Production and Characterization of Semi-Solid Formulations for the Delivery of the Cosmetic Peptide Palmitoyl-GHK
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Formulations
2.2.1. Ethosomes
2.2.2. Ethosomal Gel
2.2.3. Organogel
2.2.4. Poloxamer Gel
2.2.5. Poloxamer Organogel
2.3. Characterization of the Formulations
2.3.1. Size Analysis of Ethosomes
2.3.2. Spreadability of Semi-Solid Formulations
2.3.3. Determination of the Occlusive Factor
2.3.4. Analysis of Palm-GHK Entrapment in Ethosomes
2.4. Skin Hydration Test
2.5. In Vitro Palm-GHK Release Studies
2.6. Patch Test
3. Results
3.1. Preparation of the Formulations
3.1.1. ETO
3.1.2. ORG
3.1.3. POL
3.1.4. POL-ORG
3.2. Characterization of Viscous Formulations
3.3. In Vivo Skin Hydration Test
3.4. Preparation of Palm-GHK Formulations
3.5. In Vitro Palm-GHK Release Studies
3.6. In Vivo Comparative Irritation Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, S.-I.; Heo, S.H.; Lee, J.; Cha, H.; Shin, M.S. A Clinical Study on Anti-Wrinkle Efficacy of a Cosmetics Containing Oligoarginine Conjugation of Palmitoyl-GHK Peptide for Skin Penetrating. Turk. J. Comput. Math. Educ. 2021, 12, 401–406. [Google Scholar] [CrossRef]
- Schagen, S.K. Topical Peptide Treatments with Effective Anti-Aging Results. Cosmetics 2017, 4, 16. [Google Scholar] [CrossRef]
- Maquart, F.X.; Bellon, G.; Pasco, S.; Monboisse, J.C. Matrikines in the Regulation of Extracellular Matrix Degradation. Biochimie 2005, 87, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the Extracellular Matrix in Development and Disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Clinical Evaluation of Matrikynes®: A Novel Cosmetic Ingredient Comprised of Matrikine Peptides. Available online: https://xylyxbio.com/wp-content/uploads/2023/04/white-paper_clinical-evaluation-of-matrikynes.pdf (accessed on 7 March 2025).
- Pickart, L.; Vasquez-Soltero, J.M.; Margolina, A. GHK Peptide as a Natural Modulator of Multiple Cellular Pathways in Skin Regeneration. BioMed Res. Int. 2015, 2015, 648108. [Google Scholar] [CrossRef]
- Maquart, F.X.; Pickart, L.; Laurent, M.; Gillery, P.; Monboisse, J.C.; Borel, J.P. Stimulation of Collagen Synthesis in Fibroblast Cultures by the Tripeptide-Copper Complex Glycyl-L-Histidyl-L-Lysine-Cu2+. FEBS Lett. 1988, 238, 343–346. [Google Scholar] [CrossRef]
- MacLeman, E. Why Are Palmitoyl Oligopeptides Used in Skincare? Available online: https://thedermreview.com/palmitoyl-oligopeptide/ (accessed on 26 August 2024).
- Vintiloiu, A.; Leroux, J.C. Organogels and Their Use in Drug Delivery—A Review. J. Control Release 2008, 125, 179–192. [Google Scholar] [CrossRef]
- Kim, C.; Shim, J.; Han, S.; Chang, I. The Skin-Permeation-Enhancing Effect of Phosphatidylcholine: Caffeine as a Model Active Ingredient. J. Cosmet. Sci. 2002, 53, 363–374. [Google Scholar]
- Touitou, E.; Natsheh, H. Topical Administration of Drugs Incorporated in Carriers Containing Phospholipid Soft Vesicles for the Treatment of Skin Medical Conditions. Pharmaceutics 2021, 13, 2129. [Google Scholar] [CrossRef]
- Godin, B.; Touitou, E. Ethosomes: New Prospects in Transdermal Delivery. Crit. Rev. Ther. Drug Carrier Syst. 2003, 20, 63–102. [Google Scholar] [CrossRef]
- Natsheh, H.; Vettorato, E.; Touitou, E. Ethosomes for Dermal Administration of Natural Active Molecules. Curr. Pharm. Des. 2019, 25, 2338–2348. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Pathak, K. Therapeutic and Cosmeceutical Potential of Ethosomes: An Overview. J. Adv. Pharm. Technol. Res. 2010, 1, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Nsengiyumva, E.M.; Alexandridis, P. Xanthan Gum in Aqueous Solutions: Fundamentals and Applications. Int. J. Biol. Macromol. 2022, 216, 583–604. [Google Scholar] [CrossRef]
- Kumar, R.; Katare, O.P. Lecithin Organogels as a Potential Phospholipid-Structured System for Topical Drug Delivery: A Review. AAPS PharmSciTech 2005, 6, E298–E310. [Google Scholar] [CrossRef]
- Martinez, R.M.; Rosado, C.; Velasco, M.V.R.; Lannes, S.C.S.; Baby, A.R. Main Features and Applications of Organogels in Cosmetics. Int. J. Cosmet. Sci. 2019, 41, 109–117. [Google Scholar] [CrossRef]
- Esposito, E.; Menegatti, E.; Cortesi, R. Design and Characterization of Fenretinide Containing Organogels. Mater. Sci. Eng. C 2013, 33, 383–389. [Google Scholar] [CrossRef]
- Russo, E.; Villa, C. Poloxamer Hydrogels for Biomedical Applications. Pharmaceutics 2019, 11, 671. [Google Scholar] [CrossRef]
- Escobar-Chávez, J.J.; López-Cervantes, M.; Naïk, A.; Kalia, Y.; Quintanar-Guerrero, D.; Ganem-Quintanar, A. Applications of Thermo-Reversible Pluronic F-127 Gels in Pharmaceutical Formulations. J. Pharm. Pharm. Sci. 2006, 9, 339–358. [Google Scholar]
- Hosa, L.B.; Mira, E.G.; Santana, H.; Folch, J.M.; Masip, M.M.; Prieto, Y.M.; Revuelta, A.; Di Mauro, P.P.; Veciana, J.; Sala, S.; et al. DELOS Nanovesicles-Based Hydrogels: An Advanced Formulation for Topical Use. Pharmaceutics 2022, 14, 199. [Google Scholar] [CrossRef]
- Rehman, W.U.; Asim, M.; Hussain, S.; Khan, S.A.; Khan, S.B. Hydrogel: A Promising Material in Pharmaceutics. Curr. Pharm. Des. 2020, 26, 5892–5908. [Google Scholar] [CrossRef]
- Almeida, H.; Amaral, M.H.; Lobão, P.; Lobo, J.M.S. Pluronic® F-127 and Pluronic Lecithin Organogel (PLO): Main Features and Their Applications in Topical and Transdermal Administration of Drugs. J. Pharm. Pharm. Sci. 2012, 15, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Murdan, S. A Review of Pluronic Lecithin Organogel as a Topical and Transdermal Drug Delivery System. Hosp. Pharm. 2005, 12, 267–270. [Google Scholar]
- Saha, S.; Shivarajakumar, R.; Venkata Satyanarayana Reddy Karri, V. Pluronic Lecithin Organogels: An Effective Topical and Transdermal Drug Delivery System. Int. J. Pharm. Sci. Res. 2018, 9, 4540. [Google Scholar] [CrossRef]
- Resende, D.I.S.P.; Ferreira, M.S.; Sousa-Lobo, J.M.; Sousa, E.; Almeida, I.F. Usage of Synthetic Peptides in Cosmetics for Sensitive Skin. Pharmaceuticals 2021, 14, 702. [Google Scholar] [CrossRef]
- Sicurella, M.; Sguizzato, M.; Cortesi, R.; Huang, N.; Simelière, F.; Montesi, L.; Marconi, P.; Esposito, E. Mangiferin-Loaded Smart Gels for Hsv-1 Treatment. Pharmaceutics 2021, 13, 1323. [Google Scholar] [CrossRef]
- Iqubal, M.K.; Iqubal, A.; Anjum, H.; Gupta, M.M.; Ali, J.; Baboota, S. Determination of in Vivo Virtue of Dermal Targeted Combinatorial Lipid Nanocolloidal Based Formulation of 5-Fluorouracil and Resveratrol against Skin Cancer. Int. J. Pharm. 2021, 610, 121179. [Google Scholar] [CrossRef]
- Miranda, M.; Pais, A.A.C.C.; Cardoso, C.; Vitorino, C. AQbD as a Platform for IVRT Method Development—A Regulatory Oriented Approach. Int. J. Pharm. 2019, 572, 118695. [Google Scholar] [CrossRef]
- Esposito, E.; Calderan, L.; Galvan, A.; Cappellozza, E.; Drechsler, M.; Mariani, P.; Pepe, A.; Sguizzato, M.; Vigato, E.; Dalla Pozza, E.; et al. Ex Vivo Evaluation of Ethosomes and Transethosomes Applied on Human Skin: A Comparative Study. Int. J. Mol. Sci. 2022, 23, 15112. [Google Scholar] [CrossRef]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A Review of Poloxamer 407 Pharmaceutical and Pharmacological Characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Elewa, R.; Makrantonaki, E. Aesthetic Aspects of Skin Aging, Prevention, and Local Treatment. Clin. Dermatol. 2019, 37, 365–372. [Google Scholar] [CrossRef]
- Salamanca, C.H.; Barrera-Ocampo, A.; Lasso, J.C.; Camacho, N.; Yarce, C.J. Franz Diffusion Cell Approach for Pre-Formulation Characterisation of Ketoprofen Semi-Solid Dosage Forms. Pharmaceutics 2018, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F.; Benedusi, M.; Sguizzato, M.; Cortesi, R.; Baldisserotto, A.; Buzzi, R.; Valacchi, G.; Esposito, E. Ethosomes and Transethosomes as Cutaneous Delivery Systems for Quercetin: A Preliminary Study on Melanoma Cells. Pharmaceutics 2022, 14, 1038. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Toalá, J.E.; Hernández-Mendoza, A.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Liceaga, A.M. Potential Role of Natural Bioactive Peptides for Development of Cosmeceutical Skin Products. Peptides 2019, 122, 170170. [Google Scholar] [CrossRef] [PubMed]
- Ngoc, L.T.N.; Moon, J.Y.; Lee, Y.C. Insights into Bioactive Peptides in Cosmetics. Cosmetics 2023, 10, 111. [Google Scholar] [CrossRef]
- Lupo, M.P.; Cole, A.L. Cosmeceutical peptides. Dermatol. Ther. 2007, 20, 343–349. [Google Scholar] [CrossRef]
- Safety Assessment of Palmitoyl Oligopeptides Ingredients as Used in Cosmetics. Available online: https://www.cir-safety.org/panelbook/safety-assessment-palmitoyl-oligopeptides-ingredients-used-cosmetics (accessed on 26 August 2024).
- Lintner, K.; Peschard, O. Biologically Active Peptides: From a Laboratory Bench Curiosity to a Functional Skin Care Product. Int. J. Cosmet. Sci. 2000, 22, 207–218. [Google Scholar] [CrossRef]


 control,
control,  ETO-GEL,
ETO-GEL,  POL-GEL,
POL-GEL,  POL, and
POL, and  ORG.
ORG.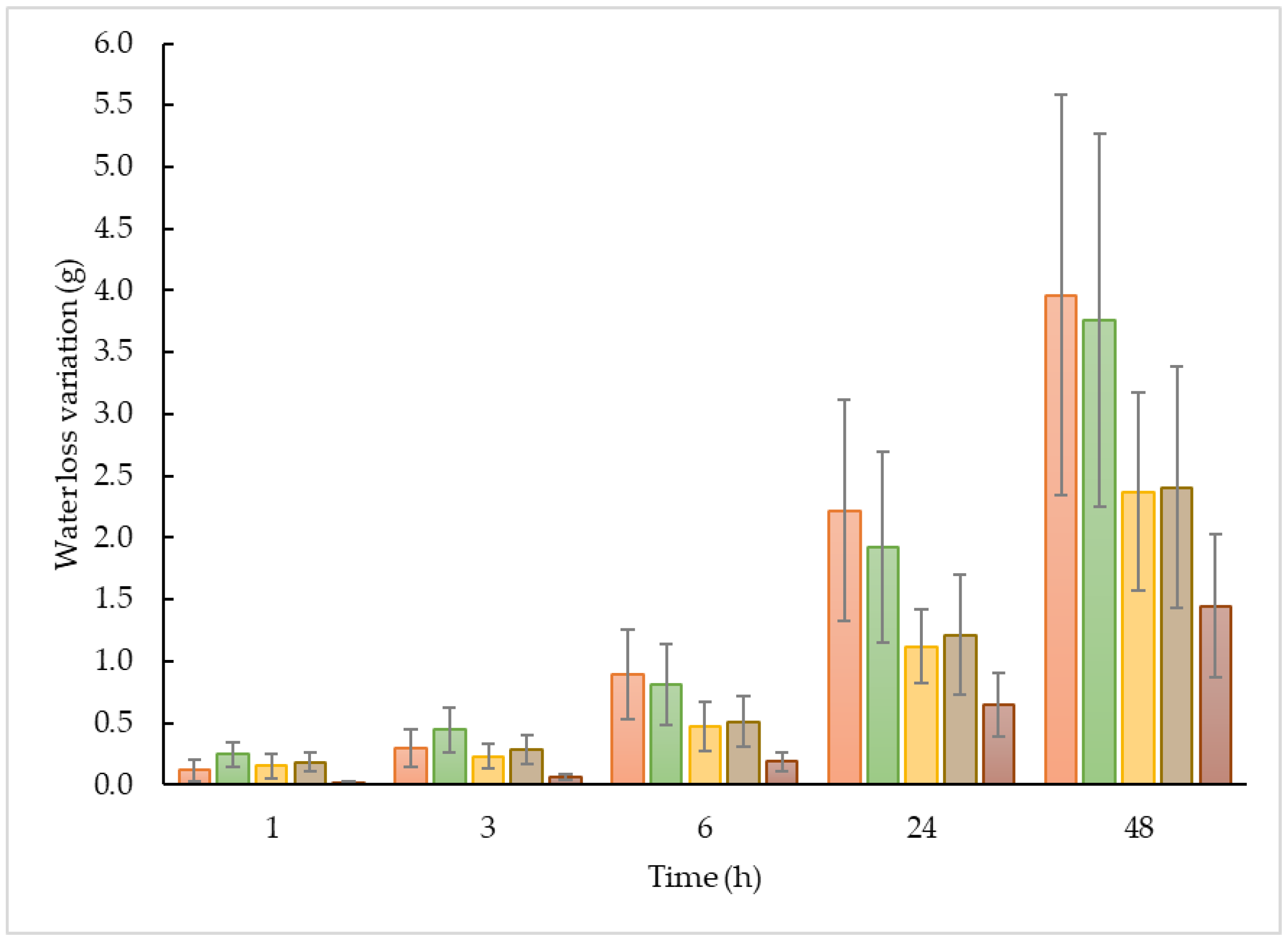
 control,
control,  ETO-GEL,
ETO-GEL,  POL-ORG, and
POL-ORG, and  ORG; 00 corresponds to the skin hydration before the application of formulation, 0 to the skin immediately after application of formulation. The values are the mean of 3 measurements taken on 5 healthy volunteers before and after the application of the formulations.
ORG; 00 corresponds to the skin hydration before the application of formulation, 0 to the skin immediately after application of formulation. The values are the mean of 3 measurements taken on 5 healthy volunteers before and after the application of the formulations.
 control,
control,  ETO-GEL,
ETO-GEL,  POL-ORG, and
POL-ORG, and  ORG; 00 corresponds to the skin hydration before the application of formulation, 0 to the skin immediately after application of formulation. The values are the mean of 3 measurements taken on 5 healthy volunteers before and after the application of the formulations.
ORG; 00 corresponds to the skin hydration before the application of formulation, 0 to the skin immediately after application of formulation. The values are the mean of 3 measurements taken on 5 healthy volunteers before and after the application of the formulations.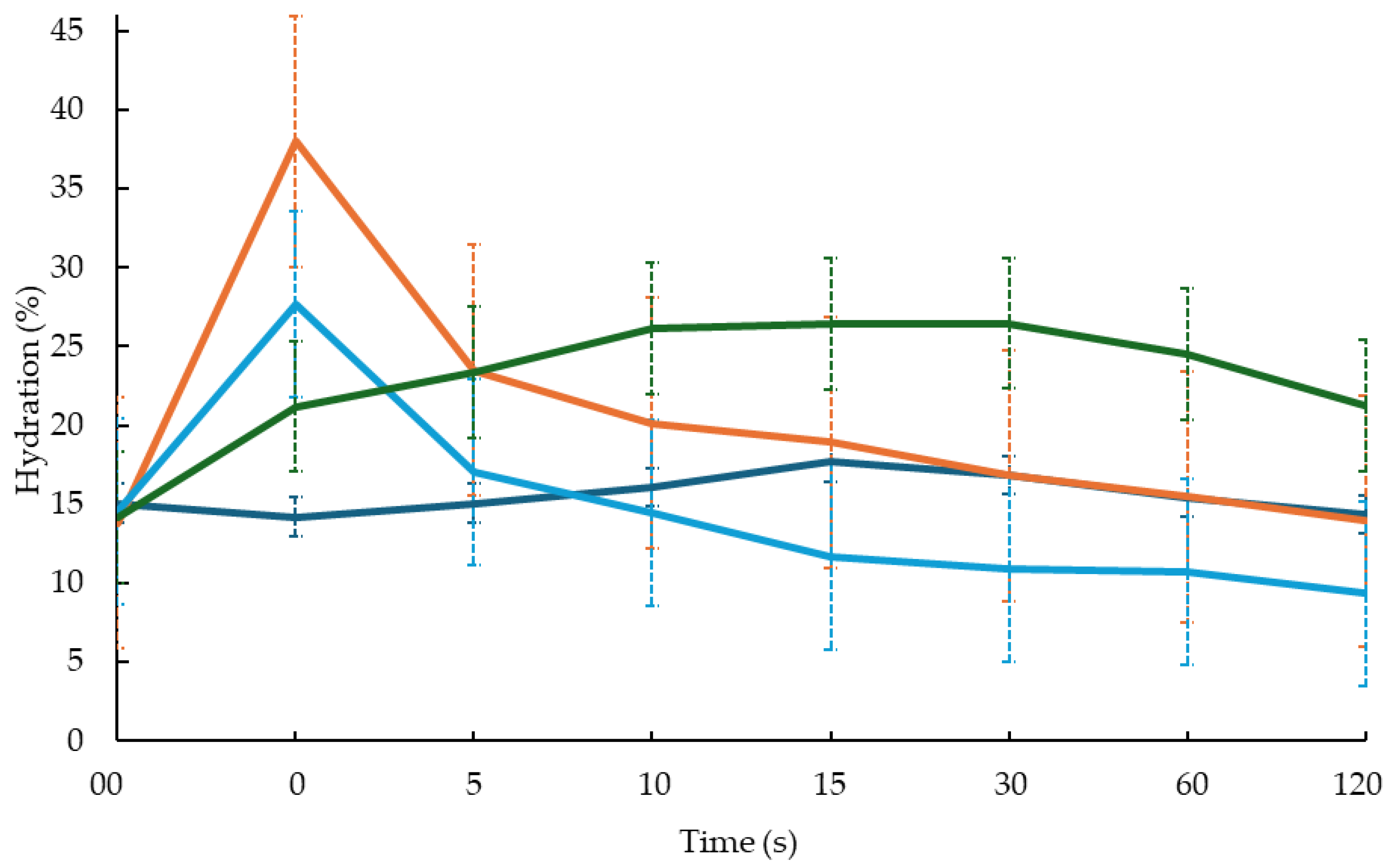
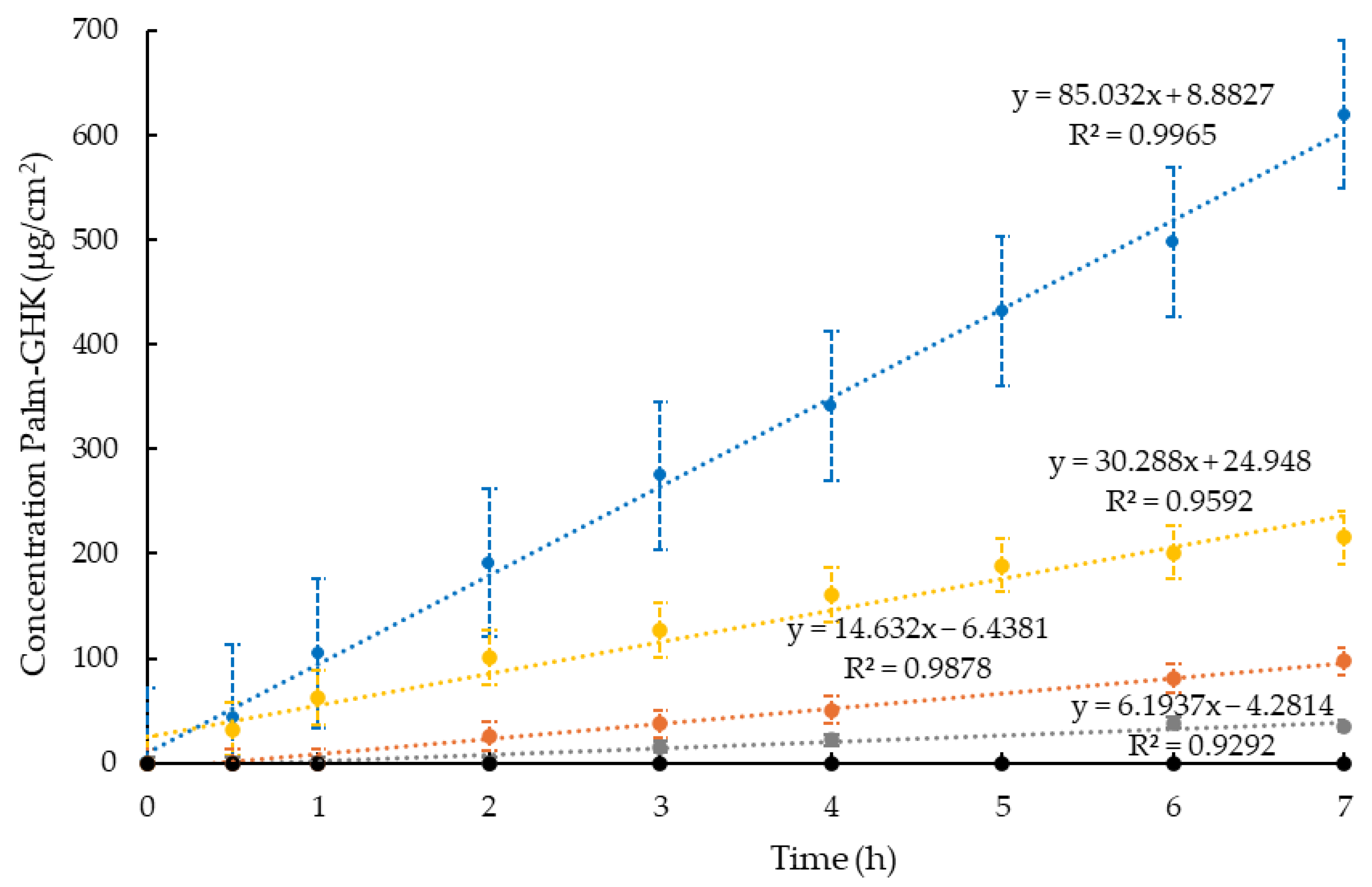
| Formulation | PC 1 %w/w | EtOH%w/w | IPP 2 %w/w | POL 3 %w/w | H2O %w/w | X-GUM 4 %w/w |
|---|---|---|---|---|---|---|
| ETO | 0.90 | 29.10 | - | - | 70.00 | - |
| ETO-GEL | 0.90 | 29.10 | - | - | 69.00 | 1.00 |
| POL | - | - | - | 20.00 | 80.00 | - |
| ORG | 15.60 | - | 82.96 | - | 1.44 | - |
| POL-ORG | 4.68 | - | 25.32 | 14.00 | 56.00 | - |
| Formulation | Z-Average (nm ± S.D.) | Intensity (nm) | PDI ± S.D. |
|---|---|---|---|
| ETO | 206.5 ± 0.0 | 100% | 0.105 ± 0.00 |
| ETO-GHK | 102.1 ± 6.4 | 100% | 0.142 ± 0.02 |
| Formulation | Spreadability (g·cm·s–1) ± S.D. | Occlusive Factor % ± S.D. |
|---|---|---|
| ETO-GEL | 19.17 ± 1.02 | 4 ± 0.096 |
| POL-ORG | 9.17 ± 0.41 | 39 ± 0.076 |
| ORG | 15.00 ± 0.00 | 63 ± 0.048 |
| POL | 15.00 ± 0.10 | 32 ± 6.030 |
| Formulation | Concentration Palm-GHK (mg/mL) | Jo (μg/cm2/h) ± S.D. | Flux (cm/h) ± S.D. |
|---|---|---|---|
| ETO-GHK | 0.92 | 27.14 ± 4.21 | 29.50 ± 4.57 |
| ETO-GEL-GHK | 0.92 | 0.68 ± 0.02 | 0.74 ± 0.02 |
| POL-GHK | 0.94 | 18.71 ± 1.97 | 19.90 ± 2.10 |
| POL-ORG-GHK | 0.94 | 7.68 ± 0.68 | 8.17 ± 0.72 |
| SOL-GHK | 1.00 | 77.71 ± 17.85 | 77.71 ± 17.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzyhovskyi, V.; Santamaria, F.; Marzola, E.; Montesi, L.; Donelli, I.; Manfredini, S.; Guerrini, R.; Esposito, E. Production and Characterization of Semi-Solid Formulations for the Delivery of the Cosmetic Peptide Palmitoyl-GHK. Cosmetics 2025, 12, 50. https://doi.org/10.3390/cosmetics12020050
Dzyhovskyi V, Santamaria F, Marzola E, Montesi L, Donelli I, Manfredini S, Guerrini R, Esposito E. Production and Characterization of Semi-Solid Formulations for the Delivery of the Cosmetic Peptide Palmitoyl-GHK. Cosmetics. 2025; 12(2):50. https://doi.org/10.3390/cosmetics12020050
Chicago/Turabian StyleDzyhovskyi, Valentyn, Federico Santamaria, Erika Marzola, Leda Montesi, Irene Donelli, Stefano Manfredini, Remo Guerrini, and Elisabetta Esposito. 2025. "Production and Characterization of Semi-Solid Formulations for the Delivery of the Cosmetic Peptide Palmitoyl-GHK" Cosmetics 12, no. 2: 50. https://doi.org/10.3390/cosmetics12020050
APA StyleDzyhovskyi, V., Santamaria, F., Marzola, E., Montesi, L., Donelli, I., Manfredini, S., Guerrini, R., & Esposito, E. (2025). Production and Characterization of Semi-Solid Formulations for the Delivery of the Cosmetic Peptide Palmitoyl-GHK. Cosmetics, 12(2), 50. https://doi.org/10.3390/cosmetics12020050








