1. Introduction
Transdermal drug delivery systems (TDDSs) present significant advantages, such as enhanced patient compliance and self-administration convenience [
1]. These systems can be customized to optimize drug bioavailability and enhance physiological and pharmacological responses, allowing for adaptable treatment duration and kinetics through exposure time modulation and the controlled release of active ingredients [
2]. The transdermal application method reduces side effects by minimizing systemic toxicity and circumventing hepatic first-pass metabolism and gastric system incompatibilities [
3]. However, challenges in transdermal drug administration must be addressed in the formulation process, considering factors like the physical and chemical parameters of active ingredients, such as size and hydrophilic/lipophilic balance, impacting their skin penetration [
4,
5]. These challenges may be exacerbated by poor skin adhesiveness or low patient compliance with treatment, resulting in limited systemic drug circulation [
6,
7].
Hydrogels, extensively investigated as biomaterials for drug delivery systems, boast high biocompatibility and hydrophilic 3D network structures. The porosity inherent in hydrogel matrices facilitates the loading of drugs and therapeutic agents [
8,
9,
10]. Furthermore, these hydrogels can be engineered to exhibit diverse functionalities, including stimuli-responsive, tissue adhesive, and electroconductive properties, derived from their main polymers [
11,
12]. The semisolid morphology and porous gel matrix enable drug or cell incorporation into the hydrogel and subsequent release. With considerable water content, hydrogels are highly biocompatible and stable, serving as sterile aqueous gels that are non-toxic and non-resorbable. Additionally, hydrogels present mechanical and chemical advantages, responding to a wide range of stresses with linear strain and displaying rapid and complete recovery upon stress removal [
13,
14,
15]. Leveraging these traits, hydrogels find application in both local and systematic drug delivery, capitalizing on swelling dynamics and drug release via diffusion mechanisms. However, despite their myriad benefits, the adhesive properties of hydrogels are insufficient for direct skin adherence. The development of a drug-in-adhesive system based on hydrogels necessitates the drug-loaded hydrogel to function as both the drug reservoir and adhesive layer for effective application to the skin surface [
16,
17].
The adhesive factors derived from common adhesion mechanisms can be categorized into two spatial scale-based groups: intermolecular interaction and molecule–network interaction [
18]. Intermolecular interactions rely on either non-specific adhesion groups or specific molecular pairings, such as the catechol-rich mussel foot protein in nature, which demonstrates adhesion through various interactions like hydrogen bonds, coordination bonds, hydrophobic interactions, and π-π interactions [
19,
20]. Many adhesives have been engineered by incorporating catechol-related groups, utilizing methods like direct incorporation of small molecules, covalent grafting, and incorporation of nanoparticles. Polydopamine (PDA), inspired by mussel foot protein, has gained considerable attention due to its remarkable adhesive properties, capable of strongly attaching to diverse substrates through reversible noncovalent or irreversible covalent interactions. PDA’s high adhesion stems from its catechol group, facilitating binding with diverse substrates through chemical interactions like Michael addition and Schiff base reactions, as well as physical interactions with imidazole groups on the skin surface [
21,
22,
23]. Moreover, PDA-coated materials exhibit excellent cell adhesion, spreading, and high viability for various mammalian cell types, rendering them promising in biomedical applications [
24,
25]. These advantages position PDA for diverse applications in the biomedical field, illustrated by PAM/PDA hydrogels that showcase controllable adhesiveness based on the quantity of PDA and other constituents [
26,
27]. Herein, we propose a multifunctional transdermal drug delivery system by integrating polyacrylamide (PAM), polydopamine (PDA), and poly(lactic-co-glycolic acid) (PLGA)-based nanoparticles. While PAM hydrogels have been widely explored for their hydrophilicity and mechanical tunability, their weak skin adhesiveness has posed limitations for transdermal applications. To overcome this, PDA was incorporated into the hydrogel matrix, leveraging its catechol-based chemistry to enhance bioadhesion and interface strength. Additionally, hydrophobic vitamin E was selected as a model active compound and encapsulated into PLGA nanoparticles to facilitate sustained delivery within the hydrogel. This composite hydrogel system is novel in that it (1) combines PDA-mediated tissue adhesion with (2) nanoparticle-assisted hydrophobic drug release and (3) maintains structural compatibility with skin application. Unlike previous studies that address either hydrogel adhesion or drug loading in isolation, our work presents a cohesive design integrating adhesive and delivery functionalities into a single system. This approach holds promise not only for vitamin E-based cosmetics but also as a modular platform for future transdermal formulations incorporating other bioactive.
Extensive research has investigated the potential health benefits and therapeutic applications of vitamin E derivatives [
28]. Their unique physico-chemical and biological properties have also contributed to the development of diverse drug delivery systems [
29]. Recognized for its potent antioxidant activity, vitamin E plays a vital role in protecting cell membranes and polyunsaturated lipids from reactive oxygen species (ROS) by modulating various signal transduction pathways [
30,
31,
32]. While primarily known for its antioxidant function, vitamin E serves broader biological roles, including maintaining the structural integrity of nearly all human cells through its influence on cell signaling [
33]. Additionally, it regulates connective tissue growth factor expression, modulates gene expression and transcription, and aids in wound protection against infections. Thus, beyond its antioxidant properties, vitamin E’s multifaceted functions highlight its significance in cellular health and immune defense [
34].
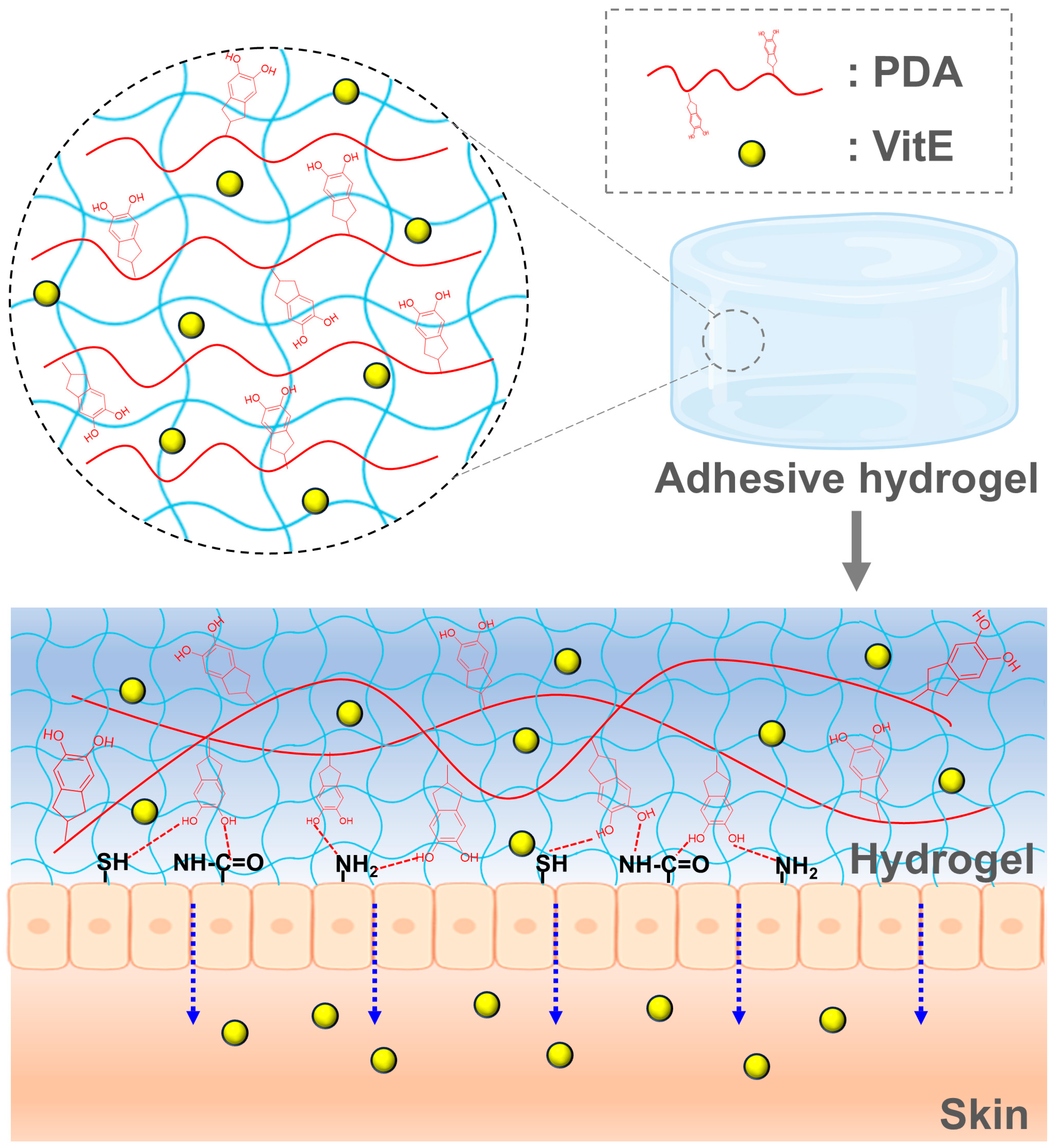
In this study, we developed skin-adhesive hydrogels using PAM and PDA formulations, leveraging the strong adhesive properties of polydopamine derived from mussel shells. Comparative analysis demonstrated that PAM/PDA hydrogels exhibited a higher swelling ratio than those without PDA. To enhance functionality, these hydrogels were integrated with PLGA nanoparticles encapsulating the hydrophobic vitamin E. The resulting hydrogel system enabled the sustained release of α-tocopherol under physiological conditions, highlighting its potential as a promising platform for transdermal vitamin E delivery. To emphasize the role of the polymeric hydrogel matrix itself, this study focused on the structural and functional integration of PAM and PDA networks, which contributes not only to mechanical robustness and skin adhesion but also to sustained delivery capability. While vitamin E was selected as a model hydrophobic compound, the hydrogel platform is designed to be broadly applicable for cosmetic actives that benefit from prolonged transdermal administration. The conceptual schematic of the adhesive hydrogel system is illustrated in
Figure 1, depicting the dual-network hydrogel composed of PAM and PDA, as well as the embedded vitamin E-loaded PLGA nanoparticles (VitE). The PAM/PDA@VitE hydrogel matrix enables strong tissue adhesion through catechol-mediated interactions, while simultaneously supporting the sustained transdermal delivery of hydrophobic drug molecules such as α-tocopherol.
2. Materials and Methods
2.1. Materials
The following materials were used in this study: dopamine hydrochloride (DA; Alfa Aesar, Ward Hill, MA, USA), acrylamide (AM; DUKSAN, Ansan-si, Republic of Korea), N,N′-methylenebisacrylamide (BIS; Sigma-Aldrich, St. Louis, MO, USA), N,N,N′,N′-tetramethylethylenediamine (TMEDA; Sigma-Aldrich, USA), ammonium persulfate (APS; Sigma-Aldrich, USA), (±)-α-tocopherol (αT; Sigma-Aldrich, USA), poly(D,L-lactide-co-glycolide) (PLGA; Sigma-Aldrich, USA), sodium hydroxide (NaOH; SAMCHUN, Seoul, Republic of Korea), polyvinyl alcohol (PVA; DAEJUNG, Jangseong-gun, Republic of Korea), Dulbecco’s Modified Eagle’s Medium (DMEM; Welgene, Gyeongsan-si, Republic of Korea), penicillin-streptomycin (Welgene, Republic of Korea), fetal bovine serum (FBS; Welgene, Republic of Korea), and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT; Amresco, Solon, OH, USA).
2.2. Synthesis of PAM Hydrogel
Polyacrylamide (PAM) hydrogels were synthesized via free-radical polymerization. Specifically, acrylamide (AM, 2.5 g), ammonium persulfate (APS, 0.25 g; used as the initiator), and N,N′-methylenebisacrylamide (BIS, 0.006 g; used as the crosslinker) were completely dissolved in 10 mL of 0.1 M sodium hydroxide (NaOH) solution that was pre-adjusted to pH 11. The solution was stirred for 15 min in an ice bath to prevent premature polymerization and ensure complete mixing. Subsequently, 1 mL of the pre-polymer solution was dispensed into cylindrical silicone molds (diameter: 1 cm; height: 0.5 cm), and N,N,N′,N′-tetramethylethylenediamine (TMEDA, 1 µL or 0.000775 g) was immediately added to each mold as a polymerization accelerator. After gentle mixing, the samples were left to polymerize at room temperature (22–25 °C) for approximately 10–15 min until gelation was complete. The formed PAM hydrogels were carefully removed from the molds and rinsed in distilled water (DW) overnight to eliminate any residual monomers and reagents. To ensure reproducibility and purity, the washing process was repeated three times with fresh DW. For structural characterization, the hydrogels were freeze-dried and sectioned, and the internal microstructure was examined by scanning electron microscopy (SEM, MAIA III, TESCAN, Brno, Czech Republic) under a high vacuum at an accelerating voltage of 10 kV. This PAM hydrogel served as the control sample for subsequent comparison with PDA-containing formulations.
2.3. Synthesis of α-Tocopherol-Loaded PLGA Nanoparticles (VitE)
α-Tocopherol-loaded poly(lactic-co-glycolic acid) (PLGA) nanoparticles were prepared using a solvent evaporation method. In brief, PLGA (75 mg) and α-tocopherol (37.5 mg) were co-dissolved in 7.5 mL of acetone as the organic phase. Separately, 1% (w/v) polyvinyl alcohol (PVA) was prepared by dissolving PVA in 75 mL of distilled water at 80 °C under constant stirring until a clear solution was obtained, and then cooled to room temperature. The PLGA/α-tocopherol acetone solution was then added dropwise into the aqueous PVA solution at a flow rate of ~0.5 mL/min under constant stirring at 1000 rpm using a magnetic stirrer at ambient conditions (22–25 °C). After complete addition, stirring was continued for 10 min to allow for initial nanoparticle formation. Subsequently, the stirring speed was reduced to 350 rpm, and the mixture was maintained for an extended stirring period of 48 h at room temperature to ensure complete solvent evaporation and stabilization of the nanoparticles. The resulting suspension was diluted with 200 mL of distilled water and centrifuged at 12,000 rpm for 15 min (4 °C) to remove unencapsulated compounds and excess surfactant. This washing step was repeated twice. The final nanoparticle pellet was collected and freeze-dried for 48 h to obtain a dry powder. For morphological characterization, the freeze-dried samples were suspended in distilled water, drop-cast on carbon-coated grids, and visualized under a transmission electron microscope (TEM; JEM-2100, JEOL, Tokyo, Japan) operated at 200 kV. The encapsulation efficiency (EE%) of vitamin E in PLGA nanoparticles was determined to be 81.2% ± 2.4% (n = 3). This result indicates the effective entrapment of the hydrophobic vitamin E within the nanoparticle matrix and is in good agreement with previous reports using similar polymeric systems.
2.4. Synthesis of PAM/PDA@VitE Hydrogel
To prepare the adhesive hydrogel matrix, polydopamine (PDA) was incorporated into a polyacrylamide (PAM) network. Initially, dopamine hydrochloride (4 mg) was dissolved in 10 mL of sodium hydroxide (NaOH) solution (pH 11) and stirred at 1000 rpm for 20 min to promote the oxidative self-polymerization of dopamine. Separately, an acrylamide monomer (AM, 2.5 g), ammonium persulfate (APS, 0.25 g; initiator), N,N′-methylenebisacrylamide (BIS, 6 mg; crosslinker), and N,N,N′,N′-tetramethylethylenediamine (TMEDA, 20 µL; accelerator) were combined and immediately added to the dopamine solution. The final pre-gel solution was gently stirred for an additional 10 min in an ice bath under a nitrogen atmosphere to minimize premature dopamine oxidation and acrylamide polymerization. Then, 5 mL of the mixture was transferred into silicone molds and allowed to cure in a convection oven at 60 °C for 4 h. After polymerization, the hydrogels were carefully removed from the molds and soaked in excess distilled water overnight with several washing cycles to remove unreacted monomers, oxidized byproducts, and low-molecular-weight impurities. The washed hydrogels were air-dried at room temperature under sterile conditions. To incorporate vitamin E-loaded nanoparticles (VitE), the dried PAM/PDA hydrogels were immersed in a phosphate-buffered saline (PBS) solution containing VitE nanoparticles (10 mg/mL) and incubated for 6 h at 37 °C under gentle shaking. After loading, the hydrogels were rinsed briefly to remove loosely adhered nanoparticles and stored at 4 °C until further use. For microstructural evaluation, the cross-sections of freeze-dried hydrogel samples were analyzed using scanning electron microscopy (SEM; MAIA III, TESCAN, Czech Republic). The concentration of polydopamine (PDA) used in the hydrogel formulation (0.004 g in 10 mL NaOH, ~0.16 wt% relative to acrylamide) was selected based on previously optimized protocols reported in the literature. Liang et al. demonstrated that PDA concentrations in the range of 0.1–0.2 wt% resulted in hydrogels with significantly improved adhesion and mechanical strength [
35]. Thus, our formulation adopts this validated PDA loading to ensure consistent performance in terms of adhesion and structural integrity.
2.5. Mechanical Adhesive Properties of Hydrogel
To evaluate the mechanical and adhesive performance of the hydrogels, a series of rheological, compression, and peel adhesion tests were conducted. Rheological measurements were performed using a rotational rheometer (Discovery HR-2, TA Instruments, New Castle, DE, USA) with a parallel plate configuration (diameter: 25 mm, gap: 1 mm). Oscillatory strain sweeps (0.1–100%) and frequency sweeps (0.1–10 Hz) were conducted at 25 °C to assess viscoelastic behavior. The compressive mechanical strength was tested using a universal testing machine (UTM; QC-548M1F, Cometech, Taichung City, Taiwan) equipped with a 100 N load cell. Hydrogels were molded into rectangular specimens (30 mm × 30 mm × 5 mm) and compressed at a constant rate of 5 mm/min until failure. The stress–strain curves were used to calculate the compressive modulus and fracture strength. The adhesive strength was quantified via a 90° peel test using the same UTM. Hydrogel patches (30 mm × 30 mm) were bonded onto polyethylene terephthalate (PET) backing films and then attached to a flat stainless-steel substrate. The peel test was performed at a crosshead speed of 10 mm/min, and the force required to detach the hydrogel from the substrate was recorded. The adhesion strength was calculated as the maximum peel force divided by the contact area of the interface (N/cm2). Each test was performed in triplicate, and the results were expressed as mean ± standard deviation (SD). All measurements were conducted under ambient laboratory conditions (temperature 22 ± 2 °C, humidity 50–60%).
2.6. Moisture Content
PAM/PDA@VitE hydrogels were prepared in dimensions of 1 cm × 1 cm. The initial weight of each dried hydrogel was recorded prior to water absorption. The samples were then immersed in an excess amount of distilled water and kept at room temperature for 3 days. After the incubation period, the hydrogels were removed, gently blotted to remove surface moisture, and weighed again. The water absorption capacity was subsequently calculated based on the weight difference using the following formula:
W = Wet weight;
D = Dry weight.
2.7. Release Test
The release profile of α-tocopherol (VitE) from PAM/PDA@VitE hydrogels was investigated under simulated physiological conditions. Hydrogel samples (10 mm × 10 mm × 2 mm) were immersed in 10 mL of phosphate-buffered saline (PBS, pH 7.4) and incubated at 37 °C with gentle agitation (100 rpm) using an orbital shaker to mimic in vivo conditions. To maintain sink conditions, 1 mL of the release medium was withdrawn at predetermined intervals (0.5, 1, 2, 4, 6, 8, 10, and 24 h) and replaced with an equal volume of fresh PBS. The collected samples were analyzed for α-tocopherol content using a UV-Vis spectrophotometer (Cary 3500, Agilent Technologies, USA) at a wavelength of 295 nm, corresponding to the maximum absorbance of VitE in PBS. A calibration curve was generated using known VitE concentrations to quantify the amount released at each time point. The release results were expressed as a cumulative percentage of total loaded VitE and plotted over time. All experiments were conducted in triplicate (n = 3), and data were reported as mean ± standard deviation (SD). No burst release was observed, and the release followed a sustained profile, consistent with dual-barrier (nanoparticle + hydrogel) controlled release kinetics.
2.8. Cell Culture
The mouse muscle fibroblast cell line (BLO-11) was obtained from the Korean Cell Line Bank (Seoul, Republic of Korea). BLO-11 cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin, and maintained at 37 °C in a humidified atmosphere containing 5% CO2.
2.9. In Vitro Cytotoxicity Tests of PAM/PDA@VitE
BLO-11 cells were seeded in a 12-well plate with 2 mL of the medium. Each well contained 3.0 × 105 cells. After overnight incubation, the cells were incubated with PDA and PLGA/PDA hydrogels for 24 h. Then, hydrogels were removed from each well, the cells were incubated with MTT solution (200 µL, 5 mg/mL) after 4 h, the medium was washed, and 1 mL DMSO solution was added to each well. The absorbance of the solution was measured at 550 nm wavelength using a microplate reader.
2.10. In Vitro Diffusion Study Using Agarose Gel
To visualize the time-dependent diffusion behavior of nanoparticles from the hydrogel matrix, an agarose-based in vitro release model was employed. A 2% (
w/
v) agarose gel was prepared by dissolving agarose powder (Sigma-Aldrich, USA) in distilled water and heating until fully melted. The hot solution was poured into 35 mm glass-bottom dishes and allowed to solidify at room temperature. FITC-loaded PLGA nanoparticles were incorporated into the PAM/PDA hydrogel during synthesis, following the same procedure as described in
Section 2.4, with FITC replacing vitamin E as the model fluorescent compound. Square hydrogel specimens (5 mm × 5 mm × 2 mm) were gently placed on top of the solidified agarose layer to ensure direct contact between the hydrogel surface and the gel matrix. The dishes were incubated at 37 °C in a humidified chamber to simulate physiological temperature. At selected time points (0, 1, 3, 6, 12, and 24 h), the hydrogel–agarose interface and the underlying agarose region were imaged using a fluorescence microscope (Axio Vert.A1, Carl Zeiss, Oberkochen, Germany) equipped with FITC filters (excitation: 495 nm, emission: 519 nm). Fluorescence intensity profiles were analyzed using ImageJ software, version 1.54f (NIH, Bethesda, MD, USA) to assess the spatial diffusion of FITC into the agarose gel. This model enabled visual tracking of nanoparticle diffusion in a hydrated, tissue-mimicking environment, providing insight into the sustained release characteristics of the hydrogel system.
3. Results and Discussion
To develop a hydrogel-based transdermal delivery system with enhanced adhesion and sustained drug release capabilities, we systematically investigated the structural and functional integration of multiple components within the hydrogel matrix. The design focused on achieving a balance between mechanical robustness, biocompatibility, and strong interfacial interaction with the skin surface. Initial efforts were directed toward selecting an appropriate hydrogel backbone material and optimizing its formulation to meet the essential criteria for transdermal application, including flexibility, durability, and compatibility with bioactive compounds.
In this study, PAM was selected as the hydrogel backbone owing to its widespread use in biological applications, excellent biocompatibility, resistance to enzymatic degradation, and its chemical versatility that allows for the facile incorporation of adhesive functional groups [
36]. The hydrogel network was synthesized via the free-radical vinyl addition polymerization of acrylamide monomers, resulting in the formation of a stable PAM matrix. Notably, the mechanical toughness of PAM, as quantified by its fracture energy, closely matches that of human skin [
26], thereby supporting its suitability for transdermal drug delivery applications. To further enhance the adhesive properties of the hydrogel, PDA was incorporated into the PAM network. PDA, inspired by the mussel adhesive protein, contains catechol groups that can form strong non-covalent and covalent interactions with biological tissues. By integrating PDA during the gelation process, the resulting hydrogel demonstrated significantly improved interfacial adhesion to skin tissue, without compromising the inherent mechanical integrity of the PAM matrix. This dual-network strategy of combining PAM’s toughness with PDA’s adhesive functionality provides a promising platform for stable and long-lasting transdermal drug delivery.
Figure S1 illustrates the two-step synthesis process of the PAM/PDA@VitE hydrogel. In the first step, PAM is polymerized in a PDA solution under the presence of an initiator and a catalyst. Polymerization is initiated immediately upon the addition of the initiator, while the catalyst accelerates the reaction, significantly reducing the overall polymerization time. Simultaneously, dopamine undergoes alkali-induced oxidation, resulting in the formation of PDA chains through catechol–quinone coupling reactions. As PDA undergoes polymerization, a distinct color transition occurs in the hydrogel, changing from colorless to light brown and eventually to dark brown (
Figure 2A). This visual change serves as a clear indicator of PDA formation and its successful incorporation into the PAM hydrogel matrix [
26].
To investigate the internal structure of the hydrogels, freeze-dried samples at an equilibrium swelling state were examined using scanning electron microscopy (SEM). The microstructures of both PAM and PAM/PDA@VitE hydrogels are presented in
Figure S2 and
Figure 2B. The pure PAM hydrogel exhibited a relatively smooth and uniform cross-sectional morphology. In contrast, the PAM/PDA@VitE hydrogel displayed a network of densely intertwined microfibers. These microfibrous structures are likely formed through π–π stacking and hydrogen bonding interactions between PDA molecules and the PAM network, specifically involving the phenolic hydroxyl groups of PDA and the amide groups of PAM [
37]. The incorporation of PDA leads to the formation of a more interconnected and porous microstructure, where the microfibers serve as physical bridges within the hydrogel matrix. This enhanced microarchitecture contributes to improved self-healing properties and mechanical strength, particularly tensile resistance, compared to the PAM hydrogel [
38]. In addition, the pore structure of the hydrogel was notably affected by the incorporation of PDA. The pore size distribution in the SEM images of PAM and PAM/PDA@VitE hydrogels reveals a clear reduction in pore size upon the addition of PDA. At a constant PAM concentration, the incorporation of PDA resulted in a decrease in pore size and a thickening of the pore walls. This microstructural transition is known to enhance the hydrogel’s ability to dissipate applied stress through a more robust and interconnected network, thereby contributing to improved tensile strength. Similar observations have been reported by Park et al., who demonstrated that reduced pore size and increased wall thickness in PDA-modified hydrogels are closely associated with enhanced mechanical properties, particularly tensile strength [
39,
40].
Water content analysis revealed that the PAM/PDA@VitE hydrogel exhibited a significantly higher water content compared to the PAM hydrogel (
Figure 2C). This increase can be attributed to the hydrophilic nature of polydopamine, which contains abundant catechol and amine functional groups capable of forming hydrogen bonds with water molecules. The incorporation of PDA into the PAM network introduces additional water-binding sites, thereby enhancing the hydrogel’s ability to retain moisture. Furthermore, the porous microfiber structure observed in the PAM/PDA@VitE hydrogel may also contribute to the increased water uptake by providing more surface area for water interaction. These findings suggest that PDA not only enhances the mechanical and adhesive properties of the hydrogel but also improves its hydration capacity, which is advantageous for transdermal drug delivery applications where sustained moisture retention is desirable.
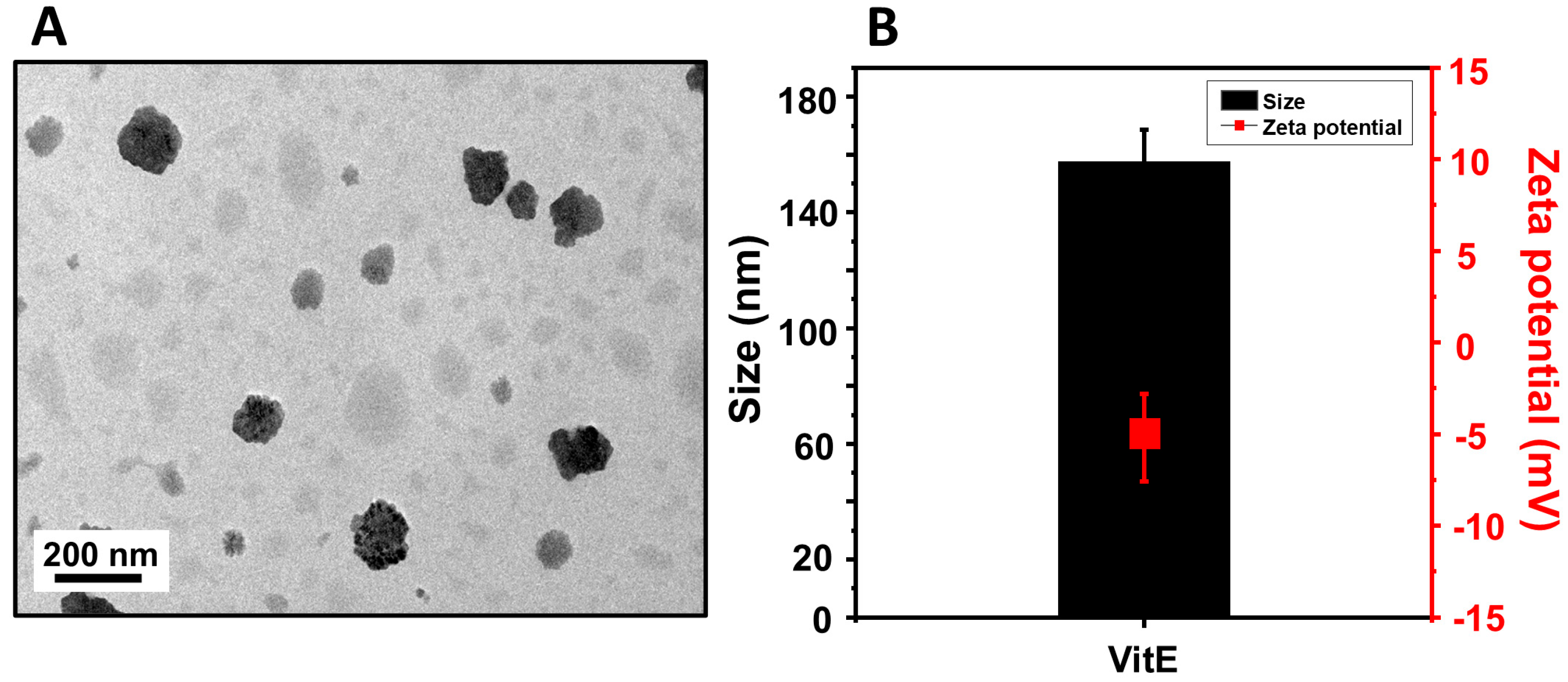
The morphology and structural integrity of the VitE were initially evaluated using transmission electron microscopy (TEM), as shown in
Figure 3A. The TEM image reveals that the nanoparticles are generally spherical with a relatively uniform distribution. Most particles appear well-dispersed without significant aggregation, and their sizes are in the nanometer range, consistent with scale bar measurements indicating sizes under 200 nm. The well-defined, dense contrast regions observed in the image suggest successful encapsulation of the hydrophobic vitamin E within the polymer matrix. Dynamic light scattering (DLS) further confirmed that the average hydrodynamic diameter of the nanoparticles was approximately 150 nm, and their surface zeta potential was measured to be −8.0 mV (
Figure 3B). This size is favorable for enhanced penetration through the stratum corneum and potential cellular uptake, while the slight negative surface charge contributes to moderate colloidal stability by preventing aggregation. The encapsulation of vitamin E into PLGA offers distinct advantages for cosmetic and dermatological applications. PLGA protects vitamin E from oxidative degradation and provides a controlled release profile, enhancing its stability and efficacy. Vitamin E is widely recognized for its role in improving skin hydration, reducing inflammation, and offering protection against UV-induced damage. Delivery via nanoparticles further improves its bioavailability and skin retention, suggesting that PLGA-based nanoformulations can serve as effective carriers for vitamin E in topical applications. Although direct stability testing of the PLGA-encapsulated vitamin E nanoparticles was not performed in this study, previous research has demonstrated the physical stability of similar formulations under physiological conditions. Varga et al. reported that α-tocopherol-loaded PLA/PLGA nanoparticles maintained their size and polydispersity index over a 30-day period in phosphate-buffered saline (PBS, pH 7.4) at 25 °C and 37 °C [
41]. These findings suggest that the encapsulated vitamin E remains stable in aqueous environments and supports the expected physicochemical integrity of our VitE nanoparticles under similar conditions.
To evaluate the impact of PDA incorporation on the adhesive performance of PAM/PDA@VitE hydrogels, both mechanical adhesion tests and rheological characterizations were conducted.
Figure 4A–C demonstrate the lap shear strength, tensile adhesion strength, and 180° peel strength, respectively, for PAM hydrogels and PAM/PDA@VitE hydrogels. All three measurements clearly show a substantial increase in adhesion performance upon the incorporation of PDA. In particular, the PAM/PDA@VitE hydrogel exhibited nearly double the lap shear strength and markedly enhanced peel resistance compared to the PAM hydrogel. This dramatic improvement is attributed to the introduction of catechol groups from PDA, which are known to strongly interact with both organic and inorganic surfaces through hydrogen bonding, metal coordination, and π–π interactions—mimicking the adhesion mechanism of mussel foot proteins. The PDA molecules are oxidized to form polydopamine chains that can covalently and non-covalently bond to the PAM matrix as well as the target substrate. These catechol functionalities increase surface binding affinity, allowing the hydrogel to form stronger and more stable contacts with skin or other biological tissues. Moreover, the PDA network contributes to an interpenetrating structure within the hydrogel, which enhances not only adhesion but also internal cohesion, improving overall mechanical integrity under stress. Although our study did not perform chemical-speciation or TOF SIMS analysis, previous reports using PDA/AM ratios close to ours (~0.1–0.2 wt%) have confirmed a uniform PDA distribution in the PAM network, the formation of catechol–amine cross links via FT IR/XPS, and the presence of fibrillar microstructures in SEM images [
26]. These studies also demonstrated significantly improved fracture toughness and adhesion in PDA/PAM hydrogels compared to PAM alone [
42]. Therefore, we are confident that our material exhibits similar structural and mechanical behaviors.
Figure 4D presents the oscillatory strain–stress behavior of the PAM and PAM/PDA@VitE hydrogels. The PAM/PDA@VitE hydrogel showed a steeper stress increase over the same strain range compared to PAM hydrogel, indicating improved elastic response and load-bearing capability. This behavior suggests enhanced internal crosslinking or physical entanglements within the network structure due to the presence of PDA. Such viscoelastic reinforcement further supports the observation that PDA enhances the structural resilience of the hydrogel, which is critical for applications involving dynamic skin contact, such as wearable patches or drug delivery interfaces. Together, these results indicate that the incorporation of PDA into PAM hydrogels not only significantly enhances surface adhesion through the introduction of versatile catechol chemistry but also strengthens the overall mechanical stability and dynamic response of the hydrogel system. Although the adhesive strength of PAM/PDA hydrogels was evaluated using polyethylene terephthalate (PET) films for standardized and reproducible measurements, it is important to recognize that this synthetic substrate does not fully replicate the mechanical and biochemical complexity of biological tissues. The flat and inert surface of PET lacks the proteinaceous and lipid components present in human or animal skin, which are critical to understanding real-world adhesive interactions. Therefore, future studies should incorporate biological substrates, such as porcine skin or reconstructed human epidermis, to more accurately assess the hydrogel’s adhesive performance in a physiologically relevant environment. These properties are especially advantageous for biomedical applications, such as transdermal drug delivery or bioelectronic interfaces, where firm yet conformable adhesion to moist and irregular skin surfaces is required.
To investigate the controlled release behavior of vitamin E from the hydrogel system, a release test was performed over time (
Figure 5A). In this system, vitamin E was first encapsulated within PLGA nanoparticles, which were subsequently embedded within a polyacrylamide-based hydrogel matrix, as schematically illustrated in
Figure 1. This two-step encapsulation strategy (vitamin E inside PLGA nanoparticles and PLGA nanoparticles dispersed within the hydrogel) was designed to achieve a sustained and controlled release profile. The release behavior observed demonstrated that vitamin E was gradually released over time without an initial burst release, indicating that the dual barriers (PLGA nanoparticle and hydrogel matrix) effectively modulated the diffusion of vitamin E into the surrounding medium. The PLGA nanoparticles acted as a primary reservoir, providing a controlled release of vitamin E as the polymer matrix underwent hydrolytic degradation. Meanwhile, the hydrogel network served as a secondary barrier, further slowing down the diffusion of the released vitamin E molecules into the external environment. Over the monitored time points, cumulative release profiles showed a steady, sustained release of vitamin E, with an approximately 50.7% release observed at 24 h. This controlled release behavior is attributed to the hydrophobic nature of PLGA, which slows down water penetration and polymer degradation, combined with the semi-permeable structure of the hydrogel matrix that retards molecule diffusion. Such a release profile is highly advantageous for transdermal delivery applications, particularly in cosmetic and dermatological fields where a prolonged antioxidant supply to the skin is desired to protect against oxidative stress and promote skin regeneration. Overall, these results confirm that the incorporation of VitE into hydrogels provides an effective platform for the sustained and controlled release of hydrophobic bioactives such as vitamin E, enhancing their therapeutic potential and applicability for long-term skin care formulations. In comparison to emulsion-based nanoparticle delivery systems, the integration of nanoparticles into a hydrogel matrix provides multiple advantages for transdermal applications. This hybrid configuration not only improves skin adhesion and formulation stability but also enables a more sustained and localized release profile, owing to the barrier function of the gel matrix and its strong water retention. Previous studies have reported that hydrogel–nanoparticle composites outperform standalone delivery systems in both drug retention and controlled release kinetics [
43]. For instance, PLGA nanoparticles embedded in a hydrogel have been shown to avoid rapid diffusion associated with aqueous emulsions and offer improved patient compliance and prolonged skin residence time. These synergistic effects make the hydrogel–nanoparticle strategy a promising platform, particularly for the topical and cosmetic delivery of antioxidant agents such as vitamin E. Although the long-term physicochemical stability of the PAM/PDA@VitE hydrogel system was not assessed in this study, future investigations will include thermal, storage, and structural stability analyses to support its viability as a transdermal formulation under typical usage conditions.
To investigate the bioactivity and cytocompatibility of VitE embedded in the hydrogel, a transwell assay was performed using BLO-11 cells (
Figure 5B). Two experimental groups were established: one with a PAM/PDA hydrogel and another with a PAM/PDA@VitE hydrogel. The hydrogels were placed on the upper chamber of a transwell insert, allowing only the released molecules to diffuse toward the BLO-11 cells cultured in the lower chamber, thus eliminating direct physical contact between the hydrogel and the cells. As shown in
Figure 5B, the cell proliferation was significantly higher in the group treated with the PAM/PDA@VitE hydrogel compared to the control group. This result clearly indicates that vitamin E released from the PLGA nanoparticles exerted a positive effect on cell viability and proliferation. Vitamin E, a potent lipid-soluble antioxidant, is known to protect cells against oxidative stress by scavenging reactive oxygen species (ROS) and stabilizing cellular membranes. Numerous studies have demonstrated that vitamin E can enhance fibroblast proliferation, promote angiogenesis, and accelerate wound healing [
44,
45]. Specifically, it has been reported that vitamin E supports the maintenance of cell membrane integrity and modulates intracellular signaling pathways involved in cell growth and survival. In the context of this study, the gradual release of vitamin E from the PLGA nanoparticles likely provided continuous antioxidant protection, reducing oxidative stress and creating a favorable environment for BLO-11 cell proliferation. Furthermore, the transwell system used in this study ensured that only the diffusible vitamin E molecules influenced the cells, confirming the biological activity of the released cargo without mechanical artifacts. The significant increase in cell viability highlights the therapeutic potential of vitamin E-loaded nanoparticle/hydrogel systems in applications requiring enhanced cell proliferation, such as skin regeneration and cosmetic formulations. These findings reinforce that incorporating vitamin E into a nanoparticle–hydrogel composite system can be an effective strategy to promote cellular health and tissue regeneration.
To evaluate the release behavior and delivery capability of PAM/PDA@VitE hydrogel, a model system was developed using agarose gel as the recipient matrix. Instead of encapsulating biologically active agents such as vitamin E, fluorescein isothiocyanate (FITC) was loaded into PLGA nanoparticles to enable the fluorescent tracking of diffusion and release profiles. Although porcine skin is commonly used for in vitro skin delivery studies due to its anatomical and physiological similarities to human skin, agarose gel was chosen in this experiment to establish a simplified, reproducible, and controllable diffusion environment. Agarose gel offers several advantages in early-stage release profiling: it provides a uniform and inert matrix that mimics the hydrated environment of biological tissue without the complexities of lipid barriers and cellular interactions inherent in porcine skin. This enables a more direct observation of diffusion-driven transport phenomena and facilitates standardization across time points [
46,
47]. Therefore, the current system is especially useful for screening nanoparticle release kinetics from hydrogels and comparing delivery performance between different formulations before advancing to animal or ex vivo models. Fluorescence microscopy images (
Figure 6) show the time-dependent penetration of FITC-labeled PLGA nanoparticles from the hydrogel into the agarose gel. At 0 h, no fluorescence signal was detected in the agarose layer, confirming the absence of nanoparticle diffusion. After 1 h, a faint fluorescent signal began to emerge at the hydrogel–agarose interface. Over time, the fluorescence intensity gradually increased and extended deeper into the agarose layer. At 6 h, a more defined diffusion front was observed, and by 12 h and 24 h, the FITC signal had penetrated substantially into the agarose matrix. These results suggest that the PAM/PDA hydrogel effectively supports the sustained release of encapsulated nanoparticles and allows for gradual diffusion into a surrounding gel matrix to occur. The progressive diffusion pattern supports the hypothesis that the hydrogel–nanoparticle system exhibits controlled and prolonged release characteristics, which is desirable in transdermal delivery applications. Although the diffusion depth and kinetics in agarose gel do not directly replicate those of actual skin tissue—owing to the lack of a stratum corneum and other structural barriers—the data nonetheless provide valuable preliminary insight. Although Franz diffusion cell assays are widely used in transdermal drug testing, we employed an agarose-based model as a simplified in vitro system to observe diffusion kinetics without the complexities of lipid and protein layers. In future studies, Franz cell-based assays with ex vivo skin will be conducted to further validate the delivery potential of PAM/PDA hydrogel systems under cosmetic application conditions. Specifically, they allow for the comparison of release rates, hydrogel–nanoparticle interactions, and stability of the carrier system over time under simplified conditions. Future work involving porcine skin or reconstructed human epidermis will be necessary to validate whether similar delivery trends occur in biologically relevant barriers. Although direct in vivo testing was not conducted in this study, previous reports have demonstrated that PDA-based hydrogel systems exhibit excellent biocompatibility in biological environments. PDA-functionalized hydrogels have been applied in various in vivo wound healing models without inducing irritation or inflammatory responses, and they have supported tissue regeneration and healing [
48]. Moreover, PDA-based adhesives have shown good skin compatibility even in human applications, with strong interfacial adhesion and minimal adverse reactions [
49]. Some studies also highlight the antibacterial and anti-inflammatory properties of PDA-containing hydrogels, which further enhance their suitability for skin-contact applications [
50]. These findings collectively suggest that the PAM/PDA hydrogel system described in this study would similarly offer a favorable safety profile, even for individuals with sensitive or compromised skin. Nevertheless, future in vivo studies, particularly using atopic or couperose-prone skin models, will be critical to comprehensively validate the system’s compatibility for clinical or cosmetic use.