Personalized Beauty: How Clinical Insights Shape Tailored Aesthetic Treatments
Abstract
:1. Introduction
Literature Review
2. Materials and Methods
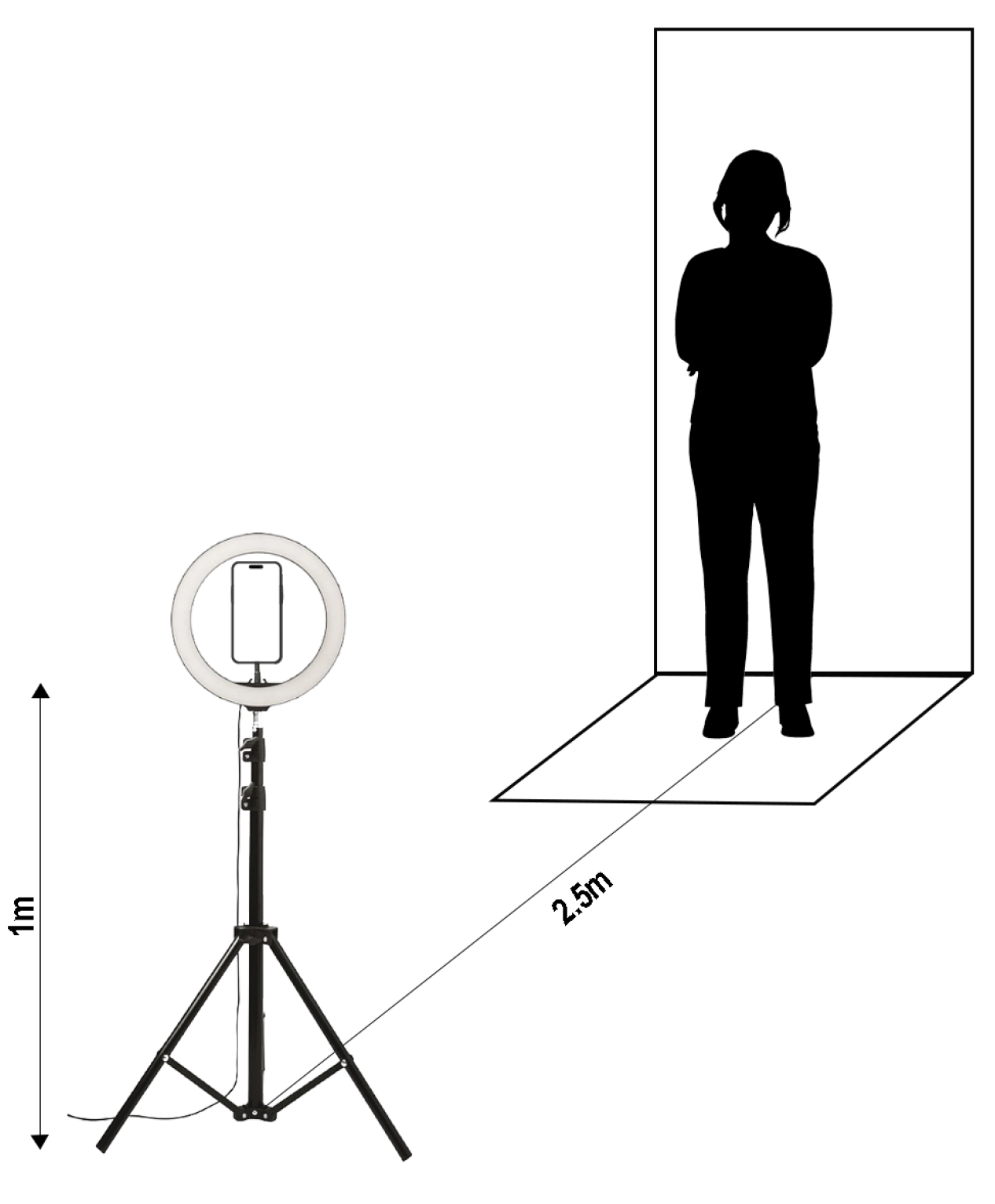
2.1. Devices
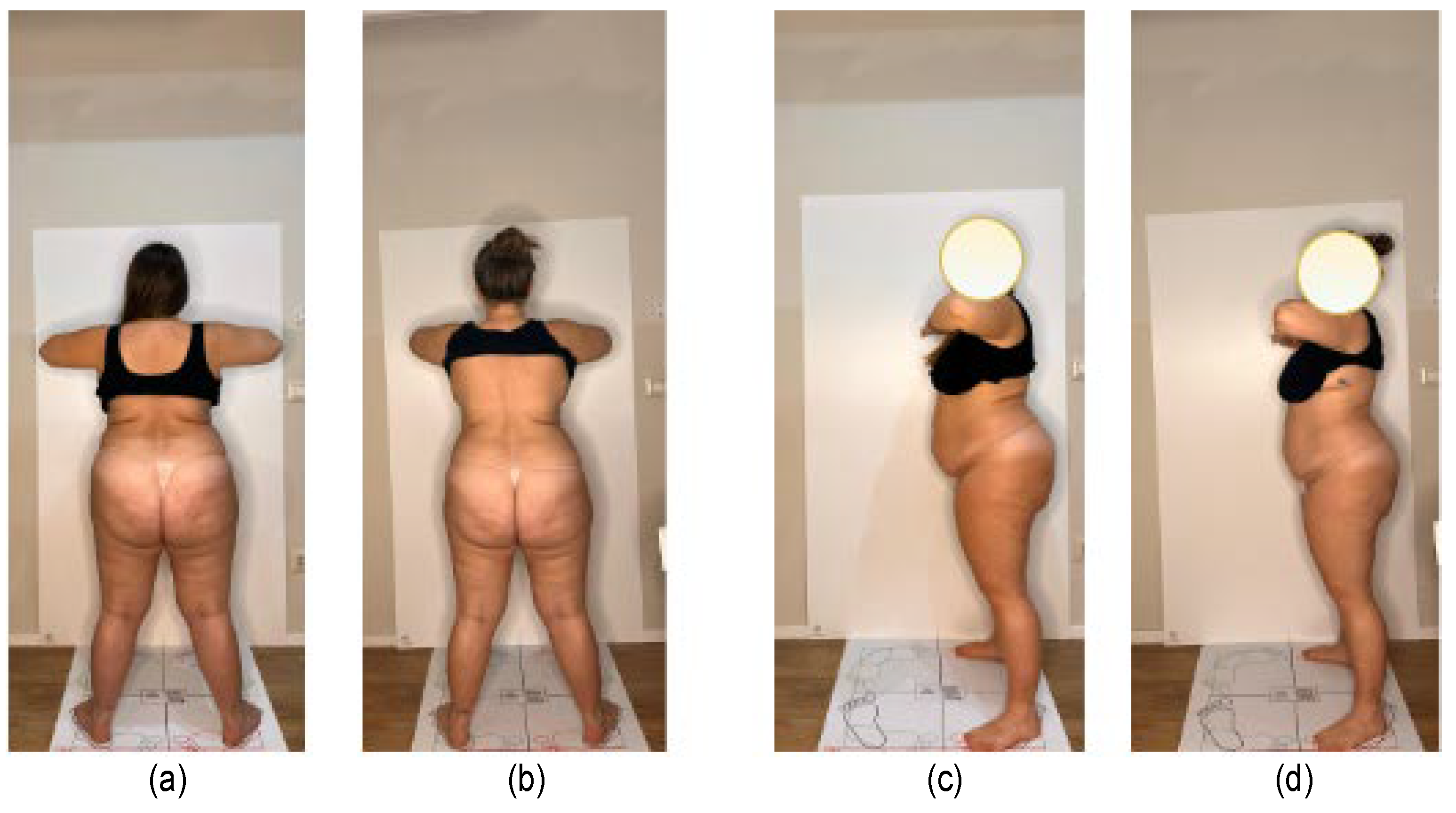
2.2. Data Acquisition
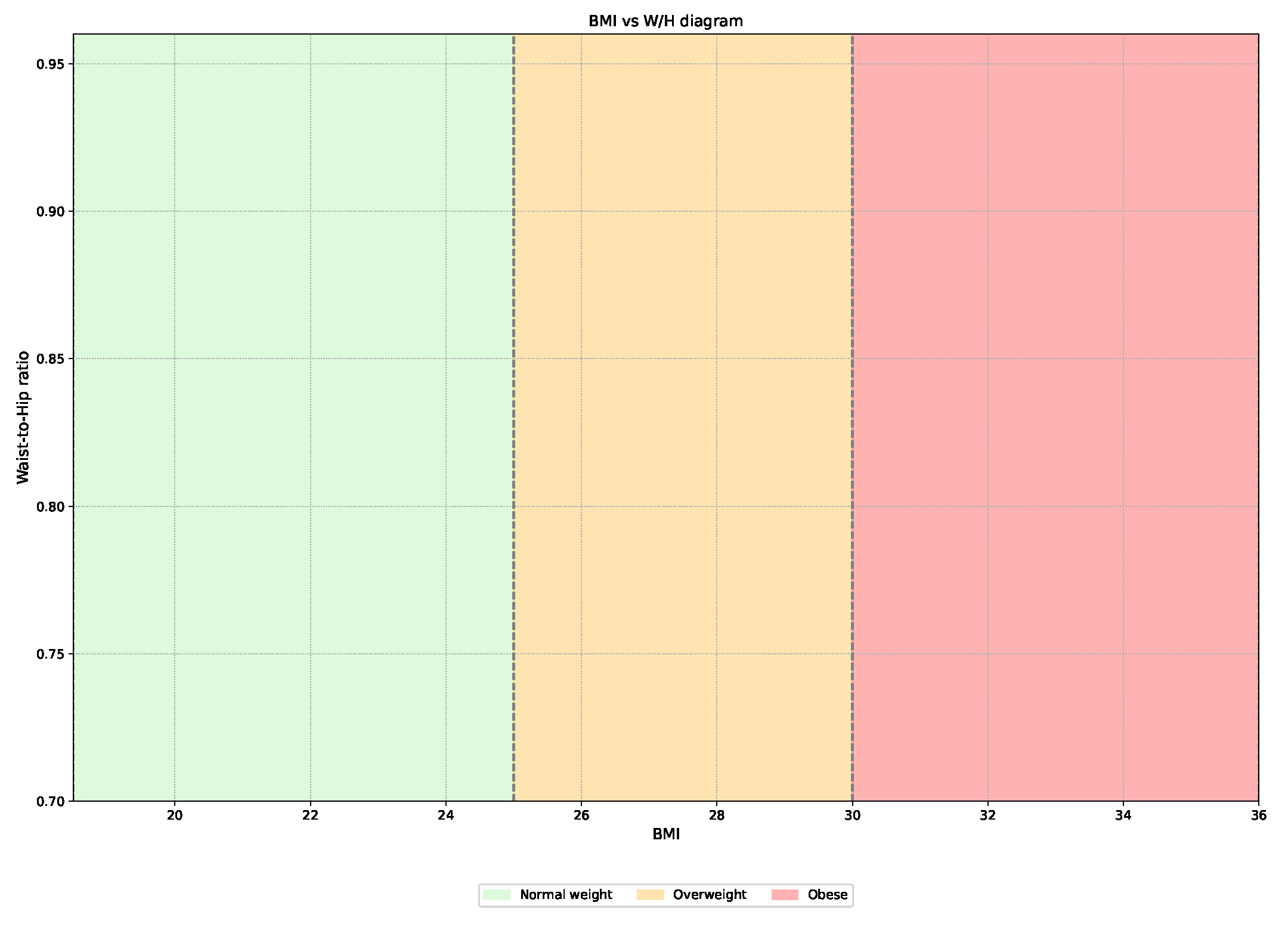
2.3. Data Analysis
3. Results and Discussion
3.1. Data Acquisition and Analysis
3.2. Statistical Analysis
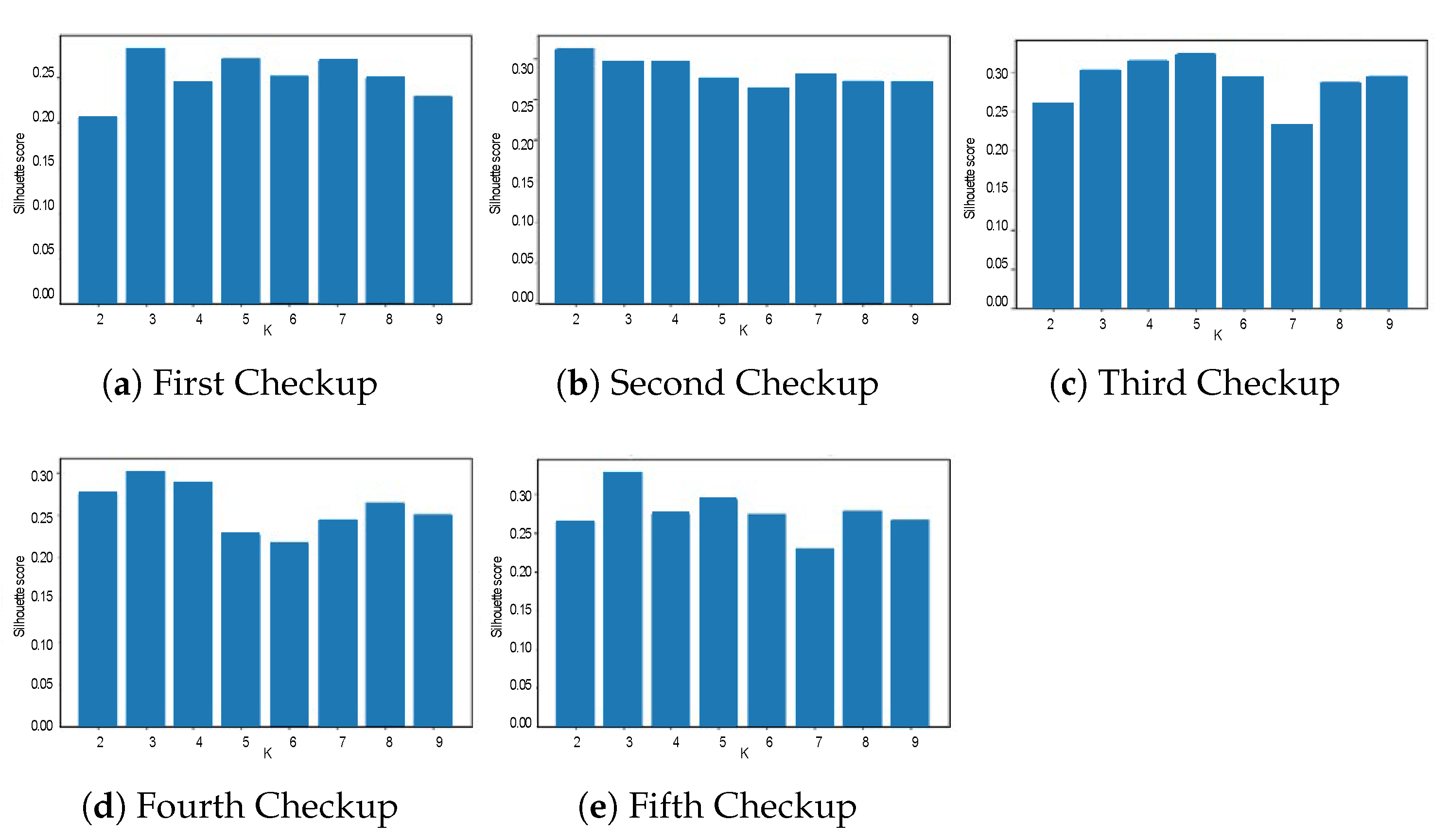
3.3. Clustering Results
- Path 1: Cluster 1 → Cluster 3 → Cluster 3 → Cluster 1 → Cluster 1.
- Path 2: Cluster 2 → Cluster 1 → Cluster 1 → Cluster 3 → Cluster 3.
- Path 3: Cluster 3 → Cluster 2 → Cluster 2 → Cluster 2 → Cluster 2.
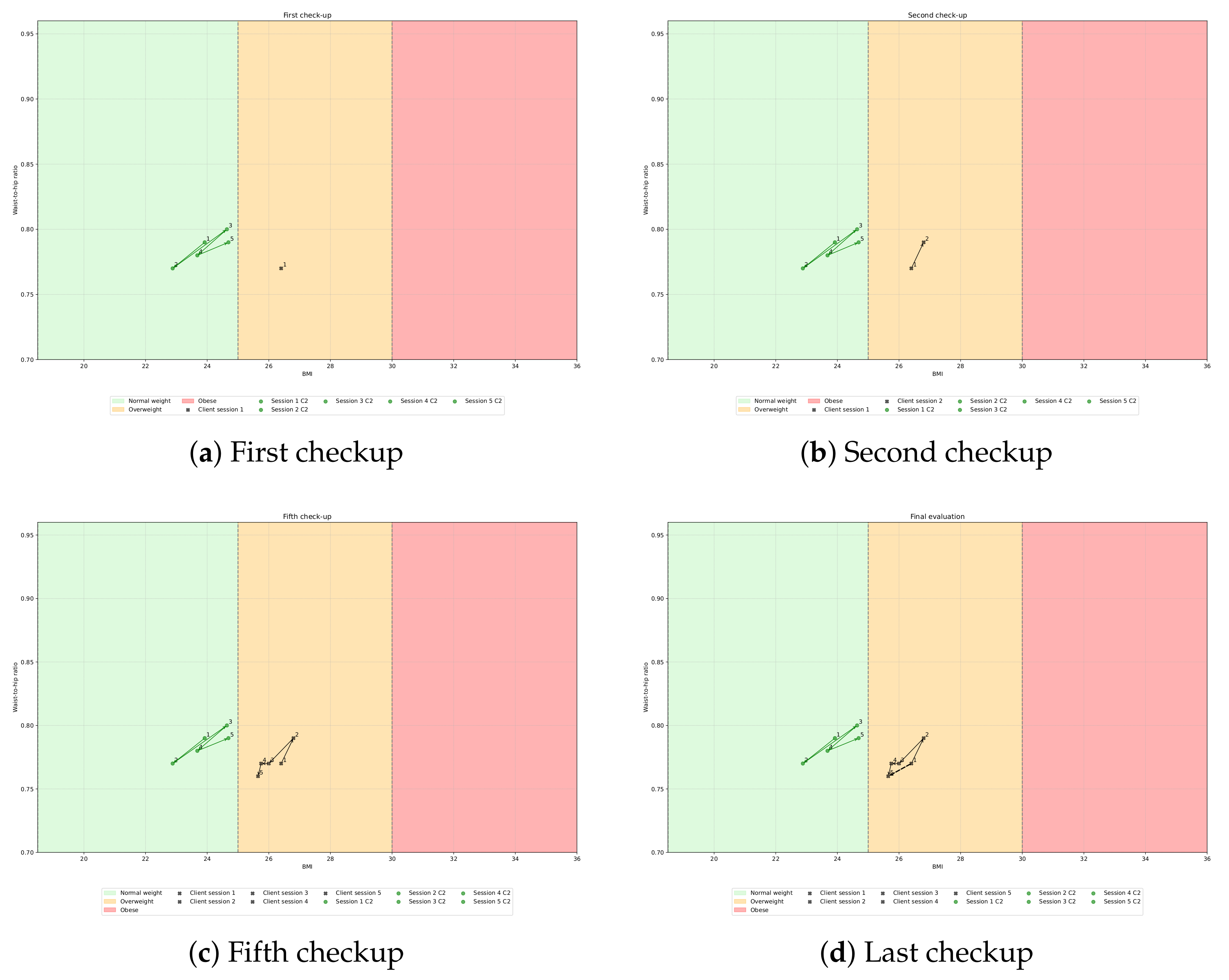
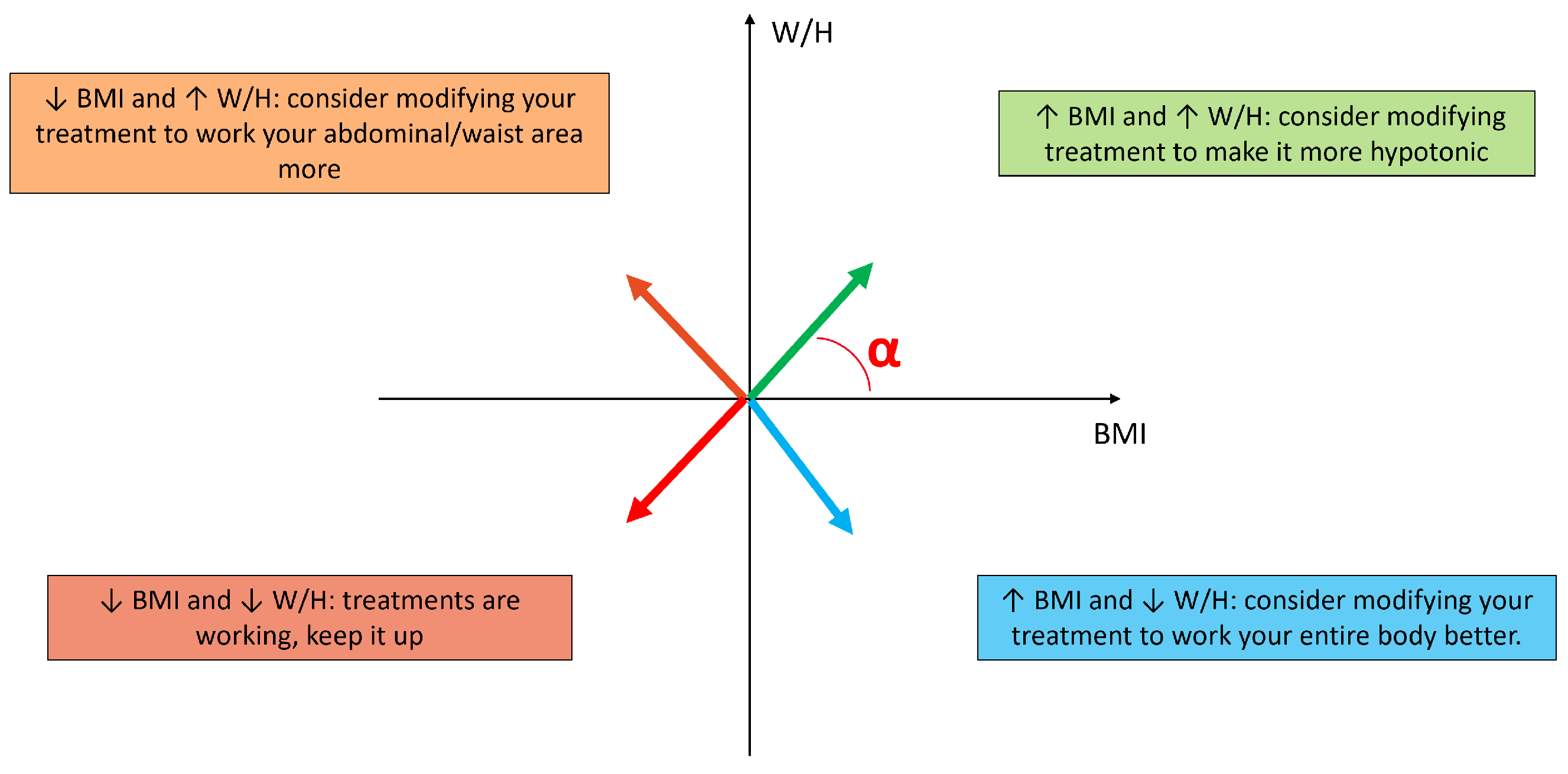
3.4. Treatment Suggestion Algorithm
- Angle between 0 and : ↑ BMI and ↑ W/H consider modifying treatment to make it more hypotonic;
- Angle between and : ↓ BMI and ↑ W/H consider modifying your treatment to work your abdominal/waist area more;
- Angle between and : ↓ BMI and ↓ W/H treatments are working, keep it up.
- Angle between and : ↑ BMI and ↓ W/H consider modifying your treatment to work your entire body better.
3.5. Limitations and Future Works
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence | BMI | Body Mass Index |
| DSS | Decision Support System | ECW | Extracellular Water |
| BIA | Bioelectrical Impedance Analysis | MM | Muscle Mass |
| TBW | Total Body Water | FM | Fat Mass |
| FFM | Fat Free Mass | BCM | Body Cell Mass |
| ICW | Intracellular Water | W/H | Waist to Hip ratio |
| BM | Basal Metabolic Rate |
Appendix A
| Variable | Check Up 1 | Check Up 2 | Check Up 3 | Check Up 4 | Check Up 5 |
|---|---|---|---|---|---|
| FM | 15.97 (13.65–18.29) | 15.25 (12.97–17.53) | 16.01 (13.86–18.16) | 15.09 (13.21–16.98) | 15.65 (14.05–17.26) |
| FFM | 43.24 (37.87–48.62) | 44.07 (41.61–46.53) | 44.67 (41.55–47.79) | 46.10 (43.58–48.62) | 45.34 (42.87–47.82) |
| MM | 21.46 (19.49–23.43) | 20.91 (19.07–22.74) | 20.74 (18.22–23.25) | 22.57 (20.87–24.26) | 21.93 (20.33–23.54) |
| BCM | 21.33 (20.00–22.66) | 20.92 (19.73–22.12) | 19.81 (16.76–22.86) | 21.73 (20.55–22.92) | 21.38 (20.21–22.54) |
| TBW | 33.05 (30.30–35.80) | 32.44 (29.68–35.20) | 32.10 (28.52–35.67) | 34.55 (32.32–36.79) | 33.67 (31.39–35.95) |
| ICW | 18.91 (17.20–20.61) | 18.45 (16.95–19.95) | 18.39 (16.33–20.45) | 19.73 (18.33–21.12) | 19.25 (18.11–20.40) |
| ECW | 14.37 (13.22–15.53) | 15.92 (11.43–20.41) | 13.70 (12.11–15.29) | 14.23 (12.28–16.19) | 14.59 (13.42–15.76) |
| BMI | 22.68 (21.81–23.55) | 22.19 (21.34–23.04) | 22.54 (21.71–23.37) | 22.71 (21.90–23.51) | 22.64 (21.76–23.53) |
| Metabolism | 1171.93 (1023.13–1320.72) | 1235.50 (1208.68–1262.33) | 1268.29 (1211.10–1325.48) | 1264.39 (1231.61–1297.18) | 1258.06 (1224.26–1291.85) |
| Waist | 78.50 (74.42–82.58) | 71.04 (66.28–75.79) | 73.03 (68.18–77.88) | 73.29 (68.52–78.06) | 73.00 (67.80–78.20) |
| Hip | 100.06 (95.63–104.50) | 94.57 (89.76–99.38) | 94.12 (88.50–99.75) | 94.82 (89.14–100.51) | 94.50 (88.51–100.49) |
| Height | 164.25 (161.35–167.15) | 163.57 (160.43–166.72) | 164.12 (161.14–167.11) | 164.18 (161.39–166.96) | 164.18 (161.39–166.96) |
| W/H | 0.77 (0.75–0.80) | 0.75 (0.72–0.78) | 0.78 (0.75–0.81) | 0.77 (0.75–0.80) | 0.77 (0.74–0.80) |
| Age | 33.75 (29.80–37.70) | 33.79 (29.45–38.13) | 33.50 (29.72–37.28) | 32.59 (28.56–36.62) | 32.59 (28.56–36.62) |
| Variable | Check Up 1 | Check Up 2 | Check Up 3 | Check Up 4 | Check Up 5 |
|---|---|---|---|---|---|
| FM | 21.53 (11.50–31.56) | 23.20 (15.48–30.91) | 22.18 (12.57–31.78) | 28.11 (18.90–37.31) | 25.43 (18.27–32.59) |
| FFM | 47.70 (39.78–55.63) | 46.62 (40.25–52.98) | 47.95 (40.80–55.09) | 42.61 (38.00–47.23) | 45.99 (38.15–53.84) |
| MM | 22.48 (17.15–27.81) | 23.38 (18.83–27.93) | 23.10 (18.77–27.43) | 20.10 (17.80–22.40) | 21.72 (17.78–25.67) |
| BCM | 22.49 (18.75–26.22) | 21.98 (18.98–24.98) | 22.61 (19.24–25.98) | 20.09 (17.91–22.27) | 21.69 (17.99–25.38) |
| TBW | 35.54 (28.65–42.43) | 32.77 (21.35–44.19) | 36.44 (31.42–41.46) | 32.77 (29.86–35.69) | 34.82 (30.55–39.08) |
| ICW | 19.66 (15.11–24.21) | 20.10 (16.28–23.92) | 20.21 (16.74–23.69) | 17.52 (15.57–19.46) | 19.17 (16.63–21.70) |
| ECW | 15.88 (13.21–18.55) | 16.42 (14.37–18.47) | 16.23 (14.35–18.10) | 15.26 (13.62–16.91) | 15.65 (13.76–17.54) |
| BMI | 27.64 (26.09–29.19) | 26.75 (25.29–28.20) | 27.03 (25.08–28.98) | 27.50 (25.03–29.96) | 27.78 (25.44–30.13) |
| Metabolism | 1421.39 (1231.14–1611.64) | 1394.48 (1270.41–1518.55) | 1437.41 (1278.50–1596.32) | 1342.70 (1203.28–1482.12) | 1402.44 (1210.02–1594.86) |
| Waist | 86.83 (82.46–91.21) | 86.81 (83.29–90.33) | 85.08 (81.41–88.76) | 84.20 (77.15–91.25) | 85.00 (79.73–90.27) |
| Hip | 109.33 (105.97–112.70) | 106.00 (102.34–109.66) | 107.83 (103.77–111.89) | 106.60 (100.74–112.46) | 108.00 (102.66–113.34) |
| Height | 160.50 (154.14–166.86) | 162.62 (157.15–168.10) | 160.83 (154.66–167.00) | 160.00 (152.35–167.65) | 160.00 (152.35–167.65) |
| W/H | 0.79 (0.76–0.82) | 0.82 (0.78–0.86) | 0.79 (0.76–0.82) | 0.79 (0.75–0.83) | 0.79 (0.77–0.81) |
| Age | 34.33 (23.78–44.88) | 34.12 (26.42–41.83) | 35.00 (23.85–46.15) | 38.40 (29.25–47.55) | 38.40 (29.25–47.55) |
| Variable | Check Up 1 | Check Up 2 | Check Up 3 | Check Up 4 | Check Up 5 |
|---|---|---|---|---|---|
| FM | 47.43 (46.53–48.34) | 45.08 (44.22–45.94) | 45.17 (42.96–47.39) | 43.81 (42.36–45.26) | 44.72 (39.46–49.99) |
| FFM | 39.00 (38.47–39.52) | 40.64 (39.47–41.82) | 41.04 (38.83–43.25) | 40.34 (39.56–41.13) | 40.85 (38.59–43.10) |
| MM | 19.85 (19.33–20.37) | 20.36 (19.62–21.10) | 20.94 (20.25–21.62) | 20.88 (20.04–21.72) | 20.92 (20.21–21.63) |
| BCM | 18.19 (17.82–18.56) | 19.25 (18.57–19.93) | 19.22 (18.12–20.33) | 19.26 (18.81–19.71) | 19.23 (18.10–20.36) |
| TBW | 32.87 (31.85–33.88) | 34.04 (32.51–35.57) | 34.10 (31.96–36.24) | 33.64 (32.90–34.37) | 34.15 (32.31–35.98) |
| ICW | 17.39 (17.04–17.73) | 17.91 (17.18–18.65) | 18.35 (17.12–19.58) | 17.90 (17.50–18.30) | 18.07 (17.15–19.00) |
| ECW | 15.20 (14.47–15.93) | 15.69 (14.99–16.39) | 15.59 (14.81–16.38) | 15.85 (15.57–16.14) | 16.08 (15.17–16.99) |
| BMI | 33.17 (30.19–36.16) | 32.97 (30.30–35.65) | 32.90 (30.34–35.46) | 32.61 (30.11–35.11) | 32.62 (30.36–34.87) |
| Metabolism | 1419.70 (1418.27–1421.14) | 1440.83 (1437.86–1443.80) | 1434.57 (1421.35–1447.80) | 1201.75 (587.35–1816.16) | 1434.78 (1430.75–1438.82) |
| Waist | 113.75 (112.71–114.79) | 115.00 (112.52–117.48) | 114.08 (110.93–117.24) | 111.08 (107.93–114.24) | 104.38 (102.33–106.42) |
| Hip | 121.25 (118.40–124.10) | 120.79 (118.40–123.19) | 120.29 (117.77–122.81) | 118.29 (115.77–120.81) | 114.46 (111.45–117.47) |
| Height | 157.83 (153.94–161.73) | 157.83 (153.94–161.73) | 157.83 (153.94–161.73) | 157.83 (153.94–161.73) | 157.83 (153.94–161.73) |
| W/H | 0.93 (0.91–0.95) | 0.95 (0.94–0.96) | 0.95 (0.94–0.96) | 0.94 (0.93–0.95) | 0.91 (0.90–0.93) |
| Age | 31.17 (24.82–37.51) | 31.17 (24.82–37.51) | 31.17 (24.82–37.51) | 31.17 (24.82–37.51) | 31.17 (24.82–37.51) |
| Metrics | Check-Up 1 | Check-Up 2 | Check-Up 3 | Check-Up 4 | Check-Up 5 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K = 2 | K = 3 | K = 4 | K = 2 | K = 3 | K = 4 | K = 2 | K = 3 | K = 4 | K = 2 | K = 3 | K = 4 | K = 2 | K = 3 | K = 4 | |
| Calinski-Harabatz | 80.50 | 157.23 | 187.65 | 64.40 | 78.39 | 99.63 | 68.91 | 76.96 | 101.33 | 163.76 | 457.41 | 567.42 | 77.38 | 69.19 | 82.22 |
| Davis-Boulding | 0.08 | 0.37 | 0.26 | 0.48 | 0.32 | 0.43 | 0.54 | 0.55 | 0.56 | 0.068 | 0.27 | 0.48 | 0.46 | 0.50 | 0.62 |
| Path | C | FM | FFM | MM | BCM | TBW | ICW | ECW | BMI | BM | WAIST | HIP | HEIGHT | W/H | AGE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Path 1 | C1 | 17.13 ± 3.3 | 39.20 ± 9.8 | 19.16 ± 2.2 | 19.76 ± 1.22 | 30.20 ± 3.29 | 17.21 ± 1.99 | 13.05 ± 1.55 | 23.72 ± 2.14 | 1223.19 ± 47.34 | 78.27 ± 7.32 | 101.54 ± 7.27 | 159.45 ± 4.65 | 0.76 ± 0.04 | 34.27 ± 7.55 |
| C3 | 12.53 ± 7.57 | 52.61 ± 12.45 | 28.32 ± 9.47 | 25.81 ± 4.45 | 42.44 ± 13.05 | 24.33 ± 8.21 | 31.61 ± 14.25 | 24.65 ± 0.77 | 1481.88 ± 300.25 | 100.00 ± 14.1 | 76.0 ± 18.3 | 162.5 ± 3.53 | 0.75 ± 0.07 | 22.5 ± 6.36 | |
| C3 | 17.7 ± 3.55 | 41.13 ± 2.39 | 18.33 ± 3.27 | 17.57 ± 5.58 | 28.85 ± 4.55 | 16.38 ± 2.35 | 12.46 ± 2.37 | 22.53 ± 1.96 | 1250 ± 120 | 91.45 ± 10.7 | 69.63 ± 7.73 | 161.8 ± 5.2 | 0.76 ± 0.05 | 32 ± 6.40 | |
| C1 | 17.22 ± 3.58 | 42.01 ± 2.48 | 19.99 ± 1.67 | 19.81 ± 1.17 | 31.31 ± 2.09 | 17.66 ± 1.31 | 12.55 ± 3.84 | 22.91 ± 1.96 | 1233.6 ± 33.92 | 92.18 ± 11.40 | 70.31 ± 8.53 | 161.09 ± 6.31 | 0.76 ± 0.05 | 32.63 ± 7.04 | |
| C1 | 16.58 ± 3.69 | 41.96 ± 2.86 | 19.98 ± 2.07 | 19.78 ± 1.35 | 31.16 ± 2.5 | 17.93 ± 1.59 | 13.47 ± 1.54 | 22.72 ± 2.20 | 1229.3 ± 2.20 | 91.5 ± 11.41 | 69.33 ± 7.93 | 160.83 ± 6.08 | 0.76 ± 0.05 | 32.83 ± 6.75 | |
| Path 2 | C2 | 16.42 ± 7.7 | 50.48 ± 5.41 | 24.78 ± 3.62 | 23.90 ± 2.45 | 37.63 ± 5.21 | 21.37 ± 3.59 | 16.55 ± 1.57 | 23.92 ± 3.17 | 1249.31 ± 417.77 | 82.50 ± 7.67 | 102.5 ± 9.25 | 167.60 ± 3.65 | 0.79 ± 0.03 | 32.40 ± 8.24 |
| C1 | 16.30 ± 4.54 | 44.13 ± 4.77 | 21.11 ± 3.26 | 21.11 ± 2.25 | 20.81 ± 4.62 | 32.81 ± 2.58 | 14.21 ± 2.24 | 22.88 ± 2.01 | 1249.96 ± 60.61 | 96.7 ± 813 | 74.88 ± 9.66 | 163.23 ± 6.44 | 0.77 ± 0.05 | 35 ± 7.32 | |
| C1 | 16.22 ± 7.22 | 50.8 ± 5.05 | 24.98 ± 3.29 | 23.98 ± 2.38 | 38.23 ± 4.72 | 21.83 ± 2.96 | 16.40 ± 2.06 | 24.6 ± 2.48 | 1383 ± 139.07 | 103.4 ± 6.93 | 82.7 ± 6.22 | 165 ± 6.25 | 0.80 ± 0.03 | 34.9 ± 9.10 | |
| C3 | 15.37 ± 6.47 | 49.86 ± 3.54 | 24.97 ± 2.38 | 23.51 ± 1.69 | 38.01 ± 2.79 | 21.73 ± 1.97 | 16.49 ± 1.28 | 23.60 ± 2.20 | 1320.7 ± 90.83 | 101.1 ± 7.92 | 79.72 ± 7.39 | 165.8 ± 4.59 | 0.78 ± 0.04 | 34.44 ± 9.26 | |
| C3 | 18.18 ± 6.37 | 50.31 ± 3.18 | 24.74 ± 1.70 | 23.72 ± 1.50 | 37.77 ± 2.32 | 21.13 ± 1.26 | 16.64 ± 1.52 | 34.69 ± 2.81 | 1363.4 ± 124.14 | 103.94 ± 7.65 | 82.88 ± 7.65 | 166.66 ± 3.57 | 0.79 ± 0.04 | 34.1 ± 9.29 | |
| Path 3 | C3 | 45.23 ± 5.87 | 39.42 ± 1.21 | 19.82 ± 0.45 | 18.41 ± 0.68 | 32.94 ± 0.90 | 17.38 ± 0.29 | 15.32 ± 0.72 | 32.51 ± 3.13 | 1407.02 ± 33.56 | 110.50 ± 8.64 | 120.35 ± 3.42 | 158.28 ± 3.59 | 0.91 ± 0.05 | 33.14 ± 7.60 |
| C2 | 40.82 ± 6.52 | 42.01 ± 2.88 | 20.69 ± 1.59 | 19.87 ± 1.36 | 30.88 ± 10.56 | 17.95 ± 1.30 | 15.95 ± 0.93 | 31.56 ± 2.91 | 1431.63 ± 46.48 | 116.97 ± 6.33 | 106.0 ± 13.62 | 159.77 ± 4.29 | 0.90 ± 0.07 | 32.5 ± 6.72 | |
| C2 | 43.32 ± 32 | 41.13 ± 1.93 | 20.64 ± 0.96 | 19.28 ± 0.97 | 33.85 ± 1.96 | 18.15 ± 1.18 | 15.57 ± 0.68 | 32.28 ± 2.76 | 1419 ± 42.39 | 119.25 ± 3.52 | 110.07 ± 10.96 | 158.28 ± 3.59 | 0.92 ± 0.07 | 33.14 ± 7.60 | |
| C2 | 41.51 ± 4.45 | 40.97 ± 1.51 | 20.58 ± 1.06 | 19.49 ± 0.66 | 33.32 ± 0.85 | 17.55 ± 0.71 | 15.85 ± 0.36 | 31.74 ± 2.57 | 1245.3 ± 502.01 | 116.34 ± 4.34 | 105.31 ± 11.18 | 159.12 ± 4.06 | 0.90 ± 0.06 | 33.0 ± 7.05 | |
| C2 | 42.7 ± 7.04 | 41.35 ± 2.38 | 20.63 ± 0.97 | 19.47 ± 1.17 | 33.91 ± 1.7 | 18.01 ± 0.81 | 15.90 ± 0.91 | 32.09 ± 2.40 | 1426 ± 22.40 | 114.25 ± 2.67 | 102.03 ± 6.43 | 158.28 ± 3.59 | 0.89 ± 0.05 | 33.14 ± 7.60 |
References
- Grech, V.S.; Kefala, V.; Rallis, E. Cosmetology in the Era of Artificial Intelligence. Cosmetics 2024, 11, 135. [Google Scholar] [CrossRef]
- Ashley, E.; Parmar, A.; Novintan, S.; Watson, L.; Chadha, P. Exploring the psychological and social motivations for aesthetic treatments in the post-COVID era. J. Cosmet. Med. 2024, 8, 34–40. [Google Scholar] [CrossRef]
- Barone, M.; De Bernardis, R.; Persichetti, P. Aesthetic medicine across generations: Evolving trends and influences. Aesthetic Plast. Surg. 2024, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Vatiwutipong, P.; Vachmanus, S.; Noraset, T.; Tuarob, S. Artificial Intelligence in Cosmetic Dermatology: A Systematic Literature Review. IEEE Access 2023, 11, 71407–71425. [Google Scholar] [CrossRef]
- Haykal, D.; Garibyan, L.; Flament, F.; Cartier, H. Hybrid cosmetic dermatology: AI generated horizon. Skin Res. Technol. 2024, 30, e13721. [Google Scholar] [CrossRef]
- Elder, A.; Cappelli, M.O.; Ring, C.; Saedi, N. Artificial intelligence in cosmetic dermatology: An update on current trends. Clin. Dermatol. 2024, 42, 216–220. [Google Scholar] [CrossRef]
- Kania, B.; Montecinos, K.; Goldberg, D.J. Artificial intelligence in cosmetic dermatology. J. Cosmet. Dermatol. 2024, 23, 3305–3311. [Google Scholar] [CrossRef]
- Vora, L.K.; Gholap, A.D.; Jetha, K.; Thakur, R.R.S.; Solanki, H.K.; Chavda, V.P. Artificial intelligence in pharmaceutical technology and drug delivery design. Pharmaceutics 2023, 15, 1916. [Google Scholar] [CrossRef]
- Kalicińska, J.; Wiśniowska, B.; Polak, S.; Spiewak, R. Artificial Intelligence That Predicts Sensitizing Potential of Cosmetic Ingredients with Accuracy Comparable to Animal and In Vitro Tests—How Does the Infotechnomics Compare to Other “Omics” in the Cosmetics Safety Assessment? Int. J. Mol. Sci. 2023, 24, 6801. [Google Scholar] [CrossRef]
- Obwegeser, D.; Timofte, R.; Mayer, C.; Eliades, T.; Bornstein, M.M.; Schätzle, M.A.; Patcas, R. Using artificial intelligence to determine the influence of dental aesthetics on facial attractiveness in comparison to other facial modifications. Eur. J. Orthod. 2022, 44, 445–451. [Google Scholar] [CrossRef]
- Patcas, R.; Bernini, D.A.; Volokitin, A.; Agustsson, E.; Rothe, R.; Timofte, R. Applying artificial intelligence to assess the impact of orthognathic treatment on facial attractiveness and estimated age. Int. J. Oral Maxillofac. Surg. 2019, 48, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Tian, B. Tele-aesthetics in South Asia. J. Cosmet. Dermatol. 2017, 16, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Flament, F.; Jiang, R.; Houghton, J.; Zhang, Y.; Kroely, C.; Jablonski, N.G.; Jean, A.; Clarke, J.; Steeg, J.; Sehgal, C.; et al. Accuracy and clinical relevance of an automated, algorithm-based analysis of facial signs from selfie images of women in the United States of various ages, ancestries and phototypes: A cross-sectional observational study. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Fliorent, R.; Fardman, B.; Podwojniak, A.; Javaid, K.; Tan, I.J.; Ghani, H.; Truong, T.M.; Rao, B.; Heath, C. Artificial intelligence in dermatology: Advancements and challenges in skin of color. Int. J. Dermatol. 2024, 63, 455–461. [Google Scholar] [CrossRef]
- Junayed, M.S.; Jeny, A.A.; Atik, S.T.; Neehal, N.; Karim, A.; Azam, S.; Shanmugam, B. AcneNet—A deep CNN based classification approach for acne classes. In Proceedings of the 2019 12th International Conference on Information & Communication Technology and System (ICTS), Surabaya, Indonesia, 18 July 2019; pp. 203–208. [Google Scholar]
- Yadav, R.; Jain, A.; Sharma, S. Acne Detection Care System using Deep Learning. In Proceedings of the 2024 11th International Conference on Reliability, Infocom Technologies and Optimization (Trends and Future Directions) (ICRITO), Noida, India, 14–15 March 2024; pp. 1–5. [Google Scholar]
- Navarro-Barrientos, J.E.; Rivera, D.E.; Collins, L.M. A dynamical model for describing behavioural interventions for weight loss and body composition change. Math. Comput. Model. Dyn. Syst. 2011, 17, 183–203. [Google Scholar] [CrossRef]
- Figueiredo, D.M.; Duarte, R.P.; Cunha, C.A. Predictive Model for Estimating Body Weight Based on Artificial Intelligence: An Integrated Approach to Pre-processing and Evaluation. In Proceedings of the International Conference on Disruptive Technologies, Tech Ethics and Artificial Intelligence; Springer: Cham, Switzerland, 2024; pp. 28–39. [Google Scholar]
- Juhász, M.; Korta, D.; Mesinkovska, N.A. A review of the use of ultrasound for skin tightening, body contouring, and cellulite reduction in dermatology. Dermatol. Surg. 2018, 44, 949–963. [Google Scholar] [CrossRef]
- Taher, A.A.M.; Al-Sultani, A.H. Electrical muscle stimulation and its advantages. J. Humanit. Soc. Sci. Res. 2023, 2. [Google Scholar]
- Paul, A.; Usman, J.; Ahmad, M.Y.; Hamidreza, M.; Maryam, H.; Ong, Z.C.; Hasikin, K.; Lai, K.W. Health efficacy of electrically operated automated massage on muscle properties, peripheral circulation, and physio-psychological variables: A narrative review. EURASIP J. Adv. Signal Process. 2021, 2021, 80. [Google Scholar] [CrossRef]
- Duren, D.L.; Sherwood, R.J.; Czerwinski, S.A.; Lee, M.; Choh, A.C.; Siervogel, R.M.; Chumlea, W.C. Body composition methods: Comparisons and interpretation. J. Diabetes Sci. Technol. 2008, 2, 1139–1146. [Google Scholar] [CrossRef]
- Aldobali, M.; Pal, K. Bioelectrical Impedance Analysis for Evaluation of Body Composition: A Review. In Proceedings of the 2021 International Congress of Advanced Technology and Engineering (ICOTEN), Taiz, Yemen, 4–5 July 2021; pp. 1–10. [Google Scholar]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.C.; Pirlich, M.; et al. Bioelectrical impedance analysis—Part I: Review of principles and methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef]
- Schoeller, D.A. Bioelectrical impedance analysis what does it measure? Ann. New York Acad. Sci. 2000, 904, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Kułaga, Z.; Świąder-Leśniak, A.; Kotowska, A.; Litwin, M. Population-based references for waist and hip circumferences, waist-to-hip and waist-to-height ratios for children and adolescents, and evaluation of their predictive ability. Eur. J. Pediatr. 2023, 182, 3217–3229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.L.; Ren, J.X.; Zhang, P.; Jin, H.; Qu, Y.; Yu, Y.; Guo, Z.N.; Yang, Y. Strong association of waist circumference (WC), body mass index (BMI), waist-to-height ratio (WHtR), and waist-to-hip ratio (WHR) with diabetes: A population-based cross-sectional study in Jilin Province, China. J. Diabetes Res. 2021, 2021, 8812431. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Seraj, R.; Islam, S.M.S. The k-means algorithm: A comprehensive survey and performance evaluation. Electronics 2020, 9, 1295. [Google Scholar] [CrossRef]
- Jain, A.K. Data clustering: 50 years beyond K-means. Pattern Recognit. Lett. 2010, 31, 651–666. [Google Scholar] [CrossRef]
- Sharma, N.; Bajpai, A.; Litoriya, M.R. Comparison the various clustering algorithms of weka tools. Facilities 2012, 4, 78–80. [Google Scholar]
- Shahapure, K.R.; Nicholas, C. Cluster quality analysis using silhouette score. In Proceedings of the 2020 IEEE 7th international conference on data science and advanced analytics (DSAA), Sydney, NSW, Australia, 6–9 October 2020; pp. 747–748. [Google Scholar]
- Lind, L.; Ahmad, S.; Elmståhl, S.; Fall, T. The metabolic profile of waist to hip ratio—A multi-cohort study. PLoS ONE 2023, 18, e0282433. [Google Scholar] [CrossRef]


| Variable | Normal Weight | Overweight | Obese |
|---|---|---|---|
| FM | +1.41 | +1.39 | −5.71 |
| FFM | +0.17 | −0.55 | +4.74 |
| MM | −1.94 | +2.43 | +5.35 |
| BCM | −0.47 | +0.50 | +5.74 |
| TBW | +0.24 | +4.20 | +3.90 |
| ICW | −2.69 | +4.35 | +3.96 |
| ECW | −1.85 | −0.18 | +5.79 |
| BMI | +1.35 | +0.11 | −1.6 |
| MB | −0.07 | −1.00 | +1.06 |
| HIP | −2.11 | +0.46 | +5.79 |
| WAIST | −4.10 | −2.32 | −8.24 |
| Variable | p-Value | |
|---|---|---|
| Check-Up 1 | Check-Up 5 | |
| FM | ||
| FFM | ||
| MM | ||
| BCM | ||
| TBW | ||
| ICW | ||
| ECW | ||
| BMI | ||
| Metabolism | ||
| Waist | ||
| Hip | ||
| Height | ||
| W/H | ||
| Age | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campanella, S.; Palma, L. Personalized Beauty: How Clinical Insights Shape Tailored Aesthetic Treatments. Cosmetics 2025, 12, 94. https://doi.org/10.3390/cosmetics12030094
Campanella S, Palma L. Personalized Beauty: How Clinical Insights Shape Tailored Aesthetic Treatments. Cosmetics. 2025; 12(3):94. https://doi.org/10.3390/cosmetics12030094
Chicago/Turabian StyleCampanella, Sara, and Lorenzo Palma. 2025. "Personalized Beauty: How Clinical Insights Shape Tailored Aesthetic Treatments" Cosmetics 12, no. 3: 94. https://doi.org/10.3390/cosmetics12030094
APA StyleCampanella, S., & Palma, L. (2025). Personalized Beauty: How Clinical Insights Shape Tailored Aesthetic Treatments. Cosmetics, 12(3), 94. https://doi.org/10.3390/cosmetics12030094







