Sun Protection Products Protect Against UV-Induced Mitochondrial DNA Damage and Blue Light-Induced Cell Decline in Human Dermal Fibroblast Skin Cell Viability
Abstract
1. Introduction
- Do commercial sun protection products provide protection by reducing the amount of UV-induced mtDNA damage in human dermal fibroblast skin cells?
- Do commercial sun protection products protect against blue light induced decrease in the viability of human dermal fibroblast skin cells?
2. Methods
2.1. UV-Induced Mitochondrial DNA Damage Experiment
2.2. MTS Blue Light Method
2.3. Statistical Analysis
3. Results
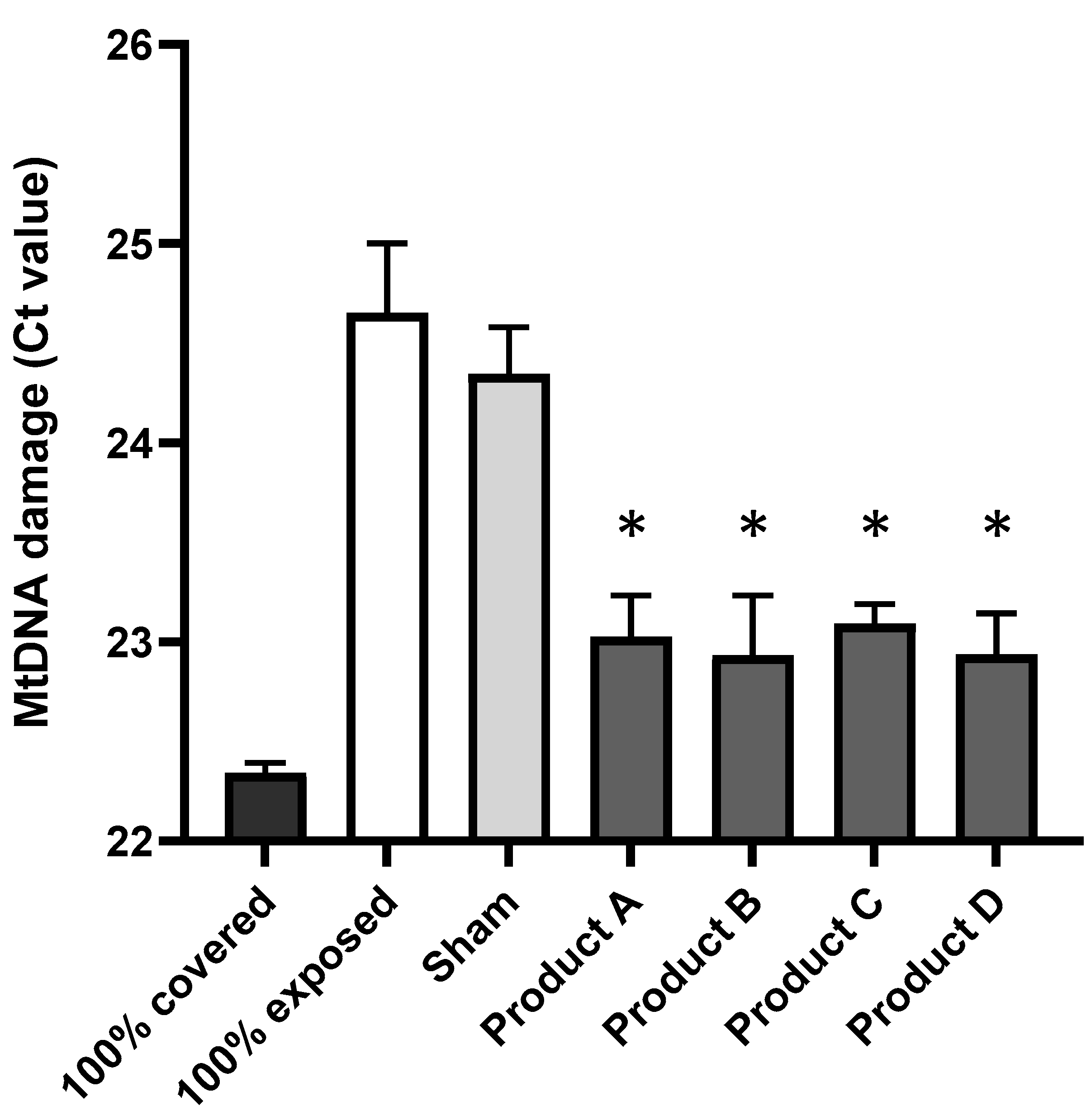
3.1. Commercial Sun Protection Products at SPF 50 Provide Protection by Reducing the Amount of UV-Induced mtDNA Damage in Human Dermal Fibroblast Skin Cells
3.2. Commercial Sun Protection SPF 50 Products Protect Against Blue Light-Induced Decrease in the Viability of Human Dermal Fibroblast Skin Cells
4. Discussion
- Four commercial sun protection SPF 50 products provide significant protection by reducing the amount of UV-induced mtDNA damage in human dermal fibroblast skin cells.
- Commercial sun protection SPF 50 products protect against blue light induced decrease in the viability of human dermal fibroblast skin cells.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soter, N.A. Acute effects of ultraviolet radiation on the skin. Semin. Dermatol. 1990, 9, 11–15. [Google Scholar] [PubMed]
- Parrado, C.; Mercado-Saenz, S.; Perez-Davo, A.; Gilaberte, Y.; Gonzalez, S.; Juarranz, A. Environmental stressors on skin aging. Mech. Insights Front. Pharmacol. 2019, 10, 759. [Google Scholar] [CrossRef]
- Diffey, B. Erythema and Acclimatization Following Repeated Sun Exposure: A Modeling Study. Photochem. Photobiol. 2021, 97, 1558–1567. [Google Scholar] [CrossRef] [PubMed]
- Birch-Machin, M.A.; Stout, R. Sun Exposure and Pollution: International Patent Application No. PCT/GB2023/050834; A qPCR Method to Analyse mtDNA Mainly in Skin Cells. WO 2023/187386 A1, 30 March 2023. Available online: https://patents.google.com/patent/WO2023187386A1/en?inventor=Mark+Birch-Machin&oq=Mark+Birch-Machin+&page=1 (accessed on 23 December 2024).
- Clatici, V.G.; Racoceanu, D.; Dalle, C.; Voicu, C.; Tomas-Aragones, L.; Marron, S.E.; Wollina, U.; Fica, S. Perceived age and life style. The specific contributions of seven factors involved in health and beauty. Maedica 2017, 12, 191. [Google Scholar]
- Birch-Machin, M.A.; Moor, J.A. Mitochondria as a Skin Health Biomarker. Aesthet. J. 2024, 11, 32–35. [Google Scholar]
- Green, A.C.; Williams, G.M.; Logan, V.; Strutton, G.M. Reduced melanoma after regular sunscreen use: Randomized trial follow-up. J. Clin. Oncol. 2011, 29, 257–263. [Google Scholar] [CrossRef]
- Mahendra, C.K.; Ser, H.-L.; Pusparajah, P.; Htar, T.T.; Chuah, L.-H.; Yap, W.H.; Tang, Y.-Q.; Zengin, G.; Tang, S.Y.; Lee, W.L.; et al. Cosmeceutical therapy: Engaging the repercussions of UVR photoaging on the skin’s circadian rhythm. Int. J. Mol. Sci. 2022, 23, 2884. [Google Scholar] [CrossRef]
- Trifunovic, A.; Hansson, A.; Wredenberg, A.; Rovio, A.T.; Dufour, E.; Khvorostov, I.; Spelbrink, J.N.; Wibom, R.; Jacobs, H.T.; Larsson, N.-G. Somatic mtDNA mutations cause aging phenotypes without affecting reactive oxygen species production. Proc. Natl. Acad. Sci. USA 2005, 102, 17993–17998. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Lee, H.C.; Lin, K.J.; Wei, Y.H. A specific 4977-bp deletion of mitochondrial DNA in human ageing skin. Arch. Dermatol. Res. 1994, 286, 386–390. [Google Scholar] [CrossRef]
- Birch-Machin, M.A. Mitochondria and Skin Disease. Clin. Exp. Dermatol. 2000, 25, 141–146. [Google Scholar] [CrossRef]
- Anderson, S.; Bankier, A.T.; Barrell, B.G.; De Bruijn, M.H.L.; Coulson, A.R.; Drouin, J.; Eperon, I.C.; Nierlich, D.P.; Roe, B.A.; Sanger, F.; et al. Sequence and organization of the human mitochondrial genome. Nature 1981, 290, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.Y.; Lee, H.C.; Yang, J.H.; Wei, Y.H. Human skin mitochondrial DNA deletions associated with light exposure. Arch. Biochem. Biophys. 1994, 312, 534–538. [Google Scholar] [CrossRef]
- Addor, F.A.S.A. Beyond photoaging: Additional factors involved in the process of skin aging. Clin. Cosmet. Investig. Dermatol. 2018, 11, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.; Goyarts, E.; Pelle, E.; Trivero, J.; Pernodet, N. Blue light disrupts the circadian rhythm and create damage in skin cells. Int. J. Cosmet. Sci. 2019, 41, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Nabila, Y.A.; Damayanti, D.; Handayani, S.; Setyaningrum, T. The effect of lifestyle on skin aging. Berk. Ilmu Kesehat. Kulit Dan Kelamin 2021, 33, 110–115. [Google Scholar] [CrossRef]
- Suitthimeathegorn, O.; Yang, C.; Ma, Y.; Liu, W. Direct and Indirect Effects of Blue Light Exposure on Skin: A Review of Published Literature. Ski. Pharmacol. Physiol. 2022, 35, 305–318. [Google Scholar] [CrossRef]
- Nakyai, W.; Tissot, M.; Humbert, P.; Grandmottet, F.; Viyoch, J.; Viennet, C. Effects of Repeated UVA Irradiation on Human Skin Fibroblasts Embedded in 3D Tense Collagen Matrix. Photochem. Photobiol. 2018, 94, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Liebel, F.; Kaur, S.; Ruvolo, E.; Kollias, N.; Southall, M.D. Irradiation of skin with visible light induces reactive oxygen species and matrix-degrading enzymes. J. Investig. Dermatol. 2012, 132, 1901–1907. [Google Scholar] [CrossRef]
- Opländer, C.; Hidding, S.; Werners, F.B.; Born, M.; Pallua, N.; Suschek, C.V. Effects of blue light irradiation on human dermal fibroblasts. J. Photochem. Photobiol. B Biol. 2011, 103, 118–125. [Google Scholar] [CrossRef]
- Liebmann, J.; Born, M.; Kolb-Bachofen, V. Blue-light irradiation regulates proliferation and differentiation in human skin cells. J. Investig. Dermatol. 2010, 130, 259–269. [Google Scholar] [CrossRef]
- Latimer, J.; Matts, P.; Diffey, B.; Lloyd, J.; Birch-Machin, M.A. Determination of the action spectrum of UVR-induced mitochondrial DNA damage in human skin cells. J. Investig. Dermatol. 2015, 135, 2512–2518. [Google Scholar] [CrossRef] [PubMed]
- Schrand, A.M.; Lin, J.B.; Hussain, S.M. Assessment of cytotoxicity of carbon nanoparticles using 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) cell viability assay. Methods Mol Biol. 2012, 906, 395–402. [Google Scholar] [PubMed]
- Koch, H.; Wittern, K.-P.; Bergemann, J. In human keratinocytes the Common Deletion reflects donor variabilities rather than chronologic aging and can be induced by ultraviolet A irradiation. J. Investig. Dermatol. 2001, 117, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Lagouge, M.; Larsson, N.G. The role of mitochondrial DNA mutations and free radicals in disease and ageing. J. Intern. Med. 2013, 273, 529–543. [Google Scholar] [CrossRef]
- Smijs, T.G.; Pavel, S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: Focus on their safety and effectiveness. Nanotechnol. Sci. Appl. 2011, 4, 95. [Google Scholar] [CrossRef]
- Summers, B.; Summers, R. The prescribing of sunscreen preparations: A guide for the general practitioner. Off. J. S. Afr. Acad. Fam. Pract. Care. 2012, 8, 440–446. [Google Scholar]
- Bens, G. Sunscreens. Adv. Exp. Med. Biol. 2014, 810, 429–463. [Google Scholar]

| A (Approx 25% Total of Filters Below) | B (Approx 21% Total of Filters Below) | C (Approx 11% Total of Filters Below) | D (Approx 20% Total of Filters Below) | |
|---|---|---|---|---|
| Mineral Filters | Zinc Oxide (UVA + UVB) | Zinc Oxide 5% (UVA + UVB) Titanium Dioxide 6% (UVA + UVB) | Titanium Dioxide (UVA + UVB) | |
| Organic Filters | Homosalate (UVB primarily) Octisalate (UVB primarily) Octocrylene (UVB, short UVA) | Avobenzone (UVA) Octisalate (UVB primarily) Ethylhexyl Triazone (UVB primarily) Octocrylene (UVB, short UVA) Drometrizole Trisiloxane (UVA + UVB) Tinosorb WPGL (UVA + UVB) Tinosorb S (UVA + UVB) | Octinoxate (UVB primarily) Mexoryl XL (UVA + UVB) Diethylamino Hydroxybenzoyl Hexyl Benzoate (UVA) Bemotrizinol (UVA + UVB) | |
| FDA Approved | Yes | No Contains: Ethylhexyl Triazone, Drometrizole Trisiloxane, Tinosorb WPGL, Tinosorb S | Yes | No Contains: Mexoryl XL, Diethylamino Hydroxybenzoyl Hexyl-Benzoate, Bemotrizinol |
| Condition | p-Value |
|---|---|
| 100% exposed vs. 100% covered | 0.0028 ** |
| 100% exposed vs. Sham (SPF0) | 0.5102 |
| 100% exposed vs. Product A | 0.0161 * |
| 100% exposed vs. Product B | 0.0203 * |
| 100% exposed vs. Product C | 0.0157 * |
| 100% exposed vs. Product D | 0.0133 * |
| Unpaired t-Test to Compare to 100% Exposed Sample Where (*): p < 0.05, (**): p < 0.01. | |
|---|---|
| Condition | p-Value |
| 100% Exposed (Tape, no product) vs. Sham (SPF0) | 0.0537 |
| 100% Exposed (Tape, no product) vs. Product B | 0.0134 * |
| 100% Exposed (Tape, no product) vs. Product C | 0.0012 ** |
| Sham (SPF0) vs. Product C | 0.0023 ** |
| Sham (SPF0) vs. Product B | 0.0695 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moor, J.; Bowman, A.; Choudhary, H.; Brookes, J.; Brieva, P.; Birch-Machin, M.A. Sun Protection Products Protect Against UV-Induced Mitochondrial DNA Damage and Blue Light-Induced Cell Decline in Human Dermal Fibroblast Skin Cell Viability. Cosmetics 2025, 12, 128. https://doi.org/10.3390/cosmetics12030128
Moor J, Bowman A, Choudhary H, Brookes J, Brieva P, Birch-Machin MA. Sun Protection Products Protect Against UV-Induced Mitochondrial DNA Damage and Blue Light-Induced Cell Decline in Human Dermal Fibroblast Skin Cell Viability. Cosmetics. 2025; 12(3):128. https://doi.org/10.3390/cosmetics12030128
Chicago/Turabian StyleMoor, Jessica, Amy Bowman, Hina Choudhary, Jonathan Brookes, Patricia Brieva, and Mark Anthony Birch-Machin. 2025. "Sun Protection Products Protect Against UV-Induced Mitochondrial DNA Damage and Blue Light-Induced Cell Decline in Human Dermal Fibroblast Skin Cell Viability" Cosmetics 12, no. 3: 128. https://doi.org/10.3390/cosmetics12030128
APA StyleMoor, J., Bowman, A., Choudhary, H., Brookes, J., Brieva, P., & Birch-Machin, M. A. (2025). Sun Protection Products Protect Against UV-Induced Mitochondrial DNA Damage and Blue Light-Induced Cell Decline in Human Dermal Fibroblast Skin Cell Viability. Cosmetics, 12(3), 128. https://doi.org/10.3390/cosmetics12030128





