Abstract
Unwanted abdominal fat is a common aesthetic concern treated through various interventions, including surgical and energy-based devices, often leading to inconsistent results. This study aimed to evaluate the feasibility of a localized, non-invasive microwave (MW) device for preferential heating of subcutaneous adipose tissue using a controlled electromagnetic field. Five female volunteers scheduled for abdominoplasty were enrolled, each undergoing a single MW treatment session five days prior to surgery. Histological analyses of adipose tissue and skin samples were conducted using Hematoxylin and Eosin staining and immunohistochemistry for Perilipin-1 and CD68. Epidermal and dermal layers remained unaffected, as evidenced by unaltered morphology in treated samples. In contrast, the absence of Perilipin-1 expression in disrupted fat cell membranes indicated adipocyte non-viability and irreversible injury. Inflammatory responses, including CD68-positive macrophages surrounding damaged adipocytes, were observed, suggesting the activation of the monocyte/macrophage system for the clearance of adipocyte residues. Microscopic and immunohistochemical findings demonstrate the effectiveness of the MW device in reducing subcutaneous fat. This study also discussed the underlying mechanisms involved in macrophage recruitment and the removal of adipocyte residues.
1. Introduction
Unwanted abdominal fat may result in an unattractive body profile and dissatisfaction with appearance. Subcutaneous adipose tissue (SAT) deposits of the central abdomen are especially common areas of concern for both males and females. They are due to the reduced lipolytic sensitivity of its adipocytes. An approved and rigorously tested non-surgical method for abdominal fat reduction is still not available. Many procedures, including surgery, lasers, and medical approaches, have been developed with satisfactory results. Many therapeutic options are available for the treatment of central abdominal adiposity. These include cryolipolysis, high-intensity focused ultrasound, non-thermal ultrasound, radiofrequency, and injection adipolysis. They all lead to adipocyte destruction through multiple different mechanisms [1]. Researchers presented theoretical and experimental works using different technology to reduce abdominal adipose tissue.
Interestingly, a recent paper [2] considered the properties of some tissues related to the absorption of microwave energy in different models using equivalent phantom and ex vivo measurements. Most of the experimental analyses of fat reduction found in the literature have been conducted in vitro, which only rarely accurately reflect the complex dynamics found in tissues and organs of living organisms.
Recent studies demonstrate the feasibility of a non-invasive, localized microwave (MW) device to induce thermal modifications into subcutaneous adipose tissue only by a controlled electromagnetic field that preferentially heats fat. Microwaves refer to electromagnetic waves at 2.5 kHz which produce thermal energy by increasing the temperature of the adipose tissue and thereby stimulating the adipocyte apoptotic pathway. They are very specific for the treatment of adipose subcutaneous fat [3,4].
Clinical studies confirmed the efficacy of microwaves on body contouring and reduction of SAT by using specific devices. They are provided with appropriately cooled special handpieces used directly on the body surface, providing a calibrated energy transfer by microwaves [5,6].
Regarding its mechanism of action, microwave technology may induce adipocyte damage in response to the functional surcharge in the transporting mechanisms through membranes of the peripheral cytoplasm. The movement of fatty acids into and out of the adipocytes is controlled by a complex network of proteins and enzymes, as well as many hormonal and metabolic factors [7].
It is possible to measure fat cell damage directly or indirectly; the viability of the adipocyte’s outer plasma membrane or inner lipid droplet membrane may be directly evaluated [8].
Perilipin, a phosphoprotein that is closely related to the adipocyte lipid droplet, is a crucial regulator of lipolysis in adipocytes. It performs essential tasks in the control of both hormonally stimulated and basal lipolysis [9,10]. Perilipin enhances triglyceride accumulation in baseline settings by limiting cytosolic lipases’ access to lipid droplets. When there is an energy deficiency, protein kinase A phosphorylates perilipin, enabling hormone-sensitive lipase and adipose triglyceride lipase to maximize lipolysis. Therefore, phosphorylation of perilipin is essential for the mobilization of fats in adipose tissue [11].
Hence, the surface of the lipid storage droplet has emerged as a central site of regulation of lipolysis. Perilipin-1 may be considered one of the major lipid droplet-binding proteins and is highly expressed in adipocytes.
Studies on the destiny of adipocytes have revealed that contrary to conventional H&E labelling, immunohistochemistry for perilipin can discriminate between live and dead adipocytes. So, during adipose fat remodeling, perilipin staining has become a common technique for determining adipocyte viability [12,13,14].
In this study, we provided morphological findings on subcutaneous adipose tissue after skin exposure to microwaves. A controlled electromagnetic field (EMF) @2.45 GHz, perpendicularly applied to the skin, is selectively absorbed by the subdermal fat layer thanks to the dielectric properties of the different tissues (epidermis, dermis, fat) crossed by the applied EMF. Due to the fat layer’s absorption properties at a set frequency, the high-controlled emission of the EMF can only create a perfect coupling in the presence of this layer. The selection of the 2.45 GHz frequency is based on its dielectric properties, which allow selective energy absorption in adipose tissue rather than in the superficial skin layers. Previous studies have demonstrated that 2.45 GHz microwaves penetrate approximately 1.2–1.5 cm into subcutaneous fat, delivering sufficient thermal energy for adipocyte disruption while minimizing epidermal heating [15,16]. While lower frequencies, such as 915 MHz and 433.9 MHz, penetrate deeper, they require higher power densities and may reduce targeting precision. Therefore, 2.45 GHz provides an optimal balance between penetration depth and controlled energy delivery for non-invasive fat reduction [4].
In summary, this study investigated adipose tissue response to microwave application on SAT in selected patients using Hematoxylin and Eosin (H&E) staining and immunohistochemistry for perilipin and CD68, a specific marker of monocyte/macrophage lineage cells, widely used to identify macrophages involved in phagocytosis and the removal of cellular debris. In adipose tissue, the presence of CD68-positive macrophages indicates immune system activation in response to adipocyte damage, and this immune response may play a fundamental role in understanding the processes behind adipose tissue remodeling and the decrease in subcutaneous fat volume. Indeed, the CD68 antigen is found in the cytoplasmic granules of a range of different blood cells. It is particularly useful as a marker for the various cells of the monocyte/macrophage lineage, including macrophages and monocytes [17,18].
2. Materials and Methods
To analyze the results carefully, we shall start by explaining that in the present study, 5 volunteers with a very specific characteristic, i.e., localized abdominal subcutaneous fat (with a skin fold thicker than 2 cm with a layer of subcutaneous fat of at least 1 cm), aged >18 years, were enrolled; an abdominoplasty had already been scheduled for the volunteers. Clearly, before proceeding with the operation, it is advisable to proceed with the evaluation of the patient’s suitability for the operation to evaluate whether she is suitable for abdominoplasty based on the inclusion and exclusion criteria; however, while it is critical to make this assessment prior to any intervention, these exams are beyond the scope of this study. Another category of patients, i.e., patients with permanent implants, such as metal/plastic plates, prostheses, and screws, or injected with chemical or autologous substances, fat injections, or prostheses in the abdominal area, were also excluded from the study.
This study was performed according to the Declaration of Helsinki and approved by the local Institutional Review Board. After being informed about the aim of the study, patients gave their written informed consent. All the enrolled volunteers were properly informed about the treatment steps and procedures to be performed.
For each enrolled patient, upon the first visit, demographic data and body mass index (BMI) were collected.
The mean BMI of the participants was 28.5 kg/m2 (range: 27.0–30.2 kg/m2), which falls within the overweight category according to the World Health Organization (WHO) classification. BMI data were analyzed to assess any potential influence on treatment outcomes.
Let us start by saying that in the pre-operative phase, the patient is subjected to a treatment five days before the scheduled date for the abdominoplasty operation. The patient underwent one session with the ONDA Plus system (DEKA M.E.L.A, Florence, Italy), a platform that has precise characteristics, namely that it uses two handpieces that produce microwaves at 2.45 GHz, generating localized and controlled heat in both deep and superficial subcutaneous tissues to thermally induce adipocyte damage [19]. In addition to the special design of the handpieces that conveniently channels the microwaves, the system can be equipped with a temperature sensor for further safety reasons.
An effective contact cooling system included in both handpieces is implemented to avoid thermal rebound damage to the surface layers during microwave transmission, ensuring total patient comfort and minimizing side effects and inflammation. These are aspects fundamentals that must always be considered in every treatment. In fact, if possible, we must always guarantee the greatest possible comfort to the patient to minimize both side effects and inflammation.
Based on the previous literature, microwave-based treatments have been shown to induce adipocyte disruption at temperatures ranging from approximately 45 °C to 50 °C without compromising the overlying skin. While direct temperature measurements of the subcutaneous fat were not performed in this study, the observed histological changes, including the loss of Perilipin-1 expression and the presence of CD68-positive macrophages, strongly suggest that the treatment induced a controlled thermal effect consistent with adipocyte injury.
The microwave treatment was performed five days before abdominoplasty, based on preliminary observations indicating that this timeframe allows for the early onset of adipocyte disruption and macrophage infiltration while minimizing the impact of prolonged inflammatory processes that could complicate histological interpretation. A longer interval might lead to tissue remodeling processes that could mask the direct effects of the microwave treatment on adipose tissue.
As with any plastic surgery procedure, in the pre-operative phase, it is advisable to identify the areas that will be affected. In particular, for each patient enrolled in this study, the abdomen was identified as the region that would be affected by the abdominoplasty, with 2 square areas of 15 × 15 cm2 that were symmetrical with respect to the sagittal plane. The navel was excluded from both squares. In particular, the perimeter of the squares was marked with a dermatological pen and then photographed to always ensure that these areas could be identified with certainty.
An important and interesting aspect is the differentiation between the area of the right and left square; in particular, the area in the right square was treated with microwaves while the area in the left square was considered as a “control group” and has not been treated. Before starting the treatment, a thin layer of pure vaseline oil was spread over the entire area to be treated for correct contact of the handpiece with the skin and smoothness of movements. During the session, by applying the Deep handpiece to the skin surface (heating focus 1.2 cm deep), microwaves were delivered into the abdominal subcutaneous adipose tissue with a total dose of 130,000 J delivered at 130 W power.
Sample collection: Two biopsy samples of adipose tissue and skin with an average diameter of 2–3 cm were taken from the tissue that was removed during the abdominoplasty procedure, in agreement with the region that the dermatographic pen had previously designated. The intervention sample was taken from the right square (treated), and the control sample was taken from the left square (untreated).
Histological procedure: The biopsy samples were fixed in 10% neutral formalin for histological investigation, followed by histochemical processing and paraffin embedding. Blocks were cut into sections of 5 μm thickness with a rotary microtome and the resulting sections were stained with H&E for examination under a light microscope. A Nikon Eclipse 80i light microscope was used to examine the samples (Nikon, Shinagawa, Tokyo, Japan).
Immunohistochemical procedures: Immunohistochemical reactions were performed on 3 μm-thick sections, arranged on marked slides, that were deparaffinized with xylene, rehydrated in alcohol and water and permeabilized for 30′ at 37 °C using Triton-X100 0.3% (Sigma-Aldrich, Milano, Italy) diluted in phosphate buffer saline (PBS). After blocking nonspecific binding sites with 1% bovine serum albumin (BSA) (Sigma-Aldrich, Milano, Italy) in PBS 30′ at 37 °C, primary monoclonal antibodies against the following antigens were applied overnight at 4 °C at the indicated dilutions: anti-Perilipin-1 (1:200) (GeneTex, Inc., Alton Pkwy, Irvine, CA, USA) and CD68 (1:200) (Sino Biological Europe, Eschborn, Germany), both in 0.1% BSA solution in PBS. The omission of primary antibodies was used as the negative control.
The next day, slices were treated with hydrogen peroxide blocking solution (Abcam, Cambridge, UK) for 10′ at room temperature, followed by incubation with Peroxidase-conjugated AffiniPure goat anti-mouse (IgG) secondary antibody (1:500) (Jackson ImmunoResearch Europe, Ely, UK) diluted in for 0.1% BSA solution in PBS, 1 h at 37 °C. Diaminobenzidine (DAB substrate) chromogen (Abcam, Cambridge, UK) dye was used as a marking detection system; sections were counterstained with hematoxylin, then dehydrated and mounted with Gel/Mount (Bioptica, Milano, Italy). Specimens were evaluated with a Nikon Eclipse 80i light microscopy; images were recorded through a microscope digital camera (Nikon Digital Sight DS-U1; Nikon) connected to a personal computer containing the software Nis Elements D 3.2 (Nikon).
3. Results
Histological analysis: Thanks to the skin cooling caused by the device handpiece, there are no differences in the epidermis and derma between the control (Figure 1A) and treated samples (Figure 1B).
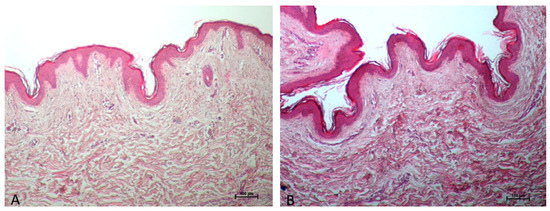
Figure 1.
The treated sample’s epidermis (B) does not differ from the control sample (A) in any way; no changes can be noticed in epidermis immediately after treatment (B) because of skin cooling performed by the device handpiece. Additionally, the dermis’s collagen seems to be strongly colored as a result of the heat-induced shrinkage or tightening of the collagen, which increases eosinophilia. ((A,B) 10× Magnifications).
Adipocyte membrane ruptures are seen at the level of adipose tissue in the treated samples compared to the control (Figure 2A,B). Additionally, the treated sample also exhibits vascular hyperaemia and immune system cells, most likely because of the monocyte–macrophage line that have entered the stromal interstitium (Figure 2C).
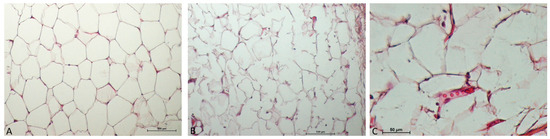
Figure 2.
In the adipose tissue immediately after treatment (B,C), the following are observed: wavy adipocitary membranes (weakening of membranes), some rupture of the plasma membrane, and initial hyperemia with dilation of blood vessels (C). In the control sample (A), packed adipocytes. ((A,B) 10× Magnification; (C), 40× Magnification).
Immunohistochemical analysis: The complete staining of the fat cell wall with an anti-perilipin antibody is an indicator of cell viability [20]. In the treated samples, Perilipin-1 is not expressed in the ruptured membranes (1.2–1.4 cm from the epidermis), despite being highly expressed on the membranes of the adipocytes in the control group. Perilipin-1 is still expressed more than 2 cm from the epidermis in the wavy adipocitary membranes, showing that there are still functional adipocytes in the deep fat layers and there has been no interference with perilipin. The absence of Perilipin-1 expression in the treated samples denotes the non-viability of the adipocytes, and it is indicative of irreversible fat cell injury (Figure 3).

Figure 3.
Adipocytes positive to Perilipin-1 in the control sample (A). After treatment with ONDA (B,C), adipocyte membranes are interrupted with negative Perilipin-1 results (non-viable adipocytes) (1.5–1.8 cm from the epidermis) (B); Perilipin-1 is still present in the wavy adipocitary membranes (more than 2 cm from the epidermis) (C). ((A–C), 40× Magnification).
Following treatment with the ONDA system, CD68-positive macrophages were detected between adipocytes, with broken or wavy membranes (Figure 4).
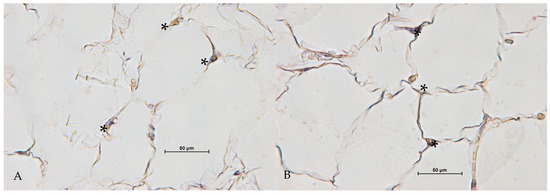
Figure 4.
CD68 positive cells (*) between adipocytes, with broken or wavy membranes after treatment with ONDA (A,B) ((A,B), 40× Magnification).
Treated tissue exhibits an inflammatory response in the fat layer, which is characteristic of injured fat. Control samples are negative for CD68, with no signs of an inflammatory response.
4. Discussion
When using a certain technology, it is necessary to evaluate its profile in terms of safety and effectiveness; in fact, the innovative microwave technology used in this study has proven to be safe and effective from a clinical point of view.
At the basis of every choice and analysis, we have seen the literature; in fact, various studies have demonstrated the clinical effectiveness and defined a protocol for the treatment of abdominal subcutaneous fat [21,22], with visible results in the reduction of skin laxity (the typical “orange peel” appearance) and in the decrease in body circumferences, but no histochemical evaluation has previously been demonstrated.
The technology used in this study is non-invasive, with clinically proven rapid results in a highly controlled and safe manner. Handpieces can provide maximum control over the depth of penetration to ensure internal organs are not damaged. The system used has very precise characteristics and allows for easy to use, safe, and painless operation.
The routine use of microwave technology before abdominoplasty offers several clinical advantages. Firstly, it facilitates the targeted disruption of adipocytes, which may contribute to easier surgical fat removal and improved contouring outcomes. The controlled thermal effect can enhance local circulation, potentially aiding in tissue healing post-surgery. Additionally, microwave treatment may induce collagen remodeling, which could lead to enhanced skin tightening effects, providing an added aesthetic benefit.
Despite these advantages, some potential complications should be considered in routine clinical practice. The primary risks associated with microwave treatment include localized erythema and transient edema, both of which are typically resolved within a few days. Additionally, variability in patient response due to differences in skin thickness, fat composition, and metabolic factors could impact treatment outcomes. Careful patient selection and individualized treatment planning are therefore fundamental to minimize adverse effects and optimize clinical results.
Compared to traditional diagnostic methods, such as histological analysis or imaging techniques, the proposed approach offers the advantage of being minimally invasive and potentially faster in providing results. However, its accuracy may depend on operator expertise and the standardization of procedures, which can pose challenges during clinical implementation. Emerging techniques, such as advanced molecular diagnostics or AI-based image analysis, often require significant investment in equipment and specialized training. In contrast, the proposed method stands out for its cost-effectiveness and ease of integration into routine practice.
Furthermore, previous research studies have shown that this technology increases cellular metabolism and local blood circulation by inducing self-regeneration processes with an increase in elastin fibers and collagen proliferation, a fundamental secondary aspect given the importance of self-regeneration processes. In the case of cellulite, the microwave energy absorbed by the connective fibrous septa causes the solubilization of collagen with consequent debridement of the dense inelastic mesh that strangles the adipose lobules. The result is a reduction in skin blemishes and a reactivation of fibroblasts to produce new collagen [3].
Our findings are aligned with those reported by Trelles et al. [23], who observed adipocyte membrane lysis and structural changes following radiofrequency treatment. While their study focused on the application of radiofrequency combined with infrared light and mechanical massage, our results demonstrated similar adipocyte disruption using microwave technology. Notably, Trelles et al. reported membrane thickening and volume reduction in adipocytes post-treatment, which parallels our findings of Perilipin-1 depletion and macrophage infiltration. These similarities support the hypothesis that various energy-based technologies can induce adipocyte remodeling through thermal effects and subsequent inflammatory responses.
Indeed, Zerbinati and colleagues [3] speculated over the ONDA’s mechanism of action, demonstrating that it is also effective for cellulite through the remodeling of collagen and for skin laxity via a tightening of collagen effect. This study showed evidence of appreciable changes in the fibrous connective tissue forming the septa with histochemical Picrosirius red staining in association with circularly polarized microscopy. The metalloproteinases family of matrix enzymes, which includes MMP-2, also known as interstitial collagenase, realistically plays a significant part in the regeneration of collagen. Due to an energetic charge from an external MW application, MMP-2 is quickly transformed from an inactive to an active molecule state. The old fibrotic bundles of collagen I start to break down as a result of this conversion, which is also preparing the connective tissue’s extracellular matrix for the renewal of fibrillar components, inducing fibroblasts to produce new native collagen (collagen III). This type is more elastic and clinically manifests with a decrease in the orange peel look of cellulite.
The clinical results led our group to focus on the mechanisms of action of microwave technology on fat cells. According to the literature, the system produces a connective matrix remodeling of adipose tissue and a subsequent microenvironmental change that regulates adipocyte metabolism [15]. The subdermal fat is modified through a series of molecular processes, stimulating a massive delivery of fat droplets. Indeed, an alteration in the homeostatic balance between connective interstitial tissue and adipocytes, responsible for the vitality of the adipose tissue, has been shown. It induces metabolic modifications due to thermal stress in the adipocytes, which are stimulated to release several lipids into the environment surrounding them in a much higher quantity than their physiological capacity. This metabolic stress induces the beginning of the “auto-adipolysis” of cells. Realistically, it may cause a functional surcharge in the transporting mechanisms across the peripheral cytoplasm membranes in adipocytes. Some essential cellular features, such as the plasma membrane, appear to be involved in adipocytes injury. That is essential for maintaining the intracellular microenvironment and the whole finely controlled transmembrane transport processes, which guarantee the correct ionic and molecular gradients and the related homeostatic equilibrium with the extracellular environment.
The interstitial connective tissue is the site of excessive molecular species transported through lipid droplets [24] in the form of fatty acids, adipokines, and pro-inflammatory molecules. This transport overtakes the normal physiologic homeostatic flow through the very thin peripheral cytoplasm of adipocytes [25].
Similar to what occurs in experimental obesity, which has been shown in mice and humans, “necrosis-like” alterations began as a result of these structural and functional processes [26]. The microparticles released by adipocytes encourage the recruitment of both local and blood-derived cells, particularly monocytes and macrophages, through a chemotactic process controlled by receptors known as “find me” receptors [27]. To all that, it follows the starting of phagocytosis through molecular triggering signals (“eat me” signals) of phosphatidylserine (a glycerophospholipid) delivered by adipocytes [28,29].
The excessive amount of free fatty acids activate the interstitial tissue’s immune system, which is represented by resident cells (a subset of macrophages also known as “dendritic cells”) via “Toll-like receptors” [30]. These cells conduct patrolling tasks in the interstitium and encourage the recall of more monocytes from the blood, like dendritic cells and Langerhans cells in the epidermis. Lipases [31], which function as enzymes on constitutive triglycerides inside adipocytes to supply glycerol and fatty acids, are also implicated in this process. In this way, these molecules are delivered by adipocytes which have already started a necrotic process, which significantly contributes to Reactive Oxygen Species formation [32,33].
These molecular species increase cytotoxic stress by rupturing cell membranes and releasing enzymes as well as cell fragments freely into the interstitial tissue. Using phagocytosis, macrophages remove these components from the interstitium here before migrating within lymphatic vessels to finish the final lysis of phagocytized components from implicated adipocytes [34].
Through changes in Perlipin-1 expression on subcutaneous abdominal adipose tissue, our study demonstrated the direct impact of microwave technology on adipocytes. Indeed, Perilipin-1 allowed us to assess the viability or not of the adipocytes. The loss of Perilipin-1 expression in the treated samples denotes the non-viability of the adipocytes and may be indicative of irreversible fat cell membrane injury due to the microwave technology used. We also showed that there was an increase in CD68+ macrophages, particularly in regions close to fatty tissue.
These processes indeed activate CD68+ macrophages responsible for the removal of adipocytes, thus resulting in subdermal fatty tissue reduction and circumference reduction. According to Cingolani and Czaja, macrophages are crucial to the lipolysis process and their presence during the cell self-destruction mechanism indicates an inflammatory process. Since adipocytes are often too large and too abundant to be digested by resident phagocytes, inflammatory cells are recruited to the area of fat injury for effective adipocyte digestion and removal [35].
Therefore, we can demonstrate for the first time that treated tissue exhibits an inflammatory response to the fat layer, which is characteristic of damaged fat and may explain the clinical efficacy of this technology.
A precise mathematical model for the distribution of microwave energy and resulting thermal effects on subcutaneous adipose tissue is fundamental for optimizing the procedure. Based on the literature, the dielectric properties of fat tissue suggest that localized temperatures between 45 °C and 55 °C are necessary to induce adipocyte apoptosis or necrosis. While the device used in this study was calibrated to deliver energy in a controlled manner, future studies should aim to model the heat diffusion and its correlation with the extent of tissue remodeling observed histologically.
5. Conclusions
This article illustrates a potential mechanism of action of microwave technology on adipocytes observed following the targeted treatment of subcutaneous fat in the abdominal region. The mechanism of action appears to be based on a combination of factors: Firstly, controlled hyperthermia, which involves a localized increase in temperature, plays a fundamental role in the process of degradation of adipose tissue. Added to this are a series of effects that are not immediately observable but equally relevant, such as the resonance of specific molecular species, both of a structural and enzymatic nature, with the frequency of the radiated waves. These phenomena contribute to inducing significant lysis of adipocytes, characterized by cellular alterations that present similarities with necrotic processes. This form of adipolysis creates, in turn, an environment that stimulates an innate immune response that actively involves monocytes and macrophages, immune cells that play a crucial role in phagocytosis, and the elimination of cellular debris.
In support of these observations, histological analyses conducted on biopsies taken from the treated areas highlighted some important alterations at a molecular level. Notably, immunohistochemistry revealed a notable decrease in the expression of Perilipin-1 protein, a marker known to be closely associated with the stability of lipid droplets in adipocytes. In parallel, an increase in the expression of inflammatory markers, in particular CD68-positive macrophages, was observed, suggesting an active infiltration of these immune cells in the areas affected by the treatment. This inflammatory response could be one of the keys to understanding the mechanisms of adipose tissue remodeling and the overall reduction of subcutaneous fat volume.
In conclusion, this preliminary immunohistochemical study seems to offer a promising basis to explain the effectiveness of microwave technology in the targeted treatment of subcutaneous adipose tissue, particularly in the central abdominal area, an area often of both clinical and aesthetic interest.
Despite its potential benefits, the proposed technique carries certain risks, including variability in sample preparation, interpretation of findings, or inherent limitations of the method in detecting specific pathologies. Another potential risk involves the reliance on specific equipment or reagents, which could limit accessibility in resource-constrained settings. Furthermore, the absence of long-term data on its clinical efficacy highlights the need for further validation studies to assess its reliability and reproducibility.
This study is limited by a small sample size and the absence of a control group, which restricts the generalizability of the findings. Additionally, the short follow-up period (five days post-treatment) does not allow us to assess the long-term effects and potential delayed adverse events. Future studies should incorporate larger, more diverse populations and include quantitative imaging techniques such as ultrasound or MRI to measure fat reduction.
Reshaping abdominal fat represents a common challenge, and the adoption of non-invasive technologies such as this can be a highly valuable therapeutic option. Although the results presented are encouraging, it remains necessary to conduct further research to confirm these preliminary observations and to deepen our understanding of the mechanisms involved. In this regard, the inclusion of this section in the manuscript could be useful, especially in contexts in which the discussion is particularly extensive or complex, thus helping to provide a more detailed and comprehensive overview.
Author Contributions
Conceptualization, L.B.; methodology, T.Z.; software, L.P.; validation, A.T., P.B. and K.H.; formal analysis, L.R.; investigation, G.F. and V.R.; data curation, L.P. and L.R.; writing—original draft preparation, E.Z. and S.B.; writing—review and editing, E.Z. and S.B.; visualization, K.H.; supervision, S.P.N.; project administration, S.P.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Calabria Centro (373/19, date of approval 4 December 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Available upon reasonable request from the corresponding author.
Conflicts of Interest
Authors T.Z., L.P., and L.R. are employed by El.En Group. The other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Friedmann, D.P. A review of the aesthetic treatment of abdominal subcutaneous adipose tissue: Background, implications, and therapeutic options. Dermatol. Surg. 2015, 41, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Asan, N.B.; Noreland, D.; Hassan, E.; Redzwan Mohd Shah, S.; Rydberg, A.; Blokhuis, T.J.; Carlsson, P.O.; Voigt, T.; Augustine, R. Intra-body microwave communication through adipose tissue. Heal. Technol. Lett. 2017, 4, 115–121. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zerbinati, N.; d’Este, E.; Farina, A.; Cornaglia, A.I.; Jafferany, M.; Golubovic, M.; Binic, I.; Sigova, J.; Van Thuong, N.; Tirant, M.; et al. Remodeling of collagen constituting interlobular septa of subcutaneous adipose tissue following microwaves application. Dermatol. Ther. 2020, 33, e13362. [Google Scholar] [CrossRef] [PubMed]
- Pahlavani, N.; Nattagh-Eshtivani, E.; Amanollahi, A.; Ranjbar, G.; Aghdaei, H.A.; Navashenaq, J.G.; Shabaninezhad, Z.; Sharahi, N.R.; Maleki, M.; Malekahmadi, M.; et al. Effects of microwave technology on the subcutaneous abdominal fat and anthropometric indices of overweight adults: A clinical trial. J. Cosmet. Dermatol. 2022, 21, 1482–1488. [Google Scholar] [CrossRef] [PubMed]
- Nisticò, S.P.; Bonan, P.; Coli, F.; Verdelli, A.; Fusco, I.; Gratteri, F.; Sicilia, C.; Cantisani, C.; Pellacani, G.; Bennardo, L.; et al. A New Protocol to Treat Abdominal Subcutaneous Fat Combining Microwaves and Flat magnetic stimulation. Bioengineering 2022, 9, 182. [Google Scholar] [CrossRef]
- Hoffmann, K.; Zappia, E.; Bonan, P.; Coli, F.; Bennardo, L.; Clementoni, M.T.; Pedrelli, V.; Piccolo, D.; Poleva, I.; Salsi, B.; et al. Microwave-Energy-Based Device for the Treatment of Cellulite and Localized Adiposity: Recommendations of the Onda Coolwaves International Advisory Board. Bioengineering 2024, 11, 1249. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thompson, B.R.; Lobo, S.; Bernlohr, D.A. Fatty acid flux in adipocytes: The in’s and out’s of fat cell lipid trafficking. Mol. Cell. Endocrinol. 2010, 318, 24–33. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zachary, C.B.; Burns, A.J.; Pham, L.D.; Jimenez Lozano, J.N. Clinical Study Demonstrates that Electromagnetic Muscle Stimulation Does Not Cause Injury to Fat Cells. Lasers Surg. Med. 2021, 53, 70–78. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tansey, J.T.; Sztalryd, C.; Hlavin, E.M.; Kimmel, A.R.; Londos, C. The central role of perilipin a in lipid metabolism and adipocyte lipolysis. IUBMB Life 2004, 56, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Brasaemle, D.L.; Subramanian, V.; Garcia, A.; Marcinkiewicz, A.; Rothenberg, A. Perilipin A and the control of triacylglycerol metabolism. Mol. Cell. Biochem. 2009, 326, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Brasaemle, D.L. Thematic review series: Adipocyte biology. The perilipin family of structural lipid droplet proteins: Stabilization of lipid droplets and control of lipolysis. J. Lipid Res. 2007, 48, 2547–2559. [Google Scholar] [CrossRef] [PubMed]
- Suga, H.; Eto, H.; Aoi, N.; Kato, H.; Araki, J.; Doi, K.; Higashino, T.; Yoshimura, K. Adipose tissue remodeling under ischemia: Death of adipocytes and activation of stem/progenitor cells. Plast. Reconstr. Surg. 2010, 126, 1911–1923. [Google Scholar] [CrossRef] [PubMed]
- Eto, H.; Kato, H.; Suga, H.; Aoi, N.; Doi, K.; Kuno, S.; Yoshimura, K. The fate of adipocytes after nonvascularized fat grafting: Evidence of early death and replacement of adipocytes. Plast. Reconstr. Surg. 2012, 129, 1081–1092. [Google Scholar] [CrossRef] [PubMed]
- Mashiko, T.; Yoshimura, K. How does fat survive and remodel after grafting? Clin. Plast. Surg. 2015, 42, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Foster, K.R.; Ziskin, M.C.; Balzano, Q. Thermal Response of Human Skin to Microwave Energy: A Critical Review. Health Phys. 2016, 111, 528–541. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.-L.; Zhao, D.; Hu, Z.-J.; Wang, Y.; Liang, F.; Wang, B.-Z. Increasing Microwave Penetration Depth in the Human Body by a Complex Impedance Match of Skin Interface with a Two-Layered Medium. Electronics 2024, 13, 3915. [Google Scholar] [CrossRef]
- Van der Kooij, M.A.; von der Mark, E.M.; Kruijt, J.K.; van Velzen, A.; van Berkel, T.J.; Morand, O.H. Human monocyte-derived macrophages express an approximately 120-kD Ox-LDL binding protein with strong identity to CD68. Arter. Thromb Vasc. Biol. 1997, 17, 3107–3116. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Killingsworth, M.C.; Myasoedova, V.A.; Orekhov, A.N.; Bobryshev, Y.V. CD68/macrosialin: Not just a histochemical marker. Lab. Investig. 2017, 97, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Zappia, E.; Bonan, P.; Coli, F.; Del Re, C.; Cassalia, F.; Tolone, M.; Bennardo, L.; Nisticò, S.P.; Cannarozzo, G. An innovative microwave technology for the treatment of submental skin laxity. Lasers Med. Sci. 2025, 40, 28. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sert, G.; Kucukguven, A.; Zırh, E.B.; Demirtaş, T.T.; Çakar, A.N.; Gümüşderelioğlu, M.; Calis, M. Photobiomodulation with polychromatic light (600–1200 nm) improves fat graft survival by increasing adipocyte viability, neovascularization, and reducing inflammation in a rat model. Lasers Surg. Med. 2022, 54, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, A.; Ferri, S.; Bonan, P.; Verdelli, A.; Stevan, S.; Tartaglia, C.; Perosino, E. Effectiveness of microwaves in the treatment of cellulite: A preliminary study. J. Plastic Pathol. Dermatol. 2019, 15, 3. [Google Scholar]
- Bennardo, L.; Fusco, I.; Cuciti, C.; Sicilia, C.; Salsi, B.; Cannarozzo, G.; Hoffmann, K.; Nisticò, S.P. Microwave Therapy for Cellulite: An Effective Non-Invasive Treatment. J. Clin. Med. 2022, 11, 515. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trelles, M.A.; Mordon, S.R. Adipocyte membrane lysis observed after cellulite treatment is performed with radiofrequency. Aesthetic Plast. Surg. 2009, 33, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Kranendonk, M.E.; Visseren, F.L.; van Balkom, B.W.; Nolte-’t Hoen, E.N.; van Herwaarden, J.A.; de Jager, W.; Schipper, H.S.; Brenkman, A.B.; Verhaar, M.C.; Wauben, M.H.; et al. Human adipocyte extracellular vesicles in reciprocal signaling between adipocytes and macrophages. Obesity 2014, 22, 1296–1308. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Salomon, C.; Freeman, D.J. Extracellular Vesicles from Adipose Tissue-A Potential Role in Obesity and Type 2 Diabetes? Front Endocrinol. 2017, 8, 202. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cinti, S.; Mitchell, G.; Barbatelli, G.; Murano, I.; Ceresi, E.; Faloia, E.; Wang, S.; Fortier, M.; Greenberg, A.S.; Obin, M.S. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005, 46, 2347–2355. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, A.; Mulya, A.; Lazic, M.; Radhakrishnan, D.; Berk, M.P.; Povero, D.; Gornicka, A.; Feldstein, A.E. Microparticles Release by Adipocytes Act as Find- Me Signals to Promote Macrophage Migration. PLoS ONE 2015, 10, e0123110. [Google Scholar] [CrossRef]
- Krahling, S.; Callahan, M.K.; Williamson, P.; Schlegel, R.A. Exposure of phosphatidylserine is a general feature in the phagocytosis of apoptotic lymphocytes by macrophages. Cell Death Differ. 1999, 6, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Engin, A.B. Adipocyte-Macrophage Cross-Talk in Obesity. Adv. Exp. Med. Biol. 2017, 960, 327–343. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.T.; Favelyukis, S.; Nguyen, A.K.; Reichart, D.; Scott, P.A.; Jenn, A.; Liu-Bryan, R.; Glass, C.K.; Neels, J.G.; Olefsky, J.M. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J. Biol. Chem. 2007, 282, 35279–35292. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, T.; Parton, R.G. Not just fat: The structure and function of the lipid droplet. Cold Spring Harb. Perspect. Biol. 2011, 3, a004838. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ventura, J.J.; Cogswell, P.; Flavell, R.A.; Baldwin, A.S., Jr.; Davis, R.J. JNK potentiates TNF-stimulated necrosis by increasing the production of cytotoxic reactive oxygen species. Genes Dev. 2004, 18, 2905–2915. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Green, D.R.; Galluzzi, L.; Kroemer, G. Cell biology. Metabolic control of cell death. Science 2014, 345, 1250256. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kataru, R.P.; Lee, Y.G.; Koh, G.Y. Interactions of immune cells and lymphatic vessels. Adv. Anat. Embryol. Cell Biol. 2014, 214, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Cingolani, F.; Czaja, M.J. Regulation and Functions of Autophagic Lipolysis. Trends Endocrinol. Metab. 2016, 27, 696–705. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).