Abstract
Reactive oxygen species (ROS), commonly recognized as free radicals, significantly contribute to skin damage by disrupting defense and repair mechanisms, thereby accelerating the aging process. An effective strategy to prevent and alleviate skin aging involves the application of topical formulations enriched with powerful antioxidant compounds. Sacha inchi oil (Plukenetia volubilis L.) has been reported to possess significant antioxidant activity, while its oil contains a high content of omega-3 fatty acids, offering potential anti-aging benefits. This study aims to evaluate the stability, in vitro anti-aging activity, and skin irritation assessments of a facial serum containing Sacha inchi oil (SIO) formulated as a topical anti-aging agent. The stability of the serum was assessed by analyzing its organoleptic properties, homogeneity, viscosity, spreadability, pH, microbial contamination, and heavy metal content over a three-month period under controlled climatic conditions. The in vitro anti-aging activity was evaluated through enzyme inhibition assays for neutrophil elastase and collagenase, while skin irritation was assessed via human patch testing. The results indicated that the SIO facial serum exhibits excellent stability, significant anti-aging activity, and is safe for topical application, with no irritant effects observed during skin irritation assessments.
1. Introduction
The process of skin aging is a multifaceted biological phenomenon influenced by intrinsic and extrinsic factors, such as ultraviolet (UV) radiation, environmental pollution, and free radicals [1,2]. Free radicals significantly contribute to the acceleration of skin aging through oxidative stress, which damages dermal structures, such as elastin and collagen, resulting in reduced skin elasticity and wrinkle formation [3,4,5]. Due to the increasing incidence of skin damage, there is a critical need for effective and safe preventive strategies and therapeutic interventions. One promising approach involves the use of natural extracts as raw materials in skincare formulations [6,7]. Natural ingredients offer potential advantages as they contain bioactive compounds that provide therapeutic benefits while minimizing side effects [8].
Sacha inchi oil (SIO) has been identified as a natural ingredient with significant potential as an active anti-aging agent in skincare formulations. Previous research demonstrates that SIO exhibits substantial antioxidant activity, with an IC50 value of 8.859 ppm [9]. Maya et al. (2023), Irianti et al. (2021), and Hadzich et al. (2020) reported that this antioxidant efficacy is attributed to its chemical constituents, including vitamin E, vitamin A, flavonoids, terpenoids, and steroids [9,10,11]. In addition, SIO is rich in unsaturated fatty acids, particularly omega-3 (48.5%), omega-6 (34.8%), and omega-9 (7.7%) [9]. These fatty acids contribute to skin health by enhancing cell regeneration, preserving moisture and elasticity, and promoting collagen synthesis [11]. The synergistic interaction of these components boosts the efficacy of SIO in preventing skin aging, exceeding the performance of cosmetic treatments based on a single bioactive compound [9].
The safety of SIO has been thoroughly evaluated, and it has been reported to be safe for use, non-genotoxic, and non-toxic [12,13]. Further research by Soimee et al. (2019) demonstrated that SIO possesses significant moisturizing properties and does not cause skin irritation. Their findings indicated that SIO does not stimulate the secretion of TNF-α or IL-1α, nor does it impair the integrity of keratin in the stratum corneum. A clinical study involving 13 volunteers further showed that SIO enhances skin hydration levels, with the results comparable to those achieved with olive oil, a well-known natural moisturizer. These outcomes confirm that SIO can be safely incorporated as an active ingredient in skincare formulations [14].
A review by the Centre for the Promotion of Imports from Developing Countries (CBI) identifies SIO as a promising ingredient in skincare products, attributed to its high omega-3 content, which constitutes 48.5% of its total fatty acids. This concentration is significantly higher compared to olive oil (1%) and argan oil (0.5%), highlighting its superiority as a source of essential fatty acids. Omega-3 plays a critical role in skin cell regeneration by regulating inflammation and promoting collagen synthesis, thereby making it a valuable active ingredient for skincare products, particularly in anti-aging formulations [9,15].
Facial serums are advanced cosmetic formulations designed to deliver a high concentration of active ingredients, enabling intensive penetration into the deeper layers of the skin. This characteristic allows serums to provide faster and more noticeable results compared to other types of skincare products [16]. Beyond their rapid cosmetic effects, the use of facial serums also enhances psychological satisfaction among consumers, as they deliver visible, tangible, and practical results, meeting the demands of modern skincare routines [17].
SIO is not only safe for use but also effective in neutralizing free radicals and enhancing skin hydration. Accordingly, this study aims to develop and evaluate the stability, in vitro anti-aging activity, and skin irritation assessment of a facial serum containing SIO. The findings of this research are expected to provide strong scientific evidence supporting the efficacy and safety of SIO as an active anti-aging ingredient in skincare formulations, thereby offering a promising alternative for broader application in the cosmetic industry.
2. Materials and Methods
2.1. Materials
The materials utilized in this study included Sacha inchi oil (Quilla Indonesia, Bandung, Indonesia), glyceryl stearate, ceteareth-33, stearyl alcohol (Megasetia, Jakarta, Indonesia), stearyl alcohol (Interchem Prima Mitra, Jakarta, Indonesia), polyacrylamide, C13-14 isoparaffin, laureth-7 (Avanthchem, Jakarta, Indonesia), glycerin, phenyl trimethicone, dimethicone (Kemiko Indonesia, Jakarta, Indonesia), butylated hydroxytoluene (DKSH, Jakarta, Indonesia), frangipani oil (Benberg Arome, Gresik, Indonesia), phenoxyethanol (Tentrem Artha Nugraha, Jakarta, Indonesia), collagen assay kit (MAK322), and neutrophil elastase activity assay kit (MAK246) (Merck KGaA, Darmstadt, Germany).
2.2. Methods
This study was conducted in four sequential stages, beginning with the formulation of the SIO facial serum, followed by accelerated stability studies, metalloproteinase activity assays, and irritation testing.
2.2.1. Formulation of SIO Facial Serum
The facial serum formulated in this study incorporates SIO as the active ingredient alongside excipients commonly used in cosmetic formulations, such as emollients, emulsifiers, solvents, and fragrances [18]. To maintain the formulation’s quality and stability, preservatives were also included [19,20]. The SIO facial serum was formulated as a viscous liquid, predominantly comprising an oil-based phase. During the formulation process, distilled water was divided into two parts: part 1 (L1) and part 2 (L2). The aqueous phase, consisting of L1 and components I and K, was heated to 70 °C (M1). Concurrently, the oil phase, comprising components B, C, D, F, G, and H, was heated to the same temperature (M2). The heated aqueous phase (M1) was then combined with the oil phase (M2) under mechanical stirring (IKA Eurostar Power Control) to achieve homogeneity (M3). Subsequently, component A was added to M3 and stirred until a uniform dispersion was obtained. Component E was then incorporated into the mixture with continuous stirring until the formulation thickened to the desired consistency (M4). Upon reaching the target consistency, L2 was gradually introduced to M4 while maintaining continuous stirring to ensure homogeneity. Finally, component J was added and thoroughly mixed to produce the final serum formulation containing SIO. The detailed composition of the SIO facial serum is provided in Table 1.

Table 1.
Formula of SIO facial serum.
2.2.2. Accelerated Stability Studies
The stability of the SIO facial serum was assessed through accelerated stability testing conducted over a three-month period under the controlled conditions of 40 ± 2 °C and 75 ± 5% relative humidity (RH). The primary objective of this study was to evaluate the formulation’s stability by analyzing various parameters, including the organoleptic properties, homogeneity, viscosity, spreadability, pH, microbial contamination, and heavy metal content [21,22].
2.2.3. Metalloproteinase Activity Assay
The anti-aging activities were evaluated by assessing neutrophil elastase and collagenase inhibitory activities using fluorometric methods.
- Anti-Elastase Assay
Elastase inhibition was assessed by measuring the color intensity of the solution, following the method outlined by Feldo et al. (2022), with slight modifications. The inhibition potential of matrix metalloproteinases (MMPs) was assessed using a fluorometric neutrophil elastase (NE) assay kit (Sigma-Aldrich MAK246) in accordance with the manufacturer’s protocol. Succinylalanylalanylalanylalinechloromethylketone (SPCK), a well-established elastase inhibitor, was utilized as a positive control at a concentration of 20 mM. The assays were performed in a standard 96-well microplate with a clear flat bottom, and fluorescence was measured immediately after the reaction using a microplate reader, with excitation at λ = 380 nm and emission at λ = 500 nm [23]. The inhibition of NE activity by the samples was calculated using the following Formula (1):
- 2.
- Anti-Collagenase Assay
The collagenase inhibition activity of the formulated facial serum was assessed using a fluorometric assay kit (Sigma-Aldrich MAK322) according to the manufacturer’s protocol. A collagenase inhibitor at a concentration of 10 mM was employed as a positive control. The assay was conducted in a standard 96-well microplate with a clear flat bottom, and fluorescence was measured immediately using a microplate reader, with excitation set at λ = 375 nm and emission at λ = 465 nm. The measurements were performed in kinetic mode for 30 min at 37 °C [23]. The collagenase inhibitory capacity of the facial serum was calculated using the following Formula (2):
2.2.4. Ethical Approval for Clinical Studies
This study adhered to the ethical principles outlined in the Declaration of Helsinki and the ICH Good Clinical Practice (GCP) guidelines. All study protocols and amendments were reviewed and approved by the Institutional Review Board (IRB) prior to initiation. Ethical approval was granted by the Ethics Committee of Universitas Bakti Tunas Husada Tasikmalaya (Approval No. 304-01/E.02/KEPK-BTH/IX/2024, dated 5 September 2024).
2.2.5. Skin Irritation Test
Irritation testing was conducted to assess the skin compatibility of the cosmetic product in a study involving 22 human volunteers. Skin compatibility was defined as the absence of irritation when the product was used under normal conditions, considering both objective reactions, such as redness or swelling, and subjective sensations, including stinging, burning, or itching [24,25]. The inclusion criteria for the study included healthy volunteers aged 18–50 years who were not using other cosmetic products on the test area, were willing to apply the test product as instructed, and consented to the observation of the application area as per the study protocol. Exclusion criteria included individuals with acute or chronic skin diseases, dermatological conditions, sensitive skin, known allergies to any components of the test product, and those who were pregnant or breastfeeding. The SIO facial serum was applied to the participants’ backs under occlusive patches for 48 h, with evaluations conducted at 1 h, 24 h, and 48 h post-application. Redness was visually assessed, with its severity graded, and the intensity of redness was also measured objectively. The primary irritation index (PII) was calculated by dividing the total sum of erythema and edema scores by the number of grading intervals [24,25]. The detailed results are presented in Table 2 and Table 3.

Table 2.
Skin irritation scoring system.

Table 3.
Average irritation index.
2.2.6. Statistical Analysis
Statistical analyses for the parameter stability tests were conducted using IBM SPSS software, version 29.0 for macOS. The Shapiro–Wilk test was employed to evaluate data normality, which is particularly suitable for small sample sizes. Parametric data were analyzed using two-way analysis of variance (ANOVA), while non-parametric data were assessed with the Friedman test.
3. Results
3.1. SIO Facial Serum
The formulation results of the SIO facial serum are presented in Figure 1. Three variations of the SIO facial serum were developed, differing in their concentrations of SIO. Specifically, formula 1 contains 5% SIO, formula 2 contains 7.5% SIO, and formula 3 contains 10% SIO. The SIO facial serum is characterized as a white, viscous liquid with a mild aromatic scent of frangipani. The detailed composition of each formula is provided in Table 1.

Figure 1.
SIO facial serum.
3.2. Stability of SIO Facial Serum
A stability study was carried out to evaluate the product’s durability under specified testing conditions over a defined period, ensuring its safety for application. The stability tests involved accelerated conditions over three months in climatic conditions at 40 ± 2 °C/75 ± 5% RH. The assessments encompassed organoleptic characteristics, homogeneity, viscosity, spreadability, pH, microbial contamination, and heavy metal content. Detailed results of the stability tests for the SIO facial serum are presented in Table 4, Table 5 and Table 6 and Figure 2, Figure 3 and Figure 4.

Table 4.
Accelerated stability study of SIO facial serum.

Table 5.
Results of microbial contamination analysis.

Table 6.
Results of heavy metal contamination analysis.
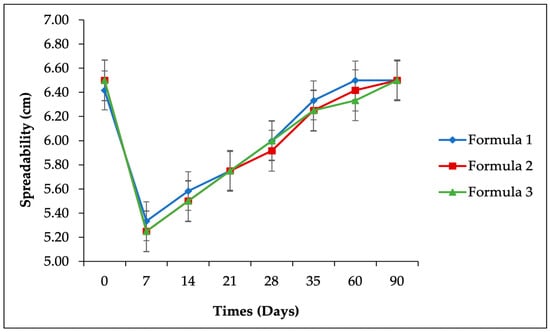
Figure 2.
Accelerated stability study results for spreadability parameters of the SIO facial serum.
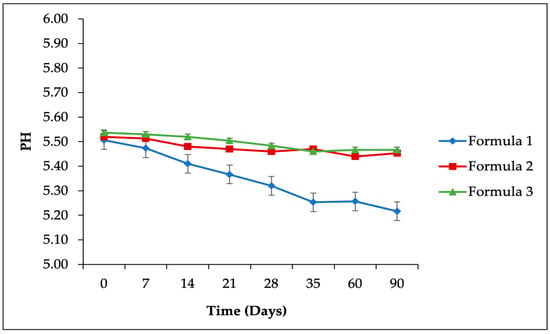
Figure 3.
Accelerated stability study results for pH parameters of the SIO facial serum.
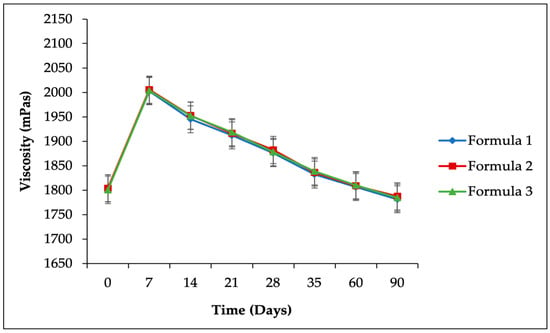
Figure 4.
Accelerated stability study results for viscosity parameters of the SIO facial serum.
3.3. Metalloproteinase Activity
The anti-aging activity of the SIO facial serum was evaluated by measuring the inhibition of neutrophil elastase and collagenase enzymes, with the results expressed as percentage inhibition, as shown in Figure 5 and Figure 6. The serum formulations were as follows: formula 1 (5% SIO), formula 2 (7.5% SIO), and formula 3 (10% SIO). The percentage inhibition of neutrophil elastase was 52.34%, 57.94%, and 71.96% for formulas 1, 2, and 3, respectively. The positive control, retinoids, exhibited a percentage inhibition of 69.16%. Regarding collagenase inhibition, the activity levels were 11.11%, 38.89%, and 66.67% for formulas 1, 2, and 3, respectively. The positive control (retinoids) resulted in a collagenase inhibition activity of 55.55%.
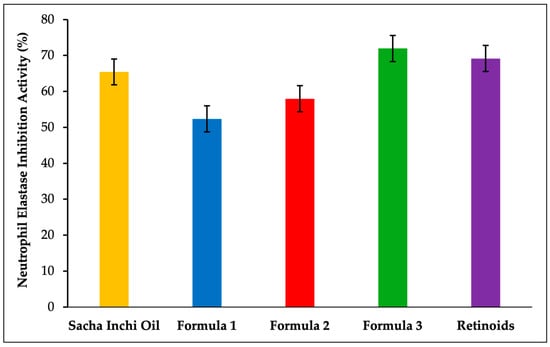
Figure 5.
Results of the neutrophil elastase inhibition activity.
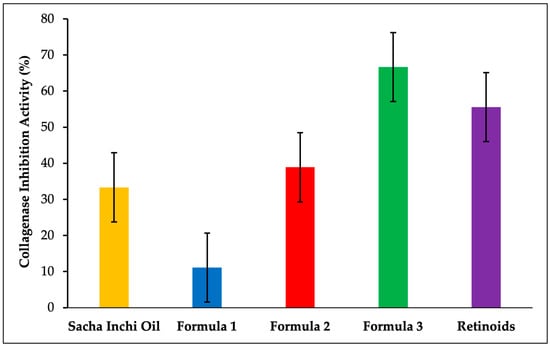
Figure 6.
Results of the collagenase inhibition activity.
3.4. Irritation Studies
The irritation test for the SIO facial serum (formula 3) was conducted to evaluate its compatibility with human skin, ensuring no irritation or adverse reactions occur under normal use conditions [26]. The study included 20 female participants with a mean age of 22 years and was conducted over a 48 h period, with observations recorded at 0, 24, and 48 h. The results were assessed using the scoring system detailed in Table 7. The findings showed that the SIO facial serum did not cause any signs of skin irritation, as no erythema, edema, or other symptoms were observed throughout the study. The primary irritation index (PII) was calculated as 0, confirming the serum’s excellent compatibility with human skin. These results validate the safety of the SIO facial serum for use on human skin.

Table 7.
Irritation test results for the SIO facial serum.
3.5. Statistical Analysis Results
Statistical analyses were performed to evaluate the significance of the experimental results, focusing on the stability parameters, including spreadability, pH, and viscosity. The analyses were conducted using IBM SPSS Statistics software (version 29.0 for macOS). Given the limited sample size, the Shapiro–Wilk test was employed to assess the normality of the data. For parametric data, such as the pH and viscosity, a two-way analysis of variance (ANOVA) was utilized, while non-parametric data, such as spreadability, were analyzed using the Friedman test. Detailed results of the stability testing are summarized in Table 8.

Table 8.
Statistical analysis results for stability studies.
4. Discussion
Various methods have been employed to prevent premature aging, including cosmetic surgical procedures and laser technologies. However, these approaches are associated with significant risks of side effects and relatively high costs [27,28]. Alternatively, aging prevention can be addressed through cosmetic care, the use of cell regulators, and topical agents enriched with antioxidants. Topical formulations containing antioxidants and cell regulators have demonstrated effectiveness in minimizing visible signs of aging, including hyperpigmentation and wrinkles [6].
The active ingredients commonly used to prevent and address the effects of aging include vitamins A, B3, C, D, and E. Vitamin A (retinol) directly influences collagen metabolism by stimulating collagen synthesis and enhancing elastin fibers. Vitamin B3 (niacinamide) inhibits the activity of extracellular matrix (ECM) degradation enzymes, thereby preserving skin structure. It also enhances collagen synthesis in dermal fibroblasts, improving skin elasticity and minimizing the appearance of fine lines and wrinkles, solidifying its role in anti-aging skincare [29,30,31]. Vitamin C is essential in addressing photoaging by enhancing collagen synthesis, stabilizing collagen structures, and mitigating their breakdown. Acting as a cofactor for the enzymes prolyl and lysyl hydroxylase, it facilitates the cross-linking and stabilization of collagen fibers, thereby maintaining their structural integrity [30,31,32,33]. Similarly, vitamin D (cholecalciferol) contributes to skin health by stimulating the production of metallothionein (MT)-mRNA, which efficiently scavenges UV-induced free radicals. This function promotes collagen synthesis and enhances skin elasticity [34,35]. Vitamin E (α-tocopherol) contributes to skin protection by reducing inflammation and inhibiting excessive cell proliferation. It also improves skin smoothness and promotes moisture retention within the stratum corneum, further supporting skin health and appearance [29,36].
Despite the availability of numerous anti-aging products in the Indonesian market, a significant proportion of the active ingredients in these formulations are imported and may not be optimally suited for the skin types and climatic conditions prevalent in Indonesia. This study, therefore, investigates the potential of SIO, a natural ingredient indigenous to Indonesia, for preventing skin aging. Previous research has demonstrated that SIO contains several bioactive compounds, including vitamin E, vitamin A, flavonoids, terpenoids, and steroids. Furthermore, SIO contains a high concentration of unsaturated fatty acids, including omega-3, omega-6, and omega-9 [9]. These fatty acids contribute to skin health by stimulating the regeneration of new skin cells, preserving moisture and elasticity, and promoting collagen production [11]. The synergistic effects of these components may increase the anti-aging efficacy of SIO, potentially exceeding the performance of cosmetic treatments that depend on a single bioactive ingredient.
The SIO facial serum was formulated as a viscous liquid with an oil-dominant phase. The prototype formulation, as detailed in Table 1, incorporates SIO as the principal active ingredient, complemented by lipid-based excipients such as glyceryl stearate, ceteareth-33, dimethicone, stearyl alcohol, and phenyl trimethicone. These components enhance the emollient and occlusive properties of the serum, thereby reinforcing the skin barrier and providing prolonged hydration. The high oil content, stabilized by emulsifiers, contributes to the formulation’s desirable rheological characteristics, including a rich and thick consistency. This composition facilitates the efficient delivery of the bioactive compounds from SIO while ensuring product stability [37].
Dimethicone fluids, widely used in skincare formulations, are recognized for their spreadability, smooth skin feel, and protective properties. Their improvement in spreadability can be achieved at relatively low concentrations (<1–2%), and they are often employed to reduce the stickiness of moisturizers containing humectants such as glycerin [38]. This oil-based formulation is specifically designed to enhance skin moisture retention and promote barrier repair, offering a targeted approach for dermatological applications. The final formulation resulted in a viscous liquid with a mild aromatic scent of frangipani and a white appearance (see Figure 1).
The quality parameters of the SIO facial serum were assessed through stability testing, in vitro anti-aging activity analysis, and safety evaluation via skin irritation testing. The stability tests encompassed parameters such as organoleptic properties, homogeneity, viscosity, spreadability, pH, microbial contamination, and heavy metal content. The organoleptic evaluation focused on the serum’s consistency, color, and odor. As presented in Table 4, all three SIO facial serum formulations maintained stable organoleptic properties over a three-month storage period under accelerated climatic conditions (40 ± 2 °C/75 ± 5% RH). The formulations retained their viscous liquid consistency, white color, and mild aromatic scent of frangipani, as illustrated in Figure 1.
Homogeneity testing was performed to assess the uniformity of the serum formulation by evaluating the consistency of particle distribution within the preparation [39]. As shown in Table 4, all three formulations (Formulas 1, 2, and 3) of the SIO facial serum did not exhibit any coarse particles when spread on a transparent glass surface. They appeared smooth and free of clumping throughout the three-month storage period at 40 ± 2 °C/75 ± 5% RH. These findings indicate that the prepared serum possesses a homogeneous composition.
The spreadability test was conducted to evaluate the formulation’s ability to spread, a critical characteristic in formulation science, as it influences the absorption and release rate of active ingredients at the target site [40]. As illustrated in Figure 2, during the three-month storage period at 40 ± 2 °C/75 ± 5% RH, a decrease in spreadability was observed in the first week, followed by an increase from the second to the twelfth week (p > 0.05) (see Table 8). Despite these fluctuations, the spreadability values remained within the range classified as good (5–7 cm) [41]. The observed increase in spreadability is likely associated with a decrease in viscosity, as a reduction in viscosity typically enhances the spreadability of a formulation [42].
Topical products, including serums, are recommended to have a pH range of 4–6 [43], aligning with the typical pH of facial skin, which is 4.5–5.5 for females and 4.0–5.5 for males [44]. The pH measurements of the SIO facial serum formulations are illustrated in Figure 3. Over the storage period at 40 ± 2 °C/75 ± 5% RH, a gradual decrease in pH was observed across all formulations. Specifically, the pH of formula 1 (5% SIO) declined from 5.51 ± 0.02 to 5.22 ± 0.02, formula 2 (7.5% SIO) decreased from 5.52 ± 0.01 to 5.45 ± 0.01, and formula 3 (10% SIO) reduced slightly from 5.52 ± 0.03 to 5.47 ± 0.02. The most significant decrease in pH was observed in formula 1, with a difference of 0.29 units (p < 0.05) (see Table 8). The observed decrease in pH is likely due to the interaction of CO2 with the aqueous phase of the serum, resulting in the formation of acidic compounds. Despite this reduction, all three formulations of the SIO facial serum maintained pH values within the acceptable range of 4–8, as specified by SNI-16-4399-1996 [45].
Viscosity measurements were performed to evaluate the consistency changes during the formulation’s storage period [46]. The viscosity data, presented in Figure 4, indicate an initial increase in viscosity after one week of storage at 40 ± 2 °C/75 ± 5% RH. This increase is attributed to the impact of shear forces applied during the mixing process, which temporarily reduced the viscosity. Over time, the polymer structure of the formulation recovered, resulting in the observed increase. However, a general decrease in viscosity was noted for formulas F1, F2, and F3 during the storage period under accelerated conditions (p > 0.05), as shown in Table 8. This reduction is likely due to elevated temperature and humidity, which increased the water content in the formulations and enhanced reactivity by allowing moisture absorption from the environment. Nevertheless, all viscosity values remained within the acceptable range of 800 to 2000 mPas [41].
In this study, the SIO facial serum was evaluated for microbial and heavy metal contamination. The microbial contamination assessment included a total plate count, yeast and mold counts, and specific tests for the presence of Pseudomonas aeruginosa, Staphylococcus aureus, and Candida albicans. These evaluations were conducted to confirm that the cosmetic serums adhered to the established contamination thresholds for cosmetic products. Microbial contamination poses significant health risks due to the presence of harmful bacteria [47]. The results of microbial contamination testing, detailed in Table 5, demonstrated compliance with the established regulatory guidelines. Additionally, the heavy metal contamination analysis, summarized in Table 6, revealed no detectable levels of cadmium (Cd), lead (Pb), arsenic (As), or mercury (Hg). Heavy metal contamination, characterized by elements with high atomic mass, is well-documented for its toxic effects on living organisms. Monitoring both microbial and heavy metal contamination is crucial to ensuring the safety and quality of cosmetic products. Repeated exposure to heavy metals in cosmetics may lead to absorption through the skin, potentially causing skin damage. Prolonged exposure may further disrupt organ function or lead to systemic diseases [48].
Anti-aging activity was assessed by evaluating neutrophil elastase and collagenase, enzymes implicated in skin aging. Neutrophil elastase plays a role in the degradation of essential extracellular matrix components, such as elastin, fibronectin, and collagen. Elastin, a critical protein for maintaining skin elasticity and the integrity of various tissues such as arteries, lungs, and ligaments, is particularly affected by this process [49]. The breakdown of elastin by elastase leads to diminished skin elasticity, a process exacerbated by UV radiation, which increases elastase activity and accelerates elastin degradation. Research suggests that effective anti-aging cosmetics often function by reducing elastase activity, thereby helping to mitigate elastin degradation and supporting the maintenance of skin elasticity [50]. The anti-aging activity results against neutrophil elastase are expressed as percentage inhibition. As illustrated in Figure 5, SIO exhibited a percentage inhibition of 65.42%. The serum formulations exhibited percentage inhibitions of 52.34%, 57.94%, and 71.96% for formulas 1, 2, and 3, respectively. The positive control, retinoids, achieved a percentage inhibition of 69.16%. These results suggest that the percentage inhibition increases with higher concentrations of SIO in the serum formulations, with formula 3 showing a higher inhibition value compared to retinoids. Compounds responsible for inhibiting neutrophil elastase activity include terpenoid compounds such as monoterpenes and triterpenes, as well as phenolic compounds like flavonoids [51]. Additionally, fatty acids, particularly those with more than 18 carbon atoms and at least one double bond, such as omega-3, exhibit inhibitory effects on elastase. Lourith et al. (2024) reported that the inhibitory activity of SIO is comparable to that of vitamin C against elastase [52]. Although the precise mechanism of neutrophil elastase inhibition is not fully elucidated, it is hypothesized that hydrophobic interactions between the enzyme and compounds in the sample may induce conformational changes in elastase [53]. Furthermore, hydroxyl groups are thought to exhibit inhibitory activity by directly binding to form an enzyme-inhibitor complex, thereby preventing the enzyme from interacting with its substrate [54].
An additional anti-aging activity test was performed using collagenase, an enzyme from the matrix metalloproteinase (MMP) family that degrades key components of the extracellular matrix, including aggrecan, elastin, fibronectin, gelatin, laminin, and collagen [55]. Collagenase plays a critical role in extracellular matrix degradation, making it a target for agents designed to maintain healthy skin by preventing dermal matrix breakdown [56]. As collagen levels naturally decline during the aging process, skin elasticity diminishes, leading to wrinkle formation. Collagen is a common ingredient in cosmetics due to its anti-aging and skin-regeneration properties. However, traditional methods for collagen measurement often require labor-intensive hydrolysis with acids or bases, expensive antibodies, and complex protocols [57]. In this study, collagen inhibition was assessed using a collagen assay kit, a straightforward, non-radioactive, and highly sensitive method for evaluating collagen inhibition. The assay begins with the enzymatic conversion of collagen in the sample into peptides. These peptides, containing terminal-N glycine, react with a dye reagent to form a fluorescent complex. The intensity of the fluorescence correlates directly with the collagen concentration in the sample. As illustrated in Figure 6, the anti-aging activity test against collagenase showed inhibition levels of 11.11%, 38.89%, and 66.67% for formulas 1, 2, and 3, respectively. Retinoids demonstrated collagenase inhibition activity of 55.55%. These results indicate that collagen inhibition increases proportionally with the concentration of SIO in the serum formulations, with formula 3 exhibiting higher collagen inhibition compared to retinoids. The anti-collagenase activity of SIO is attributed to its high content of omega-3 fatty acids (especially α-linolenic acid), as well as vitamins A and E. Omega-3 fatty acids provide anti-inflammatory properties, reducing collagenase overexpression caused by inflammation and enhancing the structural integrity of the extracellular matrix [58,59]. Vitamin A acts as a genetic regulator, modulating matrix metalloproteinase (MMP) activity, suppressing collagenase production, and stimulating collagen synthesis and skin regeneration [60,61,62]. Furthermore, vitamin D supports MMP regulation, including collagenase, while vitamin E, a robust antioxidant, protects cell membranes and proteins from oxidative damage, thereby stabilizing collagen [63,64]. The combined actions of omega-3, vitamin A, vitamin D, and vitamin E in SIO synergistically enhance its capacity to preserve collagen-rich tissues. This synergy effectively inhibits collagenase activity, maintains the structural integrity of the extracellular matrix, and promotes overall skin health [65,66].
Irritation testing was conducted to assess the compatibility of the SIO facial serum with human skin through a study involving human volunteers. Skin compatibility is characterized by the absence of irritation under normal product use conditions, accounting for both objective reactions and subjective sensations, such as stinging, burning, or itching [24,25]. This study included 20 volunteers (mean age: 22 years) with various skin types, including normal, oily, dry, and combination skin. The SIO facial serum was applied to the participants’ backs, and observations for signs of irritation, including erythema, edema, or burning sensations, were recorded at 0, 24, and 48 h over a 48 h period. The results, presented in Table 7, indicated that the SIO facial serum caused no irritation on human skin. No erythema, edema, or other signs of irritation were observed during the study, resulting in a primary irritation index (PII) value of 0. These findings are consistent with a prior study investigating the effects of SIO on inflammatory cytokine release in an ex vivo skin culture model. In that study, ex vivo cultured skin tissues treated with the oil were assessed for primary irritation by measuring keratin 1 expression and the release of TNF-α and IL-1α. Compared to the untreated samples, treated tissues exhibited no increase in TNF-α or IL-1α secretion and no disruption of keratin 1 integrity in the stratum corneum, further supporting the non-irritating properties of SIO [14].
5. Conclusions
This study focuses on the development and evaluation of the stability, in vitro anti-aging activity, and skin irritation potential of a facial serum formulated with SIO, offering valuable insights into its potential for cosmetic manufacturing. The research successfully formulated and assessed the SIO facial serum, demonstrating excellent stability, significant in vitro anti-aging efficacy, and reliable safety. The results indicated that the SIO facial serum is stable, safe for skin application, and effective in combating signs of aging by inhibiting neutrophil elastase and collagenase activity. These outcomes highlight the potential of SIO as a promising ingredient for anti-aging formulations. This study establishes a foundation for the incorporation of SIO into cosmetic products with anti-aging properties, ensuring regulatory compliance and contributing to advancements in cosmetic science for societal benefit.
6. Patents
This work has resulted in the following patent:
- Patent Number: HKI.3-HI.05.01.02.P00202405136/2024.
Author Contributions
Conceptualization, I.M., S.S. and C.K.K.; methodology, E.A. and S.R.M.; investigation, I.M. and N.A.P.; resources, S.S., H.N.S. and C.K.K.; data curation, S.R.M. and R.N.A.; writing—original draft preparation, I.M. and S.S.; writing—review and editing, I.M. and S.S.; visualization, I.M., R.N.A., M.H.N. and F.D.; supervision, N.A.P. and E.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by the Directorate General of Higher Education, Research, and Technology (DGHERT) and the Ministry of Education, Culture, Research, and Technology (MOECRT) of the Republic of Indonesia, under Grant No. 7/E1/PPK/KS.03.00/2024.
Institutional Review Board Statement
This study adhered to the principles outlined in the Declaration of Helsinki and received approval from the Institutional Review Board (Ethics Committee) of Bakti Tunas Husada Tasikmalaya (Approval No. 304-01/E.02/KEPK-BTH/IX/2024, issued on 5 September 2024).
Informed Consent Statement
Informed consent was obtained from all participants involved in the study.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
The authors thank the Ministry of Education, Culture, Research, and Technology (MOECRT) of the Republic of Indonesia, and PT. Lunaray Cahya Abadi.
Conflicts of Interest
Cahya Khairani Kusumawulan, Hadiyan Nur Sofyan, Fauzan Dzulfannazhir, and Moh Hamdan Nugraha were employed by the company. The remaining authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Lee, H.; Hong, Y.; Kim, M. Structural and Functional Changes and Possible Molecular Mechanisms in Aged Skin. Int. J. Mol. Sci. 2021, 22, 12489. [Google Scholar] [CrossRef]
- Karimi, N. Approaches in Line with Human Physiology to Prevent Skin Aging. Front. Physiol. 2023, 14, 1279371. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.A.M.E.; Michniak-Kohn, B.; Leonardi, G.R. An Overview About Oxidation in Clinical Practice of Skin Aging. An. Bras. Dermatol. 2017, 92, 367–374. [Google Scholar] [CrossRef]
- Barel, A.O.; Paye, M.; Maibach, H.I. Handbook of Cosmetic Science and Technology, 3rd ed.; Informa Healthcare USA Inc.: New York, NY, USA, 2009. [Google Scholar]
- Lee, K.-K.; Cho, J.J.; Park, E.-J.; Choi, J.-D. Anti-Elastase and Anti-Hyaluronidase of Phenolic Substance from Areca Catechu as a New Anti-Ageing Agent. Int. J. Cosmet. Sci. 2001, 23, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Irianti, T.T.; Suwijoyo, P.; Sugiyanto. Penuaan dan Pencegahannya; Universitas Gadjah Mada Press: Yogyakarta, Indonesia, 2021. [Google Scholar]
- Hoang, H.T.; Moon, J.-Y.; Lee, Y.-C. Natural Antioxidants from Plant Extracts in Skincare Cosmetics: Recent Applications, Challenges and Perspectives. Cosmetics 2021, 8, 106. [Google Scholar] [CrossRef]
- Michalak, M. Plant Extracts as Skin Care and Therapeutic Agents. Int. J. Mol. Sci. 2023, 24, 15444. [Google Scholar] [CrossRef] [PubMed]
- Maya, I.; Winardi, D.O.; Amalia, E.; Mita, S.R.; Kusumawulan, C.K.; Putriana, N.A.; Sriwidodo, S. Physicochemical Characteristics, Fatty Acid Profile, and In Vitro Antioxidant Activity Evaluation of Sacha Inchi Seed Oil from Indonesia. Cosmetics 2023, 10, 171. [Google Scholar] [CrossRef]
- Hadzich, A.; Gross, G.A.; Leimbach, M.; Ispas, A.; Bund, A.; Flores, S. Characterization of Plukenetia volubilis L. Fatty Acid-Based Alkyd Resins. Polym. Test. 2020, 82, 106296. [Google Scholar] [CrossRef]
- Chirinos, R.; Necochea, O.; Pedreschi, R.; Campos, D. Sacha Inchi (Plukenetia volubilis L.) Shell: An Alternative Source of Phenolic Compounds and Antioxidants. Int. J. Food Sci. Technol. 2016, 51, 986–993. [Google Scholar] [CrossRef]
- Herrera-Calderon, O.; Arroyo-Acevedo, J.L.; Chávez-Asmat, R.; Rojas-Armas, J.P.; Enciso-Roca, E.; Cerrate, V.C.; Franco-Quino, C.; Chacaltana-Ramos, L.; Yuli-Posadas, R.A. Effect of Sacha Inchi Oil (Plukenetia volubilis L.) on Genotoxicity in Mice (Mus musculus) and Subchronic Toxicity in Goldfish (Carassius auratus). Pharmacogn. J. 2020, 11, 1549–1557. [Google Scholar] [CrossRef]
- Arroyo-Acevedo, J.L.; Herrera-Calderon, O.; Cisneros-Hilario, C.B.; Chávez-Asmat, R.; Anampa-Guzmán, A.; Enciso-Roca, E.; Condorhuaman-Figueroa, M.; Pari-Olarte, B. Antimutagenic Effect of Plukenetia volubilis (Sacha Inchi) Oil in BALB/C Mice. Annu. Res. Rev. Biol. 2018, 24, 1–8. [Google Scholar] [CrossRef]
- Soimee, W.; Nakyai, W.; Charoensit, P.; Grandmottet, F.; Worasakwutiphong, S.; Phimnuan, P.; Viyoch, J. Evaluation of Moisturizing and Irritation Potential of Sacha Inchi Oil. J. Cosmet. Dermatol. 2019, 19, 915–924. [Google Scholar] [CrossRef]
- Brinckmann, J. Market Analysis for Three Peruvian Natural Ingredients; International Trade Center: Geneva, Switzerland, 2009. [Google Scholar]
- Budiasih, S.; Masyitah, I.; Jiyauddin, K.; Kaleemullah, M.; Samer, A.D.; Fadli, A.M.; Yusuf, E. Formulation and Characterization of Cosmetic Serum Containing Argan Oil as a Moisturizing Agent. In Proceedings of the BROMO Conference, Surabaya, Indonesia, 11–12 July 2018; pp. 11–12. [Google Scholar]
- Sasidharan, S.; Joseph, P.; Junise. Formulation and Evaluation of Fairness Serum Using Polyherbal Extracts. Int. J. Pharm. 2014, 4, 105–112. [Google Scholar]
- Waqas, M.K.; Akhtar, N.; Rasul, A.; Rashid, S.U.; Mustafa, R.; Khan, B.A.; Murtaza, G. In Vivo Evaluation of a Cosmetic Emulsion Containing Soybean Extract for Antiaging. Trop. J. Pharm. Res. 2014, 13, 1401–1406. [Google Scholar] [CrossRef][Green Version]
- Mardhiani, Y.D. Formulasi dan Stabilitas Sediaan Serum dari Ekstrak Kopi Hijau (Coffea Canephora Var. Robusta) Sebagai Antioksidan. Indones. Nat. Res. Pharm. J. 2017, 2, 19–33. [Google Scholar]
- Moravkova, T.; Filip, P. The Influence of Thickeners on the Rheological and Sensory Properties of Cosmetic Lotions. Acta Polytech. Hung. 2014, 11, 173–186. [Google Scholar] [CrossRef]
- Badan Pengawas Obat dan Makanan Republik Indonesia. Peraturan Badan Pengawas Obat dan Makanan Nomor 12 Tahun 2019 Tentang Cemaran Dalam Kosmetika; Badan Pengawas Obat dan Makanan Republik Indonesia: Jakarta, Indonesia, 2019.
- Badan Pengawas Obat dan Makanan Republik Indonesia. Peraturan Badan Pengawas Obat dan Makanan Nomor 25 Tahun 2019 Tentang Pedoman Cara Pembuatan Kosmetika Yang Baik; Badan Pengawas Obat dan Makanan Republik Indonesia: Jakarta, Indonesia, 2020.
- Feldo, M.; Wójciak, M.; Ziemlewska, A.; Dresler, S.; Sowa, I. Modulatory Effect of Diosmin and Diosmetin on Metalloproteinase Activity and Inflammatory Mediators in Human Skin Fibroblasts Treated with Lipopolysaccharide. Molecules 2022, 27, 4264. [Google Scholar] [CrossRef] [PubMed]
- The Personal Care Association. Cosmetics Europe: Product Test Guidelines for the Assessment of Human Skin Compatibility; Cosmetics Europe: Brussels, Belgium, 1997. [Google Scholar]
- Kim, D.-H.; Lim, Y.-Y.; Kim, H.-M.; Kim, S.-Y.; Kim, B.-J.; Park, S.-G.; Lee, T.-H.; Cho, S.-M. The Safety Evaluation of a Potent Angiogenic Activator, Synthetic Peptide (SFKLRY-NH2) for Skin Application. Toxicol. Res. 2012, 28, 51–56. [Google Scholar] [CrossRef] [PubMed][Green Version]
- ISO 10993-10; Biological Evaluation of Medical Devices, Part 10: Tests for Irritation and Skin Sensitization, 4th ed. International Standard ISO: Geneva, Switzerland, 2021.
- Chambers, E.S.; Vukmanovic-Stejic, M. Skin Barrier Immunity and Ageing. Immunology 2020, 160, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Zerbinati, N.; Sommatis, S.; Maccario, C.; Di Francetsco, S.; Capillo, M.C.; Rauso, R.; Herrera, M.; Bencini, P.L.; Guida, S.; Mocchi, R. The Anti-Ageing and Whitening Potential of a Cosmetic Serum Containing 3-O-Ethyl-L-Ascorbic Acid. Life 2021, 11, 406. [Google Scholar] [CrossRef]
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin Antiaging Strategies. Derm. Endocrinol. 2012, 4, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. Ascorbic Acid (Vitamin C) as a Cosmeceutical to Increase Dermal Collagen for Skin Antiaging Purposes: Emerging Combination Therapies. Antioxidants 2022, 11, 1663. [Google Scholar] [CrossRef] [PubMed]
- Jara, C.P.; Mendes, N.F.; Prado, T.P.D.; de Araújo, E.P. Bioactive Fatty Acids in the Resolution of Chronic Inflammation in Skin Wounds. Adv. Wound Care 2020, 9, 472–490. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al-Niaimi, F.; Chiang, N.Y.Z. Topical Vitamin C and the Skin: Mechanisms of Action and Clinical Applications. J. Clin. Aesthet. Dermatol. 2017, 10, 14–17. [Google Scholar]
- Ravetti, S.; Clemente, C.; Brignone, S.; Hergert, L.; Allemandi, D.; Palma, S. Ascorbic Acid in Skin Health. Cosmetics 2019, 6, 58. [Google Scholar] [CrossRef]
- Danimayostu, A.A.; Martien, R.; Lukitaningsih, E.; Danarti, R. Vitamin D3 and the Molecular Pathway of Skin Aging. Indones. J. Pharm. 2023, 34, 357–371. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D: Production, Metabolism, and Mechanisms of Action. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK278935/ (accessed on 5 September 2024).
- Joshi, M.; Hiremath, P.; John, J.; Ranadive, N.; Nandakumar, K.; Mudgal, J. Modulatory Role of Vitamins A, B3, C, D, and E on Skin Health, Immunity, Microbiome, and Diseases. Pharmacol. Rep. 2023, 75, 1096–1114. [Google Scholar] [CrossRef] [PubMed]
- Dayan, N. Handbook of Formulating Dermal Applications: A Definitive Practical Guide; John Wiley & Sons, Inc.: Hoboken, NJ, USA; Scrivener Publishing: Beverly, MA, USA, 2017; pp. 591–602. [Google Scholar]
- Girboux, A.-L.; Starch, M.S. Formulating with Silicones and Natural Lipids. Dow Corning Corp. 2008.
- Marlina; Salman; Filza, H.; Nur, E.; Poppy, A.N. Antioxidant Serum Gel Formulation with a Combination of Secretome from Mesenchymal Stem Cells and Rosemary Oil. IOP Conf. Series Earth Environ. Sci. 2023, 1228, 012036. [Google Scholar] [CrossRef]
- Khan, B.A.; Ullah, S.; Khan, M.K.; Alshahrani, S.M.; Braga, V.A. Formulation and Evaluation of Ocimum Basilicum-Based Emulgel for Wound Healing Using Animal Model. Saudi Pharm. J. 2020, 28, 1842–1850. [Google Scholar] [CrossRef] [PubMed]
- Ermawati, K.; Valeria, L. Formulasi dan Uji Stabilitas Fisik Serum Spray Ekstrak Umbi Wortel (Daucus carota L.) Sebagai Antiaging. J. Kesehat. Yamasi Makassar 2022, 6, 25–34. [Google Scholar]
- Tchienou, G.E.D.; Tsague, R.K.T.; Pega, T.F.M.; Bama, V.; Bamseck, A.; Sokeng, S.D.; Ngassoum, M.B. Multi-Response Optimization in the Formulation of a Topical Cream from Natural Ingredients. Cosmetics 2018, 5, 7. [Google Scholar] [CrossRef]
- Lukić, M.; Pantelić, I.; Savić, S.D. Towards Optimal pH of the Skin and Topical Formulations: From the Current State of the Art to Tailored Products. Cosmetics 2021, 8, 69. [Google Scholar] [CrossRef]
- Prakash, C.; Bhargava, P.; Tiwari, S.; Majumdar, B.; Bhargava, R.K. Skin Surface pH in Acne Vulgaris: Insights from an Observational Study and Review of the Literature. J. Clin. Aesthet. Dermatol. 2017, 10, 33–39. [Google Scholar]
- Badan Standarisasi Nasional. Sediaan Tabir Surya; Badan Standarisasi Nasional: Jakarta, Indonesia, 1996. [Google Scholar]
- Yousuf, M.; Khan, H.M.S.; Rasool, F.; Khan, K.u.R.; Usman, F.; Ghalloo, B.A.; Umair, M.; Babalghith, A.O.; Kamran, M.; Aadil, R.M.; et al. Chemical Profiling, Formulation Development, In Vitro Evaluation and Molecular Docking of Piper nigrum Seeds Extract Loaded Emulgel for Anti-Aging. Molecules 2022, 27, 5990. [Google Scholar] [CrossRef] [PubMed]
- Handayani, E.S.; Nugraheni, E.R.; Susilowati, A.R.I. Antibacterial and Antifungal Activities of Essential Oil of Tawangmangu Sweet Orange (Citrus sinensis) Peel at Different Altitudes. Asian J. Nat. Prod. Biochem. 2019, 1, 47–54. [Google Scholar]
- Badan Pengawas Obat dan Makanan Republik Indonesia. Waspada Keracunan Akibat Kandungan Logam Berat Pada Kosmetik; Badan Pengawas Obat dan Makanan Republik Indonesia: Jakarta, Indonesia, 2014.
- Azmi, N.; Hashim, P.; Hashim, D.M.; Halimoon, N.; Majid, N.M.N. Anti-Elastase, Anti-Tyrosinase and Matrix Metalloproteinase-1 Inhibitory Activity of Earthworm Extracts as Potential New Anti-Aging Agent. Asian Pac. J. Trop. Biomed. 2014, 4 (Suppl. S1), S348–S352. [Google Scholar] [CrossRef]
- Khan, A.; Wang, G.; Zhou, F.; Gong, L.; Zhang, J.; Qi, L.; Cui, H. Polydeoxyribonucleotide: A Promising Skin Anti-Aging Agent. Chin. J. Plast. Reconstr. Surg. 2022, 4, 187–193. [Google Scholar] [CrossRef]
- Karim, A.A.; Azlan, A.; Ismail, A.; Hashim, P.; Gani, S.S.A.; Zainudin, B.H.; Abdullah, N.A. Phenolic Composition, Antioxidant, Anti-Wrinkles, and Tyrosinase Inhibitory Activities of Cocoa Pod Extract. BMC Complement. Altern. Med. 2014, 14, 381. [Google Scholar] [CrossRef]
- Lourith, N.; Kanlayavattanakul, M.; Chaikul, P. Sacha Inchi: The Promising Source of Functional Oil for Anti-aging Products. J. Oleo Sci. 2024, 73, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Wahab, N.A.; Rahman, R.A.; Ismail, A.; Mustafa, S.; Hashim, P. Assessment of Antioxidant Capacity, Anti-Collagenase and Anti-Elastase Assays of Malaysian Unfermented Cocoa Bean for Cosmetic Application. Nat. Prod. Chem. Res. 2014, 2, 1000132. [Google Scholar]
- Kacem, R. Phenolic Compounds from Medicinal Plants as Natural Anti-Elastase Products for the Therapy of Pulmonary Emphysema. J. Med. Plants Res. 2013, 7, 3499–3507. [Google Scholar]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, X.; Liu, D. Collagen Peptides and the Related Synthetic Peptides: A Review on Improving Skin Health. J. Funct. Foods 2021, 86, 104680. [Google Scholar] [CrossRef]
- Inoue, Y.; Itoh, H.; Aoki, M.; Ogawa, S.; Yamane, T.; Baba, T.; Tachibana, N.; Kohno, M.; Oishi, Y.; Kobayashi-Hattori, K. Accelerating Effect of Soy Peptides Containing Collagen Peptides on Type I and III Collagen Levels in Rat Skin. Biosci. Biotechnol. Biochem. 2012, 76, 1549–1551. [Google Scholar] [CrossRef]
- Xie, M.; Jiang, Z.; Lin, X.; Wei, X. Application of Plant Extracts Cosmetics in the Field of Anti-Aging. J. Dermatol. Sci. Cosmet. Technol. 2024, 1, 100014. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Reddy, A.P.; Datta, S.C.; Kang, S.; Jong, Y.Y.; Chambon, P.; Voorhees, J.J. All-trans retinoic acid induces cellular retinol-binding protein in human skin in vivo. J. Investig. Dermatol. 1995, 105, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Datta, S.C.; Talwar, H.S.; Wang, Z.-Q.; Varani, J.; Kang, S.; Voorhees, J.J. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature 1996, 379, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Varani, J.; Warner, R.L.; Gharaee-Kermani, M.; Phan, S.H.; Kang, S.; Chung, J.; Wang, Z.; Datta, S.C.; Fisher, G.J.; Voorhees, J.J. Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin1. J. Investig. Dermatol. 2000, 114, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Janjetovic, Z.; Slominski, A.T. Promising Functions of Novel Vitamin D Derivatives as Cosmetics: A New Fountain of Youth in Skin Aging and Skin Protection. Cosmetics 2024, 11, 37. [Google Scholar] [CrossRef]
- Bocheva, G.; Slominski, R.M.; Slominski, A.T. The Impact of Vitamin D on Skin Aging. Int. J. Mol. Sci. 2021, 22, 9097. [Google Scholar] [CrossRef] [PubMed]
- Vo, H.V.T.; Nguyen, Y.T.; Kim, N.; Lee, H.J. Vitamin A, D, E, and K as Matrix Metalloproteinase-2/9 Regulators That Affect Expression and Enzymatic Activity. Int. J. Mol. Sci. 2023, 24, 17038. [Google Scholar] [CrossRef]
- Pandel, R.; Poljšak, B.; Godic, A.; Dahmane, R. Skin photoaging and the role of antioxidants in its prevention. ISRN Dermatol. 2013, 2013, 930164. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).