1. Introduction
Maintaining optimal skin hydration and collagen levels is crucial for preserving skin health and appearance [
1]. Skin hydration is critical for maintaining a healthy skin barrier, preventing dryness, and delaying signs of aging [
2]. Well-hydrated skin is more elastic, less prone to damage, and appears more radiant [
3]. Hyaluronic acid, a widely studied humectant, plays a significant role in maintaining skin hydration by retaining moisture in the skin layers [
4]. Collagen is another key structural protein in the skin that provides firmness and elasticity. As we age, collagen production decreases, leading to wrinkles and sagging skin. Enhancing collagen synthesis has become a focal point in anti-aging skincare research [
5].
In recent years, there has been a noticeable shift towards incorporating natural plant-based ingredients in skincare products. This growing trend has driven the development of new formulations that meet the increasing consumer demand for effective and natural plant-based skin rejuvenation solutions [
6,
7]
Astragalus membranaceus and
Centella asiatica are prominent medicinal herbs widely used in traditional Chinese medicine to improve various skin conditions [
8,
9]. These herbs are primarily composed of cycloartane-type saponins, which contribute to their therapeutic effects. Research has demonstrated that astragalosides, key active compounds in
A. membranaceus, protect human skin fibroblasts from ultraviolet A (UVA)-induced photoaging. This protection is achieved by reducing the senescence-associated β-galactosidase positive cell rate, lowering the levels of matrix metalloproteinase-1 (MMP-1), enhancing the expression of tissue inhibitor of metalloproteinase-1 (TIMP-1) and transforming growth factor-β1 (TGF-β1) [
10,
11]. Additional studies have shown that astragalosides exert anti-photoaging effects in UVB-induced premature senescence of rat dermal fibroblasts by promoting autophagy [
12].
C. asiatica is renowned for its skin-repairing and anti-inflammatory properties. The active constituents of
C. asiatica, particularly asiaticoside and madecassoside, have been extensively studied for their ability to stimulate collagen production, accelerate wound healing, and reduce scarring. These components work synergistically to enhance the skin’s structural integrity and resilience [
13]. Additionally,
C. asiatica has been found to improve skin hydration by increasing hyaluronic acid production, further supporting its role in maintaining a healthy skin barrier [
14]
Preliminary studies have suggested that a proprietary formulation combining
A. membranaceus and
C. asiatica saponins (ACS) has a rejuvenating effect on human skin in topical application [
15]. This effect is attributed to the formulation’s ability to boost collagen and hyaluronic acid synthesis in skin cells, as well as its potential to increase the uptake of proline and glucosamine in HaCaT keratinocytes and human dermal fibroblasts. These findings indicate that ACS has the potential to be an effective component for promoting skin health and combating visible signs of aging. However, while these initial results are promising, further human clinical studies are necessary to fully elucidate the benefits of ACS for skin health.
The primary objective of this study is to provide comprehensive evidence on the efficacy of ACS when used as a topical application and as an ingredient in dietary supplements. Through rigorous experimentation and analysis, this research aims to validate the potential of ACS in promoting human skin rejuvenation and health. By deepening our understanding of the effects of ACS, this study could pave the way for its broader application in skincare and dietary supplements, offering a natural and effective solution for maintaining youthful and healthy skin.
2. Materials and Methods
2.1. Plant Materials
ACS is an equal mixture of dried extracts of A. membranaceus (10:1 hydroethanolic extract) and C. Asiatica (25:1 aqueous extract) blended with maltodextrin as an excipient; its production is compliant with current Good Manufacturing Practice (cGMP). The A. membranaceus used in ACS is cultivated in Gansu province, China, and harvested during its peak season in September and October. The C. asiatica is sourced from Yunnan province and Guangxi Zhuang Autonomous Region, China, and harvested in July and August when its bioactive content is optimal.
The final ACS blend appears as an off-white to beige powder and is standardized to contain ≥0.25% total saponins and ≥0.4% asiaticoside plus madecassoside. Total saponin content is determined using a colorimetric method based on the vanillin-perchloric acid reaction, with astragaloside IV as the reference standard. The absorbance is measured at 560 ± 5 nm using a UV spectrophotometer (Shimadzu UV-2401PC, Kyoto, Japan). Asiaticoside and madecassoside contents are quantified by high-performance liquid chromatography (HPLC) with a Waters 2695 system, employing an Inertsil ODS-3 column (4.6 × 250 mm, 5 μm), with detection at 210 nm. The mobile phase is composed of a gradient of acetonitrile and water, and quantification is based on peak areas using authentic standards.
Heavy metal testing of the ACS batch (lot# FZ-C20250310) was conducted by Eurofins Technology Service (Suzhou) Co., Ltd., Suzhou, China. The results showed that lead (Pb) was <0.05 mg/kg, arsenic (As) < 0.005 mg/kg, cadmium (Cd) < 0.005 mg/kg, and mercury (Hg) was not detected (LOD = 0.001 mg/kg), all within internationally accepted safety limits. Pesticide residue analysis covering 207 compounds was performed by gas chromatography (GC) according to the internationally recognized standard BS EN 12393:2013 for multiresidue pesticide analysis, and no residues were detected (all values were <LOQ). Glyphosate and aflatoxins were also not detected. Microbiological safety was confirmed by comprehensive testing—E. coli, Staphylococcus aureus, Salmonella, Enterobacteriaceae, Coliforms, and total yeast and mold were all not detected in the tested sample, indicating microbial purity compliant with regulatory standards.
The ACS used in the studies was provided by NuLiv Science USA, Inc. (Brea, CA, USA). The ACS capsules consisted of 125 mg of the proprietary extract mixtures, while the placebo capsules contain maltodextrin. For topical application, an ACS cream formulation containing 5% ACS was used; the corresponding placebo cream was identical in composition except for the absence of ACS active ingredients.
2.2. Study Design and Subjects
This clinical research was approved by the ethics committee of the Antai Medical Care Corporation Antai Tian-Sheng Memorial Hospital (IRB No. 22–111-A); it was also registered in clinicaltrials.gov as NCT06657352. This study was conducted as a randomized, double-blind, placebo-controlled trial to evaluate the effects of a proprietary formulation of A. membranaceus and C. asiatica saponins (ACS) on skin health.
A total of 150 healthy adult subjects aged 20 years or older, with no history of chronic skin diseases, were recruited. The exclusion criteria were (i) involuntary subjects, (ii) skin disease, autoimmune skin diseases, vasculitis, liver cirrhosis or chronic renal failure, (iii) those with known cosmetic, drug or food allergies, (iv) pregnant women and nursing mothers, (v) individuals taking chronic disease medications, and (vi) those who had received laser facial treatment, fruit acid exfoliation, or experienced long-term sun exposure within 4 weeks prior to the study.
Subjects were randomly assigned to one of six groups (n = 25 per group) using a computer-generated randomization list, with group assignments sealed in envelopes to ensure allocation concealment and blinding. The topical group applied the ACS cream to the face twice daily (morning and night) for 4 weeks. The oral group ingested one ACS capsule twice daily after breakfast and dinner for 12 weeks. The combination group followed both the topical and oral regimens for 12 weeks. A placebo group, matching the appearance and texture of the ACS products but lacking active ingredients was included to ensure any observed effects were due to ACS. The six groups were: (1) topical ACS group (ACS cream applied twice daily for 4 weeks), (2) topical placebo group (placebo cream applied identically), (3) oral ACS group (ACS capsule taken twice daily after meals for 12 weeks), (4) oral placebo group (placebo capsule taken identically), (5) combination ACS group (topical ACS + oral ACS for 12 weeks), and (6) combination placebo group (topical and oral placebo for 12 weeks).
Among all participants, approximately 30% were aged between 20 and 40 years, while 70% were over 40 years old, indicating a population with a wide age distribution relevant to evaluating anti-aging or skin health interventions. Group-specific average ages ranged from 35.9 to 46.4 years. Each group comprised 3 to 6 male participants and 19 to 22 female participants, reflecting a female-dominant demographic, which is common in skin-related clinical research. This balanced design ensured the comparability of results across groups and supported the reliable evaluation of both topical and oral ACS treatments.
2.3. Skin Efficacy Assessment Methods
Thirty minutes before every skin test, subjects were instructed to remove any makeup and clean their facial skin. Skin quality testing was performed at baseline (week 0) and after 4 weeks for the topical treatment group, and at 12 weeks for the oral and combination (topical + oral) groups. Then, Soft Plus (Callegari 1930, Parma, Italy) was used to measure melanin on the subjects’ faces. Skin brightness (Chroma Meter MM-500, Minolta, Tokyo, Japan), moisture (Corneometer C0M825, Courage + Khazaka Electronic, Germany) and elasticity (Callegari 1930, Parma, Italy), and collagen density content (DermaLab
® Series SkinLab Combo, Cortex, Denmark) of each subject’s upper cheek were measured. Additionally, skin surface topography including pores and textures on the whole face was analyzed by using VISIA
® Complexion Analysis System (Canfield Scientific, Inc., Fairfield, NJ, USA). The VISIA
® System ensured consistent positioning of each subject’s head with a configurable head support. The photographic images were captured with standard light at 0-degree head positioning. The results were presented as the mean value and the relative percentage (%) to the baseline. The experimental procedure was following previously associated publications [
16].
2.4. Statistical Analysis
The experimental data analysis was first computed using the normal distribution and then assessed using the paired t-test. A significance level of p < 0.05 was considered statistically significant. Statistical significance is denoted as *, indicating p < 0.05 compared to week 0 for the group, and #, indicating p < 0.05 compared to the placebo.
3. Results
3.1. Topical Application of an ACS-Containing Cream
Table 1 summarizes the effects of 4-week topical application of an ACS-containing cream versus a placebo cream on key skin biophysical parameters. In the ACS-treated group, skin brightness (
L value) increased significantly by 2.5%, indicating a visibly lighter skin tone. This improvement was not only statistically significant compared to baseline (
p < 0.05), but also superior to the placebo group, which showed only a 1.0% increase (
p < 0.05 vs. placebo).
Skin elasticity showed a 6.5% increase in the ACS group, suggesting enhanced skin firmness and resilience. This change was statistically significant compared to the baseline and much greater than the 1.2% change seen in the placebo group. Furthermore, collagen content in the skin was elevated by 8.7% in the ACS group, compared to a 6.8% increase in the placebo group; although both groups showed statistical significance from baseline, the ACS group achieved a more pronounced improvement.
Melanin value significantly decreased by 5.2% in the ACS group, while the placebo group exhibited a slight increase (+0.2%), demonstrating that ACS application may contribute to skin lightening through reduction in melanin deposition (p < 0.05 vs. placebo). The number of visible pores also significantly dropped by 10.6%, compared to −0.9% in the placebo group, suggesting a substantial astringent and pore-refining effect.
In terms of skin texture, a parameter indicative of surface smoothness, the ACS group showed a marked 8.7% improvement, statistically better than the 2.3% seen in the placebo group (p < 0.05 vs. placebo). Although moisture retention improved in both groups, the increase was greater in the ACS group (+7.6% vs. +1.8%), albeit not reaching statistical significance.
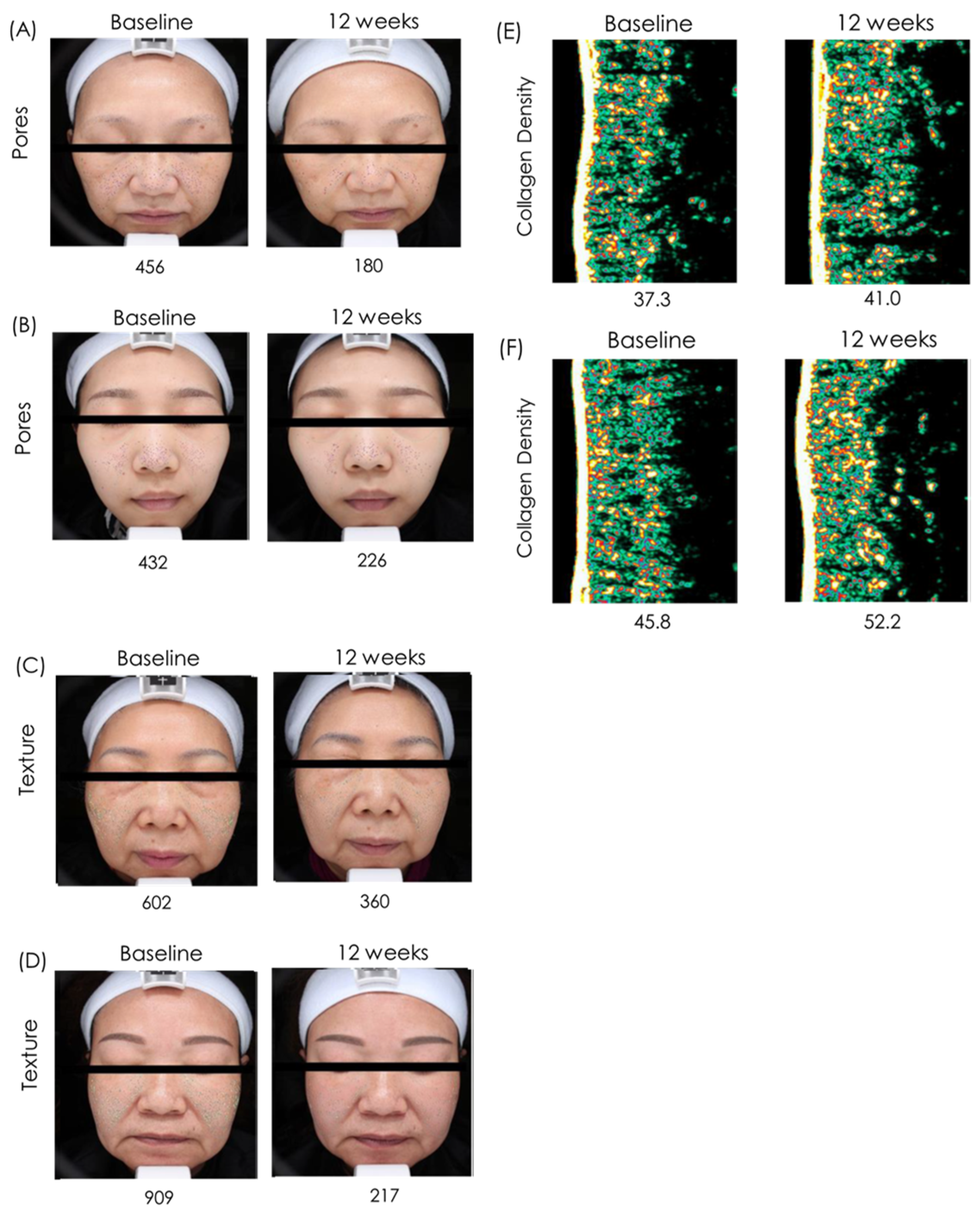
The images are representative photographs of the skin pores (
Figure 1A,B), skin texture (
Figure 1C,D) and collagen of the skin (
Figure 1E,F) in subjects who used topical ACS cream for 4 weeks. The images clearly show that topical ACS cream significantly reduced the number of pores, improved skin texture, and significantly increased collagen content compared with pre-treatment baseline. We hypothesize that these effects arise from the bioactive properties of
A. membranaceus and
C. asiatica extracts, which may upregulate the synthesis of dermal matrix components such as collagen and hyaluronic acid. This is potentially mediated by the enhanced uptake of critical skin nutrients like proline and glucosamine, essential for extracellular matrix remodeling.
Collectively, these results demonstrate that short-term (4-week) topical application of ACS-containing cream confers clinically and visually perceptible improvements in skin brightness, firmness, pigmentation balance, pore minimization, and overall skin quality.
3.2. Oral Administration of ACS Capsules
Table 2 summarizes the results of a 12-week oral supplementation with ACS capsules compared to placebo. Participants who received ACS demonstrated statistically significant improvements in multiple skin parameters. Specifically, skin brightness (
L value) increased by 2.5%, indicating a more luminous complexion. Skin elasticity improved by 4.7%, reflecting enhanced dermal resilience, and collagen content increased by 13.2%, signifying pronounced dermal remodeling. These changes were all significant compared with the pre-treatment baseline (
p < 0.05).
In addition to these benefits, melanin levels in the ACS group were significantly reduced by 6.3%, which may contribute to a more even skin tone and reduction in hyperpigmentation. Skin texture roughness showed the most dramatic improvement, decreasing by 18.5%, implying a smoother skin surface with fewer fine lines or irregularities. The number of visible pores was also reduced by 10.5%, although this change did not reach statistical significance.
In contrast, the placebo group showed no significant improvements across any of the measured parameters. On the contrary, slight deteriorations were observed in moisture retention (−2.6%), elasticity (−1.2%), and collagen content (−1.5%), suggesting a natural decline over time without supplementation.
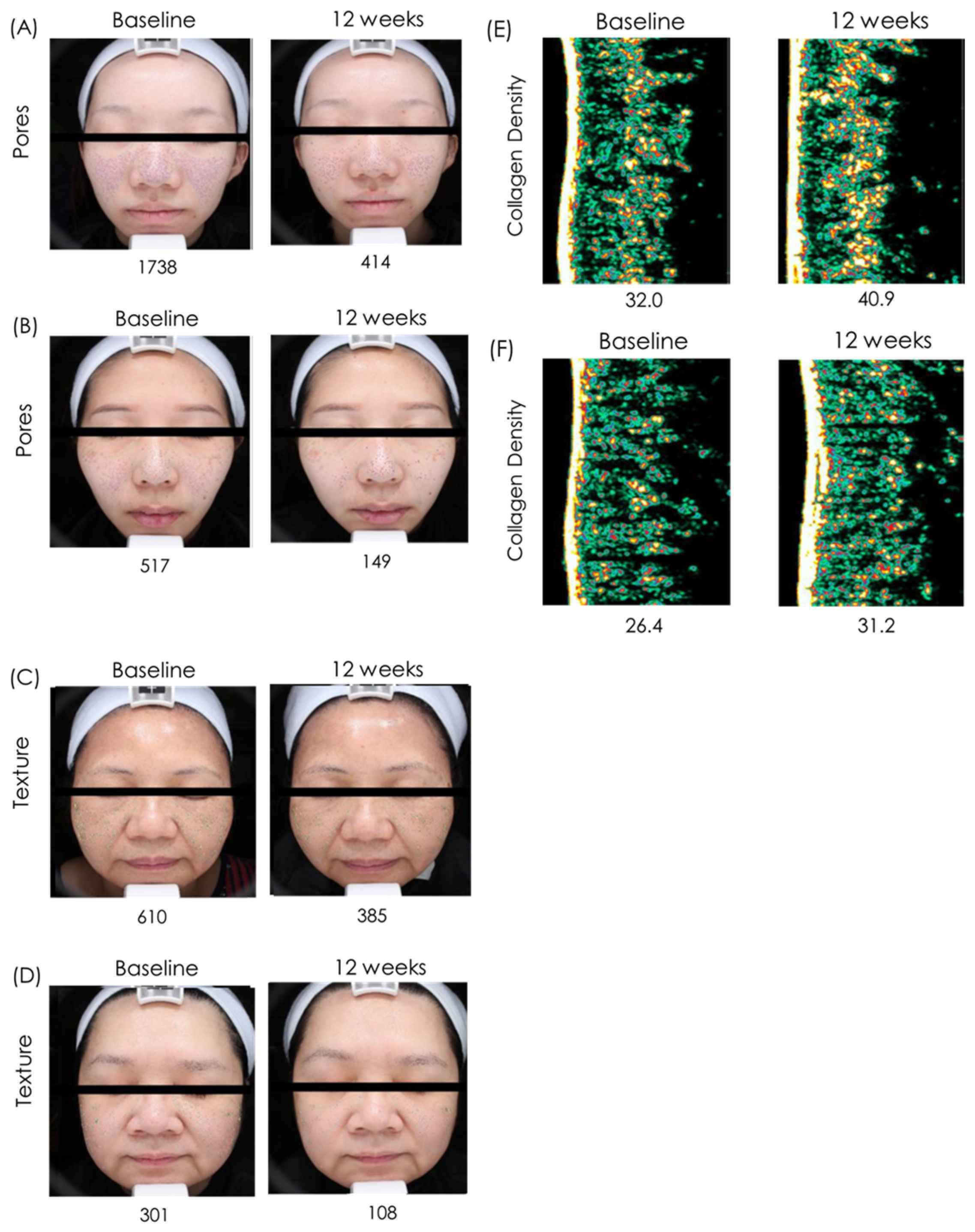
The images are representative photographs of the skin pores (
Figure 2A,B), skin texture (
Figure 2C,D) and collagen of the skin (
Figure 2E,F) in subjects who received oral administration of ACS for 12 weeks. The images clearly show that ACS capsules reduced the number of pores, significantly improved skin texture and significantly increased collagen content compared with the pre-treatment baseline.
These observations collectively indicate that the active components in ACS, such as saponins, asiaticoside, and madecassoside, may be efficiently absorbed through the gastrointestinal tract and systemically delivered to the skin. Based on the observed skin improvements after oral administration, we speculate that these active components are absorbed via the gastrointestinal tract. The biological effects are likely mediated through enhanced fibroblast activity, promotion of collagen and hyaluronic acid biosynthesis, and improved antioxidant defenses, which together contribute to visible skin improvement; however, further studies are needed to directly confirm this mechanism.
Taken together, the findings underscore the potential of ACS as an effective functional dietary supplement for promoting skin health. Its oral administration confers multifaceted dermatological benefits—including skin brightening, pigmentation control, elasticity enhancement, textural refinement, and collagen restoration—thereby supporting its value in anti-aging and skin wellness applications.
3.3. Combined Effects of Topical and Oral Administration of ACS
Table 3 outlines the synergistic effects observed after 12 weeks of concurrent topical and oral administration of ACS. Compared with the pre-treatment baseline, participants in the ACS combination group exhibited remarkable and statistically significant improvements across all measured skin parameters. Specifically, skin brightness increased by 4.2%, suggesting enhanced luminosity. Moisture retention improved significantly by 12.9%, indicating a stronger skin barrier and hydration capacity. Skin elasticity rose by 9.0%, reflecting enhanced dermal structural support, while collagen content increased by 13.7%, consistent with improved extracellular matrix remodeling.
In terms of pigmentation and texture, the melanin value decreased by 8.2%, and visible pore count decreased dramatically by 28.5%, representing the most pronounced reduction among all treatment modes. Skin texture roughness also improved by 19.8%, indicating a more refined and smooth skin surface. In comparison, the placebo group showed only modest improvements in elasticity (+2.6%), collagen (+7.9%), and pore count (−7.7%), with no significant changes in skin brightness, moisture, melanin, or texture. Statistical comparisons between groups confirmed that the ACS group significantly outperformed placebo in several key indicators—skin brightness, moisture retention, melanin reduction, pore count, and texture (p < 0.05)—indicating that the combined administration route produced additive or synergistic effects beyond those achieved by single-route application.
The images are representative photographs of the skin pores (
Figure 3A,B), skin texture (
Figure 3C,D) and collagen of the skin (
Figure 3E,F) in subjects who used both topical and oral ACS treatments for 12 weeks. The images clearly show that combined use of ACS-containing cream and capsules significantly reduced the number of pores, improved skin texture, and significantly increased collagen content compared with pre-treatment baseline. The magnitude and consistency of these improvements support the hypothesis that dual administration routes enhance bioavailability and tissue-level efficacy. While topical application ensures localized delivery to the skin’s surface and upper dermis, oral intake allows systemic distribution of bioactive compounds, such as saponins and triterpenes, which may stimulate fibroblasts, increase hyaluronic acid and collagen biosynthesis, and suppress melanogenesis.
In summary, the combined oral and topical application of ACS yielded the most comprehensive and significant benefits, improving skin hydration, brightness, firmness, pigmentation balance, pore appearance, texture, and collagen content. These results suggest that dual-route ACS supplementation represents an optimal strategy for cosmetic and dermatological intervention, delivering both esthetic and physiological enhancements to skin health.
4. Discussion
This study provides compelling evidence of the efficacy of A. membranaceus and C. asiatica (ACS) in enhancing skin health across multiple physiological parameters. Significant improvements in skin brightness, elasticity, collagen content, melanin value, pore count, and texture were consistently observed in all ACS treatment groups, with the combined oral and topical group exhibiting the most prominent benefits. Notably, after 12 weeks of dual administration, participants experienced a 4.2% increase in brightness, 12.9% improvement in moisture retention, 9.0% increase in elasticity, 8.2% decrease in melanin, 28.5% reduction in pore count, and 13.7% elevation in collagen content compared with baseline. These findings affirm the therapeutic potential of ACS not only as a topical cosmeceutical but also as a functional oral supplement for holistic skin enhancement in healthy adults.
Collagen is a structural protein found in the skin, bones, and connective tissues of the body, essential for maintaining strength, elasticity, and integrity [
1]. It works in conjunction with substances like hyaluronic acid and elastin to maintain skin elasticity, plumpness, and moisture [
17]. The increased collagen density may account for the enhanced brightness, refined pores, and improved texture of the skin. A positive correlation was found between collagen density and skin brightness; when the density of collagen fibers in the upper dermis decreases, skin color becomes less bright due to reduced light scattering [
18,
19]. Additionally, collagen and elastin are proteins that provide structural support for pores. As you age, the loss of collagen and elastin causes pores to widen [
20,
21]. Skin texture is defined by its fineness and smoothness, and when collagen levels are high, the skin remains soft, smooth, and firm [
22,
23]. In our study, increases in collagen—8.7% (topical), 13.2% (oral), and 13.7% (combined)—were accompanied by improvements in skin tone, texture, and pore visibility, confirming this interrelationship.
Research has shown that
A. membranaceus extract can prevent collagen loss caused by UV exposure in photoaged skin [
24]. Additionally, applying the extract topically has been found to boost collagen production, thereby enhancing tissue repair and the regeneration of skin wounds [
25]. Similarly, research on
C. asiatica extract have shown promising results in stimulating collagen production and enhancing skin elasticity [
26,
27]. These phytochemical properties may underpin the biological effects observed in our study. The current study observed an 8.7% increase in collagen with topical application, a 13.2% increase with oral supplementation, and a 13.7% increase with combined use. This was accompanied by improvements in skin brightness (4.2%), elasticity (9.0%), pore count (28.5%), and texture (19.8%) with the combined approach. The dual use of topical and oral ACS supplementation likely contributes to these significant enhancements. The topical cream may offer immediate benefits to the skin’s surface, while the oral capsules support deeper collagen synthesis and overall skin health.
Hydration is another critical factor in maintaining skin health and appearance. Several studies have investigated various ingredients and formulations designed to enhance skin moisture [
28]. For instance, topical applications of hyaluronic acid, a widely recognized humectant, significantly improved skin hydration levels in participants [
4]. Similarly, research on ceramides has shown promising results in improving skin barrier function and preventing moisture loss. Studies also shown that
A. membranaceus and
C. asiatica have skin-moisturizing effects that help boost levels of hyaluronic acid [
14,
29]. In the present study, the combined use of ACS-containing cream and capsules resulted in a 12.9% increase in skin moisture retention over 12 weeks. This suggests that ACS’s formulation could be more effective in attracting and retaining moisture in the skin, thus providing superior hydration benefits.
Melanin reduction is important for achieving an even skin tone and reducing hyperpigmentation. Various ingredients, such as vitamin C, niacinamide, and hydroquinone, have been extensively studied for their ability to lighten skin and reduce melanin production [
30,
31,
32]. For instance, a combination of vitamin C serum and niacinamide cream has been shown to effectively brighten the skin and fade dark spots over time [
33]. Additionally, traditional Chinese medicine has been frequently used as skin lightning agents [
34]. Among them,
C. asiatica has shown efficacy in treating hyperpigmentation disorders and promoting skin lightening [
35,
36]. Our results reflect this, with significant reductions in melanin value observed across all ACS groups: −5.2% (topical), −6.3% (oral), and −8.2% (combined), indicating a dose–effect response with enhanced efficacy via combined administration.
It is important to note that each group in this study consisted of 25 participants, with approximately 30% of all subjects aged between 20 and 40 years and about 70% over 40 years old. This indicates a study population skewed toward middle-aged and older adults, which is appropriate for assessing anti-aging and skin restoration effects. However, due to the relatively small sample size within each age bracket, subgroup analyses by age were not conducted in this study. Therefore, we presented the data as an integrated discussion across the entire cohort. Additionally, the statistical analysis applied has certain limitations given the study design and data characteristics. Future studies should consider increasing sample sizes per group, stratifying participants by age to more precisely evaluate age-specific responses to ACS treatment, and employing more comprehensive analytical methods to obtain more robust and interpretable results.
5. Conclusions
This randomized, double-blind, placebo-controlled clinical study provides compelling evidence supporting the efficacy of A. membranaceus and C. asiatica (ACS) as a multifunctional botanical formulation for enhancing skin health. Whether administered topically, orally, or in combination, ACS significantly improved multiple dermatological parameters—including skin brightness, moisture retention, elasticity, melanin content, pore count, and collagen synthesis—in healthy adult subjects.
Among the three treatment groups, the combined topical and oral ACS regimen yielded the most pronounced and comprehensive benefits, achieving a 4.2% increase in skin brightness, 12.9% enhancement in moisture retention, 9.0% improvement in elasticity, 8.2% reduction in melanin, 28.5% reduction in visible pores, and a 13.7% increase in collagen content compared to baseline. These improvements not only surpassed those observed in the placebo group but also exceeded the outcomes of either single-mode treatment, highlighting the synergistic potential of dual administration.
The results of this study suggest that ACS exerts its skin-rejuvenating effects through multiple biological mechanisms, including the stimulation of dermal collagen synthesis, enhancement of skin hydration, suppression of melanogenesis, and reinforcement of overall dermal structure and barrier function. This multimodal activity highlights ACS as a versatile and efficacious agent capable of addressing both superficial and structural signs of skin aging. Taken together, ACS represents a promising natural solution for individuals seeking a holistic, evidence-based strategy to support and enhance skin health. Its demonstrated efficacy through both topical and oral routes of administration makes it particularly well suited for incorporation into cosmeceutical formulations and functional health supplements. Future research may further explore its applications in broader populations or in combination with other synergistic bioactives, thereby expanding its clinical relevance and commercial potential.