Heat-Treated Probiotics’ Role in Counteraction of Skin UVs-Induced Damage In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Heat-Treated Probiotics
2.2. Human Immortalized HaCaT Keratinocyte Cell Line Culture
2.3. Primary Normal Human Epidermal Keratinocytes
2.4. 3D Phenion® Full-Thickness Skin Model
2.5. UVA/UVB Light Damage of HaCaT Cell Line and 3D Phenion® Full-Thickness Skin Model
2.6. Study of Recovery from UVA/UVB Light Damage of HaCatT Cell Line and 3D Phenion® Full-Thickness Skin Model with MTT Assay
2.7. RNA Extraction and Real-Time-PCR of HMOX1, IL-6, and TP53 Genes on 3D Phenion® Full-Thickness Skin Model
2.8. UVC Light Damage of NHEK
2.9. Statistical Analysis
3. Results
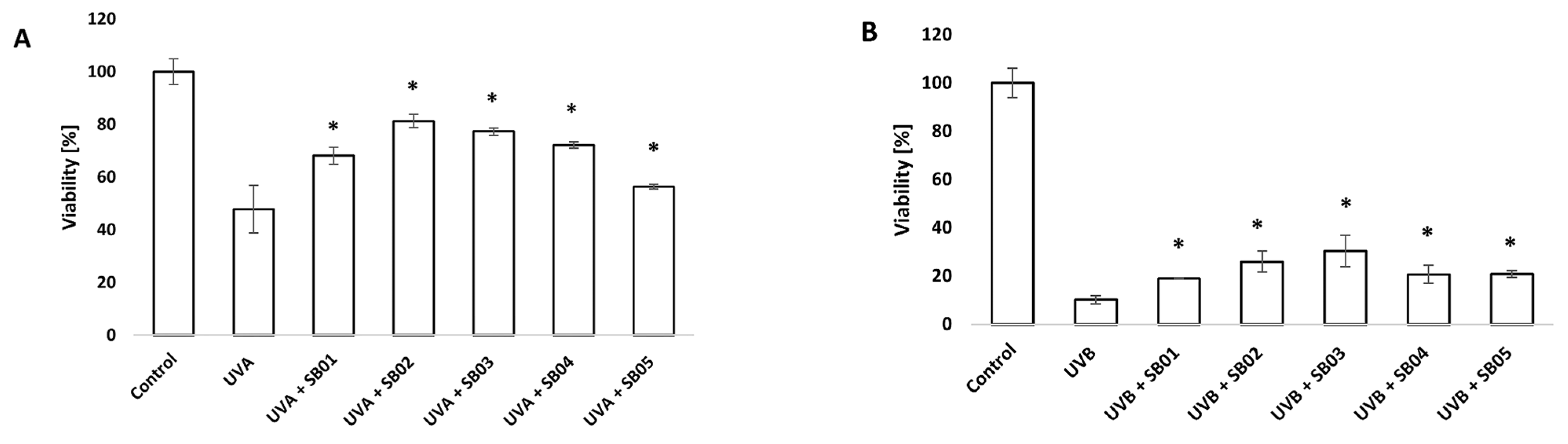
3.1. Study of Recovery from UVA/UVB Light Damage of HaCaT Cell Line with MTT Assay
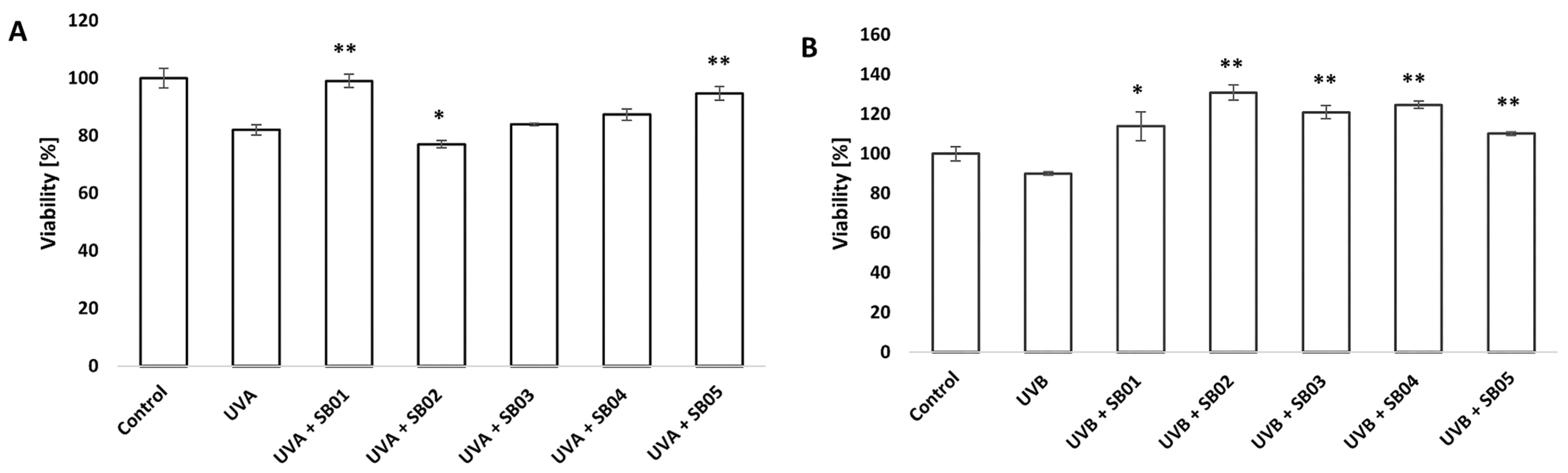
3.2. Study of Recovery from UVA/UVB Light Damage of 3D Phenion® Full-Thickness Skin Model with MTT Assay
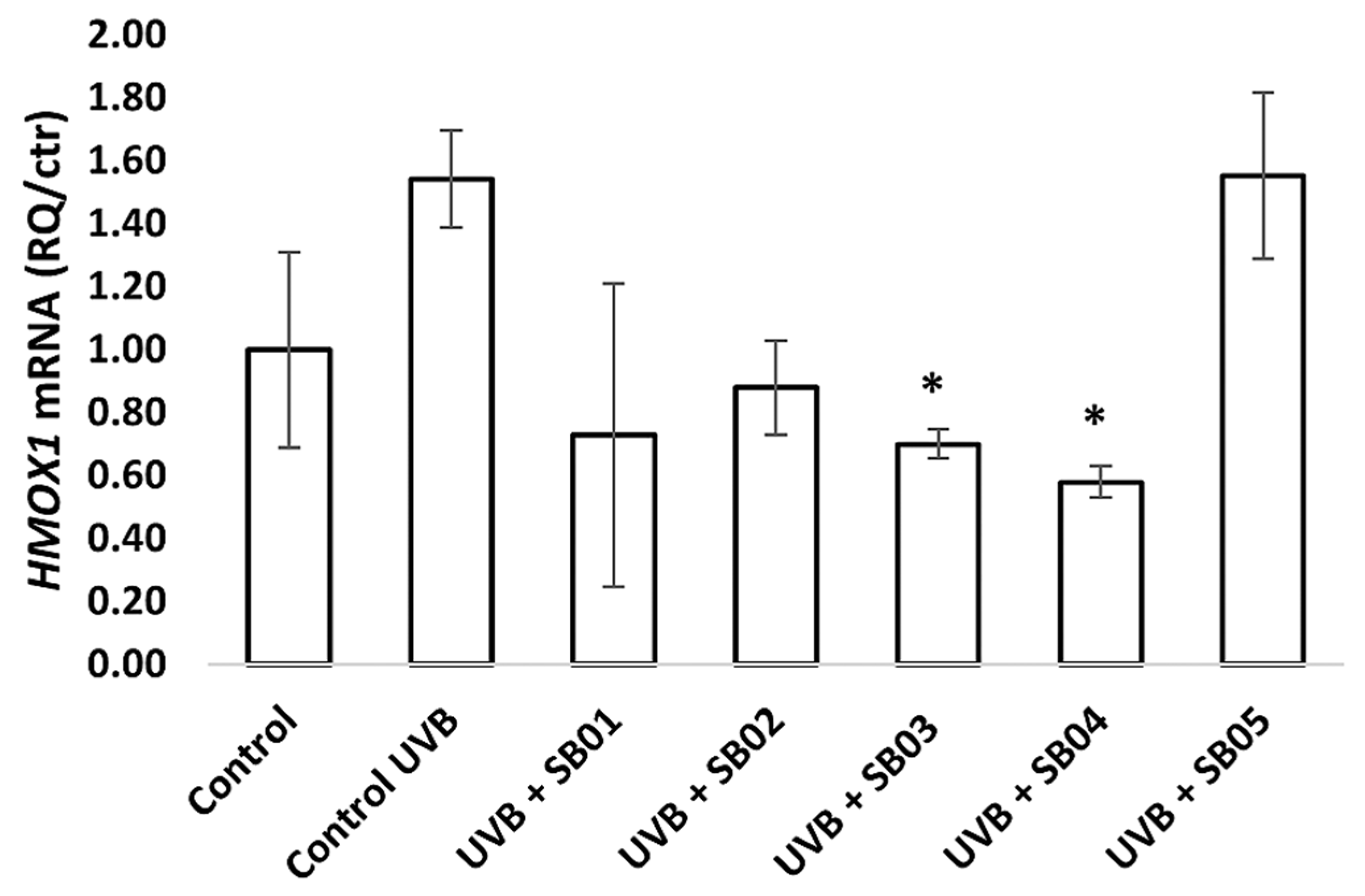
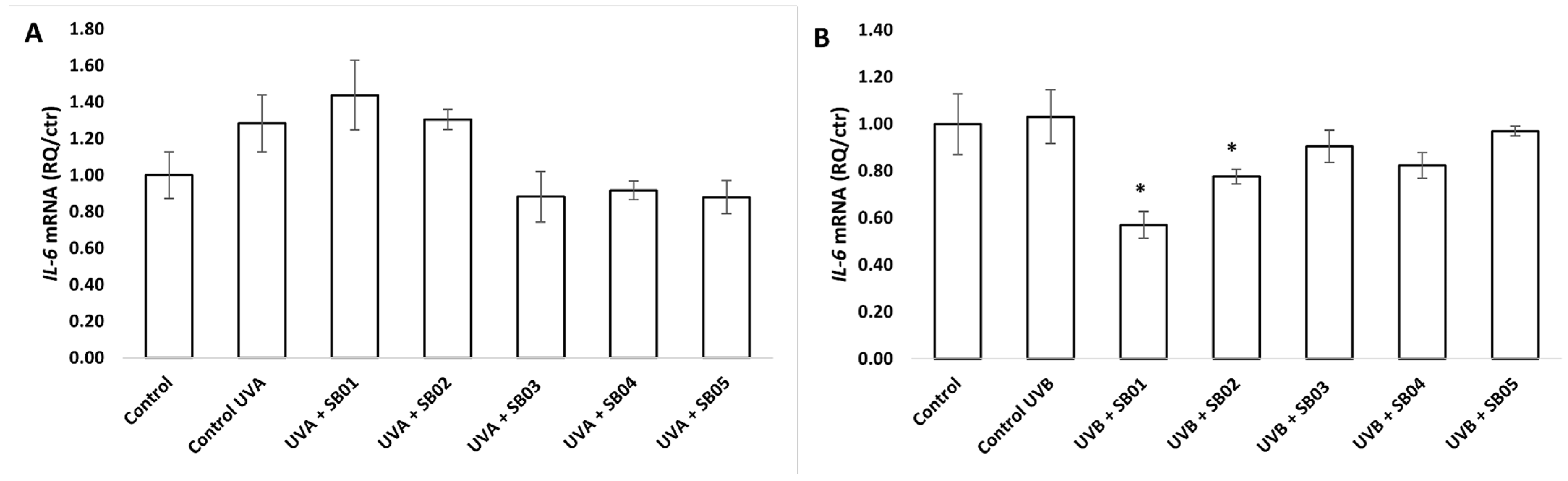
3.3. Gene Expression of HMOX1, IL-6, and TP53 in the 3D Phenion® Full-Thickness Skin Model
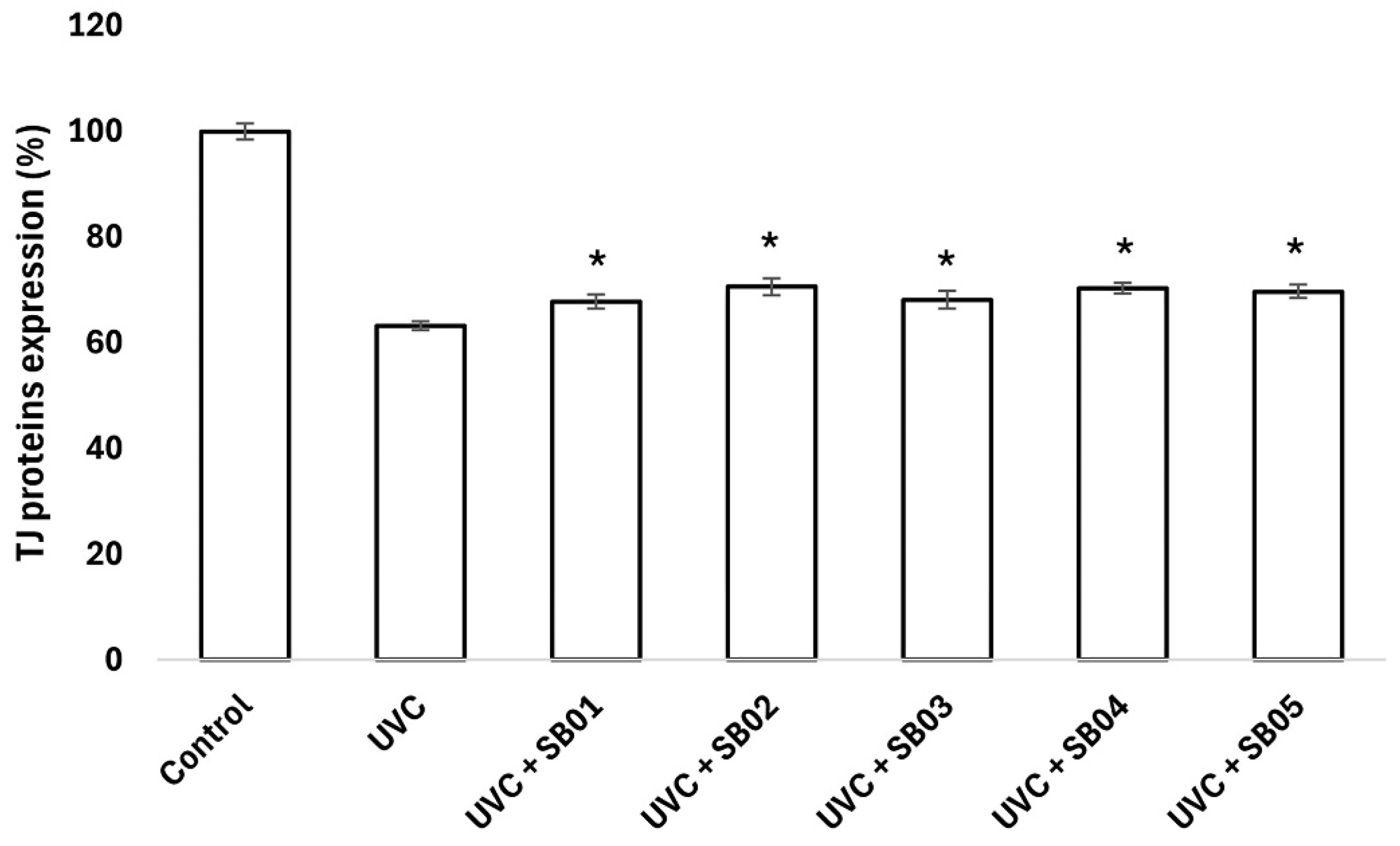
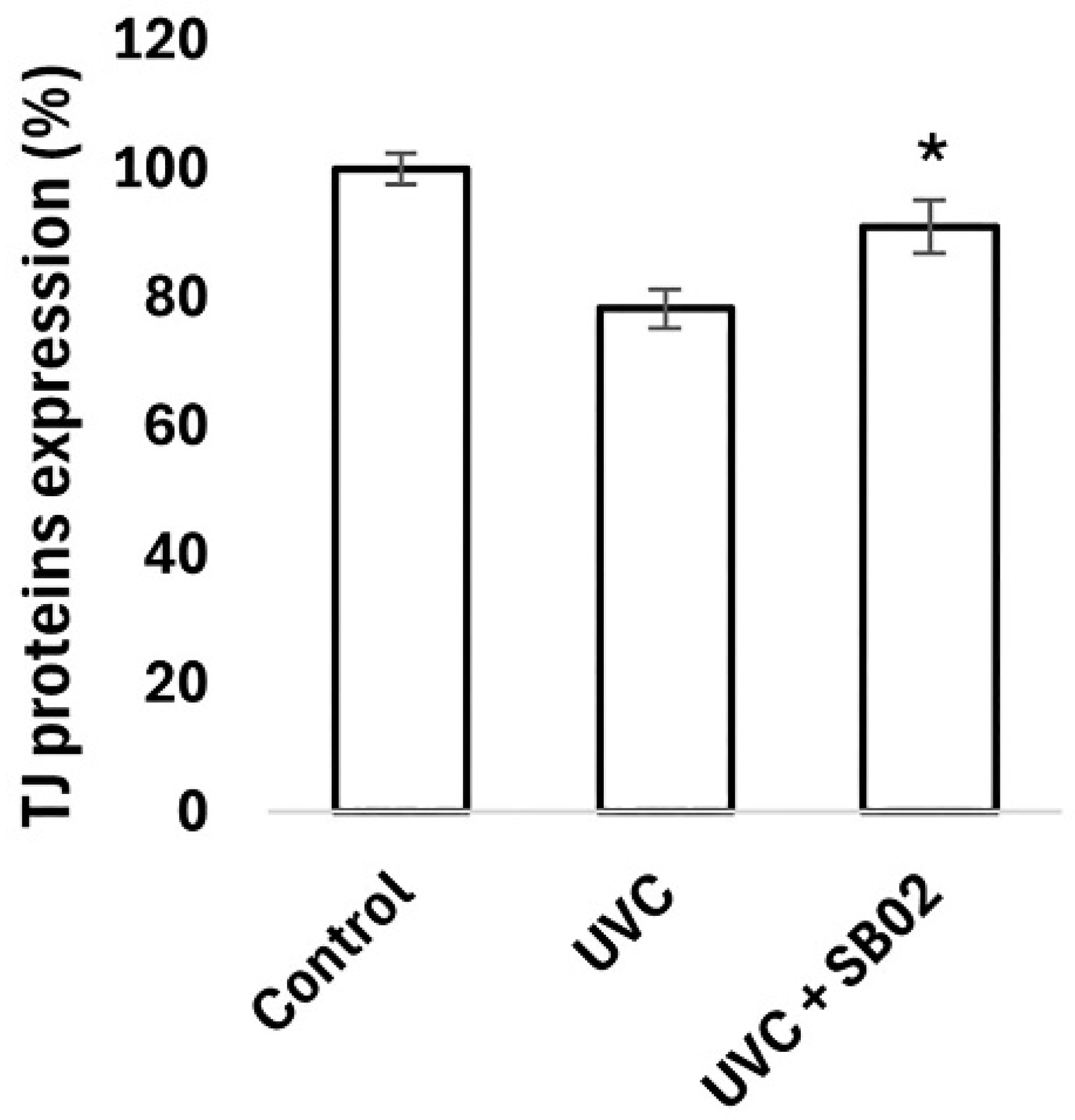
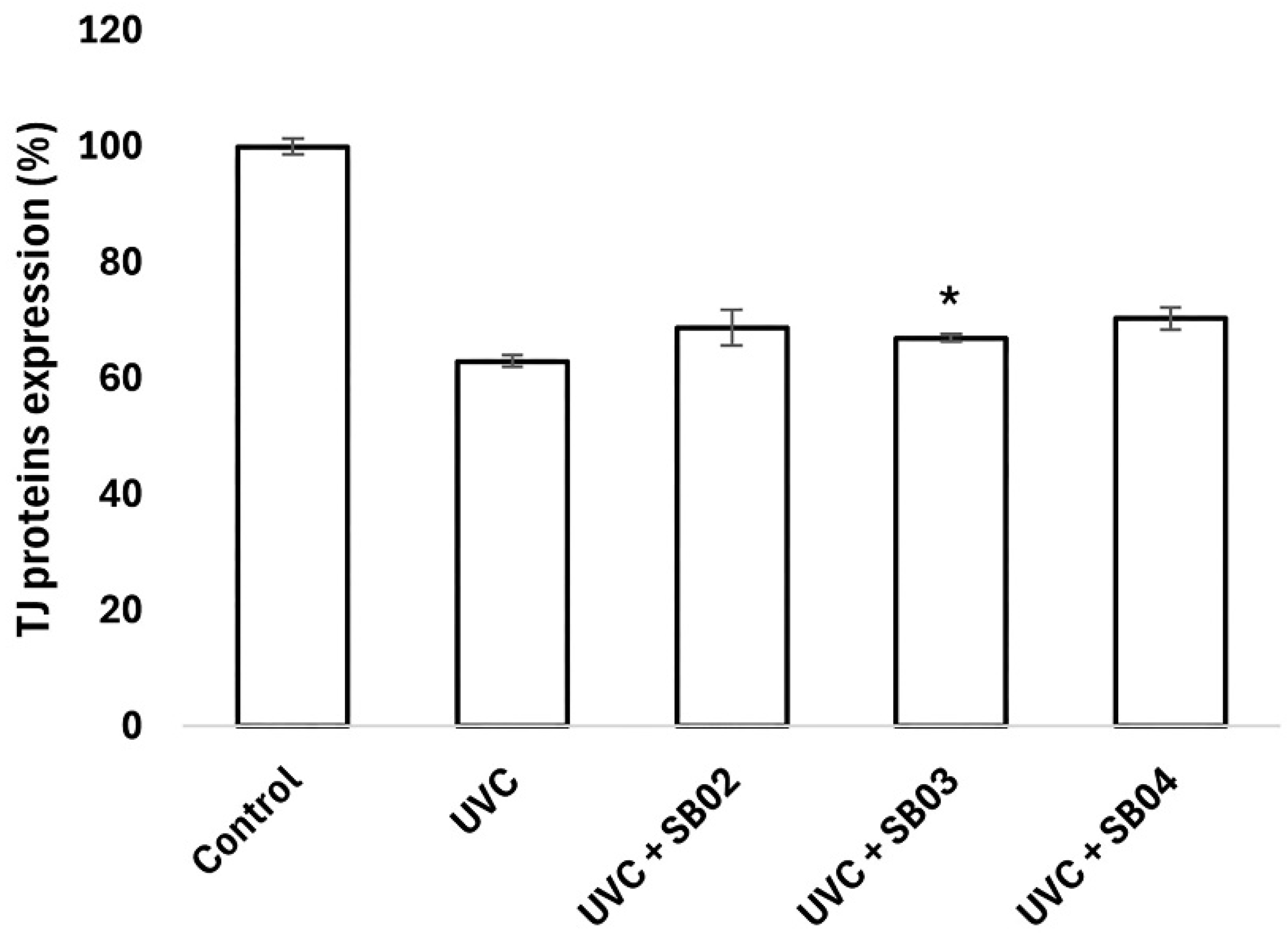
3.4. Study of Protection from UVC Light Damage of NHEK
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, D.; Ai, X.; Li, Y.; Li, Y.; Ao, Y.; Rong, J.; Li, G. Protective effects of Cu/Zn-SOD and Mn-SOD on UVC radiation-induced damage in NIH/3T3 cells and murine skin. Acta Histochem. 2023, 125, 152030. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Bouloc, A.; Sore, G.; Bernard, B.A.; Passeron, T. The skin aging exposome. J. Dermatol. Sci. 2017, 85, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Berman, B.; Cockerell, C.J. Pathobiology of actinic keratosis: Ultraviolet-dependent keratinocyte proliferation. J. Am. Acad. Dermatol. 2013, 68, S10–S19. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, M.; Zhang, Y.; Liang, Y.; Ying, W. NAD+ administration profoundly decreases UVC-induced skin damage by attenuating oxidative stress. Int. J. Physiol. Pathophysiol. Pharmacol. 2023, 15, 41–49. [Google Scholar]
- Pfeifer, G.P. Mechanisms of UV-induced mutations and skin cancer. Genome Instab. Dis. 2020, 1, 99–113. [Google Scholar] [CrossRef]
- Karapetsas, A.; Voulgaridou, G.P.; Konialis, M.; Tsochantaridis, I.; Kynigopoulos, S.; Lambropoulou, M.; Stavropoulou, M.I.; Stathopoulou, K.; Aligiannis, N.; Bozidis, P.; et al. Propolis Extracts Inhibit UV-Induced Photodamage in Human Experimental In Vitro Skin Models. Antioxidants 2019, 8, 125. [Google Scholar] [CrossRef]
- Laga, A.C.; Murphy, G.F. The Translational Basis of Human Cutaneous Photoaging. Am. J. Pathol. 2009, 174, 357–360. [Google Scholar] [CrossRef]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef]
- De Almeida, C.V.; Antiga, E.; Lulli, M. Oral and Topical Probiotics and Postbiotics in Skincare and Dermatological Therapy: A Concise Review. Microorganisms 2023, 11, 1420. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Ácsová, A.; Hojerová, J.; Martiniaková, S. Efficacy of postbiotics against free radicals and UV radiation. Chem. Pap. 2022, 76, 2357–2364. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Rawal, S.; Ali, S.A. Probiotics and postbiotics play a role in maintaining dermal health. Food Funct. 2023, 14, 3966–3981. [Google Scholar] [CrossRef]
- COSSMA. Postbiotics Anti-Ageing Care [Internet]. 2020. Available online: https://www.cossma.com/ingredients/article/postbiotics-anti-ageing-care-36102.html (accessed on 3 December 2024).
- Lu, Y.; Liu, M.; Cao, Y.; Yin, J.; Zhou, H.; Yu, W.; Liu, H.; Wang, J.; Huang, C.; Ma, P.; et al. Hydrogel sunscreen based on yeast /gelatin demonstrates excellent UV-shielding and skin protection performance. Colloids Surf. B Biointerfaces 2021, 205, 111885. [Google Scholar] [CrossRef]
- Chen, H.; Li, Y.; Xie, X.; Chen, M.; Xue, L.; Wang, J.; Ye, Q.; Wu, S.; Yang, R.; Zhao, H.; et al. Exploration of the Molecular Mechanisms Underlying the Anti-Photoaging Effect of Limosilactobacillus fermentum XJC60. Front. Cell. Infect. Microbiol. 2022, 12, 838060. [Google Scholar] [CrossRef]
- Kaur, K.; Rath, G. Formulation and evaluation of UV protective synbiotic skin care topical formulation. J. Cosmet. Laser Ther. 2019, 21, 332–342. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Chomczynski, P.; Mackey, K. Short technical reports. Modification of the TRI reagent procedure for isolation of RNA from polysaccharide- and proteoglycan-rich sources. Biotechniques 1995, 19, 942–945. [Google Scholar]
- Vigetti, D.; Viola, M.; Karousou, E.; Rizzi, M.; Moretto, P.; Genasetti, A.; Clerici, M.; Hascall, V.C.; De Luca, G.; Passi, A. Hyaluronan-CD44-ERK1/2 regulate human aortic smooth muscle cell motility during aging. J. Biol. Chem. 2008, 283, 4448–4458. [Google Scholar] [CrossRef]
- Poss, K.D.; Tonegawa, S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc. Natl. Acad. Sci. USA 1997, 94, 10925–10930. [Google Scholar] [CrossRef]
- Xia, J.; Song, X.; Bi, Z.; Chu, W.; Wan, Y. UV-induced NF-kappaB activation and expression of IL-6 is attenuated by (-)-epigallocatechin-3-gallate in cultured human keratinocytes in vitro. Int. J. Mol. Med. 2005, 16, 943–950. [Google Scholar] [PubMed]
- Soehnge, H.; Ouhtit, A.; Ananthaswamy, O.N. Mechanisms of induction of skin cancer by UV radiation. Front. Biosci. 1997, 2, d538–d551. [Google Scholar] [PubMed]
- Nichols, J.A.; Katiyar, S.K. Skin photoprotection by natural polyphenols: Anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef]
- Souak, D.; Barreau, M.; Courtois, A.; André, V.; Duclairoir Poc, C.; Feuilloley, M.G.J.; Gault, M. Challenging Cosmetic Innovation: The Skin Microbiota and Probiotics Protect the Skin from UV-Induced Damage. Microorganisms 2021, 9, 936. [Google Scholar] [CrossRef]
- The Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef]
- Jain, S.; Prajapat, G.; Abrar, M.; Ledwani, L.; Singh, A.; Agrawal, A. Cyanobacteria as efficient producers of mycosporine-like amino acids. J. Basic Microbiol. 2017, 57, 715–727. [Google Scholar] [CrossRef]
- Nakatsuji, T.; Chen, T.H.; Butcher, A.M.; Trzoss, L.L.; Nam, S.J.; Shirakawa, K.T.; Zhou, W.; Oh, J.; Otto, M.; Fenical, W.; et al. A commensal strain of Staphylococcus epidermidis protects against skin neoplasia. Sci. Adv. 2018, 4, eaao4502. [Google Scholar] [CrossRef]
- Bhatia, N.; Berman, B.; Ceilley, R.I.; Kircik, L.H. Understanding the Role of Photolyases: Photoprotection and Beyond. J. Drugs Dermatol. 2017, 16, 61–66. [Google Scholar]
- Allhorn, M.; Arve, S.; Brüggemann, H.; Lood, R. A novel enzyme with antioxidant capacity produced by the ubiquitous skin colonizer Propionibacterium acnes. Sci. Rep. 2016, 6, 36412. [Google Scholar] [CrossRef]
- Pinto, D.; Trink, A.; Giuliani, G.; Rinaldi, F. Protective Effects of Sunscreen (50+) and Octatrienoic Acid 0.1% in Actinic Keratosis and UV Damages. J. Investig. Med. 2022, 70, 92–98. [Google Scholar] [CrossRef]
- Chen, S.; Wang, X.; Nisar, M.F.; Lin, M.; Zhong, J.L. Heme Oxygenases: Cellular Multifunctional and Protective Molecules against UV-Induced Oxidative Stress. Oxidative Med. Cell. Longev. 2019, 2019, 5416728. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, N.; Brandner, J.M. Barriers and more: Functions of tight junction proteins in the skin. Ann. N. Y. Acad. Sci. 2012, 1257, 158–166. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV Radiation and the Skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, D.L.; Saladi, R.N.; Fox, J.L. Ultraviolet radiation and skin cancer. Int. J. Dermatol. 2010, 49, 978–986. [Google Scholar] [CrossRef]
- Grossman, J. The product stress testing approach. Pharm. Dev. Technol. 2004, 9, 189–194. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mondadori, G.; Amoruso, A.; Visciglia, A.; Deusebio, G.; Pinto, D.; Pane, M.; Rinaldi, F. Heat-Treated Probiotics’ Role in Counteraction of Skin UVs-Induced Damage In Vitro. Cosmetics 2025, 12, 121. https://doi.org/10.3390/cosmetics12030121
Mondadori G, Amoruso A, Visciglia A, Deusebio G, Pinto D, Pane M, Rinaldi F. Heat-Treated Probiotics’ Role in Counteraction of Skin UVs-Induced Damage In Vitro. Cosmetics. 2025; 12(3):121. https://doi.org/10.3390/cosmetics12030121
Chicago/Turabian StyleMondadori, Giorgia, Angela Amoruso, Annalisa Visciglia, Giovanni Deusebio, Daniela Pinto, Marco Pane, and Fabio Rinaldi. 2025. "Heat-Treated Probiotics’ Role in Counteraction of Skin UVs-Induced Damage In Vitro" Cosmetics 12, no. 3: 121. https://doi.org/10.3390/cosmetics12030121
APA StyleMondadori, G., Amoruso, A., Visciglia, A., Deusebio, G., Pinto, D., Pane, M., & Rinaldi, F. (2025). Heat-Treated Probiotics’ Role in Counteraction of Skin UVs-Induced Damage In Vitro. Cosmetics, 12(3), 121. https://doi.org/10.3390/cosmetics12030121





