In Vitro Examination of Fungal and Root Extracts Inspired by Traditional Medicine for Potential Periorbital Eye Infrastructure Treatments
Abstract
1. Introduction
2. Materials and Methods
2.1. Extract Preparations
2.1.1. Phellinus linteus Mushroom Extract
2.1.2. Angelica polymorpha sinensis Root Extract
2.2. High-Performance Liquid Chromatography/Mass Spectral (HPLC/QTOF-MS) Analysis of Blend
2.3. In Vitro Tissue Testing
2.3.1. Phellinus linteus Mushroom Extract Human Microarray Studies
2.3.2. Phellinus linteus Mushroom Extract Protein Studies
2.4. Angelica polymorpha sinensis Root Extract Human Microarray and Protein Studies
2.5. Statistical Analysis
3. Results and Discussion
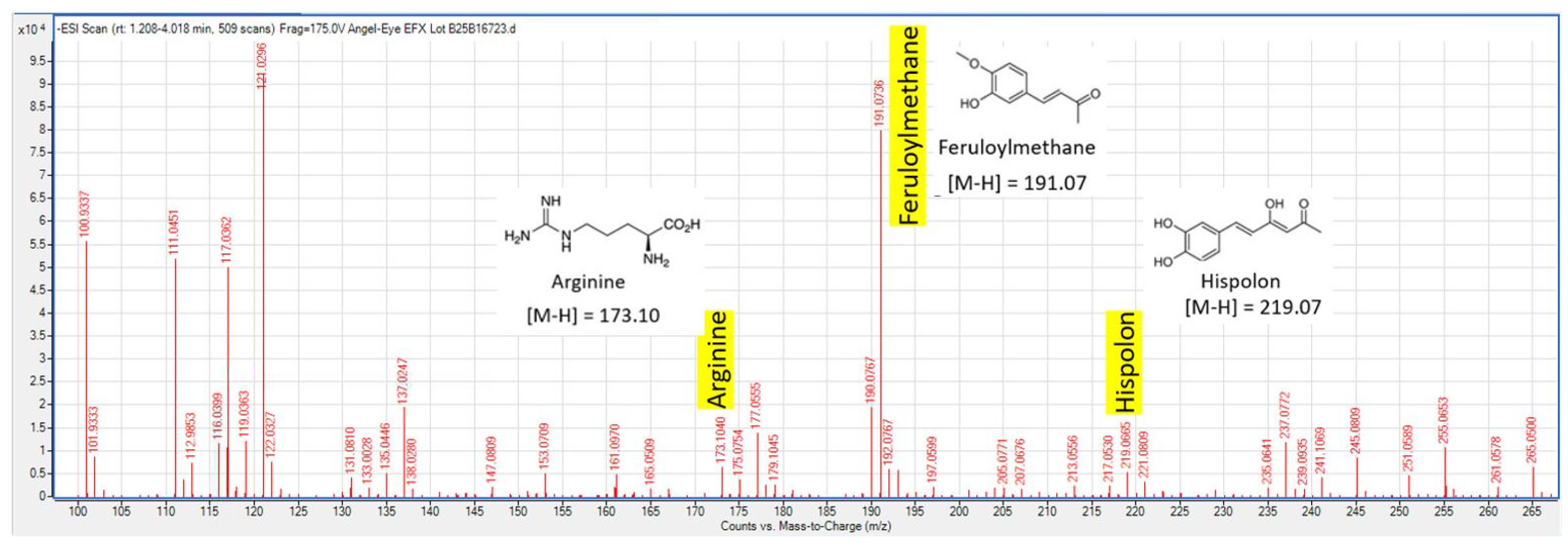
3.1. Liquid Chromatography/Mass Spectral (LC/MS) Analysis of Blended Product
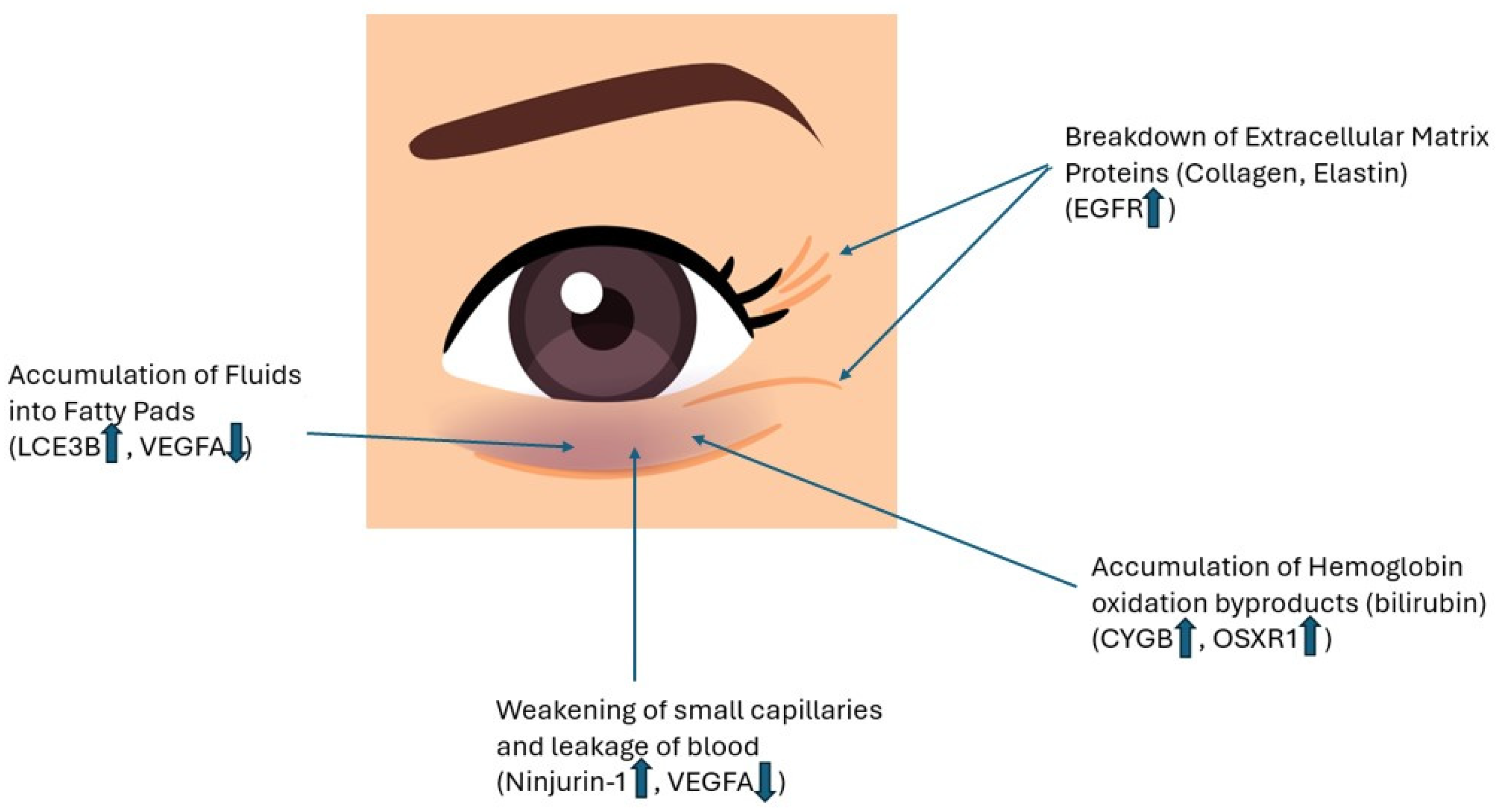
3.2. Results of In Vitro Testing
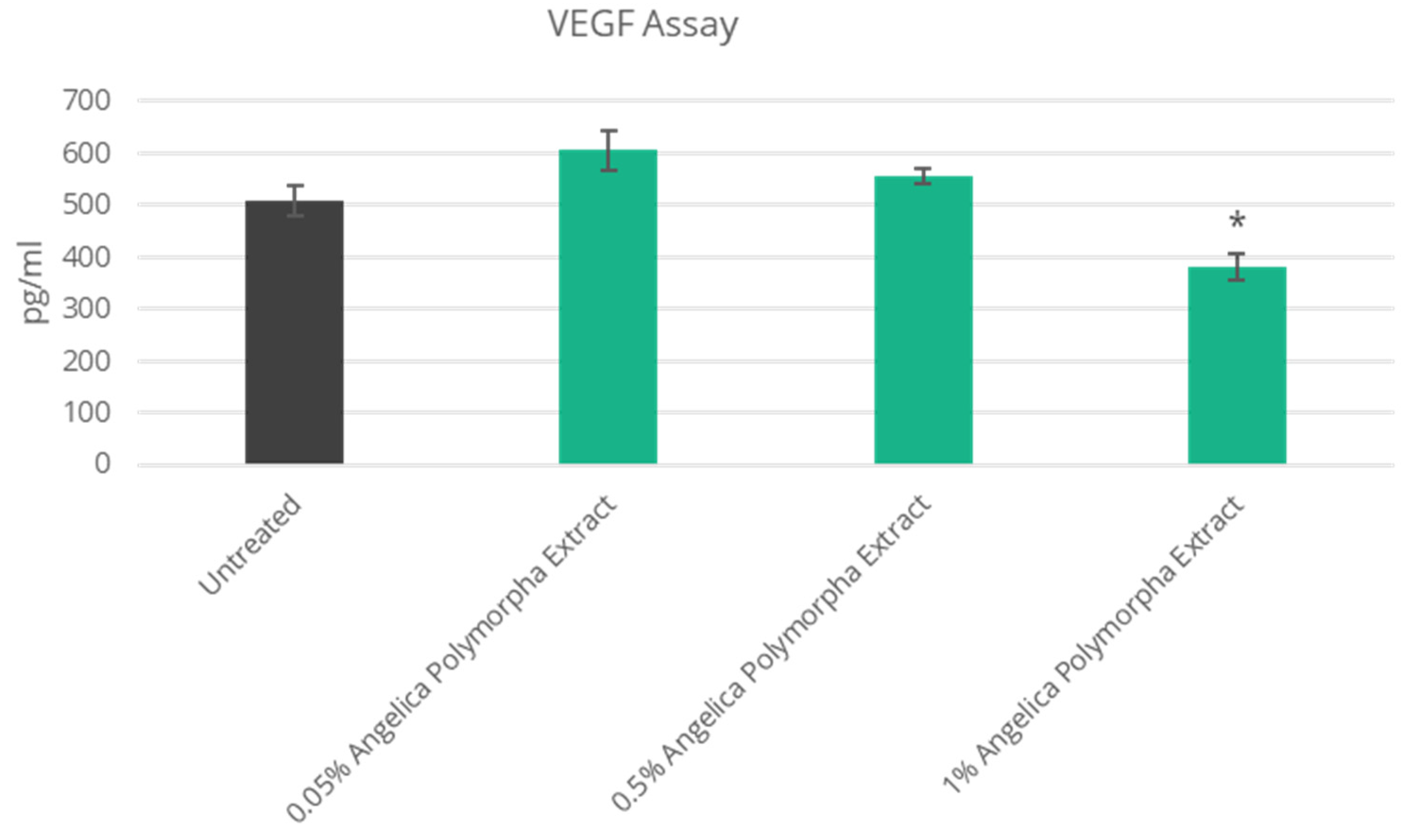
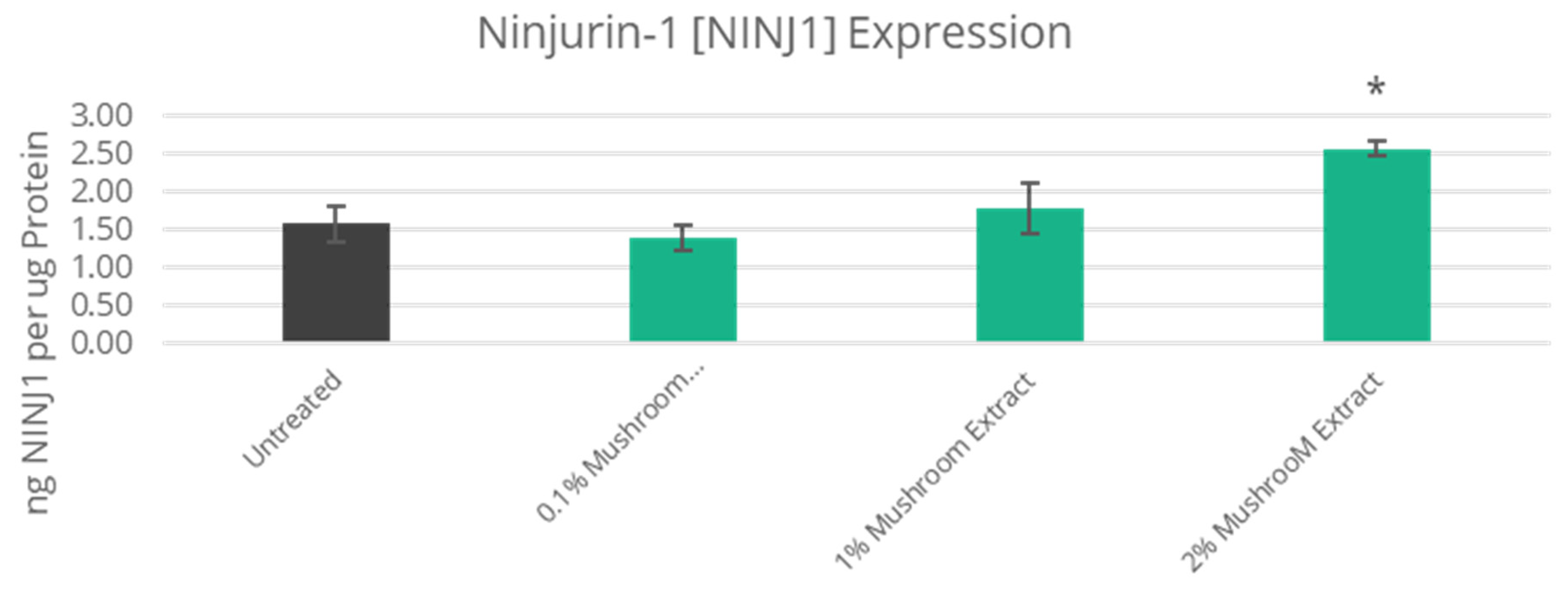
3.3. Improved Skin Vascularization and Fatty Pad Deposits
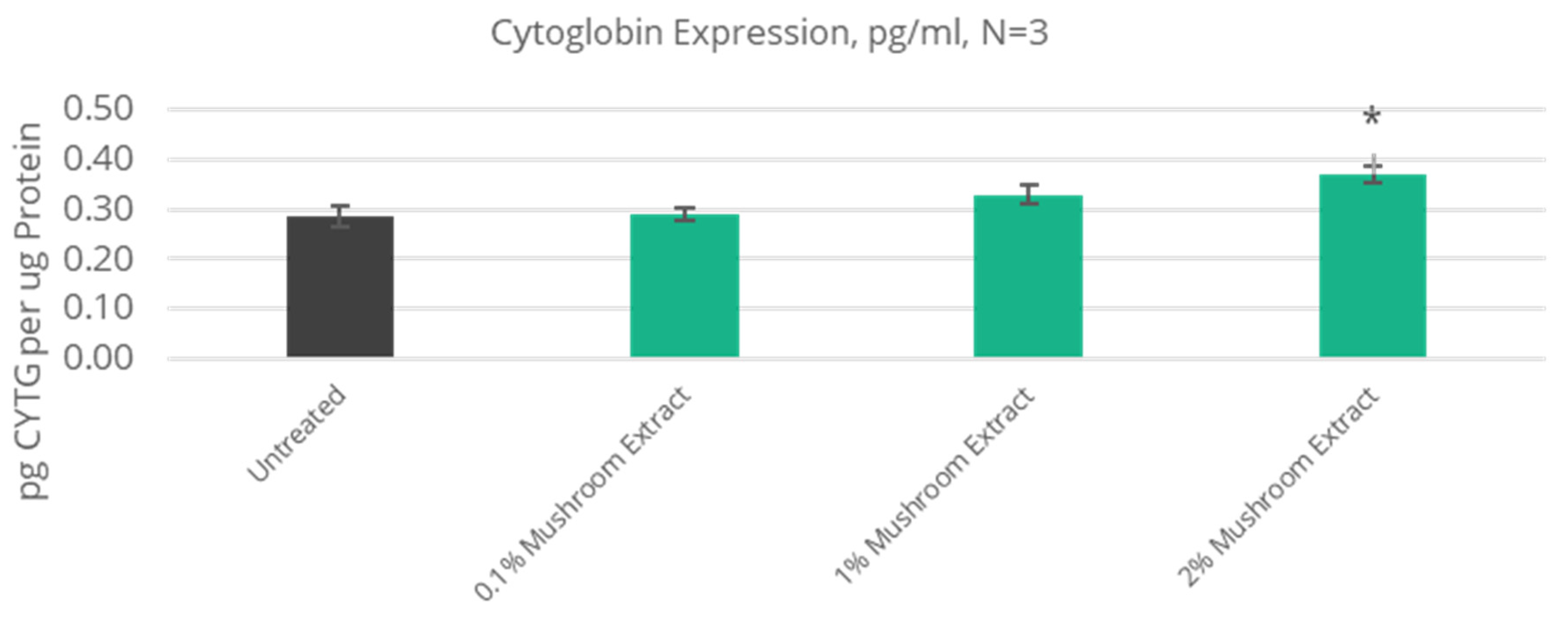
3.4. Improved Antioxidant Defense
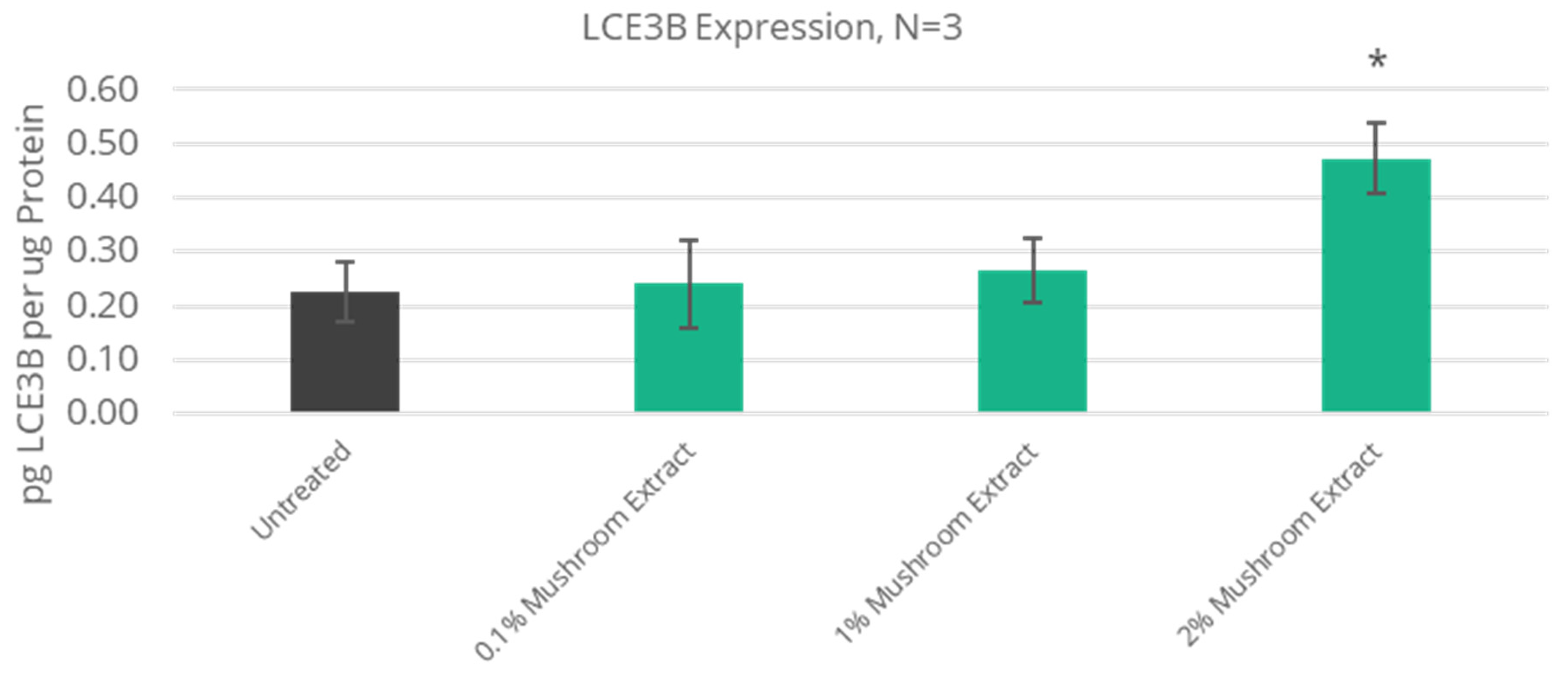
3.5. Improved Extracellular Matrix Protein Expression
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Society of Plastic Surgeons 2023 Procedural Statistics Release. Available online: https://www.plasticsurgery.org/documents/news/statistics/2023/plastic-surgery-statistics-report-2023.pdf (accessed on 5 February 2025).
- Love, L.P.; Farrior, E.H. Periocular anatomy and aging. Facial Plast. Surg. Clin. N. Am. 2010, 18, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Vrcek, I.; Ozgur, O.; Nakra, T. Infraorbital dark circles: A review of the pathogenesis, evaluation, and treatment. J. Cutan. Aesthet. Surg. 2016, 9, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, R.; Ranjan, R.; Garg, S.; Garg, Y.K.; Sonthalia, S.; Bansal, S. Periorbital hyperpigmentation: A comprehensive review. J. Clin. Aesthet. Dermatol. 2016, 9, 49–55. [Google Scholar] [PubMed]
- Ryan, T. The ageing of the blood supply and the lymphatic drainage of the skin. Micron 2004, 35, 161–171. [Google Scholar] [CrossRef]
- Grishchenko, S.V.; Borkhunova, E.N.; Filatova, A.; Vissarionova, V. Ageing eyelid: Clinical, morphological manifestations and peculiarities of microcirculation. Adv. Gerontol. 2011, 24, 331–339. [Google Scholar]
- Berggren, J.V.; Tenland, K.; Bunke, J.; Albinsson, J.; Hult, J.; Merdasa, A.; Sheikh, R.; Lindstedt, S.; Malmsjo, M. Blood perfusion is better in mycutaneous than in cutaneous flaps. Ophthalmic Plast. Reconstr. Surg. 2022, 38, 166–169. [Google Scholar] [CrossRef]
- Manner, G.E.; Wardell, K.; Wolfley, D.E.; Nilsson, G.E. Laser Doppler perfusion imaging of eyelid skin. Ophthalmic Plast. Reconstr. Surg. 1996, 12, 178–185. [Google Scholar] [CrossRef]
- Figueiras, E.; Campos, R.; Sernedo, S.; Oliveira, R.; Ferreira, L.F.R.; Humeau-Heurtier, A. A new laser Doppler flowmeter prototype for depth dependent monitoring of skin microcirculation. Rev. Sci. Instrum. 2012, 83, 034302. [Google Scholar] [CrossRef]
- Brown, D.A.; Canning, M.T.; Nay, S.L.; Pena, A.V.; Yarosh, D.B. Bicyclic monoterpene diols stimulate release of nitric oxide from skin cells, increase microcirculation and elevate skin temperature. Nitric Oxide 2006, 151, 70–76. [Google Scholar] [CrossRef]
- Ludwig, P.; Muia, F.; Bennett, S.; Gruber, J.V. Natural extract adds radiance via skin chromophores. Pers. Care 2012, 9, 44–48. [Google Scholar]
- Sparavigna, A.; Tenconi, B.; Ponti, I.D.; Guglielmini, G. Evaluation of the activity and tolerability of a cosmetic treatment for the periorbital area on the ageing face: Controlled clinical and instrumental evaluation vs. placebo. Cosmetics 2014, 1, 105–116. [Google Scholar] [CrossRef]
- Namkoong, J.; Kern, D.; Knaggs, H.E. Assessment of human skin gene expression by different blends of plant extracts with implications of periorbital skin aging. Int. J. Mol. Sci. 2018, 19, 3349. [Google Scholar] [CrossRef] [PubMed]
- Berthon, J.Y.; Cabannes, M.; Bouton, C.; Carre, M.; Bridon, E.; Filaire, E. In vitro, ex vivo and clinical approaches to evaluate the potential effect of Gentiana lutea extract on skin. Int. J. Mol. Sci. 2023, 45, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, B.; Melero, A.; Montoro, A.; Merino-Torres, J.F.; Soriano, J.M.; Onofre, N.S. A Narrative Review of the Herbal Preparation of Ayurvedic, Traditional Chinese, and Kampo Medicines Applied as Radioprotectors. Antioxidants 2023, 12, 1437. [Google Scholar] [CrossRef]
- Leung, P.C.; Ko, E.C.; Siu, W.S.; Pang, E.S.; Lau, C.B. Selected topical agents used in Traditional Chinese Medicine in the treatment of minor injuries-A review. Front. Pharmacol. 2016, 7, 16. [Google Scholar] [CrossRef]
- Zhu, T.; Kim, S.H.; Chen, C.Y. A medicinal mushroom: Phellinus Linteus. Curr. Med. Chem. 2008, 15, 1330–1335. [Google Scholar] [CrossRef]
- Chen, H.; Tian, T.; Miao, H.; Zhao, Y.Y. Traditional uses, fermentation, phytochemistry and pharmacology of Phellinus linteus: A review. Fitoterpia 2016, 113, 6–26. [Google Scholar] [CrossRef]
- Chen, W.; Tan, H.; Liu, Q.; Zheng, X.; Zhang, H.; Liu, Y.; Xu, L. A review: The bioactives and pharmacological applications of Phellinus linteus. Molecules 2019, 24, 1888. [Google Scholar] [CrossRef]
- Kwon, Y.; Haam, C.E.; Byeon, S.; Choi, S.J.; Shin, D.H.; Choi, S.K.; Lee, Y.H. Vasodilatory effect of Phellinus linteus extract in rat mesenteric arteries. Molecules 2020, 25, 3160. [Google Scholar] [CrossRef]
- Cheon, S.J.; Jang, M.J.; Jang, Y.A.; Choi, E.Y.; Jun, D.H.; Kim, Y.H.; Cho, W.A.; Jeong, Y.S.; Kwon, H.B.; Kim, T.H.; et al. Anti-wrinkle effect of Cambodian Phellinus linteus extracts. J. Life Sci. 2008, 12, 1718–1722. [Google Scholar] [CrossRef]
- Sarfraz, A.; Rasul, A.; Sarfraz, I.; Shah, M.A.; Hussain, G.; Shafiq, N.; Masood, M.; Adem, S.; Sarker, S.D.; Li, X. Hispolon: A natural polyphenol and emerging cancer killer by multiple signaling pathways. Environ. Res. 2020, 190, 110017. [Google Scholar] [CrossRef] [PubMed]
- Kolla, J.N.; Bollikolla, H.B. Biological activity of hispolon. Caribb. J. Sci. Tech. 2022, 10, 1–8. [Google Scholar] [CrossRef]
- Tang, W.; Chen, Y.; Guo, F. Comparative analysis of roots from Vicatia thibetica de Boiss and Angelica sinensis based on chemical composition, antioxidant, nitrite-scavenging and enzyme inhibition activities. Molecules 2023, 28, 1942. [Google Scholar] [CrossRef]
- Zhu, T.; Zhang, M.; Su, H.; Li, M.; Wang, Y.; Jin, L.; Li, M. Integrated metabolomic and transcriptomic analysis reveals differential mechanisms of flavonoid biosynthesis in two cultivars of Angelica sinensis. Molecules 2022, 27, 306. [Google Scholar] [CrossRef]
- Mapoung, S.; Suzuki, S.; Fuji, S.; Naiki, A.; Kato, H.; Yodkeeree, S.; Sakorn, N.; Ovatlarnporn, C.; Takahashi, S.; Limtrakul, P. Dehydrozingerone, a Curcumin analog, as a potential anti-prostate cancer inhibitor in vitro and in vivo. Molecule 2020, 25, 2737. [Google Scholar] [CrossRef]
- Gruber, J.V.; Holtz, R.; Roach, M. Examining the genomic influence of topically applied probiotics in vitro. Int. J. Cosmet. Sci. 2024, 46, 995–1003. [Google Scholar] [CrossRef]
- Matsuki, M.M.; Kabara, M.; Saito, Y.; Shimamura, K.; Minoshima, A.; Nishimura, M.; Aonuma, T.; Takehara, N.; Hasebe, N.; Kawabe, J. Ninjurin-1 is a novel factor to regulate angiogenesis through the function of pericytes. Circul. J. 2015, 79, 1363–1376. [Google Scholar] [CrossRef]
- Matsuo, R.; Kishibe, M.; Horiuchi, K.; Kano, K.; Tatsukawa, T.; Hayasaka, T.; Kabara, M.; Iinuma, S.; Eguchi, R.; Igawa, G.; et al. Ninjurin1 deletion in NG2-positive pericytes prevents microvessel maturation and delays wound healing. JID Innov. 2022, 2, 100141. [Google Scholar] [CrossRef]
- Niehues, H.; van Vlijmen-Willems, I.M.J.J.; Bergboer, J.G.M.; Kersten, F.F.J.; Narita, M.; van den Bogaard, W.J.A.J.E.H.; Zeeuwen, P.L.J.M.; Schalkwijk, J. Late cornified envelope (LCE) proteins: Distinct expression patterns of LCE2 and LCE3 members suggest nonredundant roles in human epidermis and other epithelia. Brit. J. Dermatol. 2016, 174, 795–802. [Google Scholar] [CrossRef]
- Bashir, S.; Hassan, I.; Majid, S.; Bhat, Y.J.; Farooq, R. Feasibility of establishing deletion of the late cornified envelope genes LCE3B and LCE3C as a susceptibility factor for psoriasis. Adv. Biomed. Res. 2016, 5, 109. [Google Scholar] [CrossRef]
- Hankeln, T.; Wystub, S.; Laufs, T.; Schmidt, M.; Gerlach, F.; Saaler-Reinhardt, S.; Reuss, S.; Burmester, T. The cellular and subcellular localization of neuroglobin and cytoglobin- A clue to their function. IUBMB Life 2004, 56, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Mathai, C.; Jourd’heuil, F.L.; Lopez-Soler, R.T.; Jourd’heuil, D. Emerging perspectives on cytoglobin, beyond NO dioxygenase and peroxidase. Redox Biol. 2020, 32, 101468. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Sato-Matsubara, M.; Tsuruta, D.; Tanaka, H.; Kadono, C.; Sugawara, K.; Kawada, N.; Wakamatsu, K.; To, S.; Yoshizato, K. Cytoglobin functions as a redox regulator of melanogenesis in normal epidermal melanocytes. Pigment. Cell Melanoma Res. 2024, 37, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Reeder, B.J. Insights into the function of cytoglobin. Biochem. Soc. Trans. 2023, 51, 1907–1919. [Google Scholar] [CrossRef]
- Zweler, J.L.; Hemann, C.; Kundu, T.; Ewees, M.G.; Khaleel, S.A.; Samouilov, A.; Ilangovan, G.; El-Mahdy, M.A. Cytoglobin has potent superoxide dismutase function. Proc. Nat. Acad. Sci. USA 2021, 118, e2105053118. [Google Scholar] [CrossRef]
- Zweier, J.L.; Ilangovan, G. Regulation of nitric oxide metabolism and vascular tone by cytoglobin. Antioxid. Redox Signal. 2020, 32, 1172–1187. [Google Scholar] [CrossRef]
- Hortle, E.; Tran, V.T.L.; Wright, K.; Fontaine, A.R.M.; Hansbro, P.M.; Britton, W.J.; Oehlers, S.H. OSXR1 inhibits inflammasome activation by limiting potassium efflux during mycobacterial infection. Life Sci. Alliance. 2022, 5, e202201476. [Google Scholar] [CrossRef]
- Yang, H.J.; Kim, M.J.; Kim, S.S.; Cho, Y.W. Melatonin modulates nitric oxide regulated WNK-SPAK/OSR1-NKCC1 signaling in dorsal raphe nucleus of rats. Kor. J. Physiol. Pharmacol. 2021, 25, 449–457. [Google Scholar] [CrossRef]
- Pastore, S.; Mascia, F.; Mariani, V.; Girolomoni, G. The epidermal growth factor receptor in skin repair and inflammation. J. Investig. Dermatol. 2008, 128, 1365–1374. [Google Scholar] [CrossRef]
- Nanba, D.; Toki, F.; Barrandon, Y.; Higashiyama, S. Recent advances in the epidermal growth factor receptor/ligand system biology in skin homeostasis and keratinocyte stem cell regulation. J. Dermatol. Sci. 2013, 72, 81–86. [Google Scholar] [CrossRef]


| Phellinus Linteus Extract | Angelica Polymorpha Extract | ||||
|---|---|---|---|---|---|
| Protein Result (%) | Array Result (Fold) | Protein Symbol | Protein Name | Array Result (Fold) | Protein Result (%) |
| 28% increase | 1.4 | CYGB | Cytoglobin | N/A | NT |
| 68% Increase | 1.9 | OXSR1 | Oxidative Stress Response Kinase-1 | N/A | NT |
| 100% Increase | 1.4 | LCE3B | Late Cornified Envelope-3B | 1.7 | NT |
| NT | 1.3 | EGFR | Epidermal Growth Factor Receptor | 1.6 | 20% Increase |
| NT | NC | VEGFA | Vascular Endothelial Growth Factor-A | N/A | 25% Decrease |
| 62% Increase | N/A | NINJ1 | Ninjurin-1 | N/A | NT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gruber, J.V.; Terpak, N.; Massard, S.; Chen, X.; Craffey, J.; Holtz, R. In Vitro Examination of Fungal and Root Extracts Inspired by Traditional Medicine for Potential Periorbital Eye Infrastructure Treatments. Cosmetics 2025, 12, 95. https://doi.org/10.3390/cosmetics12030095
Gruber JV, Terpak N, Massard S, Chen X, Craffey J, Holtz R. In Vitro Examination of Fungal and Root Extracts Inspired by Traditional Medicine for Potential Periorbital Eye Infrastructure Treatments. Cosmetics. 2025; 12(3):95. https://doi.org/10.3390/cosmetics12030095
Chicago/Turabian StyleGruber, James V., Nicole Terpak, Sebastien Massard, Xiang Chen, John Craffey, and Robert Holtz. 2025. "In Vitro Examination of Fungal and Root Extracts Inspired by Traditional Medicine for Potential Periorbital Eye Infrastructure Treatments" Cosmetics 12, no. 3: 95. https://doi.org/10.3390/cosmetics12030095
APA StyleGruber, J. V., Terpak, N., Massard, S., Chen, X., Craffey, J., & Holtz, R. (2025). In Vitro Examination of Fungal and Root Extracts Inspired by Traditional Medicine for Potential Periorbital Eye Infrastructure Treatments. Cosmetics, 12(3), 95. https://doi.org/10.3390/cosmetics12030095





