The Role of African Medicinal Plants in Dermatological Treatments: A Systematic Review of Antimicrobial, Wound-Healing and Melanogenesis Inhibition
Abstract
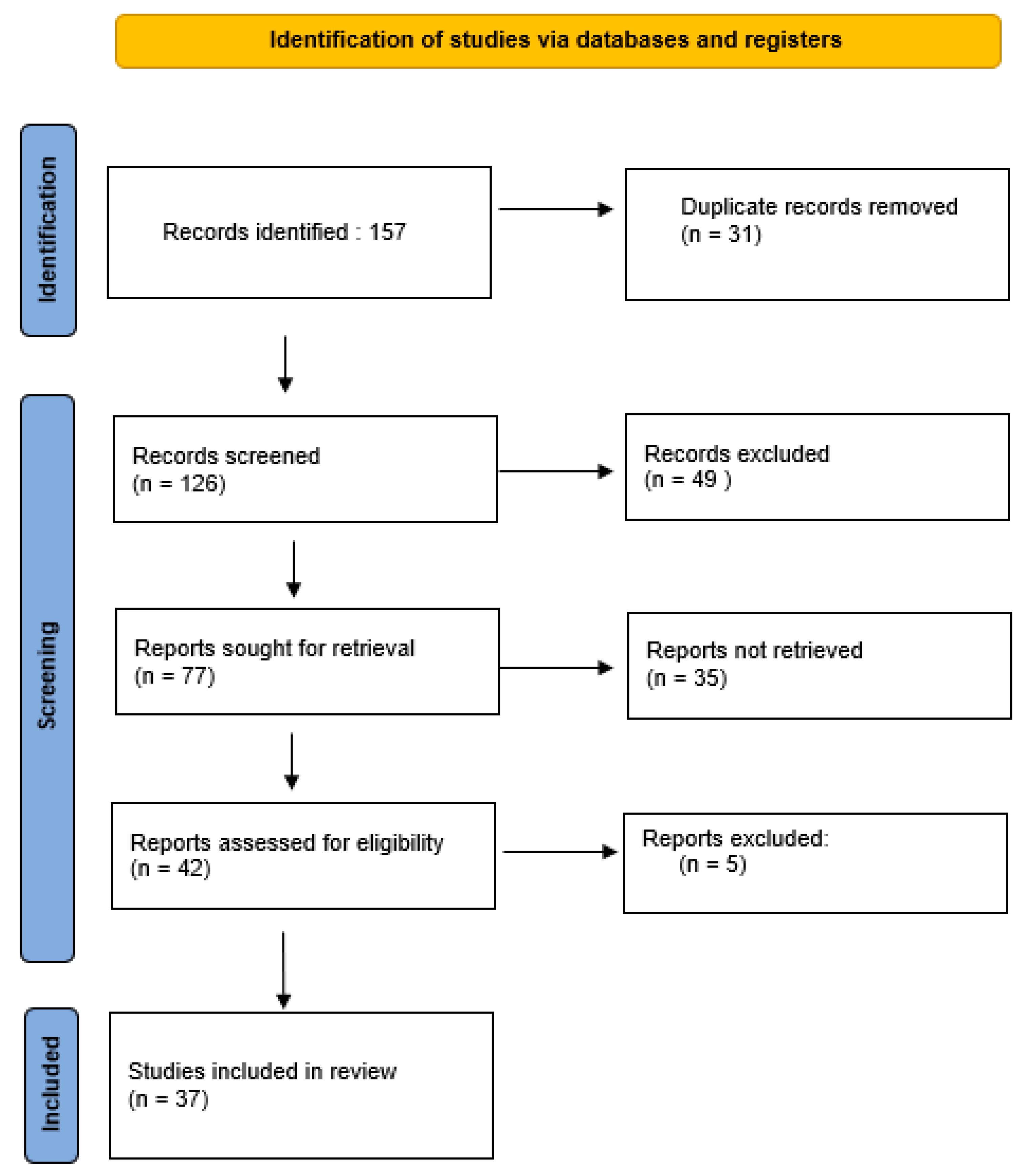
1. Introduction
2. Methodology
3. Results and Discussion
3.1. Wound Healing Potentia
Antimicrobial Activity in Wound Healing
3.2. Antimicrobial Activity
3.3. Melanogenesis Inhibition
4. Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Salmerón-Manzano, E.; Garrido-Cardenas, J.A.; Manzano-Agugliaro, F. Worldwide research trends on medicinal plants. Int. J. Environ. Res. Public Health 2020, 17, 3376. [Google Scholar] [CrossRef]
- Allkin, B. Useful plants—Medicines: At least 28,187 plant species are currently recorded as being of medicinal use. In State of the World’s Plants; Kew Bulletin: London, UK, 2017. [Google Scholar]
- Agyare, C.; Boakye, Y.D.; Bekoe, E.O.; Hensel, A.; Dapaah, S.O.; Appiah, T. African medicinal plants with wound healing properties. J. Ethnopharmacol. 2016, 177, 85–100. [Google Scholar] [CrossRef]
- Husen, A. Traditional Herbal Therapy for the Human Immune System; Chapter 5; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Parvin, S.; Kader, M.A.; Chouduri, A.U.; Rafshanjani, M.A.; Haque, M.E. Antibacterial, antifungal and insecticidal activities of the n-hexane and ethyl-acetate fractions of methanolic extract of the leaves of Calotropis gigantea Linn. J. Pharmacogn. Phytochem. 2014, 2, 47–51. [Google Scholar]
- Khatami, M.; Varma, R.S.; Zafarnia, N.; Yaghoobi, H.; Sarani, M.; Kumar, V.G. Applications of green synthesized Ag, ZnO and Ag/ZnO nanoparticles for making clinical antimicrobial wound-healing bandages. Sustain. Chem. Pharm. 2018, 10, 9–15. [Google Scholar] [CrossRef]
- Buchvald, D. Anatomy and histology of the skin. In Dermatovenerology, 1st ed.; Šimaljaková, M., Buchvald, D., Eds.; Publishing House of Comenius University: Bratislava, Slovakia, 2019; pp. 21–24. [Google Scholar]
- Ki, V.; Rotstein, C. Bacterial skin and soft tissue infections in adults: A review of their epidemiology, pathogenesis, diagnosis, treatment and site of care. Can. J. Infect. Dis. Med. Microbiol. 2008, 19, 173–184. [Google Scholar] [CrossRef]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Seo, H.S.; Lim, S.Y.; Park, K. Cutaneous immune defenses against Staphylococcus aureus infections. J. Lifestyle Med. 2014, 4, 39. [Google Scholar] [CrossRef]
- Esposito, S.; Bassetti, M.; Concia, E.; De Simone, G.; De Rosa, F.G.; Grossi, P.; Novelli, A.; Menichetti, F.; Petrosillo, N.; Tinelli, M.; et al. Diagnosis and management of skin and soft-tissue infections (SSTI). A literature review and consensus statement: An update. J. Chemother. 2017, 29, 197–214. [Google Scholar] [CrossRef]
- Desmet, C.M.; Préat, V.; Gallez, B. Nanomedicines and gene therapy for the delivery of growth factors to improve perfusion and oxygenation in wound healing. Adv. Drug Deliv. Rev. 2018, 129, 262–284. [Google Scholar] [CrossRef]
- Belachew, T.F.; Asrade, S.; Geta, M.; Fentahun, E. In vivo evaluation of wound healing and anti-inflammatory activity of 80% methanol crude flower extract of Hagenia abyssinica (Bruce) J.F. Gmel in mice. Evid. Based Complement. Altern. Med. 2020, 2020, 9645792. [Google Scholar] [CrossRef]
- Adams, S.B.; Sabesan, V.J.; Easley, M.E. Wound healing agents. Foot Ankle Clin. 2006, 11, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Gosain, A.; DiPietro, L.A. Aging and wound healing. World J. Surg. 2004, 28, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Dreifke, M.B.; Jayasuriya, A.A.; Jayasuriya, A.C. Current wound healing procedures and potential care. Mater. Sci. Eng. C 2015, 48, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Järbrink, K.; Ni, G.; Sönnergren, H.; Schmidtchen, A.; Pang, C.; Bajpai, R.; Car, J. Prevalence and incidence of chronic wounds and related complications: A protocol for a systematic review. Syst. Rev. 2016, 5, 152. [Google Scholar] [CrossRef]
- Mustoe, T. Understanding chronic wounds: A unifying hypothesis on their pathogenesis and implications for therapy. Am. J. Surg. 2004, 187, S65–S70. [Google Scholar] [CrossRef]
- Guo, S.A.; DiPietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic wound healing: A review of current management and treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef]
- Frykberg, R.G.; Banks, J. Challenges in the treatment of chronic wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef]
- Park, J.W.; Hwang, S.R.; Yoon, I.S. Advanced growth factor delivery systems in wound management and skin regeneration. Molecules 2017, 22, 1259. [Google Scholar] [CrossRef]
- Kwon, S.H.; Park, K.C. Melasma and common pigmentary dermatoses in Asian individuals and an overview of their treatment. J. Clin. Investig. Dermatol. 2014, 2, 8. [Google Scholar]
- Callender, V.D.; St Surin-Lord, S.; Davis, E.C.; Maclin, M. Postinflammatory hyperpigmentation: Etiologic and therapeutic considerations. Am. J. Clin. Dermatol. 2011, 12, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Bastiaens, M.; Hoefnagel, J.; Westendorp, R.; Vermeer, B.J.; Bouwes Bavinck, J.N. Solar lentigines are strongly related to sun exposure in contrast to ephelides. Pigment. Cell Res. 2004, 17, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Ogbechie-Godec, O.A.; Elbuluk, N. Melasma: An up-to-date comprehensive review. Dermatol. Ther. 2017, 7, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Woolery-Lloyd, H.; Kammer, J.N. Treatment of hyperpigmentation. Semin. Cutan. Med. Surg. 2011, 30, 171–175. [Google Scholar] [CrossRef]
- Giorgis, S.G.; Ambikar, D.; Tsegaw, A.; Belayneh, Y.M. Wound healing activity of 80% methanolic crude extract and solvent fractions of the leaves of Justicia schimperiana (Hochst. ex Nees) T. Anderson (Acanthaceae) in Mice. J. Exp. Pharmacol. 2022, 14, 167–183. [Google Scholar] [CrossRef]
- Amrati, F.E.; Slighoua, M.; Mssillou, I.; Chebaibi, M.; Galvão de Azevedo, R.; Boukhira, S.; Moslova, K.; Al Kamaly, O.; Saleh, A.; Correa de Oliveira, A.; et al. Lipids fraction from Caralluma europaea (Guss.): MicroTOF and HPLC analyses and exploration of its antioxidant, cytotoxic, anti-inflammatory, and wound healing effects. Separations 2023, 10, 172. [Google Scholar] [CrossRef]
- Tazeze, H.; Mequanente, S.; Nigussie, D.; Legesse, B.; Makonnen, E.; Mengie, T. Investigation of wound healing and anti-inflammatory activities of leaf gel of Aloe trigonantha LC leach in Rats. J. Inflamm. Res. 2021, 14, 5567–5580. [Google Scholar] [CrossRef]
- Hattingh, A.; Laux, J.P.; Willers, C.; Hamman, J.; Steyn, D.; Hamman, H. In vitro wound healing effects of combinations of Aloe vera gel with different extracts of Bulbine frutescens. S. Afr. J. Bot. 2023, 158, 254–264. [Google Scholar] [CrossRef]
- Matsuete-Takongmo, G. Antibacterial and wound healing properties of selected Cameroonian medicinal plants. J. Int. Res. Med. Pharm. Sci. 2023, 18, 34–51. [Google Scholar]
- Ashenafi, E.; Abula, T.; Abay, S.M.; Arayaselassie, M.; Sori, M. Evaluation of the antioxidant and wound healing properties of 80% methanol extract and solvent fractions of the leaves of Vernonia auriculifera hiern. (Asteraceae). Clin. Cosmet. Investig. Dermatol. 2023, 16, 279–299. [Google Scholar] [CrossRef]
- Hanbisa, S.; Tadesse, W.T.; Abula, T. Evaluation of wound healing activity of 80% methanol stem-bark extract and solvent fractions of Prunus africana (Hook.f.) Kalkman (Rosaceae) in mice. J. Exp. Pharmacol. 2023, 2023, 349–365. [Google Scholar] [CrossRef] [PubMed]
- Asumang, P.; Boakye, Y.D.; Agana, T.A.; Yakubu, J.; Entsie, P.; Akanwariwiak, W.G.; Adu, F.; Agyare, C. Antimicrobial, antioxidant and wound healing activities of methanol leaf extract of Bridelia micrantha (Hochst.) Baill. Sci. Afr. 2021, 14, e00980. [Google Scholar] [CrossRef]
- Aly, S.H.; El-Hassab, M.A.; Elhady, S.S.; Gad, H.A. Comparative metabolic study of Tamarindus indica L.’s various organs based on GC/MS analysis, in silico and in vitro anti-inflammatory and wound healing activities. Plants 2022, 12, 87. [Google Scholar] [CrossRef] [PubMed]
- Kuma, D.N.; Boye, A.; Kwakye-Nuako, G.; Boakye, Y.D.; Addo, J.K.; Asiamah, E.A.; Aboagye, E.A.; Martey, O.; Essuman, M.A.; Atsu Barku, V.Y. Wound healing properties and antimicrobial effects of Parkia clappertoniana keay fruit husk extract in a rat excisional wound model. BioMed Res. Int. 2022, 2022, 9709365. [Google Scholar] [CrossRef] [PubMed]
- Chinko, B.C.; Precious-Abraham, A.D. Wound healing activity of hydromethanolic Dioscorea bulbifera extract on male wistar rat excision wound models. Pharmacol. Res. Mod. Chin. Med. 2024, 11, 100425. [Google Scholar] [CrossRef]
- Yassine, K.A.; Houari, H.; Mokhtar, B.; Karim, A.; Hadjer, S.; Imane, B. A topical ointment formulation containing leaves’ powder of Lawsonia inermis accelerate excision wound healing in Wistar rats. Vet. World 2020, 13, 1280. [Google Scholar] [CrossRef]
- El Massoudi, S.; Zinedine, A.; Rocha, J.M.; Benidir, M.; Najjari, I.; El Ghadraoui, L.; Benjelloun, M.; Errachidi, F. Phenolic composition and wound healing potential assessment of Moroccan henna (Lawsonia inermis) aqueous extracts. Cosmetics 2023, 10, 92. [Google Scholar] [CrossRef]
- Shewaye, D.G.; Kahaliw, W.; Mulaw Belete, T.; Ahmed, N. Evaluation of Wound Healing and Anti-Inflammatory Activities of 80% Methanol Crude Extract and Solvent Fractions of Trichilia dregeana Sond (Meliaceae) Leaves in Mice. Evid. Based Complement. Altern. Med. 2023, 2023, 9980866. [Google Scholar] [CrossRef]
- Baidoo, M.F.; Mensah, A.Y.; Ossei, P.P.; Asante-Kwatia, E.; Amponsah, I.K. Wound healing, antimicrobial and antioxidant properties of the leaf and stem bark of Entada africana Guill. & Perr. S. Afr. J. Bot. 2021, 137, 52–59. [Google Scholar]
- Ekweogu, C.N.; Akubugwo, E.I.; Emmanuel, O.; Nosiri, C.I.; Uche, M.E.; Adurosakin, O.E.; Ijioma, S.N.; Ugbogu, E.A. Phytochemical profiling, toxicity studies, wound healing, analgesic and anti-inflammatory activities of Musa paradisiaca L. Musaceae (Plantain) stem extract in rats. J. Ethnopharmacol. 2024, 322, 117639. [Google Scholar] [CrossRef]
- Abdul-Nasir-Deen, A.Y.; Boakye, Y.D.; Osafo, N.; Agyare, C.; Boamah, D.; Boamah, V.E.; Agyei, E.K. Anti-inflammatory and wound healing properties of methanol leaf extract of Physalis angulata L. S. Afr. J. Bot. 2020, 133, 124–131. [Google Scholar]
- Benmahieddine, A.; Belyagoubi-Benhammou, N.; Belyagoubi, L.; Amari, N.O.; Zerey-Belaskri, A.E.; Gismondi, A.; Di Marco, G.; Canini, A.; Habi, S.; Atik Bekkara, F.; et al. Leaf-buds of Pistacia atlantica: A novel source of bioactive molecules with high anti-inflammatory, antioxidant, anti-tyrosinase and antimicrobial properties. Physiol. Mol. Biol. Plants 2023, 29, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Owusu, E.; Ahorlu, M.M.; Afutu, E.; Akumwena, A.; Asare, G.A. Antimicrobial activity of selected medicinal plants from a sub-Saharan African country against bacterial pathogens from post-operative wound infections. Med. Sci. 2021, 9, 23. [Google Scholar] [CrossRef]
- Lall, N.; Van Staden, A.B.; Rademan, S.; Lambrechts, I.; De Canha, M.N.; Mahore, J.; Winterboer, S.; Twilley, D. Antityrosinase and anti-acne potential of plants traditionally used in the Jongilanga community in Mpumalanga. S. Afr. J. Bot. 2019, 126, 241–249. [Google Scholar] [CrossRef]
- Lehbili, M.; Alabdul Magid, A.; Kabouche, A.; Voutquenne-Nazabadioko, L.; Abedini, A.; Morjani, H.; Gangloff, S.C.; Kabouche, Z. Antibacterial, antioxidant and cytotoxic activities of triterpenes and flavonoids from the aerial parts of Salvia barrelieri Etl. Nat. Prod. Res. 2018, 32, 2683–2691. [Google Scholar] [CrossRef] [PubMed]
- Agbo, I.A.; HlangothI, B.; Didloff, J.; Hattingh, A.C.; Venables, L.; Govender, S.; van de Venter, M. Comparative Evaluation of the Phytochemical Contents, Antioxidant and some Biological Activities of Khaya grandifoliola Methanol and Ethyl Acetate Stem Bark, Root and leaf Extracts. Trop. J. Nat. Prod. Res. 2023, 7, 2829–2836. [Google Scholar]
- Mikayoulou, M.; Mayr, F.; Temml, V.; Pandian, A.; Vermaak, I.; Chen, W.; Komane, B.; Stuppner, H.; Viljoen, A. Anti-tyrosinase activity of South African Aloe species and isolated compounds plicataloside and aloesin. Fitoterapia 2021, 150, 104828. [Google Scholar] [CrossRef]
- Chelly, S.; Chelly, M.; Occhiuto, C.; Cimino, F.; Cristani, M.; Saija, A.; Molonia, M.S.; Ruberto, G.; D’Angelo, V.; Germanò, M.P.; et al. Evaluation of antioxidant, anti-inflammatory and antityrosinase potential of extracts from different aerial parts of Rhanterium suaveolens from Tunisia. Chem. Biodivers. 2021, 18, e2100316. [Google Scholar] [CrossRef]
- Akinwunmi, O.A.; Popooola, O.K.; Nwozo, S.O.; Olanipekun, A.D.; Faleye, F.J. Total antioxidant and Anti-tyrosinase Activities of Methanol Extract of Ripe Nauclea latifolia Fruits and its Chromatographic Fractions. Trop. J. Nat. Prod. Res. 2022, 6, 806–810. [Google Scholar]
- Manjia, J.N.; Njoya, E.M.; Harishchander, A.; Munvera, A.M.; Ogundolie, F.A.; Mkounga, P.; Mcgaw, L.J.; Njayou, F.N.; Moundipa, P.F. Anti-elastase, Anti-tyrosinase, and Anti-inflammatory Activities of Three Compounds Isolated from Psorospermum aurantiacum: In Silico and In Vitro Assays. Rev. Bras. Farmacogn. 2024, 34, 1116–1128. [Google Scholar] [CrossRef]
- Tadrent, W.; Alabdul Magid, A.; Kabouche, A.; Kabouche, Z.; Sayagh, C.; Voutquenne-Nazabadioko, L. Phytochemical study, antioxidant and tyrosinase inhibitory activities of Pentzia monodiana Maire. Nat. Prod. Res. 2024, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sadeer, N.B.; Llorent-Martínez, E.J.; Bene, K.; Mahomoodally, M.F.; Mollica, A.; Sinan, K.I.; Stefanucci, A.; Ruiz-Riaguas, A.; Fernández-de Córdova, M.L.; Zengin, G. Chemical profiling, antioxidant, enzyme inhibitory and molecular modelling studies on the leaves and stem bark extracts of three African medicinal plants. J. Pharm. Biomed. Anal. 2019, 174, 19–33. [Google Scholar] [CrossRef]
- Stapelberg, J.; Nqephe, M.; Lambrechts, I.; Crampton, B.; Lall, N. Selected South African plants with tyrosinase enzyme inhibition and their effect on gene expression. S. Afr. J. Bot. 2019, 120, 280–285. [Google Scholar] [CrossRef]
- Sefi, O.; Bourgou, S.; Megdiche-Ksouri, W.; Libiad, M.; Khabbach, A.; El Haissoufi, M.; Lamchouri, F.; Krigas, N.; Ghrabi-Gammar, Z. Bioactivities and phenolic composition of Limonium boitardii Maire and L. cercinense Brullo & Erben (Plumbaginaceae): Two Tunisian strict endemic plants. Int. J. Environ. Health Res. 2022, 32, 2496–2511. [Google Scholar]
- Sinan, K.I.; Bene, K.; Zengin, G.; Diuzheva, A.; Jekő, J.; Cziáky, Z.; Picot-Allain, C.M.; Mollica, A.; Rengasamy, K.R.; Mahomoodally, M.F. A comparative study of the HPLC-MS profiles and biological efficiency of different solvent leaf extracts of two African plants: Bersama abyssinica and Scoparia dulcis. Int. J. Environ. Health Res. 2021, 31, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Kishore, N.; Twilley, D.; Blom van Staden, A.; Verma, P.; Singh, B.; Cardinali, G.; Kovacs, D.; Picardo, M.; Kumar, V.; Lall, N. Isolation of flavonoids and flavonoid glycosides from Myrsine africana and their inhibitory activities against mushroom tyrosinase. J. Nat. Prod. 2018, 81, 49–56. [Google Scholar] [CrossRef]
- Muhammad, A.; Idris, M.M.; Ali, U.; Umar, A.; Sirat, H.M. Characterization and tyrosinase activities of a mixture of β-sitosterol and stigmasterol from Bauhinia rufescens Lam. Acta Pharm. Indones. 2023, 11, 6284. [Google Scholar] [CrossRef]
- Sonka, L. Exploring Anti-Tyrosinase Bioactive Compounds from the Cape Flora; University of the Western Cape: Cape Town, South Africa, 2018. [Google Scholar]
- Yalo, M.; Makhaba, M.; Hussein, A.A.; Sharma, R.; Koki, M.; Nako, N.; Mabusela, W.T. Characterization of Four New Compounds from Protea cynaroides Leaves and Their Tyrosinase Inhibitory Potential. Plants 2022, 11, 1751. [Google Scholar] [CrossRef] [PubMed]
- Bourhim, T.; Villareal, M.O.; Gadhi, C.; Hafidi, A.; Isoda, H. Depigmenting effect of argan press-cake extract through the down-regulation of Mitf and melanogenic enzymes expression in B16 murine melanoma cells. Cytotechnology 2018, 70, 1389–1397. [Google Scholar] [CrossRef]
- Watti, O.I.; Yalo, M.; Sharma, R.; Makhaba, M.; Hussein, A.A.; Mabusela, W.T. Phytochemistry, Anti-Tyrosinase, and Anti-Diabetes Studies of Extracts and Chemical Constituents of Dicerothamnus rhinocerotis Leaves. Chemistry 2024, 6, 546–554. [Google Scholar] [CrossRef]
- Fitzmaurice, S.D.; Sivamani, R.K.; Isseroff, R.R. Antioxidant therapies for wound healing: A clinical guide to currently commercially available products. Ski. Pharmacol. Physiol. 2011, 24, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Bishop, A. Role of oxygen in wound healing. J. Wound Care 2008, 17, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Caley, M.P.; Martins, V.L.; O’Toole, E.A. Metalloproteinases and wound healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Amoo, S.O.; Aremu, A.O.; Moyo, M.; Van Staden, J. Antioxidant and acetylcholinesterase-inhibitory properties of long-term stored medicinal plants. BMC Complement. Altern. Med. 2012, 12, 87–89. [Google Scholar] [CrossRef]
- Deka, B.; Bhattacharjee, B.; Shakya, A.; Ikbal, A.M.; Goswami, C.; Sarma, S. Mechanism of action of wound healing activity of Calendula officinalis: A comprehensive review. Pharm. Biosci. J. 2021, 9, 28–44. [Google Scholar] [CrossRef]
- Khalid, K.A.; da Silva, J.T. Biology of Calendula officinalis Linn.: Focus on pharmacology, biological activities and agronomic practices. Med. Aromat. Plant Sci. Biotechnol. 2012, 6, 12–27. [Google Scholar]
- Kumari, A.; Raina, N.; Wahi, A.; Goh, K.W.; Sharma, P.; Nagpal, R.; Jain, A.; Ming, L.C.; Gupta, M. Wound-healing effects of curcumin and its nanoformulations: A comprehensive review. Pharmaceutics 2022, 14, 2288. [Google Scholar] [CrossRef]
- Salehi, B.; Stojanović-Radić, Z.; Matejić, J.; Sharifi-Rad, M.; Kumar, N.V.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of curcumin: A review of clinical trials. Eur. J. Med. Chem. 2019, 163, 527–545. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, S.; Diao, Y.; Zhang, J.; Lv, D. Fatty acid extracts from Lucilia sericata larvae promote murine cutaneous wound healing by angiogenic activity. Lipids Health Dis. 2010, 9, 24. [Google Scholar] [CrossRef]
- Khyade, M.S.; Vaikos, N.P. Wrightia tinctoria R. Br.-a review on its ethnobotany, pharmacognosy and pharmacological profile. J. Coast. Life Med. 2014, 2, 826–840. [Google Scholar]
- Attah, M.O.; Ishaya, H.B.; Chiroma, M.S.; Amaza, D.S.; Balogun, S.U.; Jacks, T.W. Effect of Tamarindus indica (Linn) on the rate of wound healing in adult rabbits. IOSR J. Dent. Med. Sci. 2015, 14, 80–84. [Google Scholar]
- Poljšak, N.; Kočevar Glavač, N. Tilia sp. seed oil—Composition, antioxidant activity and potential use. Appl. Sci. 2021, 11, 4932. [Google Scholar] [CrossRef]
- Hernández, G.R.; García, D.Y.; Sanchez, M.L. Healing cream from Tournefortia hirsutissima L. Med. Aromat. Plants 2017, 6, 4–6. [Google Scholar]
- Pereira Beserra, F.; Xue, M.; Maia, G.L.; Leite Rozza, A.; Helena Pellizzon, C.; Jackson, C.J. Lupeol, a pentacyclic triterpene, promotes migration, wound closure, and contractile effect in vitro: Possible involvement of PI3K/Akt and p38/ERK/MAPK pathways. Molecules 2018, 23, 2819. [Google Scholar] [CrossRef] [PubMed]
- Hata, K.; Hori, K.; Takahashi, S. Role of p38 MAPK in lupeol-induced B16 2F2 mouse melanoma cell differentiation. J. Biochem. 2003, 134, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Beserra, F.P.; Vieira, A.J.; Gushiken, L.F.; de Souza, E.O.; Hussni, M.F.; Hussni, C.A.; Nóbrega, R.H.; Martinez, E.R.; Jackson, C.J.; de Azevedo Maia, G.L.; et al. Lupeol, a dietary triterpene, enhances wound healing in streptozotocin-induced hyperglycemic rats with modulatory effects on inflammation, oxidative stress, and angiogenesis. Oxidative Med. Cell Longev. 2019, 2019, 3182627. [Google Scholar] [CrossRef]
- Poljšak, N.; Kreft, S.; Kočevar Glavač, N. Vegetable butters and oils in skin wound healing: Scientific evidence for new opportunities in dermatology. Phytother. Res. 2020, 34, 254–269. [Google Scholar] [CrossRef]
- Bapat, U.C.; Mhapsekar, D.R. Phytochemical investigations and antimicrobial and anticancer activities of Homonoia riparia Lour. Int. J. Pharm. Pharm. Sci. 2014, 6, 237–243. [Google Scholar]
- Fikru, A.; Makonnen, E.; Eguale, T.; Debella, A.; Mekonnen, G.A. Evaluation of in vivo wound healing activity of methanol extract of Achyranthes aspera L. J. Ethnopharmacol. 2012, 143, 469–474. [Google Scholar] [CrossRef]
- Agyare, C.; Bempah, S.B.; Boakye, Y.D.; Ayande, P.G.; Adarkwa-Yiadom, M.; Mensah, K.B. Evaluation of antimicrobial and wound healing potential of Justicia flava and Lannea welwitschii. Evid. Based Complement. Altern. Med. 2013, 2013, 632927. [Google Scholar] [CrossRef]
- Kirubanadan, S.; Bharathi, R. Histological and biochemical evaluation of wound regeneration potential of Terminalia chebula fruits. Asian J. Pharm. Clin. Res. 2016, 9, 228–233. [Google Scholar]
- Elzayat, E.M.; Auda, S.H.; Alanazi, F.K.; Al-Agamy, M.H. Evaluation of wound healing activity of henna, pomegranate and myrrh herbal ointment blend. Saudi Pharm. J. 2018, 26, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; He, Q.; Guo, G.; Zhai, C. Analysis of risk factors related to chronic non-healing wound infection and the construction of a clinical prediction model. Exp. Dermatol. 2024, 33, e15102. [Google Scholar] [CrossRef] [PubMed]
- Chaniad, P.; Tewtrakul, S.; Sudsai, T.; Langyanai, S.; Kaewdana, K. Anti-inflammatory, wound healing and antioxidant potential of compounds from Dioscorea bulbifera L. bulbils. PLoS ONE 2020, 15, e0243632. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.; Harding, K.G. Bacteria and wound healing. Curr. Opin. Infect. Dis. 2004, 17, 91–96. [Google Scholar] [CrossRef]
- Moulaoui, K.; Caddeo, C.; Manca, M.L.; Castangia, I.; Valenti, D.; Escribano, E.; Atmani, D.; Fadda, A.M.; Manconi, M. Identification and nanoentrapment of polyphenolic phytocomplex from Fraxinus angustifolia: In vitro and in vivo wound healing potential. Eur. J. Med. Chem. 2015, 89, 179–188. [Google Scholar] [CrossRef]
- Kwaji, A.; Adamu, H.; Chindo, I. Phytochemical analysis, antibacterial and antioxidant activities of Entada africana Guill. & Perr. stem bark extracts. J. Chem. Sci. 2017, 7, 10–15. [Google Scholar]
- Dash, G.K.; Murthy, P.N. Evaluation of Argemone mexicana Linn. leaves for wound healing activity. J. Nat. Prod. Plant Resour. 2011, 1, 46–56. [Google Scholar]
- Mireku, E.A.; Mensah, A.Y.; Mensah, M.L.; Ekuadzi, E.; Dickson, R.A. Antimicrobial and antioxidant activities of the stem bark of Cussonia bancoensis. J. Med. Biomed. Sci. 2014, 3, 7–13. [Google Scholar] [CrossRef]
- Douglas, K.; Gitonga, A. Antimicrobial activity of Bridelia micrantha and Grewia plagiophylla leaf extracts. Br. J. Pharm. Res. 2016, 12, 1–7. [Google Scholar] [CrossRef]
- Rex, J.R.; Muthukumar, N.M.; Selvakumar, P.M. Phytochemicals as a potential source for anti-microbial, anti-oxidant and wound healing-a review. MOJ Biorg. Org. Chem. 2018, 2, 61–70. [Google Scholar]
- Süntar, I.; Akkol, E.K.; Nahar, L.; Sarker, S.D. Wound healing and antioxidant properties: Do they coexist in plants? Free Radic. Antioxid. 2012, 2, 1–7. [Google Scholar] [CrossRef]
- Weckesser, S.; Engel, K.; Simon-Haarhaus, B.; Wittmer, A.; Pelz, K.; Schempp, C.Á. Screening of plant extracts for antimicrobial activity against bacteria and yeasts with dermatological relevance. Phytomedicine 2007, 14, 508–516. [Google Scholar] [CrossRef]
- Abd Al-Kubaisy, H.; Al-Qaysi, A.D. In vitro investigation of phytochemical, antioxidant and antimicrobial activities of Harpagophytum procumbens seeds extracts. Plant Prot. 2024, 8, 457–467. [Google Scholar]
- Anand, U.; Jacobo-Herrera, N.; Altemimi, A.; Lakhssassi, N. A comprehensive review on medicinal plants as antimicrobial therapeutics: Potential avenues of biocompatible drug discovery. Metabolites 2019, 9, 258. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.J.; Arora, D.S. Antibacterial and phytochemical screening of Anethum graveolens, Foeniculum vulgare and Trachyspermum ammi. BMC Complement. Altern. Med. 2009, 9, 30. [Google Scholar] [CrossRef]
- Rigane, G.; Ghazghazi, H.; Aouadhi, C.; Ben Salem, R.; Nasr, Z. Phenolic content, antioxidant capacity and antimicrobial activity of leaf extracts from Pistacia atlantica. Nat. Prod. Res. 2017, 31, 696–699. [Google Scholar] [CrossRef]
- Dalvand, H.; Hamdi, S.M.; Ahmadvand, H. Evaluation of antibacterial and antifungal activities of Pistacia atlantica and Pistacia khinjuk. Plant Sci. Today 2024, 11, 634–640. [Google Scholar] [CrossRef]
- Salehi, B.; Upadhyay, S.; Orhan, I.E.; Jugran, A.K.; Jayaweera, L.D.; Dias, D.; ASharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic potential of α- and β-pinene: A miracle gift of nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef]
- Cock, I.E.; Van Vuuren, S.F. A comparison of the antimicrobial activity and toxicity of six Combretum and two Terminalia species from Southern Africa. Pharmacogn. Mag. 2015, 11, 208. [Google Scholar] [CrossRef]
- Marquardt, P.; Seide, R.; Vissiennon, C.; Schubert, A.; Birkemeyer, C.; Ahyi, V.; Fester, K. Phytochemical characterization and in vitro anti-inflammatory, antioxidant and antimicrobial activity of Combretum collinum Fresen leaves extracts from Benin. Molecules 2020, 25, 288. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Kachur, K.; Suntres, Z. The antibacterial properties of phenolic isomers, carvacrol and thymol. Crit. Rev. Food Sci. Nutr. 2020, 60, 3042–3053. [Google Scholar] [CrossRef]
- Stephen, U.A.; Abiodun, F.; Osahon, O.; Ewaen, E. Phytochemical analysis and antibacterial activity of Khaya grandifoliola stem bark. Int. J. Biol. Sci. 2009, 9, 63–67. [Google Scholar] [CrossRef]
- Nordlund, J.J. The melanocyte and the epidermal melanin unit: An expanded concept. Dermatol. Clin. 2007, 25, 271–281. [Google Scholar] [CrossRef]
- Montaudié, H.; Bertolotto, C.; Ballotti, R.; Passeron, T. Fisiología del sistema pigmentario. Melanogénesis. EMC Dermatol. 2014, 48, 1–11. [Google Scholar] [CrossRef]
- Street, R.A.; Stirk, W.A.; Van Staden, J. South African traditional medicinal plant trade—Challenges in regulating quality, safety and efficacy. J. Ethnopharmacol. 2008, 119, 705–710. [Google Scholar] [CrossRef]
- Chang, T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef]
- Lai, J.S.; Lin, C.; Chiang, T.M. Tyrosinase inhibitory activity and thermostability of the flavonoid complex from Sophora japonica L (Fabaceae). Trop. J. Pharm. Res. 2014, 13, 243. [Google Scholar] [CrossRef]
- Musabayane, C.T. The effects of medicinal plants on renal function and blood pressure in diabetes mellitus. S. Afr. J. Diabetes Vasc. Dis. 2012, 9, 114–119. [Google Scholar] [CrossRef]
- Lee, S.G.; Karadeniz, F.; Seo, Y.; Kong, C.S. Anti-melanogenic effects of flavonoid glycosides from Limonium tetragonum (Thunb.) Bullock via inhibition of tyrosinase and tyrosinase related proteins. Molecules 2017, 22, 1480–1490. [Google Scholar] [CrossRef] [PubMed]
- Chiocchio, I.; Mandrone, M.; Sanna, C.; Maxia, A.; Tacchini, M.; Poli, F.J. Screening of a hundred plant extracts as tyrosinase and elastase inhibitors, two enzymatic targets of cosmetic interest. Ind. Crops Prod. 2018, 122, 498–505. [Google Scholar] [CrossRef]
- Fan, M.; Ding, H.; Zhang, G.; Hu, X.; Gong, D. Relationships of dietary flavonoid structure with its tyrosinase inhibitory activity and affinity. LWT 2019, 107, 25–34. [Google Scholar] [CrossRef]
- Si, Y.X.; Yin, S.J.; Oh, S.; Wang, Z.J.; Ye, S.; Yan, L.; Yang, J.-M.; Park, Y.-D.; Lee, J.; Qian, G.-Y. An integrated study of tyrosinase inhibition by rutin: Progress using a computational simulation. J. Biomol. Struct. Dyn. 2012, 29, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Momtaz, S.; Lall, N.; Basson, A. Inhibitory activities of mushroom tyrosine and DOPA oxidation by plant extracts. S. Afr. J. Bot. 2008, 74, 577–582. [Google Scholar] [CrossRef]
- Chen, Q.X.; Kubo, I. Kinetics of mushroom tyrosinase inhibition by quercetin. J. Agric. Food Chem. 2002, 50, 4108–4112. [Google Scholar] [CrossRef]
| Family Plant | Plant Name | Part of Plant | Extract Type | Outcomes | Control | Time Point (Day) | Reference |
|---|---|---|---|---|---|---|---|
| Acanthaceae | Justicia schimperiana (Hochst. ex Nees) T.Anderson | Leaves | Methanol | Improved wound contraction 91.29%, 92.83% and re-epithelialization in skin burns at 5% and 10% (w/w), respectively. | 0.2% Nitrofurazone (94.5%) | 16 | [28] |
| Apocynaceae | Caralluma europaea (Guss.) | Aerial part | Achloroform lipid extract | Improved wound contraction 98.20% in skin burns in the Wistar rat at 10% (w/w). | Vaseline® (75%) Madecassol® 1% (90%) | 21 | [29] |
| Asphodelaceae | Aloe trigonantha L.C.Leach | Leaves | Gel | Enhanced wound contraction and re-epithelialization 88.43%, 93.61% at 5%, 10% (w/w), respectively, anti-inflammatory activity at 400 mg/kg. | Simple ointment (81.88) and 0.2% nitrofurazone (93.6%) | 15 | [30] |
| Asphodelaceae | Aloe vera (L.) Burm.f. + Bulbine frutescens (L.) Willd. | Gel Leaves | Gel Aqueous extract | Vera gel 0.25 mg/mL with Bulbine frutescens 0.75 mg/mL enhanced wound closure and increased HaCaT migration when compared with each plant alone and the untreated group. | Untreated HaCaT cells | 24 h | [31] |
| Asteraceae | Emilia coccinea (Sims) | Leaves, roots and flowers | Ethanol | Wound closure 95%, 98.33%,100% at 1.25%, 2.25%, 5% (w/w). | Baneocin (92.50%) | 16 | [32] |
| Asteraceae | Vernonia auriculifera Hiern | Leave | Methanol | Wound contraction 96.27%, 97.75% and re-epithelialization at 2.5%, 5% (w/w) in mice. | Simple Ointment (84.88%) | 16 | [33] |
| Rosaceae | Prunus africana (Hook.f.) Kalkman | Bark | Methanol | Wound contraction 99.04%, 100% and re-epithelialization at 5%, 10% (w/w) in mice. | 0.2% Nitrofurazone 100% | 16 | [34] |
| Euphorbiaceae | Bridelia micrantha (Hochst.) Baill. | Leaves | Methanol | Enhanced wound contraction 85%, 90%, 98% at 0.625%, 2.5%, and 10% (w/w). | 1% Silver Sulphadiazine (100%) | 12 | [35] |
| Fabaceae | Tamarindus indica L. | Bark, leaves, seeds, fruits | n-hexane extract | Anti-inflammatory, wound-healing activities and enhanced fibroblast migration at 10 µg/mL. | Untreated fibroblast cells | 24 h | [36] |
| Fabaceae | Parkia clappertoniana Keay | Fruit | Ethanol | Improved epithelialization and wound contraction 75%, 85%, 90% at 0.3%, 1%, 3% (w/w). | 1% Silver sulphadiazine (92%) | 16 | [37] |
| Dioscoreaceae | Dioscorea bulbifera L. | Leaves | Hydromethanolic | Increased percentage of wound closure in the Wistar rats 87.21%, 83.81%, 71.51% at 200, 400 and 800 mg/kg. | Petroleum jelly (25.01%) | 15 | [38] |
| Lythraceae | Lawsonia inermis L. | Leaves | Aqueous | Improved wound contraction 85.97% and re-epithelization at 50% (w/w). | Ointment base only (96.37%) | 15 | [39] |
| Lythraceae | Lawsonia inermis L. | Leaves | Aqueous | Improved wound contraction 100% and re-epithelialization at 1% (w/w) in burn wounds in mice. | 1% Silver sulphadiazine (90%) | 20 | [40] |
| Meliaceae | Trichilia dregeana Sond | Leaves | Methanol | Improved wound contraction 99.56%, 100% and re-epithelialization in skin burns at 5% and 10% (w/w). | Nitrofurazone 0.2% (99.68%) | 16 | [41] |
| Mimosaceae | Entada africana Guill. & Perr. | Leaves, stem | Aqueous | Enhanced wound contraction around 99.37 to 100% and re-epithelialization at 5, 10, 15% (w/w). | Silver sulphadiazine 1% (99.94%) | 16 | [42] |
| Musaceae | Musa paradisiaca L. | Stem | Aqueous | Wound contraction 93.80% at 5%, 10% (w/w) in albino rats. | Povidone iodine (89.77%) | 16 | [43] |
| Solanaceae | Physalis angulata L | Leaves | Methanol | Wound contraction at concentrations 93%, 95% 5% and 10% (w/w) and anti-inflammatory activity at 100 and 300 mg/kg. | 1% Silver sulphadiazine (100%) | 15 | [44] |
| Family Plant | Plant Name | Part of Plant | Extract Type | Outcomes | Reference |
|---|---|---|---|---|---|
| Anacardiaceae | Pistacia atlantica Desf. subsp. | Leaves | Hydro-methanolic | Antimicrobial activity against S. aureus (MIC = 78.125 μg/mL) and L. monocytogenes, C. albicans (MIC = 39 μg/mL). | [45] |
| Asteraceae | Emilia coccinea (Sims) | Leaves, roots and flowers | Ethanol | Antimicrobial activity against S. aureus, E. coli, and P. aeruginosa (MIC = 256–512 µg/mL). | [32] |
| Acanthaceae | Justicia flava (Forssk.) Vahl. | Leaves | Aqueous /Ethanol | Antimicrobial activity against P. aeruginosa = 0.4–16.1 mm, S. aureus = 1.3–23.4 mm, E. coli = 0.7–23 mm, isolated from post-operative wounds. | [46] |
| Cecropiaceae | Myrianthus arboreus P.Beauv. | ||||
| Cucurbitaceae | Momordica charantia L. | ||||
| Euphorbiacee | Alchornea cordifolia Müll.Arg. | ||||
| Fabaceae | Parkia clappertoniana Keay | Fruit | Ethanol | Antimicrobial activitiy against K. pneumoniae (MIC = 125 μg/mL), E. coli, P. aeruginosa, and C. albicans (MIC = 250 μg/mL). | [37] |
| Pedaliaceae | Harpagophytum procumbens Burch. | Root bark | Ethanol | Antibacterial activity against C. acnes (MIC = 31.25 μg/mL), S. aureus and S. epidermidis (MIC = 10 μg/mL). | [47] |
| Anacardiaceae | Ozoroa sphaerocarpa (Sond.) R.Fern. & A.Fern. | Antibacterial activity against C. acnes (MIC = 250 μg/mL). | |||
| Combretaceae | Combretum collinum Fresen. | ||||
| Fabaceae | Schotia brachypetala Sond. | Antibacterial activity against C. acnes (MIC = 125 μg/mL). | |||
| Lamiaceae | Salvia barrelieri Etl. | Aerial parts | Ethanol | Antibacterial activity against S. aureus, S. epidermidis, P. aeruginosa, E. faecalis, and E. coli (MIC = 15.1 to 125 μg/mL). | [48] |
| Euphorbiaceae | Bridelia micrantha (Hochst.) Baill. | Leaves | Methanol | Antibacterial activity against S. pyogenes, C. albicans, E. coli, N. gonorrhoeae and S. typhi (MIC = 1.25 to 2.5 mg/mL). | [35] |
| Meliaceae | Khaya grandifoliola C.DC. | Stem, bark, root, leaves | Methanol and ethyl acetate | Methanol extract showed antimicrobial activity against S. aureus (MIC = 1–2 mg/mL) and ethyl acetate extract against S. pyogenes (MIC = 0.25 mg/mL). | [49] |
| Mimosaceae | Entada africana Guill. & Perr. | Leaves, stem | Aqueous | Antibacterial activity against S. aureus and S. pyogenes (MIC = 1.56 mg/mL). | [42] |
| Family Plant | Plant Name | Part of Plant | Extract Type | Outcomes | Control | Reference |
|---|---|---|---|---|---|---|
| Anacardiaceae | Pistacia atlantica Desf. | Leaves | Hydro-methanolic | Anti-tyrosinase (EC50 = 0.098 mg/mL). | Quercetin 0.010 mg/mL | [45] |
| Asphodelaceae | Aloe ferox Mill. | Leaves | Methanol | Anti-tyrosinase activity (IC50 = 138.2 μg/mL). | Kojic acid 87.4 μg/mL | [50] |
| Aloe spectabilis Reynolds | Anti-tyrosinase activity (IC50 = 78.9 μg/mL). | |||||
| Aloe marlothii A.Berger | Anti-tyrosinase activity (IC50 = 189.5 μg/mL). | |||||
| Aloe chabaudii Schönland | Anti-tyrosinase activity (IC50 = 224.2 μg/mL). | |||||
| Aloe excelsa A.Berger | Anti-tyrosinase activity (IC50 = 244.1 μg/mL). | |||||
| Aloe petricola Pole-Evans | Anti-tyrosinase activity (IC50 = 333.1 μg/mL). | |||||
| Aloe mitriformis Mill. | Anti-tyrosinase activity (IC50 = 395.9 μg/mL). | |||||
| Aloe candelabrum A.Berger | Anti-tyrosinase activity (IC50 = 363.7 μg/mL). | |||||
| Asteraceae | Rhanterium suaveolens Desf. | Flowers, leaves, stems | Methanol | Flower extract presented higher anti-tyrosinase activity (IC50 = 61.56 μg/mL) when compared with RSL (IC50 = 124.13 μg/mL), RSS (IC50 = 96.72 μg/mL). | Kojic acid 2.24 μg/mL | [51] |
| Rubiaceae | Nauclea latifolia smith. | Fruits | Methanol and Dichloromethane-methanol fractions | Anti-tyrosinase activity IC50 for extract = 127.3 μg/mL and fractions (NL-VII, NL-VIII) IC50 = 233.13, 124.44 μg/mL. | Kojic acid 12.01 μg/mL | [52] |
| Hypericaceae | Psorospermum aurantiacum Engl. | Stem bark | Methanol methylene chloride extract and fractions: hexane, methylene chloride, ethyl acetate, methanol | Anti-tyrosinase activity for 3-geranyloxyemodinanthrone, lipoxygenase IC50 = 65 μg/mL and 35.35 µg/mL, respectively. | Vitamin C 41.85 μg/mL | [53] |
| Combretaceae | Combretum collinum Fresen. | Root bark | Ethanol | Anti-tyrosinase activity IC50 = 47.92 μg/mL. | Kojic acid 1.38 μg/mL | [47] |
| Fabaceae | Acacia nilotica (L.) Willd. Delile | Anti-tyrosinase activity IC50 = 12.97 μg/mL. | ||||
| Schotia brachypetala Sond. | Anti-tyrosinase activity IC50 = 35.07 μg/mL. | |||||
| Rubiaceae | Vangueria infausta Burch. | Anti-tyrosinase activity IC50 = 52.81 μg/mL. | ||||
| Asteraceae | Pentzia monodiana Maire | Aerial part | Aceton | Lignanes and flavonoids exhibited anti-tyrosinase activity IC50 = 45.4 to 97.2 μM. | Kojic acid 6.4 μg/mL | [54] |
| Euphorbiaceae | Macaranga hurifolia Beille | Leaves, stem bark | Methanol | Strong anti-tyrosinase activity in the stem bark 160.42 mg KAE/g compared to the leaves 159.42 mg KAE/g. | - | [55] |
| Malvaceae | Sterculia tragacantha Lindl. | Anti-tyrosinase activity 142.28 mg KAE/g. | ||||
| Rutaceae | Zanthoxylum gilletii (De Wild.) P.G.Waterman | Anti-tyrosinase activity 128.36 mg KAE/g. | ||||
| Fabaceae | Ormocarpum trichocarpum (Taub.) Harms | Leaves, stems, roots | Ethanol | Tyrosinase inhibition at IC50 = 2.95 μg/mL. | Kojic acid 6.45 μg/mL | [56] |
| Vachellia karroo (Hayne) Banfi & Galasso | Tyrosinase inhibition at IC50 = 6.84 μg/mL. | |||||
| Myrsinaceae | Myrsine africana L. | Tyrosinase inhibition at IC50 = 27.4 μg/mL. | ||||
| Crassulaceae | Kalanchoe thyrsiflora Harv. | Tyrosinase inhibition at IC50 = 14.30 μg/mL. | ||||
| Plumbaginaceae | Limonium cercinense Brullo & Erben | Leaves | Ethanol | Anti-tyrosinase activity IC50 = 3 µg/mL. | Kojic acid 25 μg/mL | [57] |
| Plumbaginaceae | Limonium boitardii Maire | Anti-tyrosinase activity IC50 = 5 µg/mL. | ||||
| Melianthaceae | Bersama abyssinica Fresen. | Leaves | Aqueous, Methnol, Ethyl acetate | Tyrosinase inhibition 129.43 mg KAE/g in ethyl acetate extracts, methanol and water extracts 48.94 and 83.22 mg KAE/g, respectively. | - | [58] |
| Scrophulariaceae | Scoparia dulcis L. | leaves | Tyrosinase inhibition 136.47 mg KAE/g in ethyl acetate extracts, methanol and water extracts 144.73, 56.07 mg KAE/g, respectively. | - | ||
| Myrsinaceae | Myrsine africana L. | Shoots | Methanol extract, ether, CHCl3, EtOAc, and n-BuOH fractions | The extract showed tyrosinase inhibition with an IC50 of 0.12 mg/mL. The isolated compounds rutin and myricetin 3-O-α-L-rhamnopyranoside exhibited IC50 values of 0.13 ± 0.003 mM and 0.12 ± 0.002 mM, respectively. | Kojic acid 0.01 μg/mL | [59] |
| Fabaceae | Bauhinia rufescens Lam. | Stem bark | Petroleum ether extract, ether–diethyl ether–CHCl3–EtOAc–MeOH fractions. | The isolated phytosterol showed 57.1% tyrosinase inhibition at a concentration of 0.1 mg/mL. | Kojic acid 85% | [60] |
| Myricaceae | Morella quercifolia (L.) | Aerial parts | Methanol | Melanin inhibition IC50 < 6.25 μg/mL. | kojic acid < 6.25 | [61] |
| Fabaceae | Serruria furcellata R.Br. | Melanin inhibition IC50 = 7.13 μg/mL. | ||||
| Anacardiaceae | Searsia antarcticus (Willd.) | Melanin inhibition IC50 = 20.25 μg/mL. | ||||
| Asteraceae | Pentzia. ericoides (L.) | Melanin inhibition IC50 = 27.67 μg/mL. | ||||
| Rhamnaceae | Cryptolepis geifolia (L.) | Melanin inhibition IC50 = 36.88 μg/mL. | ||||
| Lamiaceae | Tetradenia riparia (Hochst.) Codd | Melanin inhibition IC50 = 43.88 μg/mL. | ||||
| Proteaceae | Protea cynaroides (L.) L. | Leaves | Methanol extract and n-hexane, DCM, EtOAc, BuOH fractions. | Anti-tyrosinase activity for extract IC50 = 85.20 μg/mL, 3,4-dihydroxybenzoic acid IC50 = 0.8776 μg/mL and 3-Hydroxy kojic acid IC50 = 0.7215 μg/mL. | kojic acid 0.8347 μg/mL | [62] |
| Sapotaceae | Argania spinosa (L.) Skeels | Fruits | Ethanol | Melanin inhibition 55% at 50 μg/mL. | Arbutin 50% | [63] |
| Asteraceae | Dicerothamnus rhinocerotis (L.f.) Koek. | Leaves | Methanol and hexane, dichloromethane, ethyl acetate, and butanol fraction. | The ethyl acetate and butanol fractions demonstrated strong anti-tyrosinase activity IC50 = 11.6 µg/mL and 13.7 µg/mL, respectively, also the isolated compound apigenin IC50 = 14.58 µM. | kojic acid 17.26 µM | [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elmahaishi, L.M.S.; Fisher, F.; Hussein, A.; Africa, C.W.J. The Role of African Medicinal Plants in Dermatological Treatments: A Systematic Review of Antimicrobial, Wound-Healing and Melanogenesis Inhibition. Cosmetics 2025, 12, 132. https://doi.org/10.3390/cosmetics12040132
Elmahaishi LMS, Fisher F, Hussein A, Africa CWJ. The Role of African Medicinal Plants in Dermatological Treatments: A Systematic Review of Antimicrobial, Wound-Healing and Melanogenesis Inhibition. Cosmetics. 2025; 12(4):132. https://doi.org/10.3390/cosmetics12040132
Chicago/Turabian StyleElmahaishi, Lubna M. S., Farzana Fisher, Ahmed Hussein, and Charlene W. J. Africa. 2025. "The Role of African Medicinal Plants in Dermatological Treatments: A Systematic Review of Antimicrobial, Wound-Healing and Melanogenesis Inhibition" Cosmetics 12, no. 4: 132. https://doi.org/10.3390/cosmetics12040132
APA StyleElmahaishi, L. M. S., Fisher, F., Hussein, A., & Africa, C. W. J. (2025). The Role of African Medicinal Plants in Dermatological Treatments: A Systematic Review of Antimicrobial, Wound-Healing and Melanogenesis Inhibition. Cosmetics, 12(4), 132. https://doi.org/10.3390/cosmetics12040132








