest Njøs; Bioforsk Vest Ullensvang; International Society for Horticultural Science; Section Pome and Stone Fruits; International Society for Horticultural Science; Working Group on Plum and Prune Genetics; Breeding and Technology; Norvegian Institute for Agricultural and Environmental Research. Proceedings of the VIIIth International Symposium on Plum and Prune Genetics, Breeding and Pomology: Lofthus, Norway, 5–9 September 2004; Vangdal, E., Sekse, L., Eds.; International Society for Horticultural Science: Leuven, Belgium, 2007.
- Wongwad, E.; Preedalikit, W.; Changprasoed, S.; Somsai, S.; Singmee, N.; Srisuksomwong, P.; Srivilai, J.; Rungsang, T.; Mungmai, L. Effects of Different Drying Processes on the Bioactivity and Rutin Content of Prunus spp. (Plums). Int. J. Food Sci. 2024, 2024, 9999731. [Google Scholar] [CrossRef]
- Cos, P.; De Bruyne, T.; Hermans, N.; Apers, S.; Berghe, D.V.; Vlietinck, A.J. Proanthocyanidins in health care: Current and new trends. Curr. Med. Chem. 2004, 11, 1345–1359. [Google Scholar] [CrossRef]
- Acero, N.; Gradillas, A.; Beltran, M.; García, A.; Muñoz Mingarro, D. Comparison of phenolic compounds profile and antioxidant properties of different sweet cherry (Prunus avium L.) varieties. Food Chem. 2019, 279, 260–271. [Google Scholar] [CrossRef]
- Enogieru, A.B.; Haylett, W.; Hiss, D.C.; Bardien, S.; Ekpo, O.E. Rutin as a Potent Antioxidant: Implications for Neurodegenerative Disorders. Oxid. Med. Cell. Longev. 2018, 2018, 6241017. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Bielawska, K.; Biernacki, M.; Dobrzyńska, I.; Skrzydlewska, E. Time-dependent effect of rutin on skin fibroblasts membrane disruption following UV radiation. Redox Biol. 2017, 12, 733–744. [Google Scholar] [CrossRef]
- Gęgotek, A.; Rybałtowska-Kawałko, P.; Skrzydlewska, E. Rutin as a Mediator of Lipid Metabolism and Cellular Signaling Pathways Interactions in Fibroblasts Altered by UVA and UVB Radiation. Oxid. Med. Cell. Longev. 2017, 2017, 4721352. [Google Scholar] [CrossRef]
- Coldman, M.F.; Poulsen, B.J.; Higuchi, T. Enhancement of percutaneous absorption by the use of volatile: Nonvolatile systems as vehicles. J. Pharm. Sci. 1969, 58, 1098–1102. [Google Scholar] [CrossRef]
- An, R.; Shi, C.; Tang, Y.; Cui, Z.; Li, Y.; Chen, Z.; Xiao, M.; Xu, L. Chitosan/rutin multifunctional hydrogel with tunable adhesion, anti-inflammatory and antibacterial properties for skin wound healing. Carbohydr. Polym. 2024, 343, 122492. [Google Scholar] [CrossRef] [PubMed]
- De Tollenaere, M.; Durduret, A.; Chapuis, E.; Lambert, C.; Lemagnen, P.; Tiguemounine, J.; Auriol, D.; Scandolera, A.; Reynaud, R. A highly soluble form of rutin for instant resolution of mask-wearing related disorders. J. Cosmet. Dermatol. 2024, 23, 1734–1744. [Google Scholar] [CrossRef]
- Chai, J.; Chen, X.; Jin, C.; Chai, F.; Tian, M. Selective enrichment of Rutin in sunscreen by boronate affinity molecularly imprinted polymer prior to determination by high performance liquid chromatography. Biochem. Eng. J. 2023, 191, 108811. [Google Scholar] [CrossRef]
- Choquenet, B.; Couteau, C.; Paparis, E.; Coiffard, L.J. Quercetin and rutin as potential sunscreen agents: Determination of efficacy by an in vitro method. J. Nat. Prod. 2008, 71, 1117–1118. [Google Scholar] [CrossRef] [PubMed]
- Tomazelli, L.C.; de Assis Ramos, M.M.; Sauce, R.; Cândido, T.M.; Sarruf, F.D.; de Oliveira Pinto, C.A.S.; de Oliveira, C.A.; Rosado, C.; Velasco, M.V.R.; Baby, A.R. SPF enhancement provided by rutin in a multifunctional sunscreen. Int. J. Pharm. 2018, 552, 401–406. [Google Scholar] [CrossRef]
- Plainfossé, H.; Burger, P.; Verger-Dubois, G.; Azoulay, S.; Fernandez, X. Design Methodology for the Development of a New Cosmetic Active Based on Prunus domestica L. Leaves Extract. Cosmetics 2019, 6, 8. [Google Scholar] [CrossRef]
- Mungmai, L.; Preedalikit, W.; Rungsang, T.; Sainakham, M. Bioactivity Determination and Development of Oil in Water Emulsion Containing Cassia fistula Bark Extract. Walailak J. Sci. Technol. (WJST) 2021, 18, 8943. [Google Scholar] [CrossRef]
- Rebaya, A.; Belghith, S.I.; Baghdikian, B.; Leddet, V.M.; Mabrouki, F.; Olivier, E.; Cherif, J.k.; Ayadi, M.T. Total Phenolic, Total Flavonoid, Tannin Content, and Antioxidant Capacity of Halimium halimifolium (Cistaceae). J. Appl. Pharm. Sci. 2015, 5, 052–057. [Google Scholar]
- Wongwad, E.; Pingyod, C.; Saesong, T.; Waranuch, N.; Wisuitiprot, W.; Sritularak, B.; Temkitthawon, P.; Ingkaninan, K. Assessment of the bioactive components, antioxidant, antiglycation and anti-inflammatory properties of Aquilaria crassna Pierre ex Lecomte leaves. Ind. Crops Prod. 2019, 138, 111448. [Google Scholar] [CrossRef]
- Saesong, T.; Allard, P.M.; Queiroz, E.F.; Marcourt, L.; Nuengchamnong, N.; Temkitthawon, P.; Khorana, N.; Wolfender, J.L.; Ingkaninan, K. Discovery of Lipid Peroxidation Inhibitors from Bacopa Species Prioritized through Multivariate Data Analysis and Multi-Informative Molecular Networking. Molecules 2019, 24, 2989. [Google Scholar] [CrossRef]
- Ariede, M.B.; Gomez Junior, W.A.; Cândido, T.M.; de Aguiar, M.M.G.B.; Rosado, C.; Rangel-Yagui, C.d.O.; Pessoa, F.V.L.S.; Velasco, M.V.R.; Baby, A.R. Would Rutin be a Feasible Strategy for Environmental-Friendly Photoprotective Samples? A Review from Stability to Skin Permeability and Efficacy in Sunscreen Systems. Cosmetics 2024, 11, 141. [Google Scholar] [CrossRef]
- Leelapornpisid, P.; Mungmai, L.; Sirithunyalug, B.; Jiranusornkul, S.; Peerapornpisal, Y. A Novel Moisturizer Extracted from Freshwater Macroalga [Rhizoclonium hieroglyphicum (C.Agardh) Kützing] for Skin Care Cosmetic. Chiang Mai J. Sci. 2014, 41, 1195–1207. [Google Scholar]
- Sainakham, M.; Promma, B.; Ngernthong, A.; Kiattisin, K.; Boonpisuttinant, K.; Wuttikul, K.; Jantrawut, P.; Ruksiriwanich, W. Preparation and stability investigation of ultrasound-assisted W/O/W multiple nanoemulsions co-loaded with hydrophobic curcumin and hydrophilic arbutin for tyrosinase inhibition. Heliyon 2024, 10, e34665. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, R.; Moreno, S.; Smythers, A.L.; Sullins, C.; Pijor, H.; Brown, G.; Trouten, A.; Richards-Waugh, L.L.; Siddig, A. Quantification of Cannabis in Infused Consumer Products and Their Residues on Skin. ACS Pharmacol. Transl. Sci. 2022, 5, 642–651. [Google Scholar] [CrossRef]
- Mungmai, L.; Preedalikit, W.; Pintha, K.; Tantipaiboonwong, P.; Aunsri, N. Collagenase and Melanogenesis Inhibitory Effects of Perilla Frutescens Pomace Extract and Its Efficacy in Topical Cosmetic Formulations. Cosmetics 2020, 7, 69. [Google Scholar] [CrossRef]
- Poomanee, W.; Yaowiwat, N.; Pattarachaidaecharuch, T.; Leelapornpisid, P. Optimized multiherbal combination and in vivo anti-skin aging potential: A randomized double blind placebo controlled study. Sci. Rep. 2023, 13, 5633. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Phenolics in cereals, fruits and vegetables: Occurrence, extraction and analysis. J. Pharm. Biomed. Anal. 2006, 41, 1523–1542. [Google Scholar] [CrossRef] [PubMed]
- Preedalikit, W.; Chittasupho, C.; Leelapornpisid, P.; Potprommanee, S.; Kiattisin, K. Comparison of Biological Activities and Protective Effects on PAH-Induced Oxidative Damage of Different Coffee Cherry Pulp Extracts. Foods 2023, 12, 4292. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, C.; García-Villanova, B.; Guerra-Hernández, E.; Verardo, V. Grape Seeds Proanthocyanidins: An Overview of In Vivo Bioactivity in Animal Models. Nutrients 2019, 11, 2435. [Google Scholar] [CrossRef]
- Habib, H.M.; El-Fakharany, E.M.; Kheadr, E.; Ibrahim, W.H. Grape seed proanthocyanidin extract inhibits DNA and protein damage and labile iron, enzyme, and cancer cell activities. Sci. Rep. 2022, 12, 12393. [Google Scholar] [CrossRef]
- Bibi, N.; Shah, M.H.; Khan, N.; Al-Hashimi, A.; Elshikh, M.S.; Iqbal, A.; Ahmad, S.; Abbasi, A.M. Variations in Total Phenolic, Total Flavonoid Contents, and Free Radicals’ Scavenging Potential of Onion Varieties Planted under Diverse Environmental Conditions. Plants 2022, 11, 950. [Google Scholar] [CrossRef] [PubMed]
- Fratianni, F.; Tucci, M.; Palma, M.D.; Pepe, R.; Nazzaro, F. Polyphenolic composition in different parts of some cultivars of globe artichoke (Cynara cardunculus L. var. scolymus (L.) Fiori). Food Chem. 2007, 104, 1282–1286. [Google Scholar] [CrossRef]
- Wijesinghe, W.A.J.P.; Ahn, G.; Lee, W.-W.; Kang, M.-C.; Kim, E.-A.; Jeon, Y.-J. Anti-inflammatory activity of phlorotannin-rich fermented Ecklonia cava processing by-product extract in lipopolysaccharide-stimulated RAW 264.7 macrophages. J. Appl. Phycol. 2013, 25, 1207–1213. [Google Scholar] [CrossRef]
- Fraga-Corral, M.; Otero, P.; Echave, J.; Garcia-Oliveira, P.; Carpena, M.; Jarboui, A.; Nuñez-Estevez, B.; Simal-Gandara, J.; Prieto, M.A. By-Products of Agri-Food Industry as Tannin-Rich Sources: A Review of Tannins’ Biological Activities and Their Potential for Valorization. Foods 2021, 10, 137. [Google Scholar] [CrossRef] [PubMed]
- Stierlin, E.; Azoulay, S.; Massi, L.; Fernandez, X.; Michel, T. Cosmetic potentials of L. leaves. J. Sci. Food Agric. 2018, 98, 726–736. [Google Scholar] [CrossRef]
- Hong, C.Y.; Wang, C.P.; Huang, S.S.; Hsu, F.L. The inhibitory effect of tannins on lipid peroxidation of rat heart mitochondria. J. Pharm. Pharmacol. 1995, 47, 138–142. [Google Scholar] [CrossRef]
- Carvalho, R.S.; Carollo, C.A.; de Magalhães, J.C.; Palumbo, J.M.C.; Boaretto, A.G.; Nunes e Sá, I.C.; Ferraz, A.C.; Lima, W.G.; de Siqueira, J.M.; Ferreira, J.M.S. Antibacterial and antifungal activities of phenolic compound-enriched ethyl acetate fraction from Cochlospermum regium (mart. Et. Schr.) Pilger roots: Mechanisms of action and synergism with tannin and gallic acid. South Afr. J. Bot. 2018, 114, 181–187. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, S.; Lin, X.; Peng, J.; Luo, D.; Wan, X.; Zhang, Y.; Dong, X.; Ma, Y. Analysis of Volatile Compounds in Different Varieties of Plum Fruits Based on Headspace Solid-Phase Microextraction-Gas Chromatography-Mass Spectrometry Technique. Horticulturae 2023, 9, 1069. [Google Scholar] [CrossRef]
- Almeida, J.S.; Lima, F.; Ros, S.D.; Bulhões, L.O.S.; de Carvalho, L.M.; Beck, R.C.R. Nanostructured Systems Containing Rutin: In Vitro Antioxidant Activity and Photostability Studies. Nanoscale Res. Lett. 2010, 5, 1603. [Google Scholar] [CrossRef]
- Tuominen, A.; Sundman, T. Stability and oxidation products of hydrolysable tannins in basic conditions detected by HPLC/DAD-ESI/QTOF/MS. Phytochem. Anal. 2013, 24, 424–435. [Google Scholar] [CrossRef]
- Khanal, R.C.; Howard, L.R.; Prior, R.L. Effect of heating on the stability of grape and blueberry pomace procyanidins and total anthocyanins. Food Res. Int. 2010, 43, 1464–1469. [Google Scholar] [CrossRef]
- de Lima Marsiglia, W.I.M.; Oliveira, L.d.S.C.; Lucas Jacinto Almeida, R.; Santos, N.C.; da Silva Neto, J.M.; Santiago, Â.M.; de Melo, B.C.A.; Honorato da Silva, F.L. Thermal stability of total phenolic compounds and antioxidant activities of jaboticaba peel: Effect of solvents and extraction methods. J. Indian Chem. Soc. 2023, 100, 100995. [Google Scholar] [CrossRef]
- Baby, A.R.; Migliato, K.F.; Maciel, C.P.M.; Zague, V.; Pinto, C.A.S.D.O.; Salgado, H.R.N.; Kaneko, T.M.; Velasco, M.V.R. Accelerated chemical stability data of O/W fluid emulsions containing the extract of Trichilia catigua Adr. Juss (and) Ptychopetalum olacoides Bentham. Rev. Bras. De Ciências Farm. 2007, 43, 405–412. [Google Scholar] [CrossRef]
- Preedalikit, W.; Chittasupho, C.; Leelapornpisid, P.; Qi, S.; Kiattisin, K. Development and Evaluation of Anti-Pollution Film-Forming Facial Spray Containing Coffee Cherry Pulp Extract. Pharmaceutics 2025, 17, 360. [Google Scholar] [CrossRef] [PubMed]
- Pyo, S.M.; Meinke, M.; Keck, C.M.; Müller, R.H. Rutin—Increased Antioxidant Activity and Skin Penetration by Nanocrystal Technology (smartCrystals). Cosmetics 2016, 3, 9. [Google Scholar] [CrossRef]
- Gullón, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- Cândido, T.M.; De Oliveira, C.A.; Ariede, M.B.; Velasco, M.V.R.; Rosado, C.; Baby, A.R. Safety and Antioxidant Efficacy Profiles of Rutin-Loaded Ethosomes for Topical Application. AAPS PharmSciTech 2018, 19, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Park, S.N.; Lee, H.J.; Gu, H.A. Enhanced skin delivery and characterization of rutin-loaded ethosomes. Korean J. Chem. Eng. 2014, 31, 485–489. [Google Scholar] [CrossRef]
- Som, I.; Bhatia, K.; Yasir, M. Status of surfactants as penetration enhancers in transdermal drug delivery. J. Pharm. Bioallied Sci. 2012, 4, 2–9. [Google Scholar] [CrossRef]
- Williams, A.C.; Barry, B.W. Urea analogues in propylene glycol as penetration enhancers in human skin. Int. J. Pharm. 1989, 56, 43–50. [Google Scholar] [CrossRef]
- Lee, C.-H.; Bhatt, P.P.; Chien, Y.W. Effect of excipient on drug release and permeation from silicone-based barrier devices. J. Control. Release 1997, 43, 283–290. [Google Scholar] [CrossRef]
- Neupane, R.; Boddu, S.H.S.; Renukuntla, J.; Babu, R.J.; Tiwari, A.K. Alternatives to Biological Skin in Permeation Studies: Current Trends and Possibilities. Pharmaceutics 2020, 12, 152. [Google Scholar] [CrossRef] [PubMed]
- Haq, A.; Goodyear, B.; Ameen, D.; Joshi, V.; Michniak-Kohn, B. Strat-M® synthetic membrane: Permeability comparison to human cadaver skin. Int. J. Pharm. 2018, 547, 432–437. [Google Scholar] [CrossRef] [PubMed]



| Ingredient | Function | Concentration (% w/w) |
|---|---|---|
| Deionized Water | Solvent | 55.0–60.0 |
| Propylene Glycol | Humectant/Penetration Enhancer | 5.0–8.0 |
| Cyclopentasiloxane and Dimethicone/Vinyl Dimethicone Crosspolymer | Emollient/Film Former | 5.0–8.0 |
| Cetearyl Olivate | Emulsifier | 5.0–8.0 |
| Cyclopentasiloxane and Dimethicone Crosspolymer | Emollient | 3.0–5.0 |
| Cyclopentasiloxane and Cyclotetrasiloxane | Emollient | 3.0–5.0 |
| Cyclopentasiloxane and Dimethiconol | Emollient | 2.0–3.0 |
| Dimethicone | Emollient | 1.0–2.0 |
| Polyacrylamide (and) C13–14 Isoparaffin (and) Laureth-7 | Thickener/Stabilizer | 1.0–2.0 |
| Ammonium Acryloyldimethyltaurate/VP Copolymer | Thickener/Stabilizer | 0.5–1.0 |
| Phenoxyethanol | Preservative | 0.5–1.0 |
| Prunus Leaf Extract (CL, Rutin = 12.1 ± 0.1% w/w) | Active Ingredient | 0.3 |
| Symbol | Grade | Clinical Description |
|---|---|---|
| - | 0 | Negative reaction |
| + | 1 | Slight erythema, either spotty or diffuse |
| ++ | 2 | Moderate uniform erythema |
| +++ | 3 | Intense erythema with edema |
| ++++ | 4 | Intense erythema with edema and vesicles |
| Sample | % Yield |
|---|---|
| EL | 8.5 ± 0.5 c |
| JL | 7.8 ± 0.8 b |
| CL | 7.6 ± 0.5 a |
| Sample | TPC (mgGAE/g Extract) | TTC (mgCAE/g Extract) | DPPH IC50 (µg/mL) | Lipid Peroxidation Inhibitory Activity (% Inhibition) |
|---|---|---|---|---|
| EL | 15.0± 2.2 a | 28.8 ± 2.0 a | 24.9 ± 1.9 a | 52.7 ± 1.9 a, b |
| JL | 16.0 ± 2.2 a | 36.0 ± 1.8 a | 24.9 ± 3.1 a | 48.3 ± 2.2 a |
| CL | 10.9 ± 1.3 b | 49.7 ± 3.2 b | 22.7 ± 3.1 a | 62.3 ± 1.0 b |
| Trolox | - | - | 4.4 ± 0.2 c | 96.6 ± 2.3 c |
| Rutin | - | - | 12.8 ± 0.4 b | 48.9 ± 1.1 a |
| Tested Substances | PDII Value | Classification of Skin Reaction |
|---|---|---|
| 2% w/v sodium lauryl sulfate | 1.83 | Slight irritation |
| Deionized water | 0.00 | Non-irritation |
| CCL | 0.14 | Non-irritation |
| CB | 0.06 | Non-irritation |
| CL extract | 0.08 | Non-irritation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mungmai, L.; Wongwad, E.; Tanamatayarat, P.; Rungsang, T.; Vivattanaseth, P.; Aunsri, N.; Preedalikit, W. Stability, Bioactivity, and Skin Penetration of Prunus Leaf Extracts in Cream Formulations: A Clinical Study on Skin Irritation. Cosmetics 2025, 12, 146. https://doi.org/10.3390/cosmetics12040146
Mungmai L, Wongwad E, Tanamatayarat P, Rungsang T, Vivattanaseth P, Aunsri N, Preedalikit W. Stability, Bioactivity, and Skin Penetration of Prunus Leaf Extracts in Cream Formulations: A Clinical Study on Skin Irritation. Cosmetics. 2025; 12(4):146. https://doi.org/10.3390/cosmetics12040146
Chicago/Turabian StyleMungmai, Lapatrada, Eakkaluk Wongwad, Patcharawan Tanamatayarat, Tammanoon Rungsang, Pattavet Vivattanaseth, Nattapol Aunsri, and Weeraya Preedalikit. 2025. "Stability, Bioactivity, and Skin Penetration of Prunus Leaf Extracts in Cream Formulations: A Clinical Study on Skin Irritation" Cosmetics 12, no. 4: 146. https://doi.org/10.3390/cosmetics12040146
APA StyleMungmai, L., Wongwad, E., Tanamatayarat, P., Rungsang, T., Vivattanaseth, P., Aunsri, N., & Preedalikit, W. (2025). Stability, Bioactivity, and Skin Penetration of Prunus Leaf Extracts in Cream Formulations: A Clinical Study on Skin Irritation. Cosmetics, 12(4), 146. https://doi.org/10.3390/cosmetics12040146








