Marine Algae Extract-Loaded Nanoemulsions: A Spectrophotometric Approach to Broad-Spectrum Photoprotection
Abstract
1. Introduction
2. Materials and Methods
2.1. Algae Harvesting
2.2. Extract Preparation
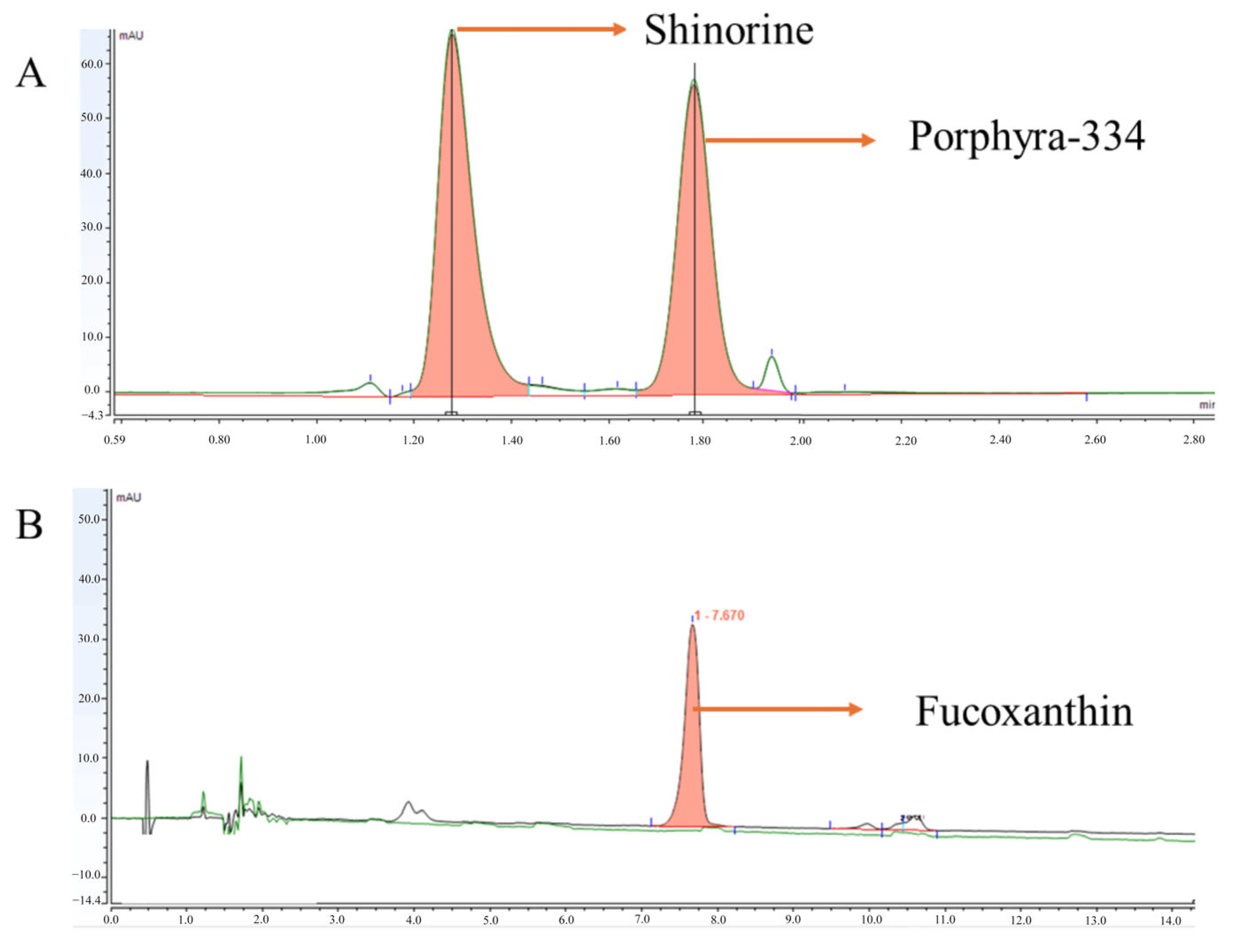
2.3. Extract Characterization
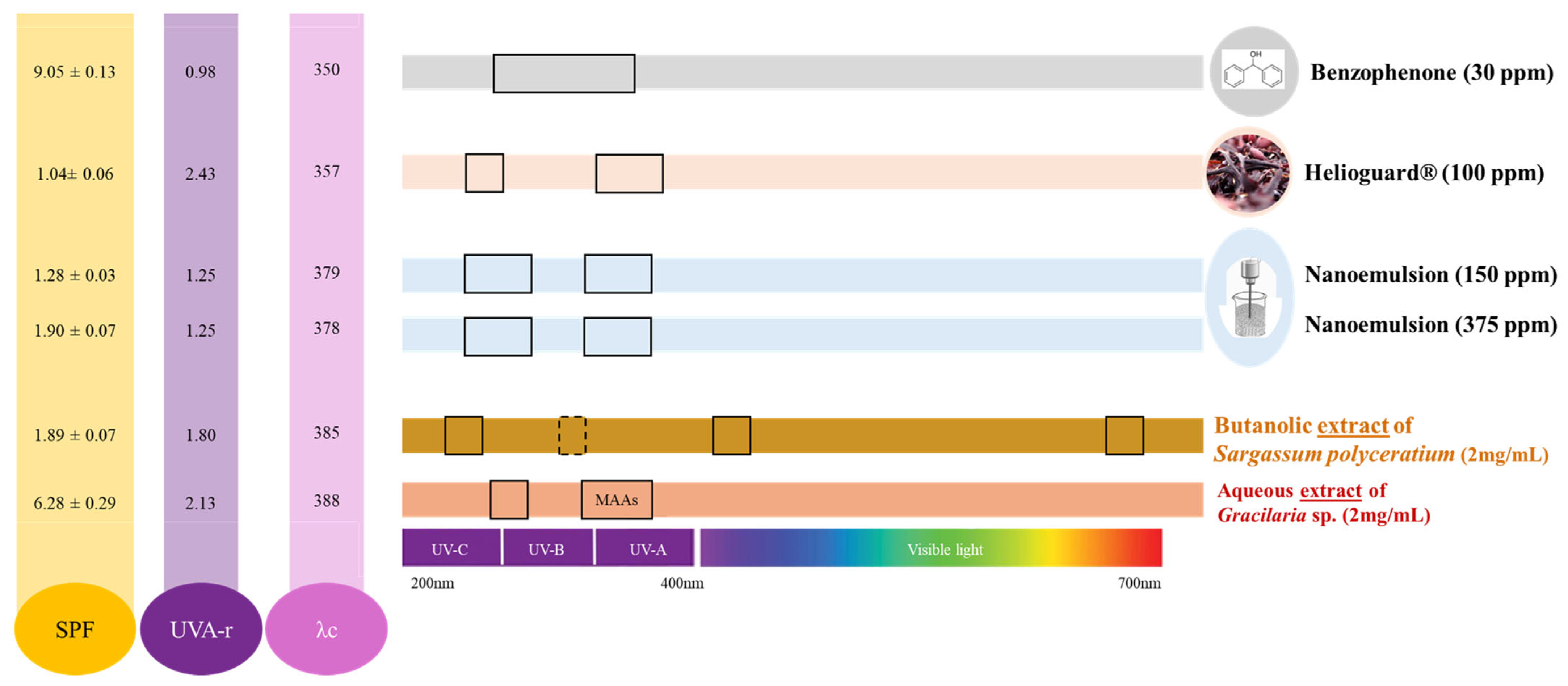
2.4. Sun Protection Factor (SPF), UVA Ratio (UVA-r), and Critical Wavelength (λc)
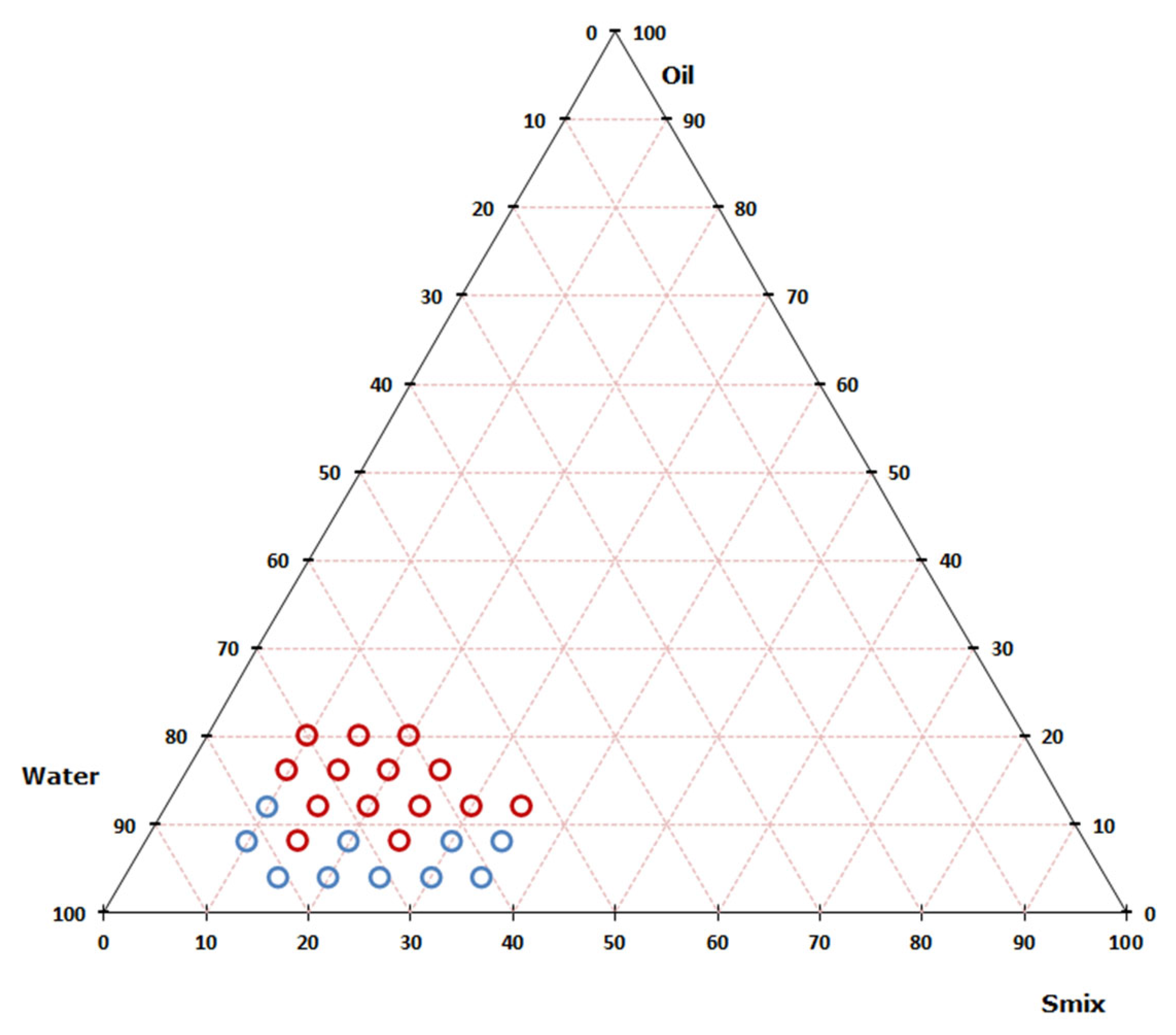
2.5. Nanoemulsion Preparation–Pseudoternary Diagram
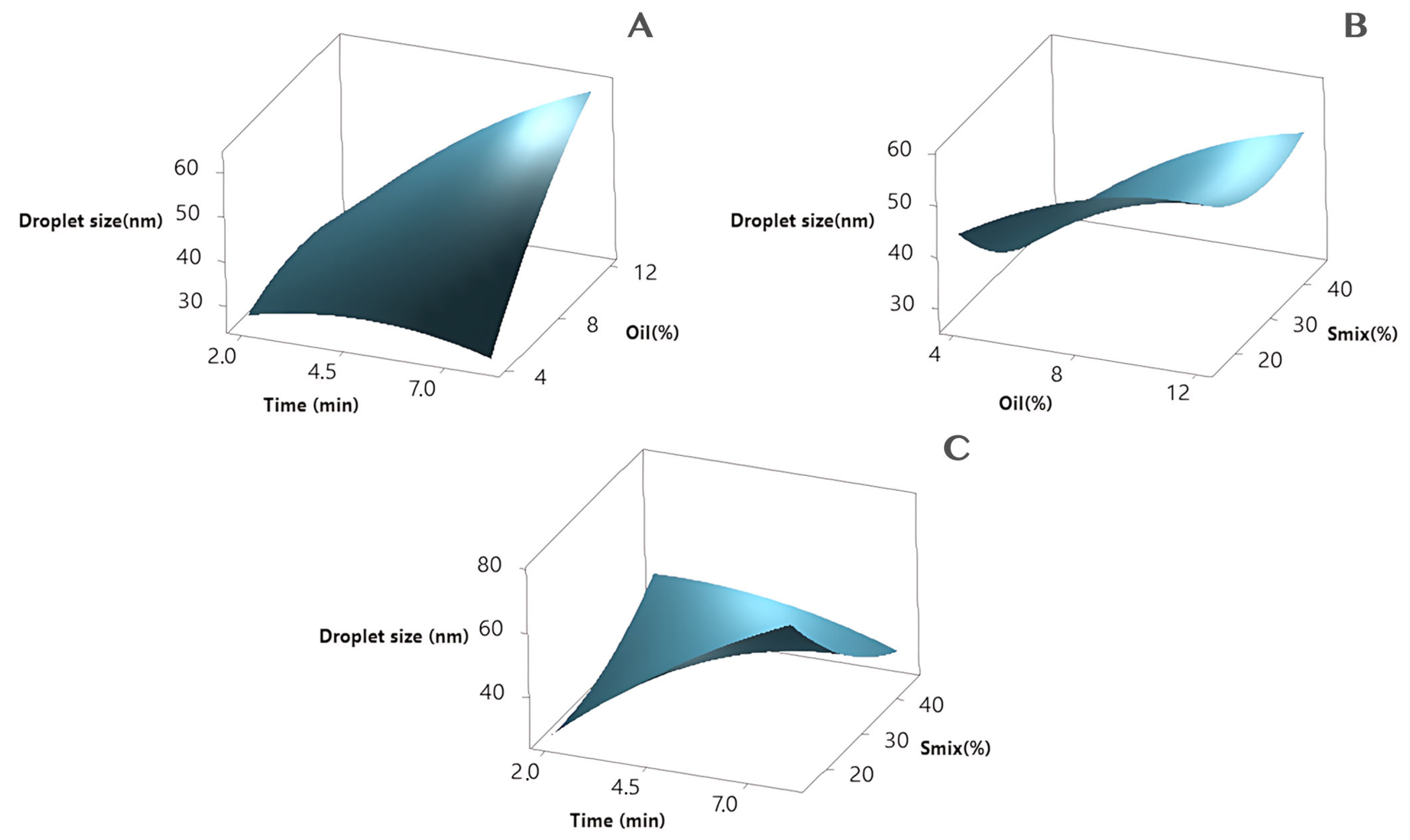
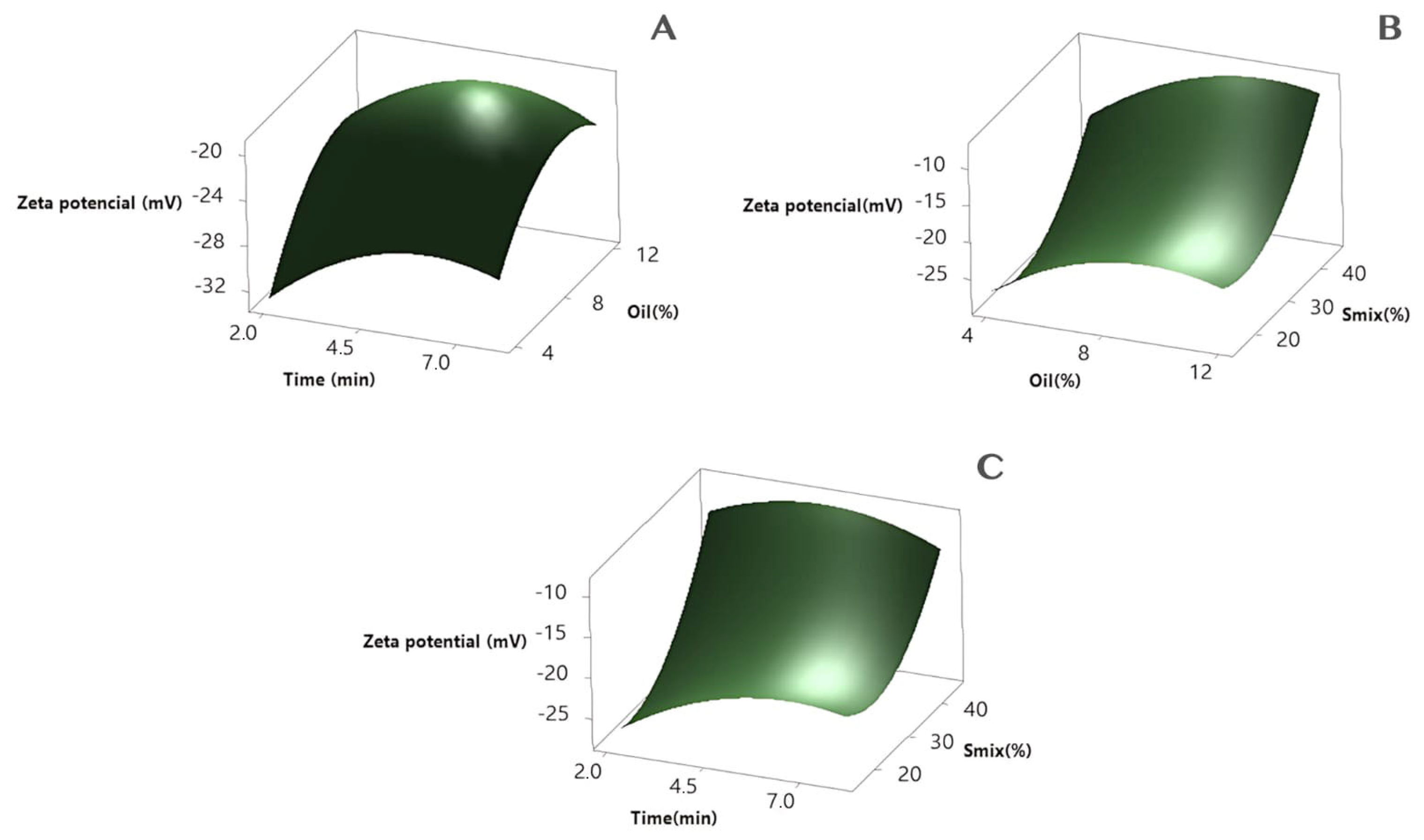
2.6. Nanoemulsion Optimization
2.7. Statistical Analysis
3. Results and Discussion
3.1. Extract Characterization
3.2. Nanoemulsion Preparation–Pseudoternary Diagram
3.3. Nanoemulsion Optimization
3.4. Sun Protection Factor (SPF), UVA Ratio (UVA-r), and Critical Wavelength (λc)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pangestuti, R.; Siahaan, E.; Kim, S.-K. Photoprotective Substances Derived from Marine Algae. Mar. Drugs 2018, 16, 399. [Google Scholar] [CrossRef]
- Torres, P.; Santos, J.P.; Chow, F.; dos Santos, D.Y. A Comprehensive Review of Traditional Uses, Bioactivity Potential, and Chemical Diversity of the Genus Gracilaria (Gracilariales, Rhodophyta). Algal Res. 2019, 37, 288–306. [Google Scholar] [CrossRef]
- Sinha, R.P.; Klisch, M.; Gröniger, A.; Häder, D.-P. Mycosporine-like Amino Acids in the Marine Red Alga Gracilaria cornea—Effects of UV and Heat. Environ. Exp. Bot. 2000, 43, 33–43. [Google Scholar] [CrossRef]
- Schneider, G.; Figueroa, F.L.; Vega, J.; Avilés, A.; Horta, P.A.; Korbee, N.; Bonomi-Barufi, J. Effects of UV–Visible Radiation on Growth, Photosynthesis, Pigment Accumulation and UV-Absorbing Compounds in the Red Macroalga Gracilaria cornea (Gracilariales, Rhodophyta). Algal Res. 2022, 64, 102702. [Google Scholar] [CrossRef]
- Qoh, A.; Amine, J.; Assobhei, O.; Etahiri, S. Investigation of UV Protection and Antiaging Properties of Mycosporinelike Amino Acids in the Moroccan Red Alga Gracilaria gracilis (Gracilariales, Rhodophyta). J. Appl. Phycol. 2025, 37, 1341–1356. [Google Scholar] [CrossRef]
- Ryu, J.; Park, S.-J.; Kim, I.-H.; Choi, Y.H.; Nam, T.-J. Protective Effect of Porphyra-334 on UVA-Induced Photoaging in Human Skin Fibroblasts. Int. J. Mol. Med. 2014, 34, 796–803. [Google Scholar] [CrossRef]
- Singh, A.; Čížková, M.; Bišová, K.; Vítová, M. Exploring Mycosporine-like Amino Acids (MAAs) as Safe and Natural Protective Agents against UV-Induced Skin Damage. Antioxidants 2021, 10, 683. [Google Scholar] [CrossRef]
- Stiger-Pouvreau, V.; Mattio, L.; De, A.; Uwai, S.; Dominguez, H.; Flórez-Fernández, N.; Connan, S.; Critchley, A.T. A Concise Review of the Highly Diverse Genus Sargassum C. Agardh with Wide Industrial Potential. J. Appl. Phycol. 2023, 35, 1453–1483. [Google Scholar] [CrossRef]
- Piao, M.J.; Yoon, W.J.; Kang, H.K.; Yoo, E.S.; Koh, Y.S.; Kim, D.S.; Lee, N.H.; Hyun, J.W. Protective Effect of the Ethyl Acetate Fraction of Sargassum muticum against Ultraviolet B–Irradiated Damage in Human Keratinocytes. Int. J. Mol. Sci. 2011, 12, 8146–8160. [Google Scholar] [CrossRef]
- Xu, Z.; Li, B.; Li, L.; Wang, N.; Wang, Y.; Wang, H.; Yan, F.; Bao, M.; Zang, S.; Wu, H.; et al. Effects of UV Radiation on Photosynthesis of Sargassum muticum. J. Exp. Mar. Biol. Ecol. 2023, 569, 151961. [Google Scholar] [CrossRef]
- Prasedya, E.S.; Syafitri, S.M.; Geraldine, B.A.F.D.; Hamdin, C.D.; Frediansyah, A.; Miyake, M.; Kobayashi, D.; Hazama, A.; Sunarpi, H. UVA Photoprotective Activity of Brown Macroalgae Sargassum Cristafolium. Biomedicines 2019, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Schneider, G.; Figueroa, F.L.; Vega, J.; Chaves, P.; Álvarez-Gómez, F.; Korbee, N.; Bonomi-Barufi, J. Photoprotection Properties of Marine Photosynthetic Organisms Grown in High Ultraviolet Exposure Areas: Cosmeceutical Applications. Algal Res. 2020, 49, 101956. [Google Scholar] [CrossRef]
- Xu, Z.; Li, L.; Jiang, H.; Yan, F.; Liu, L.; Zang, S.; Ma, Y.; Wu, H. Photosynthetic Responses of a Golden Tide Alga (Sargassum horneri) to Ultraviolet Radiation. Front. Mar. Sci. 2022, 9, 978376. [Google Scholar] [CrossRef]
- Hernández, A.R.; Sepulveda, L.; Hata, Y.; Castellanos, L.; Björklund, S.; Ruzgas, T.; Aragón, M. Algae Extract-Based Nanoemulsions for Photoprotection against UVB Radiation: An Electrical Impedance Spectroscopy Study. Sci. Rep. 2025, 15, 191. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, C.; Rojas, M.A.; Aragón, M. Optimization of Ultrasound-Assisted Emulsification of Emollient Nanoemulsions of Seed Oil of Passiflora edulis var. edulis. Cosmetics 2020, 8, 1. [Google Scholar] [CrossRef]
- Panjaitan, M.A.P.; Kasitowati, R.D.; Putri, N.; Yamindago, A.; Asmara, R.; Aliviyanti, D.; Pratiwi, D.C. Polysaccharide Isolation of Brown Seaweed: Sargassum sp and Its Photoprotection Activity. IOP Conf. Ser.Earth Environ. Sci. 2022, 1036, 012039. [Google Scholar] [CrossRef]
- Galani, E.; Galatis, D.; Tzoka, K.; Papadimitriou, V.; Sotiroudis, T.G.; Bonos, A.; Xenakis, A.; Chatzidaki, M.D. Natural Antioxidant-Loaded Nanoemulsions for Sun Protection Enhancement. Cosmetics 2023, 10, 102. [Google Scholar] [CrossRef]
- Cerqueira-Coutinho, C.; Santos-Oliveira, R.; dos Santos, E.; Mansur, C.R. Development of a Photoprotective and Antioxidant Nanoemulsion Containing Chitosan as an Agent for Improving Skin Retention. Eng. Life Sci. 2015, 15, 593–604. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase World-Wide Electronic Publication, National University of Ireland, Galway. 2025. Available online: http://www.algaebase.org (accessed on 20 February 2025).
- Sepulveda, L. Búsqueda de Compuestos con Posible Actividad Inhibitoria de Enzimas de Interés Cosmético a Partir de Algas del Caribe Colombiano. Repositorio UNAL. Available online: https://repositorio.unal.edu.co/handle/unal/84624 (accessed on 20 February 2025).
- Urrea-Victoria, V.; Costa, G.; Gavio, B.; Ramos, F.; Castellanos, L. Mycosporine-like Amino Acids Profile in Red Algae from High UV-Index Geographical Areas (San Andrés Island and La Guajira) of the Colombian Caribbean Coast. Algal Res. 2025, 86, 103927. [Google Scholar] [CrossRef]
- Foo, S.C.; Khoo, K.S.; Ooi, C.W.; Show, P.L.; Khong, N.M.H.; Yusoff, F.M. Meeting Sustainable Development Goals: Alternative Extraction Processes for Fucoxanthin in Algae. Front. Bioeng. Biotechnol. 2021, 8, 546067. [Google Scholar] [CrossRef]
- Mansur, J.S.; Breder, M.N.; Mansur, M.C.; Azulay, R.D. Determination of Sun Protection Factor by Spectrophotometry. Bras. Dermatol. 1986, 61, 121–124. [Google Scholar]
- Springsteen, A.; Yurek, R.; Frazier, M.; Carr, K.F. In Vitro measurement of sun protection factor of sunscreens by diffuse transmittance. Anal. Chim. Acta 1999, 380, 155–164. [Google Scholar] [CrossRef]
- Tadros, T.; Izquierdo, P.; Esquena, J.; Solans, C. Formation and Stability of Nano-Emulsions. Adv. Colloid Interface Sci. 2004, 108–109, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.J.; Rees, G.D. Microemulsion-Based Media as Novel Drug Delivery Systems. Adv. Drug Deliv. Rev. 2000, 45, 89–121. [Google Scholar] [CrossRef]
- Vaz, B.M.; Leite, M.S.C.; Contieri, L.S.; Mesquita, L.M.d.S.; Conde, A.; Oliveira, J.P.; Pinto, D.C.; Ventura, S.P. Efficient Extraction and Purification of Mycosporineslike Amino Acids (MAAs) Following a Multiproduct Biorefinery Approach. Sep. Purif. Technol. 2025, 363, 132200. [Google Scholar] [CrossRef]
- Ashkenazi, D.Y.; Figueroa, F.L.; Korbee, N.; García-Sánchez, M.; Vega, J.; Ben-Valid, S.; Paz, G.; Salomon, E.; Israel, Á.; Abelson, A. Enhancing Bioproducts in Seaweeds via Sustainable Aquaculture: Antioxidant and Sun-Protection Compounds. Mar. Drugs 2022, 20, 767. [Google Scholar] [CrossRef]
- Figueroa, F.L.; Álvarez-Gómez, F.; Bonomi-Barufi, J.; Vega, J.; Massocato, T.F.; Gómez-Pinchetti, J.L.; Korbee, N. Interactive Effects of Solar Radiation and Inorganic Nutrients on Biofiltration, Biomass Production, Photosynthetic Activity and the Accumulation of Bioactive Compounds in Gracilaria cornea (Rhodophyta). Algal Res. 2022, 68, 102890. [Google Scholar] [CrossRef]
- Torres, P.; Chow, F.; dos Santos, D.Y.A.C. A Chemical Investigation of the Antioxidant Capacity of Extracts from Red Macroalga Gracilaria domingensis. Phycology 2022, 2, 332–343. [Google Scholar] [CrossRef]
- Zwerger, M.; Ganzera, M. Fast and Efficient Separation of Eleven Mycosporine-like Amino Acids by UHPLC-DAD and Their Quantification in Diverse Red Algae. Mar. Drugs 2022, 20, 395. [Google Scholar] [CrossRef]
- Oliyaei, N.; Moosavi-Nasab, M. Ultrasound-Assisted Extraction of Fucoxanthin from Sargassum Angustifolium and Cystoseira Indica Brown Algae. J. Food Process. Preserv. 2021, 45, e15929. [Google Scholar] [CrossRef]
- Hong, D.; Thom, L.T.; Ha, N.C.; Hoai, T.; Minh, T.; Tam, L.T.; Dat, N.M.; Duc, T.M.; Tru, N.V.; Minh, T.; et al. Isolation of Fucoxanthin from Sargassum oligocystum Montagne, 1845 Seaweed in Vietnam and Its Neuroprotective Activity. Biomedicines 2023, 11, 2310. [Google Scholar] [CrossRef]
- Molina, G.A.; González-Reyna, M.A.; Loske, A.M.; Fernández, F.; Torres-Ortiz, D.A.; Estevez, M. Weak Shock Wave-Mediated Fucoxanthin Extraction from Sargassum spp. And Its Electrochemical Quantification. Algal Res. 2022, 68, 102891. [Google Scholar] [CrossRef]
- Torres, A.; Enk, C.D.; Hochberg, M.; Srebnik, M. Porphyra-334, a Potential Natural Source for UVA Protective Sunscreens. Photochem. Photobiol. Sci. 2006, 5, 432–435. [Google Scholar] [CrossRef]
- de la Coba, F.; Aguilera, J.; Korbee, N.; de Gálvez, M.; Herrera-Ceballos, E.; Álvarez-Gómez, F.; Figueroa, F. UVA and UVB Photoprotective Capabilities of Topical Formulations Containing Mycosporine-like Amino Acids (MAAs) through Different Biological Effective Protection Factors (BEPFs). Mar. Drugs 2019, 17, 55. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.-J.; Jeon, Y.-J. Protective Effect of Fucoxanthin Isolated from Sargassum Siliquastrum on UV-B Induced Cell Damage. J. Photochem. Photobiol. B Biol. 2009, 95, 101–107. [Google Scholar] [CrossRef]
- Chen, S.-J.; Lee, C.-J.; Lin, T.-B.; Peng, H.-Y.; Liu, H.-J.; Chen, Y.-S.; Tseng, K.-W. Protective Effects of Fucoxanthin on Ultraviolet B-Induced Corneal Denervation and Inflammatory Pain in a Rat Model. Mar. Drugs 2019, 17, 152. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ren, J.; Zhao, B.; Zhu, T.; Qi, H. Photoprotective Mechanism of Fucoxanthin in Ultraviolet B Irradiation-Induced Retinal Müller Cells Based on Lipidomics Analysis. J. Agric. Food Chem. 2022, 70, 3181–3193. [Google Scholar] [CrossRef] [PubMed]
- Kommuru, T.R.; Gurley, B.; Khan, M.A.; Reddy, I.K. Self-Emulsifying Drug Delivery Systems (SEDDS) of Coenzyme Q10: Formulation Development and Bioavailability Assessment. Int. J. Pharm. 2001, 212, 233–246. [Google Scholar] [CrossRef]
- Pouton, C.W. Formulation of Poorly Water-Soluble Drugs for Oral Administration: Physicochemical and Physiological Issues and the Lipid Formulation Classification System. Eur. J. Pharm. Sci. 2006, 29, 278–287. [Google Scholar] [CrossRef]
- Badolato, G.G.; Aguilar, F.; Schuchmann, H.P.; Sobisch, T.; Lerche, D. Evaluation of Long Term Stability of Model Emulsions by Multisample Analytical Centrifugation. In Surface and Interfacial Forces–From Fundamentals To Applications; Auernhammer, Günter, K., Butt, H.-J., Vollmer, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 66–73. [Google Scholar]
- Coelho, S.G.; Rua, D.; Miller, S.A.; Agrawal, A. Suboptimal UVA Attenuation by Broad Spectrum Sunscreens under Outdoor Solar Conditions Contributes to Lifetime UVA Burden. Photodermatol. Photoimmunol. Photomed. 2020, 36, 42–52. [Google Scholar] [CrossRef]
- Rosic, N. Mycosporine-like Amino Acids: Making the Foundation for Organic Personalised Sunscreens. Mar. Drugs 2019, 17, 638. [Google Scholar] [CrossRef] [PubMed]
- Urikura, I.; Sugawara, T.; Hirata, T. Protective Effect of Fucoxanthin against UVB-Induced Skin Photoaging in Hairless Mice. Biosci. Biotechnol. Biochem. 2011, 75, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Mumu, M.; Das, A.; Emran, T.B.; Mitra, S.; Islam, F.; Roy, A.; Karim, M.M.; Das, R.; Park, M.N.; Chandran, D.; et al. Fucoxanthin: A Promising Phytochemical on Diverse Pharmacological Targets. Front. Pharmacol. 2022, 13, 929442. [Google Scholar] [CrossRef] [PubMed]
- Chatzigianni, M.; Pavlou, P.; Siamidi, A.; Vlachou, M.; Varvaresou, A.; Papageorgiou, S. Environmental Impacts due to the Use of Sunscreen Products: A Mini-Review. Ecotoxicology 2022, 31, 1331–1345. [Google Scholar] [CrossRef]
- Resende, D.I.S.P.; Jesus, A.; Sousa Lobo, J.M.; Sousa, E.; Cruz, M.T.; Cidade, H.; Almeida, I.F. Up-To-Date Overview of the Use of Natural Ingredients in Sunscreens. Pharmaceuticals 2022, 15, 372. [Google Scholar] [CrossRef]
- Chavda, V.P.; Acharya, D.; Hala, V.; Daware, S.; Vora, L.K. Sunscreens: A Comprehensive Review with the Application of Nanotechnology. J. Drug Deliv. Sci. Technol. 2023, 86, 104720. [Google Scholar] [CrossRef]

| No | Oil (%) | Smix (%) | Water (%) | Day 1 | Day 7 | ||
|---|---|---|---|---|---|---|---|
| Droplet Size (nm) | Potential ζ (mV) | Droplet Size (nm) | Potential ζ (mV) | ||||
| 1 | 4 | 15 | 81 | 17.8 ± 0.7219 | −24.13 ± 0.83 | 17.46 ± 0.08 | −23.92 ± 0.24 |
| 2 | 4 | 20 | 76 | 27.36 ± 4.05 | −24.14 ± 0.20 | 32.38 ± 5.16 | −23.88 ± 0.36 |
| 3 | 4 | 25 | 71 | 54.27 ± 2.77 | −22.82 ± 0.32 | 21.70 ± 1.36 | −22.78 ± 0.31 |
| 4 | 4 | 30 | 66 | 18.62 ± 0.3814 | −23.56 ± 0.18 | 15.71 ± 0.14 | −23.84 ± 0.16 |
| 5 | 4 | 35 | 61 | 16.88 ± 0.1943 | −23.24 ± 0.28 | 15.09 ± 0.09 | −22.84 ± 0.31 |
| 6 | 8 | 10 | 82 | 17.59 ± 0.4099 | −22.86 ± 0.13 | 26.72 ± 0.21 | −22.42 ± 0.19 |
| 7 | 8 | 15 | 77 | 172.5 ± 1.249 | −23.31 ± 0.10 | 253.40 ± 5.99 | −23.56 ± 0,07 |
| 8 | 8 | 20 | 72 | 18.15 ± 0.07937 | −23.99 ± 0.18 | 14.67 ± 0.03 | −23.68 ± 0.42 |
| 9 | 8 | 25 | 67 | 333.9 ± 13.1 | −23.32 ± 0.16 | 280.40 ± 2.54 | −22.88 ± 0.17 |
| 10 | 8 | 30 | 62 | 31.82 ± 0.3584 | −24.07 ± 0.17 | 21.29 ± 0.03 | −24.24 ± 0.12 |
| 11 | 8 | 35 | 57 | 78.29 ± 7.053 | −24.12 ± 0.13 | 22.14 ± 0.03 | −24.68 ± 0.16 |
| 12 | 12 | 10 | 78 | 20.75 ± 0.9283 | −23.38 ± 0.21 | 18.31 ± 0.15 | −22.70 ± 0.32 |
| 13 | 12 | 15 | 73 | 322.5 ± 3.584 | −24.13 ± 0.05 | 289.20 ± 3.70 | −22.80 ± 0.32 |
| 14 | 12 | 20 | 68 | 478.1 ± 5.424 | −24.08 ± 0.10 | 340.50 ± 5.78 | −24.46 ± 0.06 |
| 15 | 12 | 25 | 63 | 316.1 ± 2.572 | −23.75 ± 0.22 | 393.70 ± 1.58 | −24.58 ± 0.16 |
| 16 | 12 | 30 | 58 | 359.8 ± 3.786 | −23.08 ± 0.34 | 124.90 ± 10.54 | −24.62 ± 0.11 |
| 17 | 12 | 35 | 53 | 949.9 ± 3.01 | −24.08 ± 0.06 | 210.30 ± 17.68 | −24.16 ± 0.13 |
| 18 | 16 | 10 | 74 | 394.6 ± 2.542 | −23.4 ± 0.02 | 378.20 ± 11.82 | −22.44 ± 0.75 |
| 19 | 16 | 15 | 69 | 380.6 ± 3.118 | 23.05 ± 0.26 | 330.30 ± 2.76 | −25.60 ± 0.29 |
| 20 | 16 | 20 | 64 | 439.3 ± 3.464 | −23.23 ± 0.38 | 399.60 ± 0.93 | −20.23 ± 0.18 |
| 21 | 16 | 25 | 59 | 390.8 ± 3.404 | −23.59 ± 0.38 | 342.50 ± 3.46 | −22.12 ± 0.09 |
| 22 | 20 | 10 | 70 | 472.1 ± 5.897 | −20.09 ± 0.08 | 420.10 ± 1.78 | −24.44 ± 0.03 |
| 23 | 20 | 15 | 65 | 258.4 ± 11.17 | −25.54 ± 0.35 | 262.30 ± 10.50 | −23.96 ± 0.17 |
| 24 | 20 | 20 | 60 | 590.2 ± 4.576 | −24.2 ± 0.33 | 435.30 ± 6.47 | −23.86 ± 0.12 |
| Independent Variables | Levels | ||
|---|---|---|---|
| −1 | 0 | −1 | |
| Oil (%) | 4 | 8 | 12 |
| Smix (%) | 15 | 30 | 45 |
| Sonication time (min) | 2 | 5 | 8 |
| No | Oil (%) | Smix (%) | Water (%) | Sonication Time (min) | Day 1 | Day 7 | ||
|---|---|---|---|---|---|---|---|---|
| Droplet Size (nm) | Potential ζ (mV) | Droplet Size (nm) | Potential ζ (mV) | |||||
| 1 | 8 | 30 | 62 | 5 | 45.72 ± 3.39 | −17.2 ± 2.0 | 52.56 ± 6.92 | −23.0 ± 2.8 |
| 2 | 8 | 30 | 62 | 5 | 34.70 ± 8.80 | −24.0 ± 0.7 | 41.50 ± 2.30 | −24.5 ± 0.6 |
| 3 | 12 | 30 | 58 | 8 | 30.09 ± 0.47 | −22.6 ± 3.0 | 33.92 ± 1.15 | −19.9 ± 2.1 |
| 4 | 4 | 45 | 51 | 5 | 38.77 ± 0.78 | −26.1 ± 0.9 | 38.32 ± 3.96 | −25.0 ± 3.1 |
| 5 | 4 | 15 | 81 | 5 | 29.33 ± 2.55 | −23.8 ± 3.2 | 89.80 ± 11.88 | −13.4 ± 0.6 |
| 6 | 8 | 30 | 62 | 5 | 40.12 ± 2.01 | −24.8 ± 3.2 | 23.44 ± 0.51 | −18.4 ± 3.0 |
| 7 | 8 | 30 | 62 | 5 | 55.96 ± 7.25 | −21.6 ± 1.7 | 26.60 ± 0.21 | −24.1 ± 2.7 |
| 8 | 8 | 45 | 47 | 8 | 31.26 ± 2.71 | −9.3 ± 0.8 | 46.17 ± 0.82 | −11.3 ± 1.7 |
| 9 | 8 | 15 | 77 | 8 | 78.01 ± 0.38 | −20.4 ± 2.0 | 125.70 ± 8.58 | −24.8 ± 0.9 |
| 10 | 8 | 45 | 47 | 2 | 40.73 ± 3.49 | −18.0 ± 0.5 | 30.95 ± 1.32 | −15.4 ± 3.5 |
| 11 | 12 | 30 | 58 | 2 | 27.04 ± 0.51 | −21.4 ± 1.2 | 24.25 ± 0.73 | −16.5 ± 2.4 |
| 12 | 8 | 30 | 62 | 5 | 50.96 ± 2.24 | −15.2 ± 2.0 | 31.56 ± 0.14 | −22.5 ± 0.4 |
| 13 | 4 | 30 | 66 | 2 | 43.42 ± 9.93 | −33.4 ± 2.1 | 22.21 ± 0.51 | −18.1 ± 2.7 |
| 14 | 12 | 45 | 43 | 5 | 55.26 ± 0.87 | −11.8 ± 1.4 | 33.09 ± 0.62 | −11.7 ± 1.6 |
| 15 | 8 | 30 | 62 | 5 | 41.19 ± 1.36 | −18.9 ± 1.4 | 29.99 ± 0.73 | −18.5 ± 2.3 |
| 16 | 8 | 15 | 77 | 2 | 28.98 ± 0.72 | −29.9 ± 3.5 | 25.41 ± 0.33 | −20.9 ± 3.2 |
| 17 | 12 | 15 | 73 | 5 | 55.78 ± 0.43 | −22.3 ± 2.4 | 89.94 ± 4.32 | −19.0 ± 2.3 |
| 18 | 4 | 30 | 66 | 8 | 26.34 ± 1.93 | −31.4 ± 0.9 | 32.14 ± 2.25 | −14.9 ± 1.3 |
| Variable | Droplet Size | Potential ζ | |||
|---|---|---|---|---|---|
| F-Value | p-Value | F-Value | p-Value | ||
| Linear terms | A | 8.61 | 0.019 | 1.28 | 0.291 |
| B | 9.84 | 0.014 | 5.35 | 0.049 | |
| C | 3.81 | 0.087 | 16.86 | 0.003 | |
| Quadratic terms | A2 | 0.84 | 0.386 | 2.63 | 0.143 |
| B2 | 0.82 | 0.391 | 3.73 | 0.089 | |
| C2 | 1.76 | 0.221 | 8.11 | 0.022 | |
| Interaction terms | AB | 4.80 | 0.060 | 0.15 | 0.704 |
| AC | 12.30 | 0.008 | 0.70 | 0.427 | |
| BC | 0.36 | 0.564 | 0.12 | 0.739 | |
| Lack-of-fit | 0.37 | 0.778 | 1.34 | 0.360 | |
| Response Variable | Experimental Value |
|---|---|
| Droplet size (nm) | 32.42 |
| Potential ζ | −26.0 |
| Final composition | |
| Kolliphor EL®: propylene glycol (%) | 20.8% |
| Capryol PGMC (%) | 4% |
| Deionized water (%) | 73.7% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tello Quiroz, J.; Rodriguez Martinez, I.A.; Urrea-Victoria, V.; Castellanos, L.; Aragón Novoa, D.M. Marine Algae Extract-Loaded Nanoemulsions: A Spectrophotometric Approach to Broad-Spectrum Photoprotection. Cosmetics 2025, 12, 101. https://doi.org/10.3390/cosmetics12030101
Tello Quiroz J, Rodriguez Martinez IA, Urrea-Victoria V, Castellanos L, Aragón Novoa DM. Marine Algae Extract-Loaded Nanoemulsions: A Spectrophotometric Approach to Broad-Spectrum Photoprotection. Cosmetics. 2025; 12(3):101. https://doi.org/10.3390/cosmetics12030101
Chicago/Turabian StyleTello Quiroz, Julian, Ingrid Andrea Rodriguez Martinez, Vanessa Urrea-Victoria, Leonardo Castellanos, and Diana Marcela Aragón Novoa. 2025. "Marine Algae Extract-Loaded Nanoemulsions: A Spectrophotometric Approach to Broad-Spectrum Photoprotection" Cosmetics 12, no. 3: 101. https://doi.org/10.3390/cosmetics12030101
APA StyleTello Quiroz, J., Rodriguez Martinez, I. A., Urrea-Victoria, V., Castellanos, L., & Aragón Novoa, D. M. (2025). Marine Algae Extract-Loaded Nanoemulsions: A Spectrophotometric Approach to Broad-Spectrum Photoprotection. Cosmetics, 12(3), 101. https://doi.org/10.3390/cosmetics12030101








