An Adhesive Peptide Derived from Mussel Protein Alleviates LL37-Induced Rosacea Through Anti-Inflammatory and Anti-Angiogenic Mechanisms
Abstract
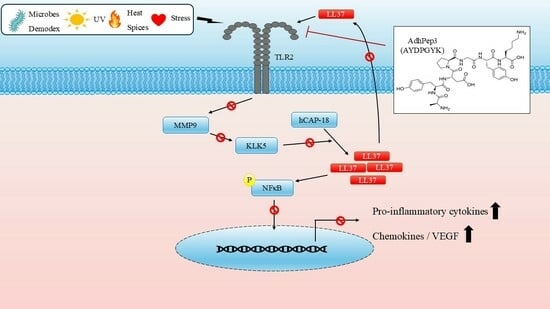
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Sample Preparation (Peptide Synthesis)
2.3. Cell Culture and Treatment
2.4. Cell Viability Assay
2.5. ELISA
2.6. Real-Time Quantitative PCR Analysis
2.7. Western Blot Analysis
2.8. Immunocytochemistry
2.9. Scratch Migration Assay
2.10. Tube Formation Assay
2.11. Statistical Analysis
3. Results
3.1. Screening and Selection of Adhesive Peptides and Cytotoxicity Assessment in HaCaT Cells
3.2. Effect of AdhPep3 on Inflammatory and Angiogenesis Markers Through Cytokine Release and mRNA Expression in LL-37-Induced HaCaT Cells
3.3. Effect of AdhPep3 on the Expression of Proteins Related to the TLR2-NFκB and AKT/ERK/JNK Pathways in LL-37-Induced HaCaT Cells
3.4. Effect of AdhPep3 on Angiogenesis Markers in LL-37-Induced HUVECs
3.5. Effect of AdhPep3 on Migration and Tube Formation in LL-37-Induced HUVECs
3.6. Effect of AdhPep3 on the Expression of Proteins Related to Tight Junctions and the Skin Barrier in HaCaT Cells
3.7. Comparison of the Rosacea Treatment Effects of Doxycycline, Metronidazole, and AdhPep3 on LL-37-Induced HaCaT Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fisher, G.W.; Travers, J.B.; Rohan, C.A. Rosacea pathogenesis and therapeutics: Current treatments and a look at future targets. Front. Med. 2023, 10, 1292722. [Google Scholar] [CrossRef]
- Rainer, B.M.; Kang, S.; Chien, A.L. Rosacea: Epidemiology, pathogenesis, and treatment. Dermatoendocrinology 2017, 9, e1361574. [Google Scholar] [CrossRef]
- Steinhoff, M.; Schauber, J.; Leyden, J.J. New insights into rosacea pathophysiology: A review of recent findings. J. Am. Acad. Dermatol. 2013, 69, S15–S26. [Google Scholar] [CrossRef]
- Chen, C.; Wang, P.; Zhang, L.; Liu, X.; Zhang, H.; Cao, Y.; Wang, X.; Zeng, Q. Exploring the Pathogenesis and Mechanism-Targeted Treatments of Rosacea: Previous Understanding and Updates. Biomedicines 2023, 11, 2153. [Google Scholar] [CrossRef]
- Lee, H.J.; Hong, Y.J.; Kim, M. Angiogenesis in Chronic Inflammatory Skin Disorders. Int. J. Mol. Sci. 2021, 22, 12035. [Google Scholar] [CrossRef]
- Steinhoff, M.; Buddenkotte, J.; Aubert, J.; Sulk, M.; Novak, P.; Schwab, V.D.; Mess, C.; Cevikbas, F.; Rivier, M.; Carlavan, I.; et al. Clinical, cellular, and molecular aspects in the pathophysiology of rosaceaJ. Investig. Dermatol. Symp. Proc. 2011, 15, 2–11. [Google Scholar] [CrossRef]
- Moreno-Angarita, A.; Aragón, C.C.; Tobón, G.J. Cathelicidin LL-37: A new important molecule in the pathophysiology of systemic lupus erythematosus. J. Transl. Autoimmun. 2020, 3, 100029. [Google Scholar] [CrossRef]
- Reinholz, M.; Ruzicka, T.; Schauber, J. Cathelicidin LL-37: An antimicrobial peptide with a role in inflammatory skin disease. Ann. Dermatol. 2012, 24, 126–135. [Google Scholar] [CrossRef]
- Verjans, E.T.; Zels, S.; Luyten, W.; Landuyt, B.; Schoofs, L. Molecular mechanisms of LL-37-induced receptor activation: An overview. Peptides 2016, 85, 16–26. [Google Scholar] [CrossRef]
- Deng, Z.; Chen, M.; Liu, Y.; Xu, S.; Ouyang, Y.; Shi, W.; Jian, D.; Wang, B.; Liu, F.; Li, J.; et al. A positive feedback loop between mTORC1 and cathelicidin promotes skin inflammation in rosacea. EMBO Mol. Med. 2021, 13, e13560. [Google Scholar] [CrossRef]
- Kuroda, K.; Okumura, K.; Isogai, H.; Isogai, E. The Human Cathelicidin Antimicrobial Peptide LL-37 and Mimics are Potential Anticancer Drugs. Front. Oncol. 2015, 5, 144. [Google Scholar] [CrossRef]
- Yang, F.; Wang, L.; Song, D.; Zhang, L.; Wang, X.; Du, D.; Jiang, X. Signaling pathways and targeted therapy for rosacea. Front. Immunol. 2024, 15, 1367994. [Google Scholar] [CrossRef]
- Addor, F.A. Skin barrier in rosacea. An. Bras. Dermatol. 2016, 91, 59–63. [Google Scholar] [CrossRef]
- Thiboutot, D.; Anderson, R.; Cook-Bolden, F.; Draelos, Z.; Gallo, R.L.; Granstein, R.D.; Kang, S.; Macsai, M.; Gold, L.S.; Tan, J. Standard management options for rosacea: The 2019 update by the National Rosacea Society Expert Committee. J. Am. Acad. Dermatol. 2020, 82, 1501–1510. [Google Scholar] [CrossRef]
- Cardwell, L.A.; Alinia, H.; Moradi Tuchayi, S.; Feldman, S.R. New developments in the treatment of rosacea—Role of once-daily ivermectin cream. Clin. Cosmet. Investig. Dermatol. 2016, 9, 71–77. [Google Scholar] [CrossRef]
- Engin, B.; Özkoca, D.; Kutlubay, Z.; Serdaroğlu, S. Conventional and Novel Treatment Modalities in Rosacea. Clin. Cosmet. Investig. Dermatol. 2020, 13, 179–186. [Google Scholar] [CrossRef]
- Martins, A.M.; Marto, J.M.; Johnson, J.L.; Graber, E.M. A Review of Systemic Minocycline Side Effects and Topical Minocycline as a Safer Alternative for Treating Acne and Rosacea. Antibiotics 2021, 10, 757. [Google Scholar] [CrossRef]
- Paiva-Santos, A.C.; Gonçalves, T.; Peixoto, D.; Pires, P.C.; Velsankar, K.; Jha, N.K.; Chavda, V.P.; Mohammad, I.S.; Cefali, L.C.; Mazzola, P.G.; et al. Rosacea Topical Treatment and Care: From Traditional to New Drug Delivery Systems. Mol. Pharm. 2023, 20, 3804–3828. [Google Scholar] [CrossRef]
- Lima, T.N.; Pedriali Moraes, C.A. Bioactive Peptides: Applications and Relevance for Cosmeceuticals. Cosmetics 2018, 5, 21. [Google Scholar] [CrossRef]
- Zhang, L.; Falla, T.J. Cosmeceuticals and peptides. Clin. Dermatol. 2009, 27, 485–494. [Google Scholar] [CrossRef]
- Rossino, G.; Marchese, E.; Galli, G.; Verde, F.; Finizio, M.; Serra, M.; Linciano, P.; Collina, S. Peptides as Therapeutic Agents: Challenges and Opportunities in the Green Transition Era. Molecules 2023, 28, 7165. [Google Scholar] [CrossRef]
- Sachdeva, S. Peptides as ‘drugs’: The journey so far. Int. J. Pept. Res. Ther. 2017, 23, 49–60. [Google Scholar] [CrossRef]
- Holten-Andersen, N.; Waite, J.H. Mussel-designed protective coatings for compliant substrates. J. Dent. Res. 2008, 87, 701–709. [Google Scholar] [CrossRef]
- Deacon, M.P.; Davis, S.S.; Waite, J.H.; Harding, S.E. Structure and Mucoadhesion of Mussel Glue Protein in Dilute Solution. Biochemistry 1998, 37, 14108–14112. [Google Scholar] [CrossRef]
- Silverman, H.G.; Roberto, F.F. Understanding marine mussel adhesion. Mar. Biotechnol. 2007, 9, 661–681. [Google Scholar] [CrossRef]
- Laursen, R.A. Reflections on the structure of mussel adhesive proteins. Results Probl. Cell Differ. 1992, 19, 55–74. [Google Scholar]
- Sengupta, D.; Heilshorn, S.C. Protein-engineered biomaterials: Highly tunable tissue engineering scaffolds. Tissue Eng. Part B Rev. 2010, 16, 285–293. [Google Scholar] [CrossRef]
- Straley, K.S.; Heilshorn, S.C. Design and adsorption of modular engineered proteins to prepare customized, neuron-compatible coatings. Front. Neuroeng. 2009, 2, 9. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, L.; Wang, H.; Zhu, P.; Jiang, S.; Qi, R.; Wu, Y.; Gao, X. Activation of aryl hydrocarbon receptor ameliorates rosacea-like eruptions in mice and suppresses the TLR signaling pathway in LL-37-induced HaCaT cells. Toxicol. Appl. Pharmacol. 2022, 451, 116189. [Google Scholar] [CrossRef]
- Zhou, L.; Zhong, Y.; Wang, Y.; Deng, Z.; Huang, Y.; Wang, Q.; Xie, H.; Zhang, Y.; Li, J. EGCG identified as an autophagy inducer for rosacea therapy. Front. Pharmacol. 2023, 14, 1092473. [Google Scholar] [CrossRef]
- Ikutama, R.; Peng, G.; Tsukamoto, S.; Umehara, Y.; Trujillo-Paez, J.V.; Yue, H.; Nguyen, H.L.T.; Takahashi, M.; Kageyama, S.; Komatsu, M.; et al. Cathelicidin LL-37 Activates Human Keratinocyte Autophagy through the P2X7, Mechanistic Target of Rapamycin, and MAPK Pathways. J. Investig. Dermatol. 2023, 143, 751–761.e757. [Google Scholar] [CrossRef]
- Tang, S.; Hu, H.; Li, M.; Zhang, K.; Wu, Q.; Liu, X.; Wu, L.; Yu, B.; Chen, X. OPN promotes pro-inflammatory cytokine expression via ERK/JNK pathway and M1 macrophage polarization in Rosacea. Front. Immunol. 2023, 14, 1285951. [Google Scholar] [CrossRef]
- Yuan, X.; Li, J.; Li, Y.; Deng, Z.; Zhou, L.; Long, J.; Tang, Y.; Zuo, Z.; Zhang, Y.; Xie, H. Artemisinin, a potential option to inhibit inflammation and angiogenesis in rosacea. Biomed. Pharmacother. 2019, 117, 109181. [Google Scholar] [CrossRef]
- Zhang, C.; Kang, Y.; Zhang, Z.; Liu, H.; Xu, H.; Cai, W.; Gao, X.; Yang, J. Long-Term Administration of LL-37 Can Induce Irreversible Rosacea-like Lesion. Curr. Issues Mol. Biol. 2023, 45, 2703–2716. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Li, Y.; Wang, Y.; Yan, S.; Xu, S.; Deng, Z.; Yang, X.; Xie, H.; Li, J. Bioinformatics and Network Pharmacology Identify the Therapeutic Role and Potential Mechanism of Melatonin in AD and Rosacea. Front. Immunol. 2021, 12, 756550. [Google Scholar] [CrossRef]
- Koczulla, R.; von Degenfeld, G.; Kupatt, C.; Krötz, F.; Zahler, S.; Gloe, T.; Issbrücker, K.; Unterberger, P.; Zaiou, M.; Lebherz, C.; et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J. Clin. Investig. 2003, 111, 1665–1672. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, M.-S.; Lee, H.Y.; Kim, S.D.; Shim, J.W.; Jo, S.H.; Lee, J.W.; Kim, J.Y.; Choi, Y.-W.; Baek, S.-H.; et al. F2L, a peptide derived from heme-binding protein, inhibits LL-37-induced cell proliferation and tube formation in human umbilical vein endothelial cells. FEBS Lett. 2008, 582, 273–278. [Google Scholar] [CrossRef]
- Kim, B.E.; Leung, D.Y.M. Significance of Skin Barrier Dysfunction in Atopic Dermatitis. Allergy Asthma Immunol. Res. 2018, 10, 207–215. [Google Scholar] [CrossRef]
- Lee, H.-J.; Kim, M. Skin Barrier Function and the Microbiome. Int. J. Mol. Sci. 2022, 23, 13071. [Google Scholar] [CrossRef]
- Proksch, E.; Fölster-Holst, R.; Jensen, J.-M. Skin barrier function, epidermal proliferation and differentiation in eczema. J. Dermatol. Sci. 2006, 43, 159–169. [Google Scholar] [CrossRef]
- Deng, Z.; Chen, M.; Xie, H.; Jian, D.; Xu, S.; Peng, Q.; Sha, K.; Liu, Y.; Zhang, Y.; Shi, W.; et al. Claudin reduction may relate to an impaired skin barrier in rosacea. J. Dermatol. 2019, 46, 314–321. [Google Scholar] [CrossRef]
- Medgyesi, B.; Dajnoki, Z.; Béke, G.; Gáspár, K.; Szabó, I.L.; Janka, E.A.; Póliska, S.; Hendrik, Z.; Méhes, G.; Törőcsik, D.; et al. Rosacea Is Characterized by a Profoundly Diminished Skin Barrier. J. Investig. Dermatol. 2020, 140, 1938–1950.e1935. [Google Scholar] [CrossRef]
- Tu, K.Y.; Jung, C.J.; Shih, Y.H.; Chang, A.L.S. Therapeutic strategies focusing on immune dysregulation and neuroinflammation in rosacea. Front. Immunol. 2024, 15, 1403798. [Google Scholar] [CrossRef]
- Wladis, E.J.; Adam, A.P. Immune signaling in rosacea. Ocul. Surf. 2021, 22, 224–229. [Google Scholar] [CrossRef]
- Pintea, A.; Manea, A.; Pintea, C.; Vlad, R.A.; Bîrsan, M.; Antonoaea, P.; Rédai, E.M.; Ciurba, A. Peptides: Emerging Candidates for the Prevention and Treatment of Skin Senescence: A Review. Biomolecules 2025, 15, 88. [Google Scholar] [CrossRef]







| Peptide Name | Sequence | Source Protein | Reference |
|---|---|---|---|
| Adhesive Pep 1 | GRALARG | Neural cell adhesion molecule | [27,28] |
| Adhesive Pep 2 | AKPTYK | Mytilus edulis Mussel foot protein-1 | [23] |
| Adhesive Pep 3 | AYDPGYK | Geukensia demissa Mussel foot protein-1 | [23,26] |
| Gene Name | Primer Sequence |
|---|---|
| Human VEGF | F: 5′-TTGCCTTGCTGCTCTACCTCCA-3′ |
| R: 5′-GATGGCAGTAGCTGCGCTGATA-3′ | |
| Human IL-1β | F: 5′-CCACAGACCTTCCAGGAGAATG-3′ |
| R: 5′-GTGCAGTTCAGTGATCGTACAGG-3′ | |
| Human IL-6 | F: 5′-AGACAGCCACTCACCTCTTCAG-3′ |
| R: 5′-TTCTGCCAGTGCCTCTTTGCTG-3′ | |
| Human IL-8 | F: 5′-GAGAGTGATTGAGAGTGGACCAC-3′ |
| R: 5′-CACAACCCTCTGCACCCAGTTT-3′ | |
| Human TNF-α | F: 5′-CTCTTCTGCCTGCTGCACTTTG-3′ |
| R: 5′-ATGGGCTACAGGCTTGTCACTC-3′ | |
| Human TLR2 | F: 5′-CTTCACTCAGGAGCAGCAAGCA-3′ |
| R: 5′-ACACCAGTGCTGTCCTGTGACA-3′ | |
| Human KLK5 | F: 5′-CGTCCCACTAAAGATGTCAGACC-3′ |
| R: 5′-TCAAGCACTGGAGGACCTTAGG-3′ | |
| Human GAPDH | F: 5′-GTCTCCTCTGACTTCAACAGCG-3′ |
| R: 5′-ACCACCCTGTTGCTGTAGCCAA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.Y.; Kim, J.H.; Lee, Y.-J.; Song, M.J.; Park, H.H.; Chung, J.H. An Adhesive Peptide Derived from Mussel Protein Alleviates LL37-Induced Rosacea Through Anti-Inflammatory and Anti-Angiogenic Mechanisms. Cosmetics 2025, 12, 143. https://doi.org/10.3390/cosmetics12040143
Kim TY, Kim JH, Lee Y-J, Song MJ, Park HH, Chung JH. An Adhesive Peptide Derived from Mussel Protein Alleviates LL37-Induced Rosacea Through Anti-Inflammatory and Anti-Angiogenic Mechanisms. Cosmetics. 2025; 12(4):143. https://doi.org/10.3390/cosmetics12040143
Chicago/Turabian StyleKim, Tae Yoon, Jin Hyeop Kim, Yeon-Jun Lee, Min Ji Song, Ha Hui Park, and Ji Hyung Chung. 2025. "An Adhesive Peptide Derived from Mussel Protein Alleviates LL37-Induced Rosacea Through Anti-Inflammatory and Anti-Angiogenic Mechanisms" Cosmetics 12, no. 4: 143. https://doi.org/10.3390/cosmetics12040143
APA StyleKim, T. Y., Kim, J. H., Lee, Y.-J., Song, M. J., Park, H. H., & Chung, J. H. (2025). An Adhesive Peptide Derived from Mussel Protein Alleviates LL37-Induced Rosacea Through Anti-Inflammatory and Anti-Angiogenic Mechanisms. Cosmetics, 12(4), 143. https://doi.org/10.3390/cosmetics12040143