Abstract
The skin microbiome is a focus for innovative skincare. This study investigated topical semi-solid balm formulations of Micrococcus luteus Q24, a live skin-native probiotic, to enhance skin quality parameters such as hydration, pores, pigmentation, wrinkles and dryness. Firstly, the compatibility and growth-promoting effects of prebiotics and functional actives on M. luteus Q24 were evaluated, identifying oil-based actives, including vitamin E and pomegranate seed oil, that significantly boosted bacterial growth compared to oatmeal, the sole effective prebiotic tested. Subsequently, a pilot cosmetic trial assessed two M. luteus Q24-enriched balms on healthy adults utilising a cutting-edge AI (Artificial Intelligence) driven skin analyser device. Balm B significantly reduced keratin levels, wrinkles, and pore size, and increased hydration, while Balm A effectively reduced spots and keratin. After 4 days of application, Balm A showed mean percentage reductions of 80% in pores, 20% in spots, 60% in wrinkles, and 100% in keratin scores, while Balm B exhibited mean percentage reductions of 100% in pores, 50% in spots, 67% in wrinkles, and 80% in keratin, with a 100% increase in hydration score. Both balms demonstrated compatibility and efficacy, highlighting the potential of M. luteus Q24 in improving skin parameters. These findings suggest that balms optimise the benefits of skin-specific probiotics for microbiome-friendly skincare. Future research with larger, placebo-controlled trials is needed to substantiate these preliminary findings.
1. Introduction
The human skin, our indispensable shield against the relentless external world, endures a constant barrage of environmental assaults and microbial threats [1,2]. This complex and vital barrier, when compromised, triggers a spectrum of skin conditions, impacting not only our physical well-being but also our psychological health, shattering confidence and diminishing quality of life [2,3,4,5]. While conventional treatments often resort to harsh chemicals and pharmaceuticals, a paradigm shift is underway. A burgeoning body of research unequivocally demonstrates the critical importance of maintaining a balanced skin microbiome for optimal health [2,3,6,7,8,9,10,11].
This intricate and dynamic ecosystem, composed of bacteria, fungi, and viruses, acts as a crucial line of defence, repelling harmful agents and regulating essential skin functions [3,4,10,11,12,13,14,15,16]. A dysbiotic microbiome, characterized by a detrimental imbalance in microbial diversity, has been directly implicated in a multitude of skin disorders, propelling us towards microbiome-friendly skincare solutions [3,6,10,11,15,16].
At the forefront of this revolution lies the exploration of topical probiotics—living microorganisms that confer profound health benefits when applied directly to the skin. Micrococcus luteus Q24, a skin-native commensal, has emerged as a promising and powerful candidate, boasting an inherent ability to thrive in the skin’s challenging environment and exhibiting potent antimicrobial activity against pathogenic bacteria [3,10,11,17]. While traditional research has primarily focused on oral administration of prebiotics, probiotics, and postbiotics for gut health, a significant expansion into topical applications for skin health is now underway. Synergistic synbiotics, the potent combination of probiotics and prebiotics, are poised to enhance the efficacy of these topical products dramatically. Furthermore, M. luteus Q24 offers a distinct approach compared to the gut-derived Bifidobacterium and Lactobacillus commonly found in cosmetics [3]. While Bifidobacterium and Lactobacillus are valued for their barrier-strengthening and anti-inflammatory properties, M. luteus Q24 stands out with its targeted antimicrobial action against skin-specific pathogens like C. acnes and S. aureus, and demonstrates efficacy in improving blemishes, hydration, and reducing signs of aging [3,11]. M. luteus Q24’s skin-native origin may confer better skin affinity, and its live delivery offers potential for active microbiome interaction, complementing the broader benefits often associated with other probiotics in promoting overall skin health and balance.
The International Scientific Association for Probiotics and Prebiotics (ISAPP, Sacramento, CA, USA, https://isappscience.org/ (accessed on 16 May 2024)) defines a synbiotic as “a mixture comprising live microorganisms and substrate(s) selectively utilized by host microorganisms that confers a health benefit on the host” [18], further categorizing them into complementary and synergistic forms. M. luteus Q24 has demonstrated remarkable potential in treating skin infections, including impetigo, acne, and tinea [10,11,17,19,20]. To unlock the full potential of a synergistic synbiotic product with M. luteus Q24, identifying prebiotics specifically utilized by this species is critical, as gut-derived prebiotics may prove ineffective [3,21,22].
This study delves into the potential of prebiotics and skin-functional ingredients [23,24,25,26,27,28,29,30] to stimulate the growth of M. luteus Q24. This foundational research lays the groundwork for selecting ingredients that exhibit both functional activity and prebiotic effects with M. luteus Q24. Crucially, subsequent investigations will be required to evaluate the impact of these ingredients on the skin microbiome, antimicrobial activity, and health outcomes. This will pave the way for the development of targeted topical formulations containing M. luteus Q24, prebiotics, and functional ingredients, leading to dramatically improved cosmetics and therapeutic outcomes. Probiotic-based interventions (prebiotics, probiotics, postbiotics, and synbiotics) hold immense promise in alleviating the burden of conventional skin treatments and significantly enhancing overall well-being [23,24,31,32].
However, simply delivering live probiotics is insufficient. A meticulously formulated balm [33], providing a protective and nourishing environment, is absolutely essential for ensuring the viability and efficacy of M. luteus Q24. Furthermore, the inclusion of prebiotics and functional actives can synergistically amplify the balm’s benefits, supporting the growth of beneficial bacteria and providing additional skin-nourishing properties [33].
This study investigates the compatibility of M. luteus Q24 with ingredients such as prebiotics and functional actives, followed by an evaluation of carefully selected ingredients, including flax seed oil, oatmeal, coconut oil, vitamin E, pomegranate seed oil, and olive squalene. These ingredients were chosen for their proven potential to support M. luteus Q24 growth, enhance skin hydration, and improve overall skin quality. It is hypothesised that a powerful synergistic approach, combining live probiotics with supportive prebiotics and functional actives in a specifically designed formulation, will effectively modulate the skin microbiome and deliver significant and demonstrable improvements in skin health. This research aims to provide a robust foundation for developing innovative, microbiome-friendly skincare solutions that address the root causes of skin imbalances and promote long-term skin vitality.
Before exploring the synergistic effects of probiotic and prebiotic combinations, this study first examined the impact of M. luteus Q24 in different semi-solid balm formulations on its own. This study was designed to comprehensively evaluate the potential of a topical probiotic balm featuring M. luteus Q24 to enhance skin health. The primary objectives were threefold: first, to determine the critical compatibility of various prebiotics and functional actives with live probiotic M. luteus Q24, ensuring the selection of ingredients that would not inhibit its viability; second, to rigorously assess the growth-promoting effects of commonly used prebiotics and functional actives, known for their skin benefits, on M. luteus Q24; and third, to conduct a pilot cosmetic trial to quantify demonstrable improvements in skin quality parameters following twice-daily application of an M. luteus Q24-enriched balm to targeted areas in healthy adult participants, utilising cutting-edge AI (Artificial Intelligence) driven skin analyser [11].
2. Materials and Methods
The materials used in this study encompassed a range of cosmetic and personal care ingredients, microbiological supplies, and general laboratory materials. Probiotic M. luteus Q24 (DSMZ 17172) [INCI: Micrococcus, Trehalose] was supplied by Blis Technologies Limited, Dunedin, NZ. Cosmetic ingredients, sourced primarily from Pure Nature (Auckland, NZ), included powders such as allantoin [INCI: Allantoin], aloe vera (200×) [INCI: Aloe Barbadensis Leaf Juice], colloidal oatmeal [Avena Sativa (Oat) Kernel Flour], manuka honey [INCI: Mel], niacinamide [INCI: Niacinamide], green tea [INCI: Camellia Sinensis Leaf Extract] (Lotus Oils, Hawkes Bay, NZ), green and gold kiwifruit [INCI: Actinidia Chinensis (Kiwi) Fruit Powder] (Anagenix, Auckland, NZ), xylitol [INCI: Xylitol] (Roquette, Lestrem, France), and fructooligosaccharides [INCI: Fructooligosaccharides] (Tata Chemicals, Mumbai, India). Oils and liquids sourced from Pure Nature (Auckland, New Zealand) comprised flaxseed [INCI: Linum Usitatissimum Seed Oil], olive squalene [INCI: Squalene], pomegranate seed [INCI: Punica Granatum Seed Oil], calendula [INCI: Calendula Officinalis Flower Extract], sweet almond Prunus Amygdalus Dulcis Oil], hyaluronic acid [INCI: Sodium Hyaluronate], lactic acid [INCI: Lactic acid]. Lumenato tomato oil [INCI: Solanum Lycopersicum (Tomato) Seed Oil] (Lycored, Branchburg, NJ, USA), and fructooligosaccharide liquid [INCI: Fructooligosaccharides] (Tata Chemicals, Mumbai, India). Additional cosmetic components were zinc ricinoleate (INCI: Zinc ricinoleate], vitamin E [INCI: Tocopherol], salicylic acid [INCI: Salicylic acid] (Tigerlillies, Waipukurau, NZ), vitamin C [INCI: Ascorbic acid] (DSM-Firmenich Nutritional Products Ltd., Basel, Switzerland), 1% retinol [INCI: Retinol] in squalene [INCI: Squalene] (The Ordinary, purchased from Farmers, Dunedin, NZ), and Neutrogena Ultra-sheer SPF 50 (Farmers, Dunedin, NZ). Microbiological and cell culture materials, obtained from Fort Richard (Auckland, NZ) and Labsupply (Dunedin, NZ), included yeast extract, various agar plates (CABK12, sheep blood, human blood + calcium), M17 broth, 24-well tissue culture plates, and phosphate buffered saline tablets (Thermofisher, Auckland, NZ). Other materials were potassium alum (University of Otago, Dunedin, NZ) and in-house produced sterile distilled water, created using a Distinction D4000 water distillation unit (Acorn Scientific, Auckland, NZ) and autoclaved (TOMY, San Diego, CA, USA) at 121 °C for 15 min.
Two novel live probiotic Balms were formulated in-house. BALM A contained oily vehicle (INCI: Capric Caprylic Triglyceride), cocoa butter (INCI: Theobroma Cacao Seed Butter) and polysorbate 80 [INCI: Polysorbate 80] (Pure Nature, Auckland, NZ), Imwitor 960K (INCI: Glyceryl Monostearate) (IOI Oleo GmbH, Hamberg, Germany), and M. luteus Q24 (DSMZ 17,172) [INCI: Micrococcus, Trehalose] (Blis Technologies Limited, Dunedin, NZ). and BALM B contained oily vehicle, white bees wax (INCI: Cera Alba), Cetostearyl alcohol (INCI: Cetaryl Alcohol) (Pure Nature, Auckland, NZ), cocoa butter, polysorbate 80 (Pure Nature, Auckland, NZ), and M. luteus Q24. The skin analyser device (dpViso) and software (Dermo Bella Expert Version 3.11.0 PMX) were purchased from Chowis Co. Ltd., Yongin-si, Republic of Korea. A Samsung Tablet 10.1 was purchased from PB Technologies, Auckland, NZ.
2.1. Probiotic-Ingredient Compatibility Study
A modified minimum inhibitory concentration (MIC) assay was conducted to evaluate the antimicrobial activity of various ingredients against M. luteus Q24 (BLIS Q24TM). Freeze-dried M. luteus Q24 powder was resuspended in 10 mL of sterile phosphate-buffered saline (PBS), homogenized, and standardized to an optical density (OD) of 0.125. CABK12 and human blood + calcium (hBaCa) agar plates were uniformly inoculated with the bacterial suspension using sterile swabs. For water-soluble ingredients, serial two-fold dilutions were prepared in sterile water, ranging from 50% to 0.3% (v/v). Oil-based ingredients were tested undiluted (100%). Ten microliters (10 µL) of each dilution or undiluted oil were spotted onto the inoculated agar plates. Additionally, approximately 0.05 g of powdered ingredients were directly applied to the centre of each plate. All plates were incubated aerobically at 37 °C for 20 h without inversion. Antimicrobial activity was determined by the presence of a clear zone of inhibition surrounding the spotted or applied ingredient, indicating inhibition of M. luteus Q24 growth.
2.2. M. luteus Q24 Growth Curves—Prebiotics and Functional Actives
Growth curves of M. luteus Q24 were determined based on live cell count measurements in the presence of ingredients. M17 broth (Difco #218561, Becton Dickinson, Auckland, NZ) was prepared without lactose following the manufacturer’s instructions. A 50 mL broth was dispensed into sterile 100 mL Schott bottles, and the desired percentage of each substance was added and mixed with a magnetic stir bar. The mixtures were autoclaved at 110 °C for 10 min to sterilize and allowed to cool. The suspensions were prewarmed to 40 °C and mixed with a magnetic stirrer immediately before dispensing. A 2 mL sample of each suspension and M17 broth only (control) were dispensed into sterile 24-well tissue culture plates. A suspension of M. luteus Q24 powder was made in sterile PBS and homogenised before adjusting to an optical density of 0.125. A 100 µL aliquot of the M. luteus Q24 suspension was added to each well and mixed by aspirating and dispensing the solution 5 times with a 1 mL pipette. The tissue culture plates were then incubated at 37 °C, 5% CO2 in air for 24 h. During incubation, the wells were mixed periodically by aspiration with a 1 mL pipette. Samples for enumeration were taken at time points 0, 6, 18, and 24 h.
Enumeration was carried out by mixing each well by aspiration and transferring 100 µL of suspension into a 1.5 mL Eppendorf tube containing 900 µL of sterile PBS (1/10 dilution). Each 1/10 dilution was vortexed horizontally for 10 min at 2600 rpm. The dilutions were serially diluted and 20 µL of each dilution, from each suspension, was spot-plated onto a sheep blood agar plate in triplicate. The spots were allowed to dry for 30 min, inverted and incubated aerobically at 37 °C for 40–48 h. The number of colonies in each spot was counted using an electronic colony counter (Q Counter, Spiral Biotech, Norwood, MA, USA) and averaged to give a final M. luteus Q24 live cell count.
2.3. M. luteus Q24 Growth Curves—Prebiotic Dose Response
The experimental protocol described in Section 2.3 was replicated, but instead of a single concentration, a range of percentages for each substance was incorporated into the analysis.
2.4. Cosmetic and Colonisation Efficacy Trial
This cosmetic efficacy study evaluated two topical balms containing live probiotics in healthy adult participants. The balms featured M. luteus Q24, a skin commensal with a documented safety profile [10,11,17] and prior marketing in New Zealand. All participants provided written informed consent, and the study was conducted in accordance with the ethical principles of the Declaration of Helsinki. The study protocol was submitted to the Health and Disability Ethics Committee (HDEC) (New Zealand) for their review of this cosmetic product study. However, the HDEC advised that their review process focuses on research with potential health-related outcomes. Since this study investigates the cosmetic effects of the product, it fell outside the HDEC’s review scope. Therefore, formal ethics committee approval was not required.
2.5. Trial Design
A single-site, single-blinded (investigator), randomised, controlled, parallel trial was conducted involving twelve participants (9 female and 2 male). Participants, aged 18 to 60, were healthy adults with self-reported dry skin and a desire to improve overall skin quality in dry areas of the elbow and chin (concerned areas). Exclusion criteria included open wounds, active infections, autoimmune disease history, and current use of antibiotics, topical steroids, or anti-inflammatory medications. Participants were instructed to apply the balm, containing >1 × 108 CFU/dose (at manufacturing) using their fingertips, twice daily (morning and evening) for seven days to the designated areas as outlined in Figure 1. The sample size for this exploratory pilot study was determined to be sufficient for initial feasibility and effect size assessment to guide future, larger trials, without a formal sample size calculation.
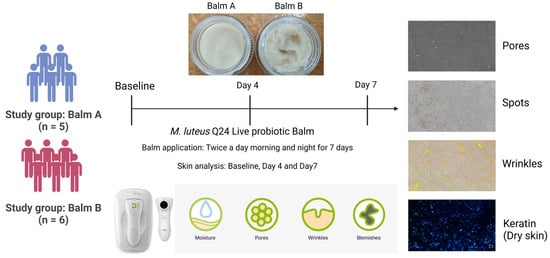
Figure 1.
Study design.
Skin parameter measurements (hydration, pigmentation/spots, wrinkles, and pores) were obtained from the designated concern areas at baseline, day 4, and day 7 using a novel AI-driven skin analysis device [11]. Keratin strip samples were collected to assess dry skin (desquamation). Strips were applied adhesive side down to the target skin area with gentle pressure and left in place for 10 s before removal. The keratin strips were then analysed using the skin analysis device. Participants were also monitored for adverse events throughout the study.
2.6. Measurement of Skin Quality
A handheld skin analyser (dP/Viso, CHOWIS, Yongin-si, Republic of Korea) was used to conduct assessments of skin parameters, including hydration, pore size, spots/pigmentation, and wrinkle depth, across the designated concern areas (chin or elbow). This device employs advanced optical technology with interchangeable lenses and artificial intelligence-driven analysis to provide detailed measurements of various skin quality attributes. Briefly, the AI skin analyser uses a moisture sensor that detects changes in capacitance, which vary depending on the skin’s moisture distribution, to measure hydration. For pores, it identifies darker, round openings, differentiating them from hair using morphology and calculating an index based on size and depth. For spots/pigmentation, it identifies darker (brown) melanin regions by brightness differences, removing hair, and calculating an index based on darkness compared to the surroundings. Wrinkles are identified by brightness differences as dark lines with a defined length and depth, with an index calculated from these dimensions. Keratin is measured by applying a special tape to the skin, capturing dead skin cells (keratin) on it. An image analysis algorithm then quantifies the amount of keratin on the tape by detecting and measuring the strong white areas. Triplicate readings were obtained from a consistent location at each time point. Data were captured as numerical scores and digital images and subsequently analysed using the Dermo Bella Expert Version 3.11.0 PMX application. Testing with the analyser was conducted under controlled environmental conditions, specifically at ambient room temperature, during consistent daylight hours, and with standardized lighting to minimise variability.
2.7. Statistical Analysis of Skin Analyser Data
Changes in skin parameters over time were statistically analysed using one-way repeated measures ANOVA. Data has been presented as graphs generated with Microsoft Excel and Prism 10.4.1 (GraphPad Software).
3. Results
3.1. Probiotic-Ingredient Compatibility Study
Thirty-one ingredients were screened for compatibility with probiotic M. luteus Q24. This included 23 functional actives, 5 conventional prebiotics, and 3 substances classified as both. Of these, green tea extract was the only substance found to be incompatible, demonstrating complete inhibition of M. luteus Q24 growth across all tested concentrations (100% to 0.3% w/w). Seven ingredients—hyaluronic acid, gold and green kiwifruit extracts, lactic acid, aloe vera, potassium alum, and salicylic acid—inhibited M. luteus Q24 growth only at high, cosmetically irrelevant concentrations. Therefore, these seven substances remain potentially suitable for prebiotic-only or synbiotic formulations (Supplementary Table S1).
3.2. M. luteus Q24 Growth Curves—Prebiotics and Functional Actives
While oatmeal, the only effective prebiotic of the four tested, increased M. luteus Q24 viable cell counts 13-fold within 24 h, oil-based functional actives exhibited a significantly greater impact (Figure 2). Specifically, vitamin E, cocoa butter, pomegranate seed oil, grape seed oil, olive squalene, olive oil, and flax seed oil boosted M. luteus growth by 18 to 224-fold over the same incubation period. Thus, these functional actives present a strong opportunity for inclusion in M. luteus Q24 formulations, aiming for positive outcomes in skin quality enhancement.
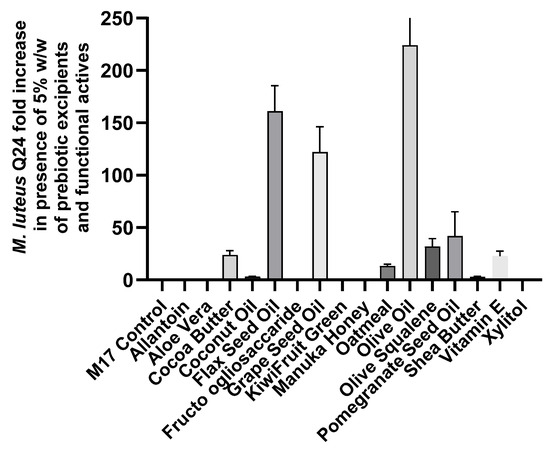
Figure 2.
Effect of prebiotic ingredients and functional actives on the growth of M. luteus Q24 after incubation at 37 °C, 5% CO2 for 24 h. Data points are means (n = 3 ± SD).
3.3. M. luteus Q24 Growth Curves—Prebiotic Dose Response
The growth curves revealed surprising concentration-dependent effects of excipients on M. luteus Q24 (Figure 3). Pomegranate seed oil, oatmeal, olive squalene, and flax seed oil unexpectedly enhanced M. luteus growth throughout the study, suggesting prebiotic potential despite their oily nature. Vitamin E showed an optimal growth-promoting concentration of 0.6%. Conversely, coconut oil displayed a biphasic response, initially stimulating growth before inhibiting it, suggesting antibacterial properties.
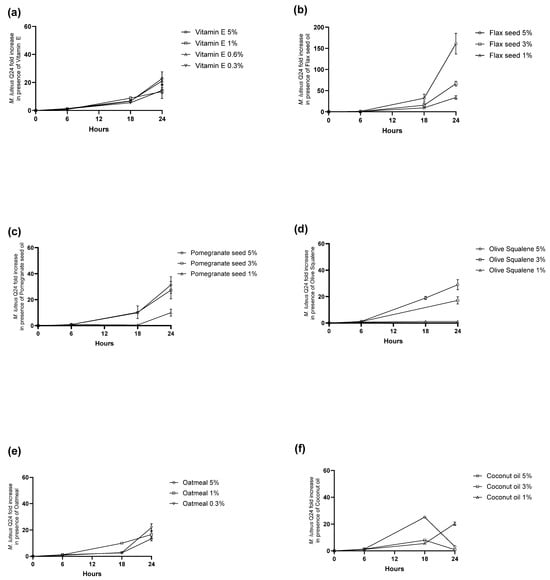
Figure 3.
Dose–response relationship of prebiotic ingredients and functional actives (a) Vitamin E (b) Flaxseed Oil (c) Pomegranate Seed Oil (d) Olive Squalene (e) Oatmeal (f) Coconut Oil on the growth of M. luteus Q24 after incubation at 37 °C, 5% CO2 for 24 h. Data points are means (n = 3 ± SD).
3.4. Cosmetic Efficacy Trial
Skin Quality Measurement
This pilot study evaluated the efficacy of two balms, A and B, on several skin parameters over seven days. Both formulations showed beneficial effects but with distinct profiles. Balm B significantly reduced keratin levels (p < 0.0001), wrinkles (p < 0.01), and pore size (p < 0.05), and also promoted a notable increase in hydration (Figure 4). Balm A, on the other hand, demonstrated a significant reduction in keratin (p < 0.001) and spots (p < 0.05), along with an increase in hydration. Balm B demonstrated a more consistent improvement across a wider range of parameters, while Balm A was particularly effective in reducing spots. Participants experienced no adverse events associated with the product.
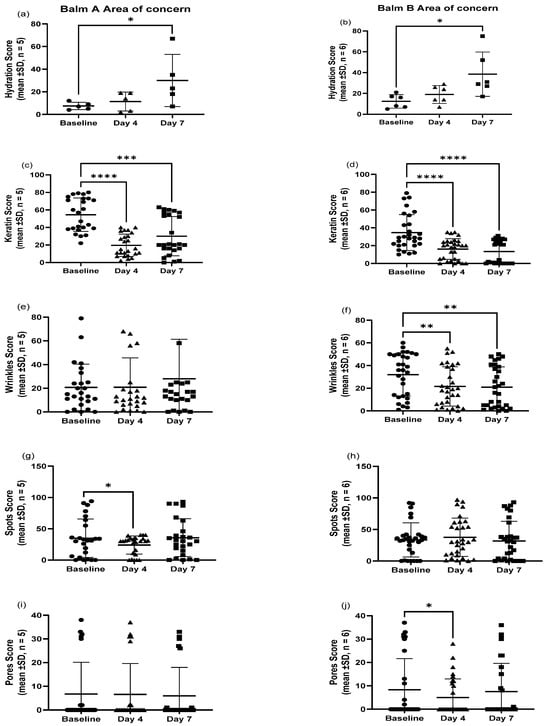
Figure 4.
Influence of M. luteus Q24 on the skin quality parameter scores of (a) hydrations, (c) keratin, (e) wrinkles, (g) spots, and (i) pores for Balm A and (b) hydrations, (d) keratin, (f) wrinkles, (h) spots, and (j) pores for Balm B following twice-daily application of live probiotic balms on the area of concern of adult participants. Statistical significance is indicated by the p-value, with lower p-values reflecting stronger evidence. p ≤ 0.05 (statistically significant *), p ≤ 0.01 (highly significant **) p ≤ 0.001 (very highly significant ***), and p ≤ 0.0001 (extremely significant ****).
Figure 5 illustrates the percentage change in skin quality parameters relative to baseline following twice-daily application of live probiotic balms (A and B) on the area of concern of adult participants. Both balms generally show a positive trend, with improvements observed across most parameters. Hydration demonstrates an increase for both balms, though the change is more pronounced for Balm B. Keratin levels show a substantial decrease for both balms, indicating smoother skin texture. Wrinkles, spots, and pores all show reductions, suggesting improvements in these areas. Specifically, compared to the baseline, after only 4 days of application, Balm A showed mean percentage reductions of 80% in pores, 20% in spots, 60% in wrinkles, and 100% in keratin scores. Balm B, after 4 days, showed mean percentage reductions of 100% in pores, 50% in spots, 67% in wrinkles, and 80% in keratin scores, along with a 100% increase in the hydration score. However, the magnitude of change varies between the balms and across the different parameters, indicating that the balms have differing effects on specific aspects of skin quality.
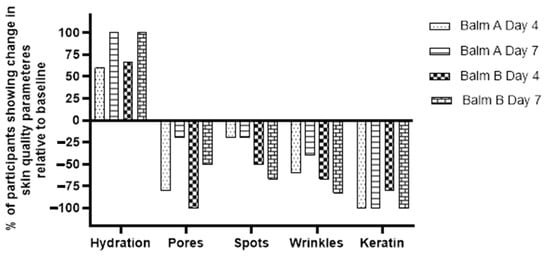
Figure 5.
Percentage change in score of skin quality parameters relative to baseline following twice-daily application of live probiotic balms (A n = 5 or B n = 6) on the area of concern of adult participants.
4. Discussion
The screening of thirty-one ingredients revealed a nuanced compatibility profile with M. luteus Q24. While green tea extract exhibited complete growth inhibition, seven other substances were only inhibitory at high, non-cosmetic concentrations, suggesting their potential in prebiotic-only or synbiotic formulations. Notably, oil-based functional actives demonstrated a significantly greater growth-promoting effect than conventional prebiotics like oatmeal, with vitamin E, cocoa butter, and several seed oils boosting M. luteus Q24 viability significantly. The growth curves also unveiled concentration-dependent effects and potential prebiotic properties in certain oily substances. Moving forward, selected substances and specific concentrations will undergo rigorous triplicate testing to confirm these initial findings before their incorporation into the final balm formulation.
The complex interplay of microorganisms residing on human skin, the skin microbiome, is crucial for skin health [2,3,6,15]. Maintaining this delicate balance is paramount, and topical formulations play a vital role in supporting a healthy microbiome. While serums have demonstrated certain benefits [10,11,17], a probiotic enriched balm [33], designed to nourish and protect, offers distinct advantages. The inherent occlusive nature of balms creates a rich, emollient barrier that effectively locks in moisture, preventing trans epidermal water loss, and establishes an ideal microenvironment for beneficial microbes, particularly the probiotic strain M. luteus Q24 [20,33]. This aligns perfectly with the skin microbiome’s dynamic [3,10,11] and intricately balanced ecosystem [23,32,34,35,36], which plays a pivotal role in maintaining skin homeostasis, acting as a vital first line of defence against external aggressors and contributing to essential skin functions such as barrier integrity and immune modulation. Maintaining this delicate equilibrium is paramount for achieving optimal skin health, and topical formulations have emerged as powerful tools for supporting a thriving microbiome [10,11,17].
M. luteus Q24, a probiotic strain naturally found on healthy skin, has a well-established safety profile [10,20], its effective antimicrobial activity against key skin pathogens [20,33], and its significant anti-inflammatory and skin rejuvenation benefits [20], highlight the clinical significance of an M. luteus Q24-enriched formulation as a microbiome-friendly cosmetic. M. luteus Q24 also demonstrated remarkable abilities to enhance skin moisture and improve overall skin quality [10,11,17]. It achieves this by interacting with skin layers, promoting hydration, and repairing the skin barrier. A balm formulation, enriched with M. luteus Q24, amplifies these benefits. The occlusive nature of the balm creates a protective layer, further preventing epidermal water loss and maintaining skin integrity. This is particularly advantageous for delivering M. luteus and its active metabolites deep into skin tissues, where they can positively influence skin barrier function, reduce pore size, and minimize wrinkles. M. luteus Q24, a commensal bacterium naturally present on healthy human skin, has demonstrated remarkable potential in enhancing skin hydration and significantly improving overall skin quality [10,11,17]. This probiotic strain interacts synergistically with skin cells, stimulating the production of essential components of the skin barrier, such as ceramides and hyaluronic acid, thereby promoting enhanced hydration and robust repair [11,19,33]. Furthermore, it has been shown that M. luteus Q24 significantly increases its relative abundance on the skin, as shown by WGS analysis of skin swabs [11]. These findings indicated that topical application of this probiotic in a serum formulation offers benefits for improving skin health quality.
The efficacy of M. luteus Q24 in improving skin hydration, and reducing wrinkles, pores, spots and keratin (dry skin), that has been previously reported [10,11,17], was evident again in this study. Advanced skin analysis techniques confirmed these improvements, demonstrating the potential of M. luteus Q24 to combat signs of skin fatigue and aging. Furthermore, as reported earlier [10,11,17] whole-genome sequencing validated the microbiome modulation mediated by M. luteus Q24 application, showing a significant increase in its relative abundance. This increase coincided with the observed improvements in skin quality, suggesting a direct link between M. luteus Q24 and the enhanced skin parameters [37,38]. Utilizing advanced skin analysis techniques, including capacitance measurements for hydration and high-resolution optical imaging for pore size and wrinkle depth, the study demonstrated significant and measurable improvements in key skin health indicators. These compelling findings underscore the significant potential of M. luteus Q24 to effectively combat the visible signs of skin fatigue and aging. This study would have been improved if a comprehensive whole-genome sequencing of skin swab samples was conducted. This would have provided more compelling evidence of microbiome modulation mediated by M. luteus Q24 application similar to that reported previously [11], where a quantifiable increase in the relative abundance of M. luteus Q24 was correlated directly with the improvements in skin quality parameters, reinforcing the direct and causal link between the probiotic strain and the enhanced skin characteristics [11]. The improvement in key skin quality parameters in this study conclusively demonstrates the balm’s effectiveness as a stable and reliable delivery method for M. luteus Q24 to thrive and exert its beneficial effects on the skin.
Unlike gut-associated probiotics, which may offer transient benefits but struggle to establish long-term colonization on the skin’s unique environment, M. luteus Q24, being a skin-specific probiotic [3], thrives in its natural habitat, resulting in a more enduring and profound positive impact on overall skin health and the development of a resilient skin barrier [11,20,33]. The balm’s formulation also aids in M. luteus Q24’s adherence to the skin’s surface and effectively shields it from potentially disruptive external environmental factors.
Despite the pilot study’s limitations, such as the lack of a placebo arm and a limited participant pool, its findings are promising. However, promising preliminary exploratory findings necessitate a future, randomised, controlled, double-blind clinical trial to establish the efficacy of this innovative probiotic balm. This trial should incorporate placebo controls and larger cohorts while exploring the impact of extended application periods and an improved formulation to generate more robust and comprehensive evidence. A further limitation of the present study is the lack of direct comparisons between the dP/Viso instrument and established skin health measurement tools such as corneometry, although an internal validation report was provided confidentially by the supplier (www.chowis.com), and our instrument use and data analysis were consistent with previous research [11]. Moving forward, these future trials, coupled with efforts to validate measurement techniques, will be critical for a more nuanced understanding of the complex interplay between M. luteus Q24, the balm formulation, and the skin microbiome, ultimately leading to targeted and personalized skincare interventions [10,11].
5. Conclusions
This research successfully demonstrated the feasibility and efficacy of a topical balm enriched with M. luteus Q24 for enhancing skin health. The study established the compatibility of various ingredients, including prebiotics and functional actives, with M. luteus Q24, and identified substances that effectively promoted its growth. The subsequent pilot cosmetic efficacy trial confirmed the balm’s ability to significantly improve skin hydration and reduce visible signs of aging, highlighting its potential as a microbiome-friendly cosmetic product. While acknowledging the limitations of the current study, these findings provide a strong foundation for future research. Specifically, larger, placebo-controlled trials are warranted to further validate the observed benefits and explore the long-term effects of M. luteus Q24-enriched balms on the skin microbiome. Future research should also consider potential improvements to the formulation for optimal delivery and explore broader clinical applications for managing skin health. Ultimately, this research contributes valuable insights towards the development of targeted skincare solutions that leverage the synergistic effects of probiotics, prebiotics, and functional actives to promote and maintain a healthy skin ecosystem.
6. Patents
Some of the work included in this paper has been part of the provisional patent WO2024047589 Topical compositions of Micrococcus luteus Q24 and use thereof [33].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cosmetics12030105/s1, Table S1: M. luteus Q24 compatibility with various tested substances.
Author Contributions
Conceptualization, S.A.M., S.S.S., R.J., A.L.V. and J.D.F.H.; methodology, S.A.M., S.S.S., R.J., A.L.V. and J.D.F.H.; formal analysis, S.A.M. and A.L.V.; investigation, S.A.M., S.S.S., A.L.V. and R.J.; writing—original draft preparation, R.J. and A.L.V.; writing—review and editing, S.A.M., S.S.S., R.J., A.L.V. and J.D.F.H.; project administration, J.D.F.H. and R.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This cosmetic product study was conducted following the ethical principles of the Declaration of Helsinki. We submitted the study protocol to the Health and Disability Ethics Committee (HDEC) (New Zealand) for review. However, the ethical review and approval were waived for this study as the review board advised that since the scope of this study is cosmetic-based rather than directed at health-related outcomes, it is outside of the scope of HDEC review and that formal ethical review and approval were not required for this study.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study, and written informed consent was obtained from the participants(s) to publish this paper.
Data Availability Statement
The data presented in this study is available on request from the corresponding author. The data is not publicly available due to restrictions, e.g., information that could compromise the privacy of research participants.
Conflicts of Interest
The authors are employees of Blis Technologies Limited, Dunedin, New Zealand. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Lax, S.; Smith, D.P.; Hampton-Marcell, J.; Owens, S.M.; Handley, K.M.; Scott, N.M.; Gibbons, S.M.; Larsen, P.; Shogan, B.D.; Weiss, S.; et al. Longitudinal analysis of microbial interaction between humans and the indoor environment. Science 2014, 345, 1048–1052. [Google Scholar] [CrossRef] [PubMed]
- Dréno, B.; Araviiskaia, E.; Berardesca, E.; Gontijo, G.; Sanchez Viera, M.; Xiang, L.F.; Martin, R.; Bieber, T. Microbiome in healthy skin, update for dermatologists. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 2038–2047. [Google Scholar] [CrossRef] [PubMed]
- McLoughlin, I.J.; Wright, E.M.; Tagg, J.R.; Jain, R.; Hale, J.D.F. Skin Microbiome—The Next Frontier for Probiotic Intervention. Probiotics Antimicrob. Proteins 2022, 14, 630–647. [Google Scholar] [CrossRef] [PubMed]
- Scharschmidt, T.C.; Fischbach, M.A. What lives on our skin: Ecology, genomics and therapeutic opportunities of the skin microbiome. Drug Discov. Today Dis. Mech. 2013, 10, e83–e89. [Google Scholar] [CrossRef]
- Skowron, K.; Bauza-kaszewska, J.; Kraszewska, Z.; Wiktorczyk-kapischke, N.; Grudlewska-buda, K.; Kwiecińska-piróg, J.; Wałecka-zacharska, E.; Radtke, L.; Gospodarek-komkowska, E. Human skin microbiome: Impact of intrinsic and extrinsic factors on skin microbiota. Microorganisms 2021, 9, 543. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.H. Skin microbiome: Genomics-based insights into the diversity and role of skin microbes. Trends Mol. Med. 2011, 17, 320–328. [Google Scholar] [CrossRef]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef]
- Wallen-Russell, C.; Wallen-Russell, S. Meta Analysis of Skin Microbiome: New Link between Skin Microbiota Diversity and Skin Health with Proposal to Use This as a Future Mechanism to Determine Whether Cosmetic Products Damage the Skin. Cosmetics 2017, 4, 14. [Google Scholar] [CrossRef]
- Oh, J.; Byrd, A.L.; Park, M.; Kong, H.H.; Segre, J.A. Temporal Stability of the Human Skin Microbiome. Cell 2016, 165, 854–866. [Google Scholar] [CrossRef]
- Jain, R.; Voss, A.L.; Tagg, J.R.; Hale, J.D.F. Evaluation of the Preliminary Safety, Tolerability and Colonisation Efficacy of Topical Probiotic Formulations Containing Micrococcus luteus Q24 in Healthy Human Adults. Cosmetics 2022, 9, 121. [Google Scholar] [CrossRef]
- McLoughlin, I.J.; Voss, A.L.; Hale, J.D.F.; Jain, R. Cosmetic Efficacy of the Topical Probiotic Micrococcus luteus Q24 in Healthy Human Adults. Cosmetics 2024, 11, 122. [Google Scholar] [CrossRef]
- França, K. Topical Probiotics in Dermatological Therapy and Skincare: A Concise Review. Dermatol. Ther. 2021, 11, 71–77. [Google Scholar] [CrossRef]
- Kloos, W.E.; Musselwhite, M.S. Distribution and persistence of Staphylococcus and Micrococcus species and other aerobic bacteria on human skin. Appl. Microbiol. 1975, 30, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.M.; Nelson, A.M. Skin microbiota: Friend or foe in pediatric skin health and skin disease. Pediatr. Dermatol. 2019, 36, 815–822. [Google Scholar] [CrossRef]
- Cooper, A.J.; Weyrich, L.S.; Dixit, S.; Farrer, A.G. The skin microbiome: Associations between altered microbial communities and disease. Australas. J. Dermatol. 2015, 56, 268–274. [Google Scholar]
- Maguire, M.; Maguire, G. The role of microbiota, and probiotics and prebiotics in skin health. Arch. Dermatol. Res. 2017, 309, 411–421. [Google Scholar] [CrossRef]
- Jain, R.; Voss, A.L.; Del Rosario, J.; Hale, J.D.F. Efficacy of a topical live probiotic in improving skin health. Int. J. Cosmet. Sci. 2025, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Hale, J.D.F.; Jain, R.; Voss, A.L. Topical Composition and Use Thereof. U.S. Patent Application US20240156718A1, 16 May 2024. [Google Scholar]
- WO2006104403A1—Skin Treatment Compositions—Google Patents. Available online: https://patents.google.com/patent/WO2006104403A1/en (accessed on 30 September 2024).
- Cani, P.D. Human gut microbiome: Hopes, threats and promises. Gut 2018, 67, 1716–1725. [Google Scholar] [CrossRef]
- Deehan, E.C.; Duar, R.M.; Armet, A.M.; Perez-Muñoz, M.E.; Jin, M.; Walter, J. Modulation of the Gastrointestinal Microbiome with Nondigestible Fermentable Carbohydrates To Improve Human Health. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Lolou, V.; Panayiotidis, M.I. Functional Role of Probiotics and Prebiotics on Skin Health and Disease. Fermentation 2019, 5, 41. [Google Scholar] [CrossRef]
- Al-Ghazzewi, F.H.; Tester, R.F. Impact of prebiotics and probiotics on skin health. Benef. Microbes 2014, 5, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Criquet, M.; Bertin, C.; Roure, R.; Dayan, L.; Nollent, V. Safety and efficacy of personal care products containing colloidal oatmeal. Clin. Cosmet. Investig. Dermatol. 2012, 5, 183–193. [Google Scholar] [CrossRef]
- Varma, S.R.; Sivaprakasam, T.O.; Arumugam, I.; Dilip, N.; Raghuraman, M.; Pavan, K.B.; Rafiq, M.; Paramesh, R. In vitro an-ti-inflammatory and skin protective properties of Virgin coconut oil. J. Tradit. Complement Med. 2018, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.L.; Valente, D.; Moreira, H.R.; Pintado, M.; Costa, P. Effect of squalane-based emulsion on polyphenols skin penetration: Ex vivo skin study. Colloids Surf. B Bio interfaces 2022, 218, 112779. [Google Scholar] [CrossRef]
- Dimitrijevic, J.; Tomovic, M.; Bradic, J.; Petrovic, A.; Jakovljevic, V.; Andjic, M.; Živković, J.; Milošević, S.Đ.; Simanic, I.; Dragicevic, N. Punica granatum L. (Pomegranate) Extracts and Their Effects on Healthy and Diseased Skin. Pharmaceutics 2024, 16, 458. [Google Scholar] [CrossRef]
- Goyal, A.; Sharma, V.; Upadhyay, N.; Gill, S.; Sihag, M. Flax and flaxseed oil: An ancient medicine & modern functional food. J. Food Sci. Technol. 2014, 51, 1633–1653. [Google Scholar]
- Keen, M.A.; Hassan, I. Vitamin E in dermatology. Indian Dermatol Online J. 2016, 7, 311. [Google Scholar] [CrossRef]
- Rawal, S.; Ali, S.A. Probiotics and postbiotics play a role in maintaining dermal health. Food Funct. 2023, 14, 3966–3981. [Google Scholar] [CrossRef]
- De Almeida, C.V.; Antiga, E.; Lulli, M. Oral and Topical Probiotics and Postbiotics in Skincare and Dermatological Therapy: A Concise Review. Microorganisms 2023, 11, 1420. [Google Scholar] [CrossRef]
- WO2024047589. Topical Compositions of Micrococcus Luteus Q24 and Use Thereof. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2024047589 (accessed on 30 September 2024).
- Xu, H.; Li, H. Acne, the Skin Microbiome, and Antibiotic Treatment. Am. J. Clin. Dermatol. 2019, 20, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef] [PubMed]
- Huttenhower, C.; Gevers, D.; Knight, R.; Abubucker, S.; Badger, J.H.; Chinwalla, A.T.; Creasy, H.H.; Earl, A.M.; Fitzgerald, M.G.; Fulton, R.S.; et al. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar]
- Draelos, Z.D. Proper Skin Hydration and Barrier Function. In Nutritional Cosmetics; Beauty from Within; William Andrew Publishing: Norwich, NY, USA, 2009; pp. 355–363. [Google Scholar]
- Purnamawati, S.; Indrastuti, N.; Danarti, R.; Saefudin, T. The Role of Moisturizers in Addressing Various Kinds of Dermatitis: A Review. Clin. Med. Res. 2017, 15, 75–87. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).