Abstract
Peloids are mixtures of clays, sediments, or peat with mineral–medicinal water or seawater, or salt-lake water used in spa therapy for different treatments, including dermatological ones. The origin of peloids can be natural; that is, they are formed in situ at the place where the thermal water emerges or on the shores of the sea or salt lake, or they are prepared ad hoc from high-quality materials, such as clays or peat. Peloids are also used as cosmeceuticals in skin care to treat different skin disorders and/or conditions, such as psoriasis, eczema, and other scaly disorders, sensitive skin, and acne. This review reports all available scientific data concerning the effects and specific activities of peloids in skin care and cosmeceuticals, providing a better understanding of the clinical and cosmetic benefits. Finally, the safety and regulation of peloids are also discussed.
1. Introduction
The skin is the largest organ of the body, with a structure organized in layers with functions mainly of protection, in addition to regulating body temperature and sensory function. Thus, the layers of the skin (epidermis, dermis, and fatty subcutaneous tissue) perform these protective functions, among which the barrier function stands out. The skin has several mechanisms to perform this barrier function. The first and most important is the epidermal one, through the stratum corneum. In addition to this physical barrier, the skin also acts as a chemical and biochemical barrier, through lipids, acids, hydrolytic enzymes, antimicrobial peptides, and macrophages. The immunological barrier is made up of the components of the cellular and humoral immunity of the immune system [1,2].
On the other hand, it is important to point out the importance of skin homeostasis in maintaining the stability of the entire internal environment. At the same time, skin homeostasis is also the basis for maintaining the function of the skin barrier, as the skin barrier is the first line of defense of the human body and its main function is to prevent water loss and the entry of harmful substances into the body. Only when skin homeostasis is well maintained can the skin barrier perform its functions properly and protect the body from external threats. Therefore, maintaining skin homeostasis is of great importance for maintaining human health [3].
To maintain the skin barrier, skin protection and hydration are essential, so the use of cosmetics has become a daily routine. Cosmetics are defined as “any substance or mixture intended to be placed in contact with the external parts of the human body (epidermis, hair system, nails, lips, and external genital organs) or with the teeth and the mucous membranes of the oral cavity with a view exclusively or mainly to cleaning them, perfuming them, changing their appearance, protecting them, keeping them in good condition, or correcting body odours” (Regulations (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products) (http://data.europa.eu/eli/reg/2009/1223/2019-08-13 (accessed on 1 September 2024)) [4].
Cosmeceuticals are defined as “a cosmetic product that is purported to have therapeutic action capable of affecting the skin positively beyond the time of its application”. The term was coined by Dr. Albert Kligman of the University of Pennsylvania describing a hybrid category of products mid-way on the spectrum of cosmetics and pharmaceuticals [5]. Cosmeceuticals have also been defined as “cosmetics having active ingredients similar to a pharmaceutical but at reduced concentrations of actives”. Despite that, they are not categorized as drugs that can cure or heal a disease condition, and they are regulated under the same regulations as cosmetics [6].
Because some synthetic ingredients in cosmetics can cause skin irritation or accumulate in the skin, producing undesirable effects, users, as well as cosmetic companies, have turned their attention to natural ingredients and products [7]. Therefore, cosmeceuticals from plants or biotechnological bioactive compounds are more popular [8]. Hence, the enlarging market for skincare formulations, especially cosmeceuticals, and the constant search for alternative natural constituents led to the production of different types of skin products focused not only on the daily routine but also on the wellness field. In this scenario, peloids or thermal muds acquire new relevance, moving from being exclusively therapeutic products in the field of balneology to being used as cosmeceuticals for skin care in different areas.
Peloids are mixtures of clays, sediments, or peat with mineral–medicinal water (MMW), seawater, or salt-lake water that are used in spa therapy for different treatments, including dermatological ones. Peloids have been used for years to treat different skin disorders, such as psoriasis, atopic dermatitis, or acne, and nowadays, they are also used as cosmeceuticals to improve skin health and conditions.
Tian et al. reviewed the medical and cosmetic applications of peloids with a particular emphasis on aging and changes in skin microbiota. This review suggests that there is a need to explore which chemical elements are involved in their effects, which peloid is most suitable for each skin condition and the mechanisms of action involved, as well as to promote regulation regarding the limits of heavy metals and harmful bacteria [9]. The present review attempts to address some of these questions and to provide answers to them as far as possible.
The aim of this review is to reveal the role of peloids in skin care and highlight their potential use as cosmeceuticals. First, an overview of the concept and composition of peloids is described to highlight their differentiating characteristics with respect to other skin care products, such as facial and body masks. Second, the relevance of each phase of the peloid is described to understand the role of each of its components in the possible actions and benefits on the skin. Finally, the review reports actual research conducted to demonstrate the effects and benefits of thermal peloids on the skin. Additionally, the safety and quality of peloids are also discussed, to determine and avoid possible toxic effects.
2. Materials and Methods
For the non-systematic literature review, PubMed, Google Scholar, and Web of Science databases were consulted, and the search terms used were “peloids or mud and skin care”, “peloids or mud and cosmetics”, “peloids or mud and cosmeceuticals”. The search was performed with data up to September 2024.
To ensure that relevant information was obtained to answer the research question, inclusion and exclusion criteria were established. The inclusion criteria were articles focusing on the problem under study, articles describing the composition of the peloids used in skin care or dermatological disorders, and articles describing the different specific activities of peloids reporting the dermotherapeutic or cosmetic use. Exclusion criteria included articles not related to the problem under study and in which the peloid or mud was not one of the main components of the skin care formulation. Thus, 145 articles were revised, and 21 articles were selected and analyzed for review.
3. Peloids: Definition, Composition and Classification
The concept of peloids is relatively modern; it was accepted in 1933 by the International Committee of Measurements as a general term for muds for medicinal use and it is interesting to mention in this regard the proposal of Judd Lewis, President of the “International Standard Measurements Committee”, who proposed to give a generic name and classify the “Semi-solid Bath media” or “Peloids” (Boue, Fango, Gyttya, Limon, Lutum, Moor, Peat, Sapropel, Schlick, Seaweed, Torf). Since the name must be of Greek or Latin origin, the Greek word “Pelòs” (πελδς) is chosen instead of the Latin “Lutum”, which also means mud, but the Greek word forms derivatives such as Peloterapia and Pelología, grammatically correct, while the Latin word would give, according to Lewis, barbarisms [10]. The word peloid thus adapts to any language in a way analogous to colloid or alkaloid. It was at the Wiesbaden Congress (Germany) of the International Society of Medical Hydrology (ISMH), in 1938, where the word “Peloid” was finally accepted [11].
The acceptance of the word peloid was immediately agreed upon by the scientific community, but not the definition. That is why, over the years, multiple definitions have emerged that reflect the variety of components and presentations that mud has.
3.1. Peloid Definition
The first definition that was proposed and discussed was that of Lewis in 1933 [12]: “A peloid is, whatever the medium, a natural product, consisting of a uniform mixture of solid matter and finely divided organic matter and water, which is applied in medical practice as a poultice for external treatment”.
The definition adopted by the International Peloids Committee of the ISMH at the Conference held in Wiesbaden in 1938 [13] states: “Peloids are substances that are formed in nature through geological processes and that when finely granulated, mixed with water, have applications in medical practice in the form of baths or poultices”.
However, the use of peloids evolved and other uses such as dermocosmetic use were included. In 2013, the Working Group led by Gomes [14] defined peloids as “Peloid is a maturated mud or muddy dispersion with healing and/or cosmetic properties, composed of a complex mixture of fine-grained natural materials of geologic and/or biologic origins, mineral water or sea water, and commonly organic compounds from biological metabolic activity”.
Initially, it was considered that to be called peloid, a mud had to have undergone a maturation process (understood as a long contact process of the solid phase with the mineral–medicinal water). During the 3rd Symposium on Thermal Mud, held in 2004 in Dax, it was agreed to distinguish between the two main types of peloids: (i) muds or clays that are just mixed with mineral water with no maturation process, the so-called extemporaneous or prepared “ad hoc” peloids; (ii) muds or clays mixed with mineral water, and naturally or artificially matured [15].
3.2. Peloid Classification
The above-mentioned Working Group also classified peloids according to their origin and applications [14]. This classification is summarized in Figure 1.
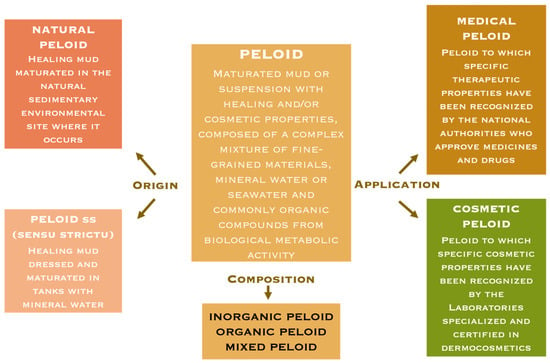
Figure 1.
Peloid definition and classification (adapted from Gomes et al., 2013) [14].
As can be seen in Figure 1, the peloid can have a natural origin, but in many thermal spa centers peloids are prepared “ad hoc” from clays, sediments, or silts, mixed with mineral–medicinal water.
Natural peloid is obtained from natural environments, such as lakes or river estuaries, and it is subjected to a sifting process. “Ad hoc” peloids are prepared by mixing the raw materials, liquid and solid phases (previously characterized), to which microalgae or cyanobacteria can be added. Finally, both peloids must undergo quality control (Figure 2).
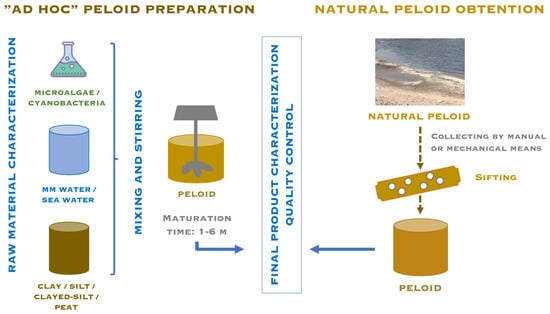
Figure 2.
“Ad hoc” peloid preparation vs natural peloid obtention.
3.3. Peloid Composition
Peloids are composed of a liquid phase which can be MMW, seawater, or salt-lake water; a solid phase, which is frequently inorganic and made of clay, silt or sediments, but also can be organic (e.g., peat); and a biological fraction consisting mainly of microalgae and cyanobacteria from the waters themselves or cultivated. The composition of the peloids is decisive in their therapeutic and cosmetic effects, but so is the method of application, especially when they are applied hot/warm, since it is seen that the penetration of the bioactive substances is facilitated (Figure 3).
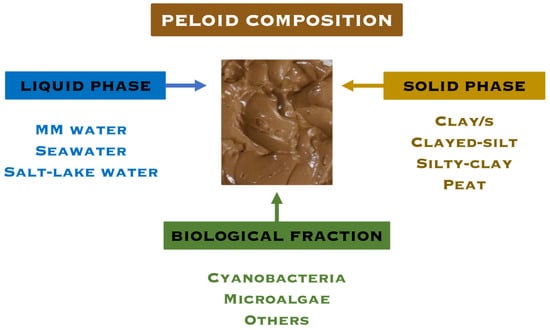
Figure 3.
Peloid composition.
The liquid phase is important as the mineral–medicinal water composition can varied from chloride, sulfate, bicarbonate, sodium, calcium, and silica MMW (among others) to seawater, or salt-lake water, and this leads to different actions on the skin due to the ions and trace elements present. MMW can be classified according to the temperature, dry residue, hardness, etc., but the one that provides the most information regarding its therapeutic use is that of mineral content. In 2009, the European Commission defined and classified natural mineral water, but this classification does not include natural mineral waters used at source for curative purposes [16]. Thus, each country regulates the use MMW; the classifications are all quite similar, the differences being mainly related to the minimum ion content. Table 1 summarizes the classification of MMW from Spanish Medical Hydrology Association (SEHM) (https://www.hidromed.org/hm/index.php/el-agua (accessed on 1 September 2024)) [17].

Table 1.
Classification of mineral–medicinal waters according to the mineral composition (SEHM) [17].
Seawater is a complex mixture with a high percentage of water depending on its origin and the rest are salts and smaller amounts of other types of substances, including dissolved organic and inorganic materials, particles, and atmospheric gases. The salinity of seawater and salt-lake water varies from around 35 g/kg in the ocean to approximately 330 g/kg in the Dead Sea; for example, in Tuz Lake (Turkey) it is 324 g/kg, and in Mar Chiquita (Argentina) it is 60 g/kg, to mention a few examples where peloids naturally occur. The most abundant elements, after oxygen and hydrogen, are chlorine and sodium, followed by magnesium, sulfur, calcium, potassium, bromine, strontium, boron, silicon, and fluorine, up to more than 80 chemical elements [18].
The solid phase is composed by different types of sediments from the mineral–medicinal waters themselves when they have high salinity or mineral content (e.g., hypersaline MMW), silt from natural deposits (fine-grained sediments), or clays (smectite, illite, kaolinite, etc.). But peat can also be used as a solid phase to prepare a more organic peloid; peat is an organic compound that comes from the slow maceration of herbs and plants deposited in particularly wet and marshy environments.
The biological fraction is also of great interest since microalgae and cyanobacteria found in peloids have been proven to generate biologically active substances (especially during the maturation process), which in turn are responsible for their beneficial effects and actions.
4. Relevance of the Components in the Formulation of Peloids and Their Therapeutic Effects
The effects of peloids derive on the one hand from their thermotherapeutic action and, on the other hand, from the components of their formulation; that is, the solid phase, the liquid phase, and the biological fraction. Before reviewing the actions and effects of peloids on the skin, we will describe the role played by the different components that make up their composition.
4.1. Relevance of Solid Phase
The most abundant research on the solid phase is related to clays and, to a lesser extent, to sediments and peat.
In 2020, Carretero [19,20] described the mineralogy and chemistry of clays for pelotherapy, as well as the organic compounds, microbiology and medical applications. The solid phase of most peloids is clay or clayey sediments (phyllosilicates, quartz, calcite, feldspars and dolomite); among phyllosilicates, different proportions of smectites, kaolinite, illite, illite–smectite mixed layer, and chlorite can be found. The review highlighted that the smectites are considered more than others to prepare peloids because they provide better thermal properties and greater cation exchange capacity. It also refers to the mineralogical, crystallochemical, and property changes that occur with maturation time, which depend on the type of mineral–medicinal water used and the phyllosilicates that make up the clays used in the study, as well as the solubility of other minerals present. It also states that the optimal maturation time is between two and three months, since after that time, the changes in the composition and properties of the peloid are minimal.
López-Galindo et al. studied the pharmacological and cosmetic use of clays as they are traditionally used as adsorbents and protectors in topical and systemic formulations in the treatment of, for example, acne, and leg ulcers [21,22,23]. The use in dermatological and cosmetic applications is based on the rheological properties, the high capacity for cation exchange and absorption, and the slow cooling rate of clays when properly prepared [21]. Viseras et al. carried out several studies to assess the suitability of various clays for use in skin care products. The results are of interest for the development of peloids for dermocosmetic use [24,25]. Clays are useful in cosmetics design simply because they are made of rigid, small, anisometric particles. Smectites are of interest due to their electrical charges that result in ion exchange capabilities and are therefore capable of loading, transporting, and delivering active ingredients in cosmetics, but also in the adsorption and removing waste substances from the skin surface. The intermediate net negative charges of smectites result in layer expansion in polar media and specific rheological properties that are very useful in cosmetic formulations. Kaolinite and mica are made of hard prismatic particles that are slightly abrasive on skin, teeth, or hair, which can be useful in deep cleansing of the skin [25].
In terms of health benefits, clays are very well known for their anti-inflammatory activity [26], bacteriostatic and bactericidal effects [27], promotion of the excretion of toxins [28], shooting and regenerative actions [29], and even the treatment of ulcers [30]. Additionally, clay minerals’ high adsorption capacity allows them to adsorb secretions, toxins, and impurities, as well as bacteria and viruses. The high cation exchange capacity can promote the release and transfer of vital chemical elements to the body that are present in its composition, such as sodium, potassium, magnesium, copper, iron, zinc, and manganese [31]. In the cosmetic field, clays are used for cleansing and moisturization of the skin and to combat compact lipodystrophies, acne, and cellulite [32], and also have anti-inflammatory properties [33].
As examples of peloids made of clay, the peloids from the Euganean Thermal District can be cited. The “virgin clay” is obtained in the Euganean Hills, which is rich in carbonates (calcite > dolomite), quartz, feldspars, and phyllosilicates; the clay fraction (<2 μm) corresponds to kaolinite and illite–smectite type. The clay is mixed with the thermal water and maturated in the spa itself in a maturation pool [34].
In France, peloids are also very popular; one of the most well-known is the peloid of Dax, elaborated in a factory that supplies mud to 17 thermal spas, and prepared from a sediment (“limon” from de Adour River) whose main composition is silica, silicates, crystallized or amorphous oxides and hydroxides, carbonates, phosphates, and sulfates [35].
Marine peloids come from a natural environment and are composed of a variety of sediment compounds, mostly silt. In addition to marine peloids of natural origin, which are scarce, peloids are increasingly being prepared from clays, such as bentonitic clays, mainly of ancient marine origin, but also others of cosmetic quality with various origins.
Some examples of natural marine peloids are from Morinje Bay and Makirina Bay (Croatia), the Igalo Bay marine peloid (Montenegro) [36,37,38] on the Adriatic coast, and the Techirghiol peloid in the Black Sea (Romania), a sapropel type containing organic carbon-rich sediments [39], and the peloid from the saline lagoon Tuz lake (Tuz Gölü) in Turkey, whose composition was a combination of clay minerals (mainly montmorillonite, kaolinite, and muscovite) and non-clay minerals (mainly quartz, calcite, dolomite, and albite) [40]. Other highly mineralized muds are those from Kazakhstan lakes, most of them sulfide-type and rich in bromine, which could also be of interest for skin disease treatment [41].
Another interesting example in the Adriatic coast is the Sečovlje Salina peloid, a hypersaline mud from the Sečovlje Salt Pans, the biggest natural park in Slovenia; these muds were characterized by very fine, sandy, medium silt in which the mud fraction greatly dominated, and contents of the major and trace elements of saline muds were comparable to their mean concentrations in surface sediment from the Central Adriatic Sea over the sand fraction. This peloid is being used for wellness purposes, and to formulate cosmetics [42].
The most studied marine peloid is the Dead Sea mud, whose main composition is quartz, calcite, and halite [43], and has been shown to exert antibacterial and anti-inflammatory activities [44]; this mud is been used to treat a wide range of dermatological conditions, especially psoriasis, atopic dermatitis, and other eczemas [45].
As it has been mentioned before, in some countries, peloids are produced from peat, although there are few studies on their composition and actions. Peat is mixed with mineral–medicinal water in different proportions to make poultices or baths as an extemporaneous peloid or undergo a maturation process, but, in some cases, mixtures of peat and clay can be found; this is the case of Caldes de Boí peloid (Spain) [46].
Peat is highly rich in minerals, enzymes, macromolecules (such as proteins, lipids, and cellulose), and decomposition products (such as humic acid, umolignin, humine, amino acids, and bitumen). These compounds have multiple biological activities, such as antibacterial, antifungal, immunomodulatory, and photoprotective activity, relevant to the use of peat in dermatology and cosmetics [47].
4.2. Relevance of Liquid Phase
MMW, in addition to constituting the liquid phase of the peloid, has a crucial importance in the actions of the peloids, since it provides the minerals present in its composition. There are several studies on the role of MMW on dermatological disease, but very few are related to minerals and trace elements. The most recent, by Mourelle et al. [48], describes their role in the treatment of different dermatological disorders and skin conditions. This research summarizes that some anions, such as chloride, sulfate, and bicarbonate, can exert anti-inflammatory actions, along with other actions. For example, chloride and bicarbonate can protect the skin against dehydration, and sulfate has antibiofilm activity but also antioxidant properties, and carbonate enhances the wound healing process. Cations can also exert important activities; for example, calcium, and sodium can improve wound healing, and magnesium improves skin barrier function and has anti-inflammatory properties. Among the trace elements, selenium and strontium possess antioxidant activity; manganese and boron promote wound healing; and zinc has been shown to exert antipruritic activity. Other compounds such as SiO2 can also exert anti-inflammatory activity and have been able to promote skin barrier recovery, sulfur and derivatives (SH−; H2S) are mainly used to reduce inflammation and scaling in psoriasis, but also in wound repair, and CO2-rich waters improve peripheral circulation [48].
Seawater has a recognized effect in the treatment of eczema, dermatosis, psoriasis, nasopharyngeal inflammation, conjunctivitis, vaginitis, and other infections of the external genital organs. In cosmetology, it has also been used to improve skin hydration and elasticity. In addition, it helps regulate sebum production (in oily skin and acne) and scalp flaking (dandruff). It also plays an important role in the absorption of saline and metal ions, favoring the excretion of toxic waste and some oxygenation of the tissues [49]. Additionally, magnesium salts, which are the prevailing minerals in Dead Sea water, are known to have anti-inflammatory activity, improving psoriasis, eczema, and other dermatological diseases [50,51,52], but also regenerative properties, moisturizing, smoothing, and anti-photoaging benefits [53,54].
4.3. Relevance of the Biological Fraction
The biological fraction of the peloids has been extensively studied since it has been proven that, in addition to providing biologically active substances on its own, it also helps to generate them within the peloid during the maturation process.
Several studies have been carried out to identify the microbial composition of Abano-Montegrotto peloids (Euganean Thermal District) and their role in the therapeutical effects, finding that, during the mud maturation process the clay surface is colonized by complex microbiota forming blue-green biofilms mainly composed of filamentous and coccoid cyanobacteria strains such as Cyanobacterium aponinum ETS-03 [55,56,57], and Phormidium ETS-05 [58,59] being the latter most abundant strain present in the mature muds [60]. Later on, Zampieri et al. [61] investigated the exopolysaccharides (EPS) produced by Phormidium sp. ETS05, which showed anti-inflammatory and pro-resolution activities in chemical and injury-induced zebrafish inflammation models, suggesting that EPS could be responsible for the anti-inflammatory effect of these peloids. Subsequent studies confirmed the anti-inflammatory and antioxidant activities of the EPS of these thermal muds [62]. Additionally, in 2021, a new filamentous cyanobacterium was found, called Thermospirulina andreolii, which is capable of synthetizing phycocyanin and exopolysaccharides, with possible antioxidant and anti-inflammatory activities, suggesting its potential for contributing to the healing properties of these muds [63].
The diversity of cyanobacteria from the thermal mud of Balaruc-les-Bains (France) has been also studied, identifying the species Pseudochroococcus coutei [64]. Additionally, Demay et al. investigated the potential beneficial activities of nine cyanobacteria isolated from this thermal mud, in terms of the production of antioxidant and/or anti-inflammatory compounds using genomic, metabolomic, and bioactivity analyses, finding that the strains Planktothricoides raciborskii PMC 877.14, Nostoc sp. PMC 881.14, and Pseudo-chroococcus couteii PMC 885.14 demonstrated strong anti-inflammatory activity, and Aliinostoc sp. PMC 882.14 showed slight wound-healing activity [65]. Halary et al. also investigated the intertwined metabolic pathways between cyanobacteria and surrounding bacteria, offering new insight into the potential role and metabolic interaction of bacteria in thermal mud-associated cyanobacterial biofilms [66].
Blue Lagoon mud is a silica-rich peloid very well-known for its microbial diversity [67,68,69,70]; filamentous bacteria that are developed in this mud have been studied to evaluate their therapeutic and dermocosmetic properties, finding that the cyanobacteria Cyanobacterium aponinum is able to secrete exopolysaccharides with anti-inflammatory properties [71,72].
Halophilic bacteria can also play a role in the therapeutic properties of hypersaline muds; thus, Dead Sea mud has been shown to exert antibacterial properties [73], and the halophilic bacteria Haloarcula marismortui (Volcani) has been identified [74]; other microorganisms present in the mud are Bacillus persicus, which showed antimicrobial activity [44], and other Bacillus species exerting antibacterial and antifungal activities [75].
Organic compounds such as humic acids, lipids, and carbohydrates as well as hydrogen sulfide found in hydrosulfide peloids are also of interest as they could increase the balneological value of the muds, as is the case with the Mongolian muds [76]. The organic compounds of several peloids from Igalo Bay (Montenegro) have also been investigated, finding that they are composed mainly of fatty acids (saturated and unsaturated) as well as essential amino acids, which have significant physiological, medical, and pharmaceutical effects [38].
5. Results
From the analysis of scientific articles, 21 studies about the peloids used to treat different skin diseases and conditions were found, as well as those studies related to the use of cosmeceuticals, which frequently are described in the same research. The studies are organized by type of peloid: natural, and “ad hoc” prepared. Natural peloids are: Blue Lagoon peloid, Dead Sea Mud, Cervia peloid, Korean Sea Mud, Ulcinj peloid, and Xiushan Island sea mud. “Ad hoc” prepared peloids are Compostela, La Toja, Saturnia, and Massaciuccoli. Table 2 summarizes these findings.

Table 2.
Studies involving peloids in skin care and cosmeceuticals.
6. Discussion
As described in the introduction, peloids include MMW, seawater, or salt-lake water in their liquid phase. Numerous studies showed the potential of mineral–medicinal waters for skin care, with the most commonly used waters for skin disorders being chlorine, sulfur, bicarbonate waters rich in magnesium and calcium, and silica-rich MMW [95,96,97]. Seawater and salt-lake water are very well-known for their high content of minerals and trace elements that can benefit the skin [98] and contribute to the peloid properties.
Clay, silt, and peat included in the solid phase can also provide minerals and/or organic compounds that also contribute to the therapeutic and dermocosmetic potential of the peloid. Several authors studied the suitability of different clays to prepared peloids; for example, Khiari et al. investigated 11 Tunisian clays traditionally used in home-made mud-packs, finding that their characteristics are similar to others used to prepare peloids (illite, kaolinite, and smectic-types), highlighting the importance of investigating the crystalline silica (quartz) fractions that could reveal uncontrolled manipulation [99]. Karakaya et al. investigated the physical and physico-chemical properties of 20 peloids from Turkey to evaluate the suitability of their use in pastes, masks, creams, and/or mud baths, concluding that the peloids with high cation exchange capacity (CEC), swelling, and absorption capacity may be suitable for removing oils, toxins, and contaminants from the skin [100].
Peloids have been used successfully for the treatment of various inflammatory conditions, mainly rheumatic diseases, but also for chronic inflammatory skin disorders, such as atopic dermatitis, and psoriasis. Peloids have also been shown to be useful in different skin conditions, such as acne or sensitive skin, and to improve barrier function or prevent aging.
On the other hand, it has been observed that peloid therapy can modify the cutaneous microbiota, and it has been hypothesized that it could have an indirect beneficial action on the immune function, which may be of interest in dermatological disorders in which an imbalance occurs in it [101].
Bathing in Blue Lagoon geothermal seawater has been reported to be beneficial for improving psoriasis and its anti-inflammatory properties [102,103]. The Blue Lagoon mud has been extensively investigated, both for therapeutic and cosmeceutical purposes, finding, as has been mentioned before, that exopolysaccharides could be responsible for the main properties of the peloid [71,72], but also the mineralogical compounds of the mud. Both silica mud and cyanobacterial extracts are used to prepare cosmeceutical formulations. Studies in vitro and in vivo were carried out showing that extracts from silica mud and one type of microalgae inhibited UVA radiation-induced upregulation of matrix metalloproteinase1 expression, and both algae as well as silica mud extracts induced collagen 1A1 and 1A2 gene expression in this cell type. An in vivo study performed in healthy volunteers showed that a galenic formulation containing silica mud and microalgae was able to reduce TEWL and strengthen the skin barrier [77].
Later studies in vitro and in vivo reported that normal human epidermal melanocytes, which had been treated with nontoxic concentrations of Blue Lagoon microalgae, showed a significantly reduced expression of α-MSH-induced expression of genes involved in melanin synthesis. Furthermore, patients treated with a serum containing Blue Lagoon mineral salts and microalgae extracts resulted in a reduction of uneven facial skin pigmentation [104].
Dead Sea water and Dead Sea peloids are probably the most studied hypersaline water and mud. Both are used for therapeutic purposes but also to prepare cosmeceuticals. In 2018, Bawab et al. reviewed the applications of Dead Sea (DS) mud in cosmetics, finding several patents for skin care products containing DS mud and salts, hydrolyzed collagen, and plant extracts; skin formulations containing urea and DS mud or salts that help in reducing skin irritation; or DS mud formulations for the prevention of dandruff [105].
In vitro and clinical studies demonstrated the benefits of DS mud in skin care. Ma’or et al., in an in vitro study, demonstrated that DS mud exerts antimicrobial activity, which might partially explain the therapeutic properties of the mud and may explain the antiacne effect attributed to facial DS mud masks [73]. Antibacterial properties of crude DS mud have been attributed to the presence of Bacillus persicus [44]. DS mud was also able to accelerate the wound-healing process by enhancing granulation, wound contraction, epithelialization, angiogenesis, and collagen deposition [78].
Two clinical studies were performed by Hamed et al. in 2018 and 2021 to assess the skin tolerance of DS mud formulations, showing that DS mud was very well tolerated, even in long-term use, did not disturb the skin barrier, and exerted a slight skin hydration improvement [79,80]. Furthermore, a cosmeceutical formulation containing DS mud, aloe vera extract, and vitamins showed in vitro antioxidant and anti-inflammatory activities, protecting against UVB irradiation [52].
Cervia liman peloid, silica-rich and prepared from hypersaline water, combined with heliotherapy, was able to reduce PASI and the use of topical drugs, as well as the psoriasis recurrences [81].
Ischia Island peloid is volcanic-derived clay mud that is used for psoriasis treatment, reducing inflammation, scaling, and erythema. Clinical studies showed that the combination of balneotherapy and mud therapy was able to reduce PASI [82]. Furthermore, Ischia Island mud was demonstrated to exert antibacterial and antibiofilm activities in vitro study [83].
Marine muds were also investigated to evaluate their potential use as cosmeceuticals. Korean sea mud, obtained from a tidal flat on the west coast in Boryeong (Chungnam, Korea), which is used to prepare different cosmetic formulations such as soap and packs, has been investigated to evaluate its anti-inflammatory properties. Humic substances (humic acid, fulvic acid, and humine) from crude sea mud were extracted and their anti-inflammatory activity was tested in human keratinocytes that had been subjected to UVB irradiation. Measurement of the levels of PGE2 (Prostaglandin E2) released from the irradiated cells demonstrated that aqueous extracts showed a higher anti-inflammatory effect than methanol extracts. Authors concluded that the mud extract could be used as a safe natural component in skin formulations aimed to either protect or calm irritated skin from sun exposure [84].
Ulcinj peloid is a marine mud from the area of Ulcinj’s Solana (Montenegro), composed of quartz, kaolinite, illite, halite, and sylvite, and is also rich in sodium, calcium, and magnesium. Microbial diversity was also studied, concluding that the main genus was Pinnularia, belonging to the division Bacillariophyta-siliceous algae. The Ulcinj peloid showed antibacterial activity [85], and previous studies demonstrated its beneficial effects on reducing TEWS, skin erythema index, and increasing stratum corneum humidity [86]. A cosmeceutical peloid consisting of 2% Ulcinj peloid and tinctures of medicinal plants was investigated in patients with acne. After application twice daily for 28 days, the results showed an improvement in the FDA-IGA (Investigator’s Global Assessment of Acne Severity) score compared to the control group [87].
Du et al. reviewed several natural muds in China (folk use). After a comprehensive comparison of several mineral muds of different origins, it was found that most of them are rich in potassium, calcium, sodium, magnesium, iron, manganese, zinc, and copper, and the effect can be related to these elements, but there is no experimental proof. The authors concluded that although good results have been achieved in the clinical application of mineral mud, there is no systematic research on its effects, and in order to make the application of mineral mud more scientific, safe, and extensive, the specific effects of mineral mud should be studied [88].
”Ad hoc” prepared peloids are mainly composed of clay (hectorite, illite, kaolinite, etc.), but peat peloids can also be found.
In Spain, two prepared “ad hoc” peloids showed their efficacy in reducing the symptoms of psoriasis. La Toja peloid is prepared from the sediment of La Toja MMW, classified as hypersaline, chloride bromo-iodic, iron-rich, and hectorite clay. A long-term study demonstrated that this peloid is able to reduce PASI and also relapsed disease related to stress, and relapsed disease intensity of affected areas, achieving an overall improvement (subjective assessment), and reducing the need for medications [89,90]. Compostela peloid (from thermal spa O Tremo) is also an “ad hoc” peloid whose main composition is sulfur MMW and hectorite clay; preliminary studies on psoriasis showed that this mud is able to reduce PASI [91].
Saturnia mud is composed of a mixture of clay mainly composed of montmorillonite with the presence of illite and kaolinite, and hydrogen sulphide-rich MMW, maturated in tanks for 12 months with the spring water constantly flowing at 37.0 °C. The scavenging activity on DPPH (Diphenyl-2-picrylhydrazyl) radical along the different stages of maturation (virgin mud, one-month, six-month, and twelve-month maturation time) showed that the antioxidant activity achieved the peak in six-month maturation [92].
Peat is also of great interest in the preparation of dermocosmetic peloids. Błonska-Sikora et al. [106] reviewed the dermocosmetic formulations whose main ingredients are peat or sulfurous water, but they only mentioned a company that combines both ingredients (BALNEO kosmetyki: https://balneokosmetyki.pl (accessed on 1 September 2024)).
Di Pascua et al. [47] studied the composition of Massaciuccoli peat (Undulna Thermae, Italy) at different stages of maturation with salty bromine–iodine water to evaluate its potential use in dermatological disorders, observing an increase in protein production throughout the maturation process, in addition to the presence of humic and fulvic acids. Previous studies have shown that this peat could be useful in the treatment of seborrheic dermatitis [93], and gynoid lipodystrophies [94].
Other studies related to the cosmeceutical use of peloids are also of interest, despite no clinical or in vitro studies having been performed to support the supposed cosmetic properties.
Vadlja et al. evaluated the microbial community within the Nin mud (Croatia) to assess its diversity and secondly to identify natural compounds exhibiting biological activity, which could be linked to the peloid’s putative healing properties, finding a high portion of detected compounds that exhibit biological activities [107].
PA Iavich et al. [108] developed cosmeceutical formulations (masks) containing 6 to 8% of Akthala mud (Georgia) based on the antibacterial and anti-inflammatory properties, as well as wound healing activity of this mud [109], but no studies have been carried out to support these dermocosmetic properties [108].
Sulfur peloids, in addition to their application in rheumatic pathology, may be of interest in dermatological disorders, given the well-known therapeutic effects of sulfur associated with anti-oxidizing mechanisms, which is the case of Copahue peloids [110]. More studies are needed to evaluate its use in skin care.
Additionally, Spiloti et al. investigated the properties of mud extracts from various thermal spa centers in Greece, studying their mineralogical content, and performing a quantitative analysis of the phenolic compounds, as well as the anti-inflammatory and antimicrobial properties. Then, they selected the most stable mud extract, as being the most suitable for incorporation into a cosmetic formulation, and investigated its effect on the functional integrity of human keratinocytes. Results showed that treatment of keratinocytes with mud extract led to a significant increase of ATP levels as well as mRNA expression of genes involved in cell protection and longevity, concluding that mud could be used as a natural antioxidant and moisturizing agent with cosmeceutical applications [111].
Finally, it is worth mentioning a pilot study on the efficacy of a seaweed mud application in the treatment of cellulite; although it is not a peloid, as it is a mixture of clay and seaweed, it can serve as a reference for the formulation of peloids for this type of disorders and thalasso-wellness treatments. After 4 weeks of seaweed mud treatment, patients showed significant restoration of dermal organization with induced collagen synthesis and reduced inflammation, edema, and lipid deposition [112].
The main limitations of this research are that there are few clinical studies related to the use of peloids in the treatment of skin dermatological disorders as well as their dermocosmetic use. Most of the research works deal with chemical composition, but there is a lack of studies on the specific mechanisms of action of the chemical elements and compounds present.
7. Safety and Security of Cosmeceutical Peloids
Preparing and manufacturing peloids requires a controlled procedure as well as exhaustive quality controls. Mourelle et al. proposed a method for the manufacture of a dermocosmetic peloid in which the selection and characterization of raw materials (MMW/seawater, solid phase, and cyanobacteria/microalgae) in the first stage, peloid characterization in the second stage, and finally, efficacy tests should be performed [113]. Ma’or et al., in a review related to the formulation of Dea Sea mud in cosmetics products, pointed out that one of the main problems when natural mud is mixed with other ingredients to formulate dermocosmetic products is to stabilize the mixture since the mud particles tend to sediment. To do this, adding anti-flocculants or water-absorbing agents is recommended; another way of stabilizing to avoid the separation of the solid–liquid phases is to reduce the particle size of the mud, thus increasing its viscosity [114].
The penetration of the chemical elements of peloids through the skin has been demonstrated [115,116]. Hence, apart from the beneficial effects on the skin, there is concern about the penetration of potentially toxic ions. Therefore, when peloids are of natural origin, it is necessary to investigate the possible toxic elements present and evaluate their safety. It is also very important to study its possible microbiological contamination.
In addition, the content of certain potentially sensitizing chemical elements such as chromium and nickel must be taken into account, establishing concentration limits [117].
Karpińska et al. investigated the contents of natural and artificial isotopes in peat mud (Poland) in order to estimate the radiation dose absorbed via skin in patients during standard peat mud treatment; it turns out that the doses were very low and did not pose a risk to the patient [114,118].
Cantaluppi et al. investigated the quantitative measures of gamma-emitting radionuclides in therapeutic muds that have been developed on about 30 thermal centers and on the virgin clays in the Euganean Thermal District. Results showed that no radiological risks have been detected for workers managing the ponds, even in the most cautious scenario. Moreover, for patients, the contribution of the Euganean mud therapy to the annual equivalent dose is insignificant [119].
Park et al. performed extensive research on natural sediments from the subsea depth of 200 m (Hupo basin of the East Sea, South Korea) to evaluate their potential use as natural peloids. The study included the mineralogical feature, thermophysical characterization, thermal and physicochemical properties, and toxic elements content, concluding that the concentrations of harmful elements in the samples were well below the thresholds for cosmetic products in South Korea, as well as other standards [120].
Evaluation of hygienic aspects of thermal mud microbiology is also a matter of interest in terms of security. Baldowin et al. investigated the microbiological quality of more than 180 samples of the Euganean Thermal District and proposed a protocol and a set of indicator parameters for evaluating the microbiological hygiene quality of thermal mud that could encompass Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, and dermatophytes. The protocol should be applied to all phases of mud preparation (virgin mud, maturation process, application, recycling). They also recommend a pasteurization step of the peloid with thermal water ≥ 60 °C just before treatment, so as to grant its hygienic quality as well [121].
In this context, Bastos et al. recently emphasized the need for specific regulation for peloids that can be considered borderline products [122,123]. In most countries, peloids are marketed under cosmetics law, although they may also be marketed as over-the-counter (OTC) products in the USA, and as quasi-drugs in Japan [124]. Bastos et al. proposed that these products require a more accurate case-by-case evaluation in order to comply with relevant legislation for medicinal products, particularly when used for therapeutic purposes [116], but also for cosmetic use.
8. Conclusions and Way Forward
Peloids have been used for years in both dermatological disorders and skin care of different skin conditions and have demonstrated this potential use as cosmeceuticals, with very good results in patients suffering from a range of conditions, specifically chronic inflammatory skin diseases such as psoriasis and atopic dermatitis, but also in sensitive skin or acne. Over recent years, several publications, both related to natural and prepared ad hoc peloids, have led to a better understanding of their effects and specific activities, such as antimicrobial activity, anti-inflammatory and antioxidant effects, improvement and acceleration of the wound-healing process, or skin barrier function improvement. Regarding its safety, although there are few studies, progress is being made in research on microbiological quality and the presence of harmful elements. The evidence to date justifies the use of peloids in skin care and cosmeceuticals, pointing out that they are also safe.
Future research should aim to delve deeper into the mechanisms of action, the bioavailability of potentially hazardous substances, and the therapeutic efficacy for various skin conditions, and in parallel to this, develop specific regulations to determine the conditions of application inside and outside the thermal centers, and for quality control and their possible commercialization as cosmeceuticals.
Author Contributions
M.L.M.: Conceptualization, methodology, investigation, writing—original draft preparation, writing—review and editing. C.P.G.: Methodology, investigation, writing—review, and editing. J.L.L.: Writing—review and editing, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Baroni, A.; Buommino, E.; De Gregorio, V.; Ruocco, E.; Ruocco, V.; Wolf, R. Structure and Function of the Epidermis Related to Barrier Properties. Clin. Dermatol. 2012, 30, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Madnani, N.; Deo, J.; Dalal, K.; Benjamin, B.; Murthy, V.V.; Hegde, R.; Shetty, T. Revitalizing the Skin: Exploring the Role of Barrier Repair Moisturizers. J. Cosmet. Dermatol. 2024, 23, 1533–1540. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Q.; Zhi, L.; You, B.; Wang, G.; Wu, N.; Jia, Y. Skin Homeostasis: Mechanism and Influencing Factors. J. Cosmet. Dermatol. 2024, 23, 1518–1526. [Google Scholar] [CrossRef] [PubMed]
- Regulations (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products. Available online: http://data.europa.eu/eli/reg/2009/1223/2019-08-13 (accessed on 1 September 2024).
- Kligman, A. The Future of Cosmeceuticals: An Interview with Albert Kligman, MD, PhD. Interview by Zoe Diana Draelos. Dermatol. Surg. 2005, 31 Pt 2, 890–891. [Google Scholar] [PubMed]
- Sathyaseelan, S.; Rao, B.H.; Anushmati, S. Cosmeceuticals: A Transit State from Synthetic to Natural. Indian J. Pharmacol. 2024, 56, 42–51. [Google Scholar] [CrossRef]
- Nguyen, J.K.; Masub, N.; Jagdeo, J. Bioactive ingredients in Korean cosmeceuticals: Trends and research evidence. J. Cosmet. Dermatol. 2020, 19, 1555–1569. [Google Scholar] [CrossRef]
- Chan, L.K.W.; Lee, K.W.A.; Lee, C.H.; Lam, K.W.P.; Lee, K.F.V.; Wu, R.; Wan, J.; Shivananjappa, S.; Sky, W.T.H.; Choi, H.; et al. Cosmeceuticals in photoaging: A review. Skin Res. Technol. 2024, 30, e13730. [Google Scholar] [CrossRef]
- Tian, X.; Zhang, Y.; Li, H.; Jiao, Y.; Wang, Q.; Zhang, Y.; Ma, N.; Wang, W. Property of mud and its application in cosmetic and medical fields: A review. Environ. Geochem. Health 2022, 44, 4235–4251. [Google Scholar] [CrossRef]
- Porlezza, C. Considerazione sui fanghi terapeutici (peloidi). Thermae 1965, II, 6–57. (In Italian) [Google Scholar]
- Maraver, F. Antecedentes históricos de la peloterapia. An. Hidrol. Médica 2006, 1, 17–42. (In Spanish) [Google Scholar]
- Lewis, J. Thermal properties of peloids. Part II. Arch. Med. Hydrol. 1935, 13, 56–57. [Google Scholar]
- Gomes, C.S.F.; Rautureau, M.; Gomes, J.H.C.; Silva, E.A.F. Interactions of Clay and Clay Minerals with the Human Health. In Minerals Latu Sensu and Human Health; Gomes, C., Rautureau, M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 271–375. [Google Scholar] [CrossRef]
- Gomes, C.; Carretero, M.I.; Pozo, M.; Maraver, F.; Cantista, P.; Armijo, F.; Legido, J.L.; Teixeira, F.; Rautureau, M.; Delgado, R. Peloids and Pelotherapy: Historical Evolution, Classification and Glossary. Appl. Clay Sci. 2013, 75–76, 28–38. [Google Scholar] [CrossRef]
- Legido, J.; Medina, C.; Lourdesmourelle, M.; Carretero, M.; Pozo, M. Comparative Study of the Cooling Rates of Bentonite, Sepiolite and Common Clays for Their Use in Pelotherapy. Appl. Clay Sci. 2007, 36, 148–160. [Google Scholar] [CrossRef]
- Directive 2009/54/EC of the European Parliament and of the Council of 18 June 2009 on the Exploitation and Marketing of Natural Mineral Waters. Available online: https://eur-lex.europa.eu/eli/dir/2009/54/oj (accessed on 1 September 2024).
- Spanish Medical Hydrology Association (SEHM). Available online: https://www.hidromed.org/hm/index.php/el-agua (accessed on 1 September 2024).
- Legido, J.L.; Gómez, C.P. Seawater: Composition, Physical and Chemical Properties. In Thalassotherapy and Cosmeceuticals; Mourelle, M.L., Kalasariya, H.S., Eds.; Aquatic Sciences series; CRC Press: Boca Raton, FL, USA, 2025; in press. [Google Scholar]
- Carretero, M.I. Clays in pelotherapy. A review. Part I: Mineralogy, chemistry, physical and physicochemical properties. Appl. Clay Sci. 2020, 189, 105526. [Google Scholar] [CrossRef]
- Carretero, M.I. Clays in pelotherapy. A review. Part II: Organic compounds, microbiology and medical applications. Appl. Clay Sci. 2020, 189, 105531. [Google Scholar] [CrossRef]
- López-Galindo, A.; Viseras, C. Pharmaceutical and Cosmetic Applications of Clays. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2004; Volume 1, pp. 267–289. [Google Scholar] [CrossRef]
- López-Galindo, A.; Viseras, C.; Cerezo, P. Compositional, Technical and Safety Specifications of Clays to Be Used as Pharmaceutical and Cosmetic Products. Appl. Clay Sci. 2007, 36, 51–63. [Google Scholar] [CrossRef]
- López-Galindo, A.; Viseras, C.; Aguzzi, C.; Cerezo, P. Pharmaceutical and Cosmetic Uses of Fibrous Clays. In Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2011; Volume 3, pp. 299–324. [Google Scholar] [CrossRef]
- Viseras, C.; Carazo, E.; Borrego-Sánchez, A.; García-Villén, F.; Sánchez-Espejo, R.; Cerezo, P.; Aguzzi, C. Clay Minerals in Skin Drug Delivery. Clays Clay Min. 2019, 67, 59–71. [Google Scholar] [CrossRef]
- Viseras, C.; Sánchez-Espejo, R.; Palumbo, R.; Liccardi, N.; García-Villén, F.; Borrego-Sánchez, A.; Massaro, M.; Riela, S.; López-Galindo, A. Clays in Cosmetics and Personal-Care Products. Clays Clay Min. 2021, 69, 561–575. [Google Scholar] [CrossRef]
- Awad, M.E.; López-Galindo, A.; El-Rahmany, M.M.; El-Desoky, H.M.; Viseras, C. Characterization of Egyptian Kaolins for Health-Care Uses. Appl. Clay Sci. 2017, 135, 176–189. [Google Scholar] [CrossRef]
- Gomes, C.F.; Gomes, J.H.; Da Silva, E.F. Bacteriostatic and Bactericidal Clays: An Overview. Environ. Geochem. Health 2020, 42, 3507–3527. [Google Scholar] [CrossRef]
- Moraes, J.D.D.; Bertolino, S.R.A.; Cuffini, S.L.; Ducart, D.F.; Bretzke, P.E.; Leonardi, G.R. Clay Minerals: Properties and Applications to Dermocosmetic Products and Perspectives of Natural Raw Materials for Therapeutic Purposes—A Review. Int. J. Pharm. 2017, 534, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Wargala, E.; Sławska, M.; Zalewska, A.; Toporowska, M. Health Effects of Dyes, Minerals, and Vitamins Used in Cosmetics. Women 2021, 1, 223–237. [Google Scholar] [CrossRef]
- Finkelman, R.B. The Influence of Clays on Human Health: A Medical Geology Perspective. Clays Clay Min. 2019, 67, 1–6. [Google Scholar] [CrossRef]
- Sarruf, F.D.; Contreras, V.J.P.; Martinez, R.M.; Velasco, M.V.R.; Baby, A.R. The Scenario of Clays and Clay Minerals Use in Cosmetics/Dermocosmetics. Cosmetics 2024, 11, 7. [Google Scholar] [CrossRef]
- Ghadiri, M.; Chrzanowski, W.; Rohanizadeh, R. Biomedical applications of cationic clay minerals. RSC Adv. 2015, 5, 29467–29481. [Google Scholar] [CrossRef]
- Carretero, M.I. Clay minerals and their beneficial effects upon human health. A review. Appl. Clay Sci. 2002, 21, 155–163. [Google Scholar] [CrossRef]
- Caichiolo, M.; Zampieri, R.M.; Adessi, A.; Ciani, M.; Caldara, F.; Dalla Valle, L.; La Rocca, N. Microbial Polysaccharides Extracted from Different Mature Muds of the Euganean Thermal District Show Similar Anti-Inflammatory Activity In Vivo. Int. J. Mol. Sci. 2024, 25, 4999. [Google Scholar] [CrossRef]
- Knorst-Fouran, A.; Casás, L.M.; Legido, J.L.; Coussine, C.; Bessières, D.; Plantier, F.; Lagière, J.; Dubourg, K. Influence of Dilution on the Thermophysical Properties of Dax Peloid (TERDAX®). Thermochim. Acta 2012, 539, 34–38. [Google Scholar] [CrossRef]
- Mihelčić, G.; Kniewald, G.; Ivanišević, G.; Čepelak, R.; Mihelčić, V.; Vdović, N. Physico-Chemical Characteristics of the Peloid Mud from Morinje Bay (Eastern Adriatic Coast, Croatia): Suitability for Use in Balneotherapy. Environ. Geochem. Health 2012, 34, 191–198. [Google Scholar] [CrossRef]
- Komar, D.; Dolenec, T.; Dolenec, M.; Vrhovnik, P.; Lojen, S.; Belak, Ž.L.; Kniewald, G.; Šmuc, N.R. Physico-chemical and geochemical characterization of Makirina Bay peloid mud and its evaluation for potential use in balneotherapy (N Dalmatia, Republic of Croatia). Indian J. Tradit. Knowl. 2015, 14, 5–12. [Google Scholar]
- Bigovic, M.; Roganovic, M.; Milasevic, I.; Djurovic, D.; Slavic, V.; Kosovic, M.; Vlahovic, M.; Perovic, S.; Perovic, A.; Kastratovic, V.; et al. Physico-chemical characterization of Igalo Bay peloid (Montenegro) and assessment of the pollution of potentially toxic elements in the sampling area. Farmacia 2020, 68, 560–571. [Google Scholar] [CrossRef]
- Baricz, A.; Levei, E.A.; Șenilă, M.; Pînzaru, S.C.; Aluaş, M.; Vulpoi, A.; Filip, C.; Tripon, C.; Dădârlat, D.; Buda, D.M.; et al. Comprehensive Mineralogical and Physicochemical Characterization of Recent Sapropels from Romanian Saline Lakes for Potential Use in Pelotherapy. Sci. Rep. 2021, 11, 18633. [Google Scholar] [CrossRef] [PubMed]
- Özay, P.; Karagülle, M.; Kardeş, S.; Karagülle, M.Z. Chemical and Mineralogical Characteristics of Peloids in Turkey. Environ. Monit. Assess. 2020, 192, 805. [Google Scholar] [CrossRef] [PubMed]
- Akimzhanova, K.; Sabitova, A.; Mussabayeva, B.; Kairbekov, Z.; Bayakhmetova, B.; Proch, J. Chemical composition and physicochemical properties of natural therapeutic mud of Kazakhstan salt lakes: A review. Environ. Geochem. Health. 2024, 46, 43. [Google Scholar] [CrossRef] [PubMed]
- Glavaš, N.; Mourelle, M.L.; Gómez, C.P.; Legido, J.L.; Rogan Šmuc, N.; Dolenec, M.; Kovač, N. The Mineralogical, Geochemical, and Thermophysical Characterization of Healing Saline Mud for Use in Pelotherapy. Appl. Clay Sci. 2017, 135, 119–128. [Google Scholar] [CrossRef]
- Kamitsou, M.D.; Sygouni, V.; Kanellopoulou, D.G.; Gardikis, K.; Koutsoukos, P.G. Physicochemical Characterization of Sterilized Muds for Pharmaceutics/Cosmetics Applications. Environ. Geochem. Health 2018, 40, 1449–1464. [Google Scholar] [CrossRef]
- Al-Karablieh, N. Antimicrobial Activity of Bacillus persicus 24-DSM Isolated from Dead Sea Mud. Open Microbiol. J. 2017, 11, 372–383. [Google Scholar] [CrossRef]
- Riyaz, N.; Arakkal, F. Spa Therapy in Dermatology. Indian J. Dermatol. Venereol. Leprol. 2011, 77, 128. [Google Scholar] [CrossRef]
- Pozo, M.; Carretero, M.I.; Maraver, F.; Pozo, E.; Gómez, I.; Armijo, F.; Rubí, J.A.M. Composition and Physico-Chemical Properties of Peloids Used in Spanish Spas: A Comparative Study. Appl. Clay Sci. 2013, 83–84, 270–279. [Google Scholar] [CrossRef]
- Di Pasqua, L.G.; Berardo, C.; Raffo, L.; Ferrigno, A.; Guffanti, E.; Vairetti, M. Analysis of Massaciuccoli Peat after Maturation in Sodium Chloride Water of Undulna Thermae. Int. J. Environ. Res. Public Health 2022, 19, 2169. [Google Scholar] [CrossRef]
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L. Unveiling the Role of Minerals and Trace Elements of Thermal Waters in Skin Health. Appl. Sci. 2024, 14, 6291. [Google Scholar] [CrossRef]
- Pereira, L. Thalassotherapy and Marine Cosmeceuticals. In Therapeutic and Nutritional Uses of Algae, 1st ed.; CRC Press/Taylor & Francis Group: Abingdon, UK, 2017; Chapter 12; pp. 503–522. [Google Scholar]
- Denda, M.; Katagiri, C.; Hirao, T.; Maruyama, N.; Takahashi, M. Some Magnesium Salts and a Mixture of Magnesium and Calcium Salts Accelerate Skin Barrier Recovery. Arch. Dermatol. Res. 1999, 291, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Proksch, E.; Nissen, H.-P.; Bremgartner, M.; Urquhart, C. Bathing in a Magnesium-Rich Dead Sea Salt Solution Improves Skin Barrier Function, Enhances Skin Hydration, and Reduces Inflammation in Atopic Dry Skin. Int. J. Dermatol. 2005, 44, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Harari, M. Beauty is not only Skin Deep: The Dead Sea features and Cosmetics. An. Hidrol. Méd. 2012, 5, 75–88. [Google Scholar] [CrossRef]
- Portugal-Cohen, M.; Cohen, D.; Ish-Shalom, E.; Laor-Costa, Y.; Ma’or, Z. Dead Sea Minerals: New Findings on Skin and the Biology Beyond. Exp. Dermatol. 2019, 28, 585–592. [Google Scholar] [CrossRef]
- Yan, X.; Bao, X.; Cheng, S.; Ba, Q.; Chang, J.; Zhou, K.; Yan, X. Anti-aging and Rejuvenating Effects and Mechanism of Dead Sea Water in Skin. Int. J. Cosmet. Sci 2024, 46, 307–317. [Google Scholar] [CrossRef]
- Moro, I.; Rascio, N.; La Rocca, N.; Di Bella, M.; Andreoli, C. Cyanobacterium aponinum, a New Cyanoprokaryote from the Microbial Mat of Euganean Thermal Springs (Padua, Italy). Algol. Stud. 2007, 123, 1–15. [Google Scholar] [CrossRef]
- Moro, I.; Rascio, N.; La Rocca, N.; Sciuto, K.; Albertano, P.; Bruno, L.; Andreoli, C. Polyphasic Characterization of a Thermo-Tolerant Filamentous Cyanobacterium Isolated from the Euganean Thermal Muds (Padua, Italy). Eur. J. Phycol. 2010, 45, 143–154. [Google Scholar] [CrossRef]
- Gris, B.; Sforza, E.; Morosinotto, T.; Bertucco, A.; La Rocca, N. Influence of Light and Temperature on Growth and High-Value Molecules Productivity from Cyanobacterium aponinum. J. Appl. Phycol. 2017, 29, 1781–1790. [Google Scholar] [CrossRef]
- Berrini, C.C.; De Appolonia, F.; Dalla Valle, L.; Komárek, J.; Andreoli, C. Morphological and Molecular Characterization of a Thermophilic Cyanobacterium (Oscillatoriales) from the Euganean Thermal Springs (Padua, Italy). Arch. Hydrobiol. Algol. Stud. 2004, 113, 73–85. [Google Scholar] [CrossRef]
- Marcolongo, G.; De Appolonia, F.; Venzo, A.; Berrie, C.P.; Carofiglio, T.; Ceschi Berrini, C. Diacylglycerolipids Isolated from a Thermophile Cyanobacterium from the Euganean Hot Springs. Nat. Prod. Res. 2006, 20, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Gris, B.; Treu, L.; Zampieri, R.M.; Caldara, F.; Romualdi, C.; Campanaro, S.; La Rocca, N. Microbiota of the Therapeutic Euganean Thermal Muds with a Focus on the Main Cyanobacteria Species. Microorganisms 2020, 8, 1590. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, R.M.; Adessi, A.; Caldara, F.; Codato, A.; Furlan, M.; Rampazzo, C.; De Philippis, R.; La Rocca, N.; Dalla Valle, L. Anti-Inflammatory Activity of Exopolysaccharides from Phormidium sp. ETS05, the Most Abundant Cyanobacterium of the Therapeutic Euganean Thermal Muds, Using the Zebrafish Model. Biomolecules 2020, 10, 582. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, R.M.; Adessi, A.; Caldara, F.; De Philippis, R.; Dalla Valle, L.; La Rocca, N. In Vivo Anti-Inflammatory and Antioxidant Effects of Microbial Polysaccharides Extracted from Euganean Therapeutic Muds. Int. J. Biol. Macromol. 2022, 209, 1710–1719. [Google Scholar] [CrossRef] [PubMed]
- Moro, I.; Fuiano, M.A.; Rascio, N.; De Philippis, R.; La Rocca, N. Phylogenetic, Morphological and Biochemical Studies on Thermospirulina andreolii gen. & sp. nov. (Cyanophyta) from the Euganean Thermal District (Italy). Phycologia 2021, 60, 487–496. [Google Scholar] [CrossRef]
- Duval, C.; Hamlaoui, S.; Piquet, B.; Toutirais, G.; Yéprémian, C.; Reinhardt, A.; Duperron, S.; Marie, B.; Demay, J.; Bernard, C. Diversity of Cyanobacteria from Thermal Muds (Balaruc-Les-Bains, France) with the Description of Pseudochroococcus Coutei gen. nov., sp. nov. FEMS Microbes 2021, 2, xtab006. [Google Scholar] [CrossRef]
- Demay, J.; Halary, S.; Knittel-Obrecht, A.; Villa, P.; Duval, C.; Hamlaoui, S.; Roussel, T.; Yéprémian, C.; Reinhardt, A.; Bernard, C.; et al. Anti-Inflammatory, Antioxidant, and Wound-Healing Properties of Cyanobacteria from Thermal Mud of Balaruc-Les-Bains, France: A Multi-Approach Study. Biomolecules 2020, 11, 28. [Google Scholar] [CrossRef]
- Halary, S.; Duperron, S.; Demay, J.; Duval, C.; Hamlaoui, S.; Piquet, B.; Reinhardt, A.; Bernard, C.; Marie, B. Metagenome-Based Exploration of Bacterial Communities Associated with Cyanobacteria Strains Isolated from Thermal Muds. Microorganisms 2022, 10, 2337. [Google Scholar] [CrossRef]
- Petursdottir, S.K.; Kristjansson, J.K. The relationship between physical and chemical conditions and low microbial diversity in the Blue Lagoon geothermal lake in Iceland. FEMS Microbiol. Ecol. 1996, 19, 39–45. [Google Scholar] [CrossRef][Green Version]
- Petursdottir, S.K.; Kristjansson, J.K. Silicibacter lacuscaerulensis gen. nov., sp. nov., a Mesophilic Moderately Halophilic Bacterium Characteristic of the Blue Lagoon Geothermal Lake in Iceland. Extremophiles 1997, 1, 94–99. [Google Scholar] [CrossRef]
- Petursdottir, S.K.; Bjornsdottir, S.H.; Hreggvidsson, G.O.; Hjorleifsdottir, S.; Kristjansson, J.K. Analysis of the Unique Geothermal Microbial Ecosystem of the Blue Lagoon. FEMS Microbiol. Ecol. 2009, 70, 425–432. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Palinska, K.A.; Vogt, J.C.; Surosz, W. Biodiversity Analysis of the Unique Geothermal Microbial Ecosystem of the Blue Lagoon (Iceland) Using next-Generation Sequencing (NGS). Hydrobiologia 2018, 811, 93–102. [Google Scholar] [CrossRef]
- Gudmundsdottir, A.B.; Omarsdottir, S.; Brynjolfsdottir, A.; Paulsen, B.S.; Olafsdottir, E.S.; Freysdottir, J. Exopolysaccharides from Cyanobacterium aponinum from the Blue Lagoon in Iceland Increase IL-10 Secretion by Human Dendritic Cells and Their Ability to Reduce the IL-17+RORγt+/IL-10+FoxP3+ Ratio in CD4+ T Cells. Immunol. Lett. 2015, 163, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsdottir, A.B.; Brynjolfsdottir, A.; Olafsdottir, E.S.; Hardardottir, I.; Freysdottir, J. Exopolysaccharides from Cyanobacterium aponinum Induce a Regulatory Dendritic Cell Phenotype and Inhibit SYK and CLEC7A Expression in Dendritic Cells, T Cells and Keratinocytes. Int. Immunopharmacol. 2019, 69, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Ma’or, Z.; Henis, Y.; Alon, Y.; Orlov, E.; Sørensen, K.B.; Oren, A. Antimicrobial Properties of Dead Sea Black Mineral Mud. Int. J. Dermatol. 2006, 45, 504–511. [Google Scholar] [CrossRef]
- Oren, A.; Ginzburg, M.; Ginzburg, B.Z.; Hochstein, L.I.; Volcani, B.E. Haloarcula marismortui (Volcani) sp. nov., nom. rev., an extremely halophilic bacterium from the Dead Sea. Int. J. Syst. Bacteriol. 1990, 40, 209–210. [Google Scholar] [CrossRef]
- Obeidat, M. Isolation and characterization of extremely halotolerant Bacillus species from Dead Sea black mud and determination of their antimicrobial and hydrolytic activities. Afr. J. Microbiol. Res. 2017, 11, 1303–1314. [Google Scholar]
- Tserenpil, S.; Dolmaa, G.; Voronkov, M.G. Organic Matters in Healing Muds from Mongolia. Appl. Clay Sci. 2010, 49, 55–63. [Google Scholar] [CrossRef]
- Grether-Beck, S.; Mühlberg, K.; Brenden, H.; Felsner, I.; Brynjólfsdóttir, Á.; Einarsson, S.; Krutmann, J. Bioactive Molecules from the Blue Lagoon: In Vitro and in Vivo Assessment of Silica Mud and Microalgae Extracts for Their Effects on Skin Barrier Function and Prevention of Skin Ageing. Exp. Dermatol. 2008, 17, 771–779. [Google Scholar] [CrossRef]
- Abu-Al-Bas, M.A. Histological Evaluation of the Healing Properties of Dead Sea Black Mud on Full-Thickness Excision Cutaneous Wounds in BALB/c Mice. Pak. J. Biol. Sci. 2012, 15, 306–315. [Google Scholar] [CrossRef]
- Hamed, S.; Almalty, A.-M. Skin Tolerance of Three Types of Dead Sea Mud on Healthy Skin: A Short-Term Study. J. Cosmet. Sci. 2018, 69, 269–278. [Google Scholar] [PubMed]
- Hamed, S.; Almalty, A.; Alkhatib, H.S. The Cutaneous Effects of Long-term Use of Dead Sea Mud on Healthy Skin: A 4-week Study. Int. J. Dermatol. 2021, 60, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Melandri, D.; Albano, V.M.; Venturi, M.; Flamigni, A.; Vairetti, M. Efficacy of Combined Liman Peloid Baths and Heliotherapy in the Treatment of Psoriasis at Cervia Spa, Emilia, Italy. Int. J. Biometeorol. 2020, 64, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Delfino, M.; Russo, N.; Migliaccio, G.; Carraturo, N. Studio sperimentale sull’efficacia dei fanghi termali dell’isola di Ischia associati a balneoterapia nella cura della psoriasi volgare a placche [Experimental study on efficacy of thermal muds of Ischia Island combined with balneotherapy in the treatment of psoriasis vulgaris with plaques]. Clin. Ter. 2003, 154, 167–171. (In Italian) [Google Scholar]
- Di Onofrio, V.; Maione, A.; Guida, M.; De Castro, O.; Liguori, R.; Carraturo, F.; Galdiero, E. Screening and Isolation of Microbes from a Mud Community of Ischia Island Thermal Springs: Preliminary Analysis of a Bioactive Compound. J. Prev. Med. Hyg. 2021, 62, E479–E488. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, J.; Lee, H.-B.; Shin, J.H.; Kim, E.-K. Water-Retentive and Anti-Inflammatory Properties of Organic and Inorganic Substances from Korean Sea Mud. Nat. Prod. Commun. 2010, 5, 1934578X1000500. [Google Scholar] [CrossRef]
- Potpara, Z.; Duborija-Kova, A. Effects of the Peloid Cream from the Montenegrin Adriatic Coast on Skin Humidity, Transepidermal Water Loss and Erythema Index, Examined with Skin Bioengeneering In Vivo Methods. Farmacia 2012, 60, 524–534. [Google Scholar]
- Potpara, Z.; Pantovic, S.; Duborija-Kovacevic, N.; Tadic, V.; Vojinovic, T.; Marstijepovic, N. The Properties of the Ulcinj Peloid Make It Unique Biochemical Laboratory Required for the Treatment of Problematic Skin and Health Care. Nat. Prod. Commun. 2017, 12, 1934578X1701200. [Google Scholar] [CrossRef]
- Potpara, Z.; Pantovic, S.; Zecevic, A.A. Peloid-Based Cosmeutics Is Effective in the Treatment of Acnotic Skin—Prospective Study from Montenegro. Res. Sq. 2024. [Google Scholar] [CrossRef]
- Du, Y.; Deng, X.; Li, L.; Dong, Y. The Investigation of Function of Mineral Mud for the Skin. Asian J. Beauty Cosmetol. 2016, 14, 98–104. [Google Scholar] [CrossRef]
- Arribas, M.; Meijide, R.; Mourelle, M.L. Long-term effects of psoriasis treatment with mineral water and peloids of la Toja. Balnea 2012, 6, 289–290. [Google Scholar]
- Arribas, M.; Gómez, C.P.; Mourelle, M.L. Nuevos casos clínicos tratados con peloide La Toja. In Proceedings of the V Congreso Iberoamericano de Peloides, Badajoz, Spain, 11–14 June 2017. (In Spanish). [Google Scholar]
- Cabana, B.; Galiñares, M.; Mourelle, L. Estudio preliminar con peloides en paciente con psoriasis. In Proceedings of the V Congreso Iberoamericano de Peloides, Badajoz, Spain, 11–14 June 2017. (In Spanish). [Google Scholar]
- Centini, M.; Tredici, M.R.; Biondi, N.; Buonocore, A.; Maffei Facino, R.; Anselmi, C. Thermal Mud Maturation: Organic Matter and Biological Activity. Int. J Cosmet. Sci. 2015, 37, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Pedrinazzi, C.; Andreoli, S.; Battistini, E.; D’Errigo, M.L.; Gregotti, C.; Richelmi, P. Efficacia di una maschera di torba e acqua termale salsobromoiodica nel trattamento della dermatite seborroica del viso. J. Plast. Dermatol. 2009, 5, 294. (In Italian) [Google Scholar]
- Rondanelli, M.; Opizzi, A.; Perna, S.; Faliva, M.A.; Buonocore, D.; Pezzoni, G.; Michelotti, A.; Marchetti, R.; Marzatico, F. Efficacia significativa del trattamento di due settimane con associazione di torba del Massaciuccoli ed acqua clorurato-sodica delle terme di Undulna sulla lipodistrofia a localizzazione ginoide in un gruppo di donne sovrappeso. [Significant two-weeks clinical efficacy of an association between Massaciuccoli peat and sodium chloride water of Undulna Thermae measured on gynoid lipodystrophy in a group of overweight female]. Ann. Ig 2012, 24, 369. (In Italian) [Google Scholar] [PubMed]
- Cacciapuoti, S.; Luciano, M.; Megna, M.; Annunziata, M.; Napolitano, M.; Patruno, C.; Scala, E.; Colicchio, R.; Pagliuca, C.; Salvatore, P.; et al. The Role of Thermal Water in Chronic Skin Diseases Management: A Review of the Literature. J. Clin. Med. 2020, 9, 3047. [Google Scholar] [CrossRef]
- Moini Jazani, A.; Ayati, M.H.; Nadiri, A.A.; Nasimi Doost Azgomi, R. Efficacy of Hydrotherapy, Spa Therapy, and Balneotherapy for Psoriasis and Atopic Dermatitis: A Systematic Review. Int. J. Dermatol. 2023, 62, 177–189. [Google Scholar] [CrossRef]
- Protano, C.; Vitali, M.; De Giorgi, A.; Marotta, D.; Crucianelli, S.; Fontana, M. Balneotherapy Using Thermal Mineral Water Baths and Dermatological Diseases: A Systematic Review. Int. J. Biometeorol. 2024, 68, 1005–1013. [Google Scholar] [CrossRef]
- Couteau, C.; Coiffard, L. Phycocosmetics and Other Marine Cosmetics, Specific Cosmetics Formulated Using Marine Resources. Mar. Drugs 2020, 18, 322. [Google Scholar] [CrossRef]
- Khiari, I.; Mefteh, S.; Sánchez-Espejo, R.; Cerezo, P.; Aguzzi, C.; López-Galindo, A.; Jamoussi, F.; Viseras Iborra, C. Study of Traditional Tunisian Medina Clays Used in Therapeutic and Cosmetic Mud-Packs. Appl. Clay Sci. 2014, 101, 141–148. [Google Scholar] [CrossRef]
- Karakaya, C.M.; Karakaya, N.; Aydin, S. The Physical and Physicochemical Properties of Some Turkish Thermal Muds and Pure Clay Minerals and Their Uses in Therapy. Turk. J. Earth Sci. 2017, 26, 395–409. [Google Scholar] [CrossRef]
- Antonelli, M.; Donelli, D. Mud Therapy and Skin Microbiome: A Review. Int. J. Biometeorol. 2018, 62, 2037–2044. [Google Scholar] [CrossRef] [PubMed]
- Eysteinsdóttir, J.H.; Sigurgeirsson, B.; Ólafsson, J.H.; Fridriksson, T.; Agnarsson, B.A.; Davíðsson, S.; Valdimarsson, H.; Lúðvíksson, B.R. The Role of Th17/Tc17 Peripheral Blood T Cells in Psoriasis and Their Positive Therapeutic Response. Scand. J. Immunol. 2013, 78, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Eysteinsdóttir, J.H.; Ólafsson, J.H.; Agnarsson, B.A.; Lúðvíksson, B.R.; Sigurgeirsson, B. Psoriasis Treatment: Faster and Long-standing Results after Bathing in Geothermal Seawater. A Randomized Trial of Three UVB Phototherapy Regimens. Photodermatol. Photoimmunol. Photomed. 2014, 30, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Grether-Beck, S.; Marini, A.; Jaenicke, T.; Brenden, H.; Felsner, I.; Aue, N.; Brynjolfsdottir, A.; Krutmann, J. Blue Lagoon Algae Improve Uneven Skin Pigmentation: Results from in Vitro Studies and from a Monocentric, Randomized, Double-Blind, Vehicle-Controlled, Split-Face Study. Skin Pharmacol. Physiol. 2022, 35, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Bawab, A.A.; Bozeya, A.; Abu-Mallouh, S.; Abu Irmaileh, B.; Daqour, I.; Abu-Zurayk, R.A. The Dead Sea Mud and Salt: A Review of Its Characterization, Contaminants, and Beneficial Effects. IOP Conf. Ser. Mater. Sci. Eng. 2018, 305, 012003. [Google Scholar] [CrossRef]
- Błońska-Sikora, E.M.; Klimek-Szczykutowicz, M.; Michalak, M.; Kulik-Siarek, K.; Wrzosek, M. Potential Possibilities of Using Peat, Humic Substances, and Sulfurous Waters in Cosmetology. Appl. Sci. 2024, 14, 6912. [Google Scholar] [CrossRef]
- Vadlja, D.; Bujak, M.; Čož-Rakovac, R.; Roje, M.; Čižmek, L.; Horvatić, A.; Svetličić, E.; Diminić, J.; Rakovac, S.; Oros, D.; et al. Bioprospecting for Microorganisms in Peloids—Extreme Environment Known for Its Healing Properties. Front. Mar. Sci. 2022, 9, 822139. [Google Scholar] [CrossRef]
- PA, I.; Kakhetelidze, M.; Gabelaya, M.; Churadze, L. Cosmeceutical masks using therapeutic mud of Akhtala (Georgia) and products from plant materials. World J. Pharm. Res. 2020, 9, 189–194. [Google Scholar]
- Bokuchava, N. Therapeutic Mud of Georgia; Publishing House “Technical University”: Tbilisi, Georgia, 2009; ISBN 978-9941-14-371-7. (In Russian) [Google Scholar]
- Baschini, M.T.; Pettinari, G.R.; Vallés, J.M.; Aguzzi, C.; Cerezo, P.; López-Galindo, A.; Setti, M.; Viseras, C. Suitability of Natural Sulphur-Rich Muds from Copahue (Argentina) for Use as Semisolid Health Care Products. Appl. Clay Sci. 2010, 49, 205–212. [Google Scholar] [CrossRef]
- Spilioti, E.; Vargiami, M.; Letsiou, S.; Gardikis, K.; Sygouni, V.; Koutsoukos, P.; Chinou, I.; Kassi, E.; Moutsatsou, P. Biological Properties of Mud Extracts Derived from Various Spa Resorts. Environ. Geochem. Health 2017, 39, 821–833. [Google Scholar] [CrossRef]
- Amuso, D.; Medoro, A.; Scapagnini, G.; Gambacorta, A.; Davinelli, S.; Iorio, E.L.; Bonetti, L.R.; Sbarbati, A. A Pilot Study on the Efficacy of a Seaweed Mud Application in the Treatment of Cellulite. J. Cosmet. Dermatol. 2024, 23, 2181–2189. [Google Scholar] [CrossRef] [PubMed]
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L. Microalgal Peloids for Cosmetic and Wellness Uses. Mar. Drugs 2021, 19, 666. [Google Scholar] [CrossRef] [PubMed]
- Ma’or, Z.; Cohen, D.; Assis, A. Formulating Dead Sea Mud in Cosmetic Products, Its Effects on Skin, and the Underlying Biological Mechanism: A Review. J. Cosmet. Dermatol. Sci. Appl. 2024, 14, 276–288. [Google Scholar] [CrossRef]
- Tateo, F.; Ravaglioli, A.; Andreoli, C.; Bonina, F.; Coiro, V.; Degetto, S.; Giaretta, A.; Menconi Orsini, A.; Puglia, C.; Summa, V. The In-Vitro Percutaneous Migration of Chemical Elements from a Thermal Mud for Healing Use. Appl. Clay Sci. 2009, 44, 83–94. [Google Scholar] [CrossRef]
- Bastos, C.M.; Rocha, F.; Patinha, C.; Marinho-Reis, P. Characterization of Percutaneous Absorption of Calcium, Magnesium, and Potentially Toxic Elements in Two Tailored Sulfurous Therapeutic Peloids: A Comprehensive in Vitro Pilot Study. Int. J. Biometeorol. 2024, 68, 1061–1072. [Google Scholar] [CrossRef]
- Ma’or, Z.; Halicz, L.; Portugal-Cohen, M.; Russo, M.Z.; Robino, F.; Vanhaecke, T.; Rogiers, V. Safety evaluation of traces of nickel and chrome in cosmetics: The case of Dead Sea mud. Regul. Toxicol. Pharmacol. 2015, 73, 797–801. [Google Scholar] [CrossRef]
- Karpińska, M.; Mnich, K.; Kapała, J.; Bielawska, A.; Kulesza, G.; Mnich, S. Radioactivity of Peat Mud Used in Therapy. J. Environ. Radioact. 2016, 152, 97–100. [Google Scholar] [CrossRef]
- Cantaluppi, C.; Carraro, A.; Tateo, F.; Fasson, A. Gamma-Emitting Radionuclides in Therapeutic Muds of the Euganean Thermal District (Padua, Italy). Appl. Clay Sci. 2023, 245, 107142. [Google Scholar] [CrossRef]
- Park, C.; Kim, J.-H.; Choi, W.; Kim, D.; No, S.-G.; Chung, D.; Lee, H.; Seo, S.; Seo, S.M. Natural Peloids Originating from Subsea Depths of 200 m in the Hupo Basin, South Korea: Physicochemical Properties for Potential Pelotherapy Applications. Environ. Geochem. Health 2024, 46, 240. [Google Scholar] [CrossRef]
- Baldovin, T.; Amoruso, I.; Caldara, F.; Buja, A.; Baldo, V.; Cocchio, S.; Bertoncello, C. Microbiological Hygiene Quality of Thermal Muds: A Pilot Study in Pelotherapy Facilities of the Euganean Thermal District (NE Italy). Int. J. Environ. Res. Public Health 2020, 17, 5040. [Google Scholar] [CrossRef]
- Bastos, C.M.; Rocha, F.; Gomes, N.; Marinho-Reis, P. The Challenge in Combining Pelotherapy and Electrotherapy (Iontophoresis) in One Single Therapeutic Modality. Appl. Sci. 2022, 12, 1509. [Google Scholar] [CrossRef]
- Bastos, C.M.; Rocha, F. Experimental Peloid Formulation Using a Portuguese Bentonite and Different Mineral-Medicinal Waters Suitable for Therapeutic and Well-Being Purposes. Clays Clay Min. 2023, 71, 684–706. [Google Scholar] [CrossRef]
- Ferreira, M.; Matos, A.; Couras, A.; Marto, J.; Ribeiro, H. Overview of Cosmetic Regulatory Frameworks around the World. Cosmetics 2022, 9, 72. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).