Antimicrobial Compounds from Food Waste in Cosmetics
Abstract
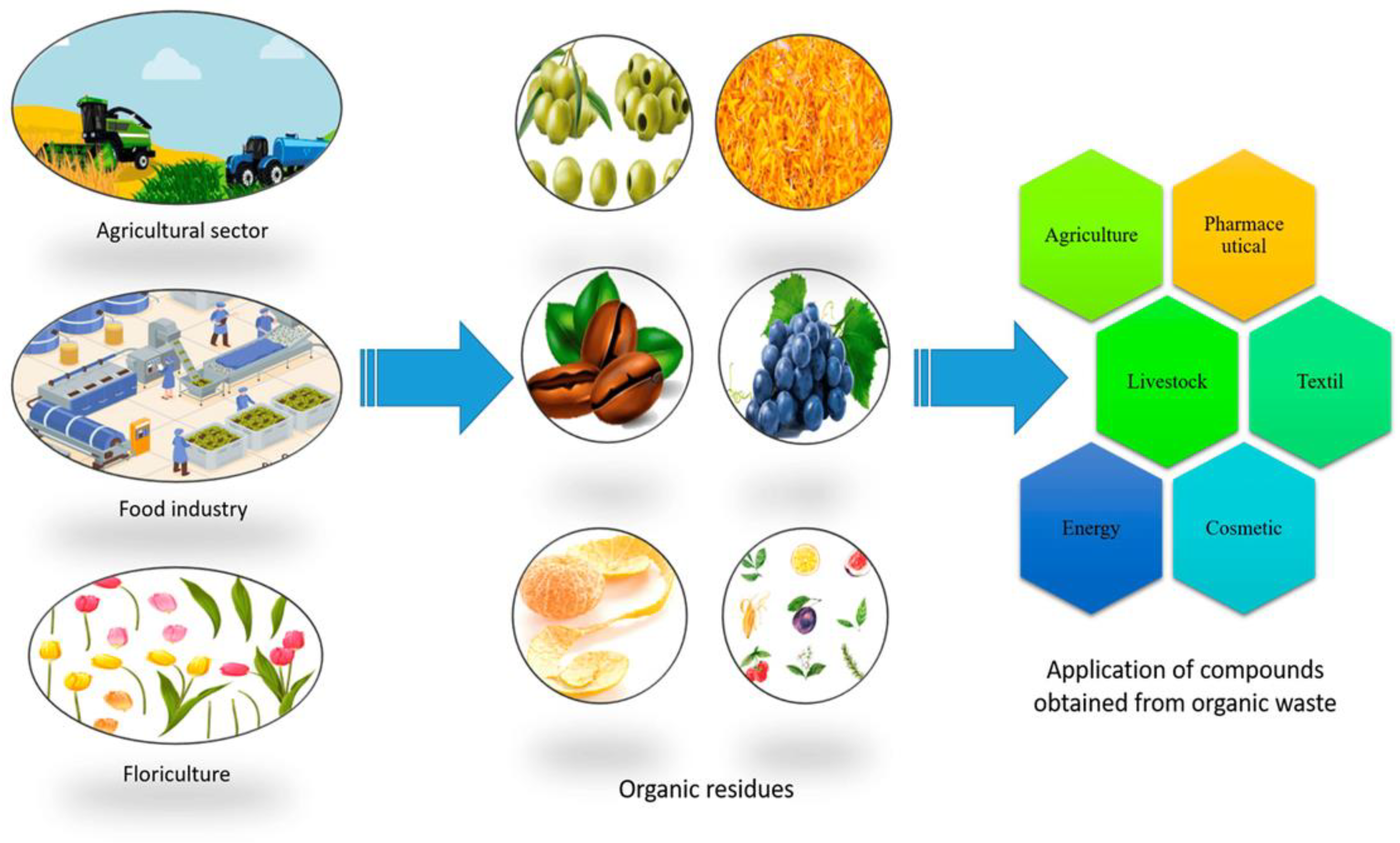
1. Introduction
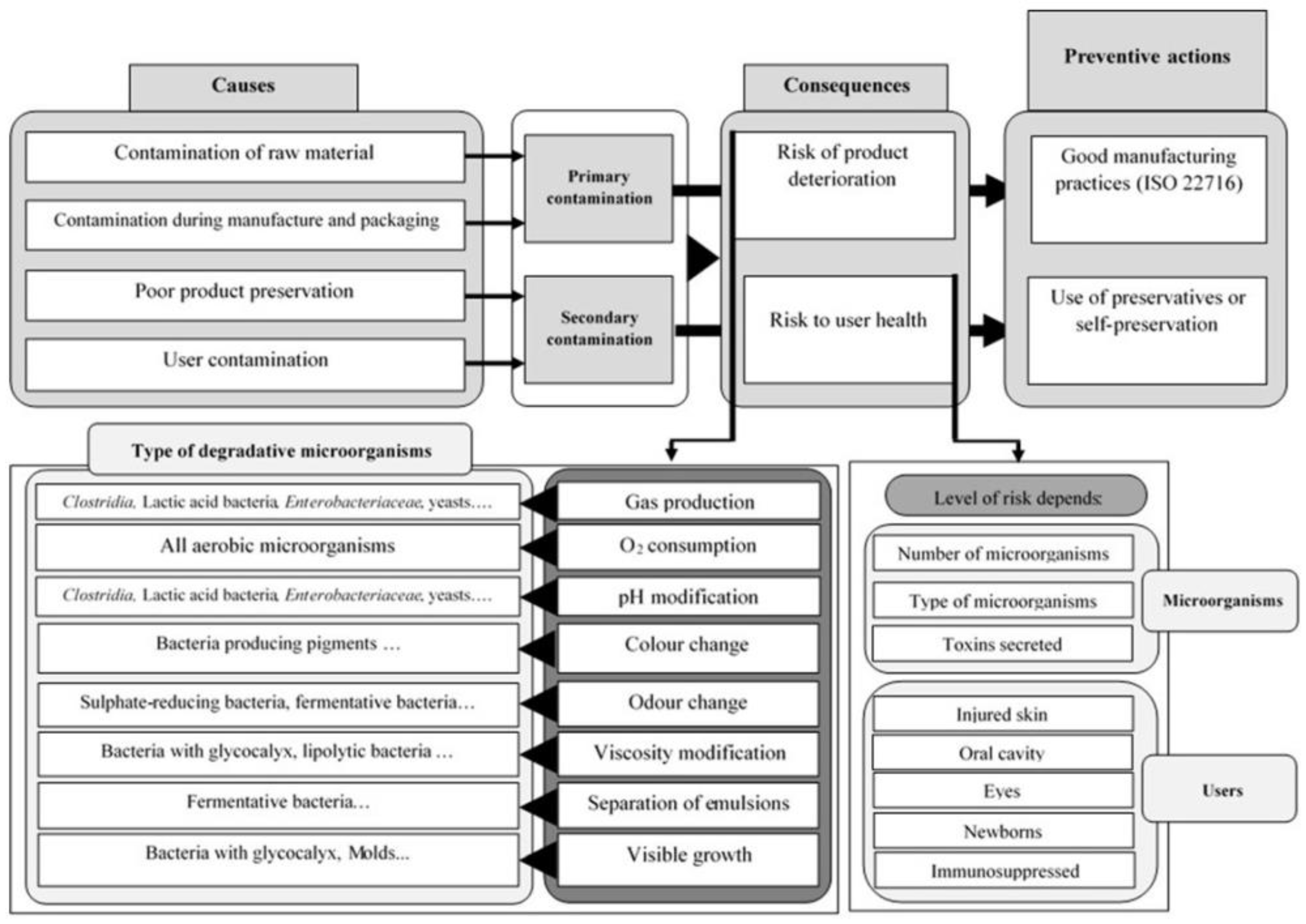
2. State-of-the-Art Preservation Strategies
3. Food Waste Natural Compounds with Antimicrobial Properties
3.1. Plant-Based Extracts
3.2. Phenolic Compound
3.3. Terpenoids
4. Limitations and Challenges
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Usmani, Z.; Sharma, M.; Awasthi, A.K.; Sharma, G.D.; Cysneiros, D.; Nayak, S.C.; Thakur, V.K.; Naidu, R.; Pandey, A.; Gupta, V.K. Minimizing hazardous impact of food waste in a circular economy—Advances in resource recovery through green strategies. J. Hazard. Mater. 2021, 416, 126154. [Google Scholar] [CrossRef]
- Chauhan, C.; Dhir, A.; Akram, M.U.; Salo, J. Food loss and waste in food supply chains. A systematic literature review and framework development approach. J. Clean. Prod. 2021, 295, 126438. [Google Scholar] [CrossRef]
- Morganti, P.; Gao, X.; Vukovic, N.; Gagliardini, A.; Lohani, A.; Morganti, G. Food Loss and Food Waste for Green Cosmetics and Medical Devices for a Cleaner Planet. Cosmetics 2022, 9, 19. [Google Scholar] [CrossRef]
- Ścieszko, E.; Budny, E.; Rotsztejn, H.; Erkiert-Polguj, A. How has the pandemic lockdown changed our daily facial skincare habits? J. Cosmet. Dermatol. 2021, 20, 3722–3726. [Google Scholar] [CrossRef]
- Lee, J.; Kwon, K.H. Changes in the use of cosmetics worldwide due to increased use of masks in the coronavirus disease-19 pandemic. J. Cosmet. Dermatol. 2022, 21, 2708–2712. [Google Scholar] [CrossRef] [PubMed]
- Morganti, P.; Lohani, A.; Gagliardini, A.; Morganti, G.; Coltelli, M.-B. Active Ingredients and Carriers in Nutritional Eco-Cosmetics. Compounds 2023, 3, 122–141. [Google Scholar] [CrossRef]
- Pinto, D.; de la Luz Cádiz-Gurrea, M.; Silva, A.M.; Delerue-Matos, C.; Rodrigues, F. Cosmetics—Food Waste Recovery. In Food Waste Recovery; Elsevier: Amsterdam, The Netherlands, 2021; pp. 503–528. [Google Scholar]
- Parra-Pacheco, B.; Cruz-Moreno, B.A.; Aguirre-Becerra, H.; García-Trejo, J.F.; Feregrino-Pérez, A.A. Bioactive Compounds from Organic Waste. Molecules 2024, 29, 2243. [Google Scholar] [CrossRef]
- Mehdizadeh, T.; Tajik, H.; Langroodi, A.M.; Molaei, R.; Mahmoudian, A. Chitosan-starch film containing pomegranate peel extract and Thymus kotschyanus essential oil can prolong the shelf life of beef. Meat Sci. 2020, 163, 108073. [Google Scholar] [CrossRef]
- Gigliobianco, M.R.; Cortese, M.; Nannini, S.; Di Nicolantonio, L.; Peregrina, D.V.; Lupidi, G.; Vitali, L.A.; Bocchietto, E.; Di Martino, P.; Censi, R. Chemical, Antioxidant, and Antimicrobial Properties of the Peel and Male Flower By-Products of Four Varieties of Punica granatum L. Cultivated in the Marche Region for Their Use in Cosmetic Products. Antioxidants 2022, 11, 768. [Google Scholar] [CrossRef]
- Houston, D.M.; Bugert, J.; Denyer, S.P.; Heard, C.M. Anti-inflammatory activity of Punica granatum L. (Pomegranate) rind extracts applied topically to ex vivo skin. Eur. J. Pharm. Biopharm. 2017, 112, 30–37. [Google Scholar] [CrossRef]
- Hamrita, B.; Emira, N.; Papetti, A.; Badraoui, R.; Bouslama, L.; Ben Tekfa, M.-I.; Hamdi, A.; Patel, M.; Elasbali, A.M.; Adnan, M. Phytochemical Analysis, Antioxidant, Antimicrobial, and Anti-Swarming Properties of Hibiscus sabdariffa L. Calyx Extracts: In Vitro and In Silico Modelling Approaches. Evid. Based Complement. Altern. Med. 2022, 2022, 1252672. [Google Scholar] [CrossRef] [PubMed]
- Primavilla, S.A.-O.; Pagano, C.A.-O.; Roila, R.A.-O.; Branciari, R.A.-O.; Ranucci, D.A.-O.; Valiani, A.; Ricci, M.A.-O.; Perioli, L.A.-O. Antibacterial Activity of Crocus sativus L. Petals Extracts against Foodborne Pathogenic and Spoilage Microorganisms, with a Special Focus on Clostridia. Life 2022, 13, 60. [Google Scholar] [CrossRef]
- Kotenkova, E.; Kupaeva, N. Comparative Antioxidant Study of Onion and Garlic Waste and Bulbs. In Proceedings of the IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; p. 012031. [Google Scholar]
- Noda, Y.; Asada, C.; Sasaki, C.; Nakamura, Y. Effects of hydrothermal methods such as steam explosion and microwave irradiation on extraction of water-soluble antioxidant materials from garlic husk. Waste Biomass Valoriz. 2019, 10, 3397–3402. [Google Scholar] [CrossRef]
- Mutua, J.K.; Imathiu, S.; Owino, W. Evaluation of the proximate composition, antioxidant potential, and antimicrobial activity of mango seed kernel extracts. Food Sci. Nutr. 2017, 5, 349–357. [Google Scholar] [CrossRef] [PubMed]
- de Elguea-Culebras, G.O.; Sánchez-Vioque, R.; Santana-Méridas, O.; Herraiz-Peñalver, D.; Carmona, M.; Berruga, M.I. In vitro antifungal activity of residues from essential oil industry against Penicillium verrucosum, a common contaminant of ripening cheeses. Lwt 2016, 73, 226–232. [Google Scholar] [CrossRef]
- Kabara, J.J.; Orth, D. Principles for Product Preservation. In Cosmetic Science and Technology Series; Elsevier: Amsterdam, The Netherlands, 1997; pp. 1–14. [Google Scholar]
- Mitsui, T. Preservation of Cosmetics. New Cosmetic Science; Elsevier: Amsterdam, The Netherlands, 1997; pp. 199–208. [Google Scholar]
- Smith, C.; Alexander, B. The relative cytotoxicity of personal care preservative systems in Balb/C 3T3 clone A31 embryonic mouse cells and the effect of selected preservative systems upon the toxicity of a standard rinse-off formulation. Toxicol. In Vitro 2005, 19, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Halla, N.; Fernandes, I.P.; Heleno, S.A.; Costa, P.; Boucherit-Otmani, Z.; Boucherit, K.; Rodrigues, A.E.; Ferreira, I.C.F.R.; Barreiro, M.F. Cosmetics Preservation: A Review on Present Strategies. Molecules 2018, 23, 1571. [Google Scholar] [CrossRef]
- Sandle, T. EU GMP Annex 1: Manufacture of Sterile Medicinal Products; European Commission: Brussels, Belgium, 2022. [Google Scholar]
- Varvaresou, A.; Papageorgiou, S.; Tsirivas, E.; Protopapa, E.; Kintziou, H.; Kefala, V.; Demetzos, C. Self-preserving cosmetics. Int. J. Cosmet. Sci. 2009, 31, 163–175. [Google Scholar] [CrossRef]
- ISO 22716:2007; Cosmetics—Good Manufacturing Practices (GMP)—Guidelines on Good Manufacturing Practices. International Organization for Standardization. Available online: https://www.iso.org/standard/36437.html (accessed on 28 August 2024).
- Parliament, E. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products. In Official Journal of the European Union; European Union: Brussels, Belgium, 2009. [Google Scholar]
- Devlieghere, F.; De Loy-Hendrickx, A.; Rademaker, M.; Pipelers, P.; Crozier, A.; De Baets, B.; Joly, L.; Keromen, S. A new protocol for evaluating the efficacy of some dispensing systems of a packaging in the microbial protection of water-based preservative-free cosmetic products. Int. J. Cosmet. Sci. 2015, 37, 627–635. [Google Scholar] [CrossRef]
- Yablonski, J.I.; Mancuso, S.E. Microbial Risks and Eco-Friendly Packaging. Formul. Packag. Mark. Nat. Cosmet. Prod. 2011, 179–211. [Google Scholar] [CrossRef]
- Geis, P.A. Cosmetic Microbiology: A Practical approach, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Kerdudo, A.; Fontaine-Vive, F.; Dingas, A.; Faure, C.; Fernandez, X. Optimization of cosmetic preservation: Water activity reduction. Int. J. Cosmet. Sci. 2015, 37, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Dao, H.; Lakhani, P.; Police, A.; Kallakunta, V.; Ajjarapu, S.S.; Wu, K.-W.; Ponkshe, P.; Repka, M.A.; Narasimha Murthy, S. Microbial stability of pharmaceutical and cosmetic products. AAPS PharmSciTech 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Donsì, F.; Ferrari, G. Essential oil nanoemulsions as antimicrobial agents in food. J. Biotechnol. 2016, 233, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Gharenaghadeh, S.; Karimi, N.; Forghani, S.; Nourazarian, M.; Gharehnaghadeh, S.; Jabbari, V.; Khiabani, M.S.; Kafil, H.S. Application of Salvia multicaulis essential oil-containing nanoemulsion against food-borne pathogens. Food Biosci. 2017, 19, 128–133. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Rojas-Graü, A.; Soliva-Fortuny, R.; Martín-Belloso, O. Physicochemical characterization and antimicrobial activity of food-grade emulsions and nanoemulsions incorporating essential oils. Food Hydrocoll. 2015, 43, 547–556. [Google Scholar] [CrossRef]
- Char, C.; Cisternas, L.; Pérez, F.; Guerrero, S. Effect of emulsification on the antimicrobial activity of carvacrol. CyTA J. Food 2016, 14, 186–192. [Google Scholar] [CrossRef]
- Buranasuksombat, U.; Kwon, Y.J.; Turner, M.; Bhandari, B. Influence of emulsion droplet size on antimicrobial properties. Food Sci. Biotechnol. 2011, 20, 793–800. [Google Scholar] [CrossRef]
- Siquet, F.; Devleeschouwer, M.J. Antibacterial Agents and Preservatives. In Handbook of Cosmetic Science and Technology; Paye, M., Barel, A.O., Maibach, H.I., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 223–231. [Google Scholar]
- Lukic, M.; Pantelic, I.; Savic, S. An overview of novel surfactants for formulation of cosmetics with certain emphasis on acidic active substances. Tenside Surfactants Deterg. 2016, 53, 7–19. [Google Scholar] [CrossRef]
- Mccarthy, T.J. Formulated factors affecting the activity of preservatives. In Cosmetic and Drug Preservation: Principles and Practice, 1st ed.; Kabara, J.J., Ed.; Marcel Dekker: New York, NY, USA, 1984; pp. 359–387. [Google Scholar]
- Truchliński, J.; Sembratowicz, I.; Gorzel, M.; Kiełtyka-Dadasiewicz, A. Allergenic potential of cosmetic ingredients. Arch. Physiother. Glob. Res. 2015, 19, 7–15. [Google Scholar] [CrossRef]
- Khang, D.T.; Tien, L.T.T.; Men, T.T.; Thuy, N.P. Potential of Fermented Fruit Peel Liquid in Cosmetics as a Skin Care Agent. Cosmetics 2021, 8, 33. [Google Scholar] [CrossRef]
- Gordobil, O.; Olaizola, P.; Banales, J.M.; Labidi, J. Lignins from Agroindustrial by-Products as Natural Ingredients for Cosmetics: Chemical Structure and In Vitro Sunscreen and Cytotoxic Activities. Molecules 2020, 25, 1131. [Google Scholar] [CrossRef]
- d’Avanzo, N.; Mancuso, A.; Mare, R.; Silletta, A.; Maurotti, S.; Parisi, O.I.; Cristiano, M.C.; Paolino, D. Olive Leaves and Citrus Peels: From Waste to Potential Resource for Cosmetic Products. Cosmetics 2024, 11, 41. [Google Scholar] [CrossRef]
- Nowak, K.; Jabłońska, E.; Ratajczak-Wrona, W. Controversy around parabens: Alternative strategies for preservative use in cosmetics and personal care products. Environ. Res. 2021, 198, 110488. [Google Scholar] [CrossRef] [PubMed]
- Marcelino, J.; Giménez-Arnau, A.M. Impact of Cosmetics and Cleansers in Atopic Dermatitis—How to Advise Patients. Curr. Treat. Options Allergy 2024, 11, 62–76. [Google Scholar] [CrossRef]
- Gonelimali, F.D.; Lin, J.; Miao, W.; Xuan, J.; Charles, F.; Chen, M.; Hatab, S.R. Antimicrobial properties and mechanism of action of some plant extracts against food pathogens and spoilage microorganisms. Front. Microbiol. 2018, 9, 1639. [Google Scholar] [CrossRef]
- Machado, A.; Zamora-Mendoza, L.; Alexis, F.; Álvarez-Suarez, J.M. Use of plant extracts, bee-derived products, and probiotic-related applications to fight multidrug-resistant pathogens in the post-antibiotic era. Future Pharmacol. 2023, 3, 535–567. [Google Scholar] [CrossRef]
- Voon, H.C.; Bhat, R.; Rusul, G. Flower extracts and their essential oils as potential antimicrobial agents for food uses and pharmaceutical applications. Compr. Rev. Food Sci. Food Saf. 2012, 11, 34–55. [Google Scholar] [CrossRef]
- El Khetabi, A.; Lahlali, R.; Ezrari, S.; Radouane, N.; Lyousfi, N.; Banani, H.; Askarne, L.; Tahiri, A.; El Ghadraoui, L.; Belmalha, S.; et al. Role of plant extracts and essential oils in fighting against postharvest fruit pathogens and extending fruit shelf life: A review. Trends Food Sci. Technol. 2022, 120, 402–417. [Google Scholar] [CrossRef]
- Clarke, D.; Tyuftin, A.A.; Cruz-Romero, M.C.; Bolton, D.; Fanning, S.; Pankaj, S.K.; Bueno-Ferrer, C.; Cullen, P.J.; Kerry, J.P. Surface attachment of active antimicrobial coatings onto conventional plastic-based laminates and performance assessment of these materials on the storage life of vacuum packaged beef sub-primals. Food Microbiol. 2017, 62, 196–201. [Google Scholar] [CrossRef]
- Charalampia, D.; Koutelidakis, A. From pomegranate processing by-products to innovative value added functional ingredients and bio-based products with several applications in food sector. BAOJ Biotech 2017, 3, 210. [Google Scholar]
- Shahamirian, M.; Eskandari, M.H.; Niakousari, M.; Esteghlal, S.; Hashemi Gahruie, H.; Mousavi Khaneghah, A. Incorporation of pomegranate rind powder extract and pomegranate juice into frozen burgers: Oxidative stability, sensorial and microbiological characteristics. J. Food Sci. Technol. 2019, 56, 1174–1183. [Google Scholar] [CrossRef] [PubMed]
- Esparza-Merino, R.M.; Macías-Rodríguez, M.E.; Cabrera-Díaz, E.; Valencia-Botín, A.J.; Estrada-Girón, Y. Utilization of by-products of Hibiscus sabdariffa L. as alternative sources for the extraction of high-quality pectin. Food Sci. Biotechnol. 2019, 28, 1003–1011. [Google Scholar] [CrossRef]
- Sáyago-Ayerdi, S.G.; Velázquez-López, C.; Montalvo-González, E.; Goñi, I. By-product from decoction process of Hibiscus sabdariffa L. calyces as a source of polyphenols and dietary fiber. J. Sci. Food Agric. 2014, 94, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Vasileva, I.; Denkova, R.; Chochkov, R.; Teneva, D.; Denkova, Z.; Dessev, T.; Denev, P.; Slavov, A. Effect of lavender (Lavandula angustifolia) and melissa (Melissa officinalis) waste on quality and shelf life of bread. Food Chem. 2018, 253, 13–21. [Google Scholar] [CrossRef]
- Shakeri, A.; Sahebkar, A.; Javadi, B. Melissa officinalis L.–A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2016, 188, 204–228. [Google Scholar] [CrossRef] [PubMed]
- Lachguer, K.; El Merzougui, S.; Boudadi, I.; Laktib, A.; Ben El Caid, M.; Ramdan, B.; Boubaker, H.; Serghini, M.A. Major Phytochemical Compounds, In Vitro Antioxidant, Antibacterial, and Antifungal Activities of Six Aqueous and Organic Extracts of Crocus sativus L. Flower Waste. Waste Biomass Valoriz. 2023, 14, 1571–1587. [Google Scholar] [CrossRef] [PubMed]
- Zara, S.; Petretto, G.L.; Mannu, A.; Zara, G.; Budroni, M.; Mannazzu, I.; Multineddu, C.; Pintore, G.; Fancello, F. Antimicrobial activity and chemical characterization of a non-polar extract of saffron stamens in food matrix. Foods 2021, 10, 703. [Google Scholar] [CrossRef]
- Jafarı-sales, A.; Pashazadeh, M. Antibacterial effect of methanolic extract of saffron petal (Crocus sativus L.) on some standard gram positive and gram negative pathogenic bacteria in vitro. Curr. Perspect. Med. Aromat. Plants 2020, 3, 1–7. [Google Scholar] [CrossRef]
- Belyagoubi, L.; Loukidi, B.; Belyagoubi-Benhammou, N.; Gismondi, A.; Di Marco, G.; D’Agostino, A.; Canini, A.; Benmahieddine, A.; Rouigueb, K.; Ben Menni, D. Valorization of Algerian saffron: Stigmas and flowers as source of bioactive compounds. Waste Biomass Valoriz. 2021, 12, 6671–6683. [Google Scholar] [CrossRef]
- Salem, M.A.; Mohamed, O.G.; Mosalam, E.M.; Elberri, A.I.; Abdel-Bar, H.M.; Hassan, M.; Al-Karmalawy, A.A.; Tripathi, A.; Ezzat, S.M.; Abo Mansour, H.E. Investigation of the phytochemical composition, antioxidant, antibacterial, anti-osteoarthritis, and wound healing activities of selected vegetable waste. Sci. Rep. 2023, 13, 13034. [Google Scholar] [CrossRef]
- Mwaurah, P.W.; Kumar, S.; Kumar, N.; Panghal, A.; Attkan, A.K.; Singh, V.K.; Garg, M.K. Physicochemical characteristics, bioactive compounds and industrial applications of mango kernel and its products: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2421–2446. [Google Scholar] [CrossRef]
- Rao, G.V.; Mukhopadhyay, T.; Rajesh, G.D. Skin Care Agent and Compositions Thereof. U.S. Patent 20110135772A1, 9 June 2011. [Google Scholar]
- Poomanee, W.; Khunkitti, W.; Chaiyana, W.; Intasai, N.; Lin, W.-C.; Lue, S.-C.; Leelapornpisid, P. Multifunctional biological properties and phytochemical constituents of Mangifera indica L. seed kernel extract for preventing skin aging. Toxicol. Res. 2021, 37, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.; Dadmohammadi, Y.; Abbaspourrad, A. Nutritional and Bioactive Components of Pomegranate Waste Used in Food and Cosmetic Applications: A Review. Foods 2021, 10, 657. [Google Scholar] [CrossRef] [PubMed]
- Bertolo, M.R.; Martins, V.C.; Horn, M.M.; Brenelli, L.B.; Plepis, A.M. Rheological and antioxidant properties of chitosan/gelatin-based materials functionalized by pomegranate peel extract. Carbohydr. Polym. 2020, 228, 115386. [Google Scholar] [CrossRef]
- Abou Zekry, S.S.; Abdellatif, A.; Azzazy, H.M.E. Fabrication of pomegranate/honey nanofibers for use as antibacterial wound dressings. Wound Med. 2020, 28, 100181. [Google Scholar] [CrossRef]
- Foss, S.R.; Nakamura, C.V.; Ueda-Nakamura, T.; Cortez, D.A.; Endo, E.H.; Dias Filho, B.P. Antifungal activity of pomegranate peel extract and isolated compound punicalagin against dermatophytes. Ann. Clin. Microbiol. Antimicrob. 2014, 13, 32. [Google Scholar] [CrossRef]
- Islam, M. Food and medicinal values of Roselle (Hibiscus sabdariffa L. Linne Malvaceae) plant parts: A review. Open J. Nutr. Food Sci. 2019, 1, 1003. [Google Scholar]
- Abu-Izneid, T.; Rauf, A.; Khalil, A.A.; Olatunde, A.; Khalid, A.; Alhumaydhi, F.A.; Aljohani, A.S.; Sahab Uddin, M.; Heydari, M.; Khayrullin, M. Nutritional and health beneficial properties of saffron (Crocus sativus L.): A comprehensive review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2683–2706. [Google Scholar] [CrossRef]
- Wali, A.F.; Alchamat, H.A.A.; Hariri, H.K.; Hariri, B.K.; Menezes, G.A.; Zehra, U.; Rehman, M.U.; Ahmad, P. Antioxidant, antimicrobial, antidiabetic and cytotoxic activity of Crocus sativus L. petals. Appl. Sci. 2020, 10, 1519. [Google Scholar] [CrossRef]
- Sirisa-ard, P.; Pholsonklam, K.; Satchachai, A.; Tragoolpua, Y.; Kaewkod, T. Antioxidant, antibacterial activities and cytotoxicity of garlic leaf extract from garlic waste. Nat. Life Sci. Commun. 2023, 22, e2023059. [Google Scholar] [CrossRef]
- Hernández-Montesinos, I.Y.; Carreón-Delgado, D.F.; Ocaranza-Sánchez, E.; Ochoa-Velasco, C.E.; Suárez-Jacobo, Á.; Ramírez-López, C. Garlic (Allium sativum) peel extracts and their potential as antioxidant and antimicrobial agents for food applications: Influence of pretreatment and extraction solvent. Int. J. Food Sci. Technol. 2023, 58, 6794–6805. [Google Scholar] [CrossRef]
- Gabriel, T.; Vestine, A.; Kim, K.D.; Kwon, S.J.; Sivanesan, I.; Chun, S.C. Antibacterial activity of nanoparticles of garlic (Allium sativum) extract against different bacteria such as Streptococcus mutans and Poryphormonas gingivalis. Appl. Sci. 2022, 12, 3491. [Google Scholar] [CrossRef]
- Rasmussen, T.B.; Bjarnsholt, T.; Skindersoe, M.E.; Hentzer, M.; Kristoffersen, P.; Kote, M.; Nielsen, J.; Eberl, L.; Givskov, M. Screening for quorum-sensing inhibitors (QSI) by use of a novel genetic system, the QSI selector. J. Bacteriol. 2005, 187, 1799–1814. [Google Scholar] [CrossRef]
- Rabbani, G.; Albert, M.J.; Rahman, A.H.; Isalm, M.M.; Islam, K.N.; Alam, K. Short-chain fatty acids improve clinical, pathologic, and microbiologic features of experimental shigellosis. J. Infect. Dis. 1999, 179, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Bhatwalkar, S.B.; Mondal, R.; Krishna, S.B.N.; Adam, J.K.; Govender, P.; Anupam, R. Antibacterial properties of organosulfur compounds of garlic (Allium sativum). Front. Microbiol. 2021, 12, 613077. [Google Scholar] [CrossRef] [PubMed]
- Arulselvan, K.; Gayas, Z.; Uday, V. Antibacterial Activity of Garlic Extract against Streptococcus mutans and Lactobacillus acidophilus: An In Vitro Study. J. South Asian Assoc. Pediatr. Dent. 2022, 5, 26–31. [Google Scholar]
- D’Ercole, S.; D’Addazio, G.; Di Lodovico, S.; Traini, T.; Di Giulio, M.; Sinjari, B. Porphyromonas gingivalis load is balanced by 0.20% chlorhexidine gel. A randomized, double-blind, controlled, microbiological and immunohistochemical human study. J. Clin. Med. 2020, 9, 284. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, M.; Nishimura, T. The preventive and therapeutic application of garlic and other plant ingredients in the treatment of periodontal diseases. Exp. Ther. Med. 2020, 19, 1507–1510. [Google Scholar] [CrossRef]
- Yadav, M.; Bohra, R.; Gupta, N. In vitro Determination of Antibacterial Effect of Garlic (Allium sativum) on Staphylococcus aureus and E. coli. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 498–506. [Google Scholar] [CrossRef]
- Kim, H.W.; Seok, Y.S.; Cho, T.J.; Rhee, M.S. Risk factors influencing contamination of customized cosmetics made on-the-spot: Evidence from the national pilot project for public health. Sci. Rep. 2020, 10, 1561. [Google Scholar] [CrossRef]
- Dadashi, L.; Dehghanzadeh, R. Investigating incidence of bacterial and fungal contamination in shared cosmetic kits available in the women beauty salons. Health Promot. Perspect. 2016, 6, 159. [Google Scholar] [CrossRef] [PubMed]
- Muhammed, H.J. Bacterial and fungal contamination in three brands of cosmetic marketed in Iraq. Iraqi J. Pharm. Sci. 2011, 20, 38–42. [Google Scholar] [CrossRef]
- Pai, S.; Platt, M. Antifungal effects of Allium sativum (garlic) extract against the Aspergillus species involved in otomycosis. Lett. Appl. Microbiol. 1995, 20, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Min, J.H.; Jeong, J.H.; Park, Y.U.; Lee, J.S.; Lee, S.J.; Chang, W.-B. Quality characteristics of garlic peel according to processing methods. Food Sci. Preserv. 2020, 27, 32–37. [Google Scholar]
- Gong, C.; Singh, A.; Singh, P.; Singh, A. Anaerobic digestion of agri-food wastes for generating biofuels. Indian J. Microbiol. 2021, 61, 427–440. [Google Scholar] [CrossRef]
- Hadj Saadoun, J.; Bertani, G.; Levante, A.; Vezzosi, F.; Ricci, A.; Bernini, V.; Lazzi, C. Fermentation of agri-food waste: A promising route for the production of aroma compounds. Foods 2021, 10, 707. [Google Scholar] [CrossRef]
- Osorio, L.L.D.R.; Flórez-López, E.; Grande-Tovar, C.D. The potential of selected agri-food loss and waste to contribute to a circular economy: Applications in the food, cosmetic and pharmaceutical industries. Molecules 2021, 26, 515. [Google Scholar] [CrossRef]
- Papaioannou, E.H.; Mazzei, R.; Bazzarelli, F.; Piacentini, E.; Giannakopoulos, V.; Roberts, M.R.; Giorno, L. Agri-food industry waste as resource of chemicals: The role of membrane technology in their sustainable recycling. Sustainability 2022, 14, 1483. [Google Scholar] [CrossRef]
- Panda, L.; Duarte-Sierra, A. Recent advancements in enhancing antimicrobial activity of plant-derived polyphenols by biochemical means. Horticulturae 2022, 8, 401. [Google Scholar] [CrossRef]
- Aziz, N.; Farag, S.; Mousa, L.; Abo-Zaid, M. Comparative antibacterial and antifungal effects of some phenolic compounds. Microbios 1998, 93, 43–54. [Google Scholar]
- Šeregelj, V.; Tumbas Šaponjac, V.; Lević, S.; Kalušević, A.; Ćetković, G.; Čanadanović-Brunet, J.; Nedović, V.; Stajčić, S.; Vulić, J.; Vidaković, A. Application of encapsulated natural bioactive compounds from red pepper waste in yogurt. J. Microencapsul. 2019, 36, 704–714. [Google Scholar] [CrossRef]
- Sharma, K.; Mahato, N.; Lee, Y.R. Systematic study on active compounds as antibacterial and antibiofilm agent in aging onions. J. Food Drug Anal. 2018, 26, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Jaisinghani, R.N. Antibacterial properties of quercetin. Microbiol. Res. 2017, 8, 6877. [Google Scholar] [CrossRef]
- Gupta, N.; Poddar, K.; Sarkar, D.; Kumari, N.; Padhan, B.; Sarkar, A. Fruit waste management by pigment production and utilization of residual as bioadsorbent. J. Environ. Manag. 2019, 244, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, V.; Maisto, M.; Schiano, E.; Iannuzzo, F.; Keivani, N.; Manuela Rigano, M.; Santini, A.; Novellino, E.; Carlo Tenore, G.; Summa, V. Phytochemical investigation and antioxidant properties of unripe tomato cultivars (Solanum lycopersicum L.). Food Chem. 2024, 438, 137863. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Yang, M.; Wang, T.; Sun, Z.; Liu, M.; Zhang, J.; Zeng, Q.; Cai, C.; Li, Y. Antibacterial mechanism of vanillic acid on physiological, morphological, and biofilm properties of carbapenem-resistant Enterobacter hormaechei. J. Food Prot. 2020, 83, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Zdziobek, P.A.-O.X.; Jodłowski, G.A.-O.X.; Strzelec, E.A.-O. Biopreservation and Bioactivation Juice from Waste Broccoli with Lactiplantibacillus plantarum. Molecules 2023, 28, 4594. [Google Scholar] [CrossRef] [PubMed]
- Lima, V.N.; Oliveira-Tintino, C.D.; Santos, E.S.; Morais, L.P.; Tintino, S.R.; Freitas, T.S.; Geraldo, Y.S.; Pereira, R.L.; Cruz, R.P.; Menezes, I.R. Antimicrobial and enhancement of the antibiotic activity by phenolic compounds: Gallic acid, caffeic acid and pyrogallol. Microb. Pathog. 2016, 99, 56–61. [Google Scholar] [CrossRef]
- El-Shahat, M.S.; Rabie, M.A.; Ragab, M.; Siliha, H.I. Changes on physicochemical and rheological properties of biscuits substituted with the peel and alcohol-insoluble solids (AIS) from cactus pear (Opuntia ficus indica). J. Food Sci. Technol. 2019, 56, 3635–3645. [Google Scholar] [CrossRef]
- Moschona, A.; Liakopoulou-Kyriakides, M. Encapsulation of biological active phenolic compounds extracted from wine wastes in alginate-chitosan microbeads. J. Microencapsul. 2018, 35, 229–240. [Google Scholar] [CrossRef]
- Zhang, D.; Nie, S.; Xie, M.; Hu, J. Antioxidant and antibacterial capabilities of phenolic compounds and organic acids from Camellia oleifera cake. Food Sci. Biotechnol. 2020, 29, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Ming, D.; Wang, D.; Cao, F.; Xiang, H.; Mu, D.; Cao, J.; Li, B.; Zhong, L.; Dong, X.; Zhong, X. Kaempferol inhibits the primary attachment phase of biofilm formation in Staphylococcus aureus. Front. Microbiol. 2017, 8, 2263. [Google Scholar] [CrossRef]
- Pereira, M.G.; Maciel, G.M.; Haminiuk, C.W.I.; Bach, F.; Hamerski, F.; de Paula Scheer, A.; Corazza, M.L. Effect of extraction process on composition, antioxidant and antibacterial activity of oil from yellow passion fruit (Passiflora edulis var. Flavicarpa) seeds. Waste Biomass Valoriz. 2019, 10, 2611–2625. [Google Scholar] [CrossRef]
- Budiati, T.; Suryaningsih, W.; Bethiana, T.N. Antimicrobial of tropical fruit and vegetable waste extract for food-borne pathogenic bacteria. Ital. J. Food Saf. 2022, 11, 10510. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, T.; Adamczak, A.; Ożarowski, M. Antibacterial Activity of Apigenin, Luteolin, and Their C-Glucosides. In Proceedings of the 5th International Electronic Conference on Medicinal Chemistry, Online, 1–30 November 2019; pp. 1–9. [Google Scholar]
- Martillanes, S.; Rocha-Pimienta, J.; Delgado-Adámez, J. Agrifood by-products as a source of phytochemical compounds. Descr. Food Sci. 2018, 3, 43–58. [Google Scholar]
- Khare, T.; Anand, U.; Dey, A.; Assaraf, Y.G.; Chen, Z.-S.; Liu, Z.; Kumar, V. Exploring phytochemicals for combating antibiotic resistance in microbial pathogens. Front. Pharmacol. 2021, 12, 720726. [Google Scholar] [CrossRef]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, S.; Bais, S. A review on protocatechuic acid and its pharmacological potential. Int. Sch. Res. Not. 2014, 2014, 952943. [Google Scholar] [CrossRef]
- Jayaraman, P.; Sakharkar, M.K.; Lim, C.S.; Tang, T.H.; Sakharkar, K.R. Activity and interactions of antibiotic and phytochemical combinations against Pseudomonas aeruginosa in vitro. Int. J. Biol. Sci. 2010, 6, 556. [Google Scholar] [CrossRef]
- Kuete, V.; Nana, F.; Ngameni, B.; Mbaveng, A.T.; Keumedjio, F.; Ngadjui, B.T. Antimicrobial activity of the crude extract, fractions and compounds from stem bark of Ficus ovata (Moraceae). J. Ethnopharmacol. 2009, 124, 556–561. [Google Scholar] [CrossRef]
- Jalali, O.; Best, M.; Wong, A.; Schaeffer, B.; Bauer, B.; Johnson, L. Protocatechuic acid as a topical antimicrobial for surgical skin antisepsis: Preclinical investigations. JBJS Open Access 2020, 5, e19. [Google Scholar] [CrossRef]
- Chen, X. A review on coffee leaves: Phytochemicals, bioactivities and applications. Crit. Rev. Food Sci. Nutr. 2019, 59, 1008–1025. [Google Scholar] [CrossRef] [PubMed]
- Segheto, L.; Santos, B.C.S.; Werneck, A.F.L.; Vilela, F.M.P.; de Sousa, O.V.; Rodarte, M.P. Antioxidant extracts of coffee leaves and its active ingredient 5-caffeoylquinic acid reduce chemically-induced inflammation in mice. Ind. Crops Prod. 2018, 126, 48–57. [Google Scholar] [CrossRef]
- Monteiro, Â.; Colomban, S.; Azinheira, H.G.; Guerra-Guimarães, L.; Do Céu Silva, M.; Navarini, L.; Resmini, M. Dietary antioxidants in coffee leaves: Impact of botanical origin and maturity on chlorogenic acids and xanthones. Antioxidants 2019, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Campa, C.; Mondolot, L.; Rakotondravao, A.; Bidel, L.P.; Gargadennec, A.; Couturon, E.; La Fisca, P.; Rakotomalala, J.-J.; Jay-Allemand, C.; Davis, A.P. A survey of mangiferin and hydroxycinnamic acid ester accumulation in coffee (Coffea) leaves: Biological implications and uses. Ann. Bot. 2012, 110, 595–613. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its main pharmacological activity and potential application in clinical medicine. Oxidative Med. Cell. Longev. 2020, 2020, 8825387. [Google Scholar] [CrossRef] [PubMed]
- Orús, P.; Gomez-Perez, L.; Leranoz, S.; Berlanga, M. Increasing antibiotic resistance in preservative-tolerant bacterial strains isolated from cosmetic products. Int. Microbiol. 2015, 18, 51–59. [Google Scholar]
- Santhosh Kumar, Y.; Kulanthaivel, L.; Hikku, G.; Saravanan, R.; Lakshmi, T.; Kirubhanand, C.; Karthikeyan, M.; Vijayalakshmi, P.; Subbaraj, G.K. Improved Antibacterial Activity of Water-Soluble Nanoformulated Kaempferol and Combretastatin Polyphenolic Compounds. Int. J. Polym. Sci. 2021, 2021, 5682182. [Google Scholar] [CrossRef]
- Badui Dergal, S. Química de los Alimentos; Pearson Educación: Mexico City, México, 2016. [Google Scholar]
- de Valle-Prieto, M.B.; Delgado-Adámez, J.; Gil, M.V.; Martillanes, S.; Franco, M.N.; Martín-Vertedor, D. Virgin olive oil enriched with lutein-zeaxanthin from Spinacia oleracea. J. Oleo Sci. 2017, 66, 463–468. [Google Scholar] [CrossRef][Green Version]
- Adeyemi, A.D.; Oluigbo, C.C.; Esan, A.O.; Bello, M.O.; Oladoye, S.O.; Emmanuel, C.P.; Effiong, E. Chemical Composition and Antimicrobial Activity of the Essential oils of 14 known Ficus species—A Concise. Biointerface Res. Appl. Chem 2021, 12, 8003–8034. [Google Scholar]
- Cunha, C.; Ribeiro, H.M.; Rodrigues, M.; Araujo, A.R. Essential oils used in dermocosmetics: Review about its biological activities. J. Cosmet. Dermatol. 2022, 21, 513–529. [Google Scholar] [CrossRef]
- Djenane, D. Chemical profile, antibacterial and antioxidant activity of Algerian citrus essential oils and their application in Sardina pilchardus. Foods 2015, 4, 208–228. [Google Scholar] [CrossRef] [PubMed]
- Farahmandfar, R.; Tirgarian, B.; Dehghan, B.; Nemati, A. Comparison of different drying methods on bitter orange (Citrus aurantium L.) peel waste: Changes in physical (density and color) and essential oil (yield, composition, antioxidant and antibacterial) properties of powders. J. Food Meas. Charact. 2020, 14, 862–875. [Google Scholar] [CrossRef]
- Hou, H.-S.; Bonku, E.M.; Zhai, R.; Zeng, R.; Hou, Y.-L.; Yang, Z.-H.; Quan, C. Extraction of essential oil from Citrus reticulate Blanco peel and its antibacterial activity against Cutibacterium acnes (formerly Propionibacterium acnes). Heliyon 2019, 5, e02947. [Google Scholar] [CrossRef] [PubMed]
- Zema, D.; Calabrò, P.; Folino, A.; Tamburino, V.; Zappia, G.; Zimbone, S. Valorisation of citrus processing waste: A review. Waste Manag. 2018, 80, 252–273. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.X.; Wang, H.C.; Chu, Y.A.-O.; Wu, Y.Z.; Liao, J.A.; Su, Z.A.-O. Essential oil from Citrus depressa peel exhibits antimicrobial, antioxidant and cancer chemopreventive effects. J. Sci. Food Agric. 2024, 104, 3982–3991. [Google Scholar] [CrossRef]
- Geraci, A.; Di Stefano, V.; Di Martino, E.; Schillaci, D.; Schicchi, R. Essential oil components of orange peels and antimicrobial activity. Nat. Prod. Res. 2017, 31, 653–659. [Google Scholar] [CrossRef]
- Quirino, A.; Giorgi, V.; Palma, E.A.-O.X.; Marascio, N.A.-O.; Morelli, P.A.-O.; Maletta, A.A.-O.X.; Divenuto, F.; De Angelis, G.; Tancrè, V.; Nucera, S.; et al. Citrus bergamia: Kinetics of Antimicrobial Activity on Clinical Isolates. Antibiotics 2022, 11, 361. [Google Scholar] [CrossRef]
- Malik, A.; Najda, A.A.-O.; Bains, A.; Nurzyńska-Wierdak, R.; Chawla, P.A.-O. Characterization of Citrus nobilis Peel Methanolic Extract for Antioxidant, Antimicrobial, and Anti-Inflammatory Activity. Molecules 2021, 26, 4310. [Google Scholar] [CrossRef] [PubMed]
- Satari, B.; Karimi, K. Citrus processing wastes: Environmental impacts, recent advances, and future perspectives in total valorization. Resour. Conserv. Recycl. 2018, 129, 153–167. [Google Scholar] [CrossRef]
- Fresh, F.C.F. Processed Statistical Bulletin 2016; FAO: Rome, Italy, 2017. [Google Scholar]
- Kim, S.Y. Chemical composition and antioxidant activity of crude polysaccharide from citron (Citrus junos Sieb. Ex TANAKA) seed. Prev. Nutr. Food Sci. 2018, 23, 335. [Google Scholar] [CrossRef] [PubMed]
- Farhat, A.; Fabiano-Tixier, A.-S.; El Maataoui, M.; Maingonnat, J.-F.; Romdhane, M.; Chemat, F. Microwave steam diffusion for extraction of essential oil from orange peel: Kinetic data, extract’s global yield and mechanism. Food Chem. 2011, 125, 255–261. [Google Scholar] [CrossRef]
- Fisher, K.; Phillips, C.A. The effect of lemon, orange and bergamot essential oils and their components on the survival of Campylobacter jejuni, Escherichia coli O157, Listeria monocytogenes, Bacillus cereus and Staphylococcus aureus in vitro and in food systems. J. Appl. Microbiol. 2006, 101, 1232–1240. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Karakaya, H.; Ozturk, F.S.; Koc, T.B.; Yasar, K. Chemical Composition and Antimicrobial Activity of Pummelo (Citrus maxima) Essential Oil Derived from Fruit Peel. J. Essent. Oil Bear. Plants 2022, 25, 524–535. [Google Scholar] [CrossRef]
- Salvatori, E.A.-O.; Morgan, L.A.-O.; Ferrarini, S.A.-O.; Zilli, G.A.-O.; Rosina, A.A.-O.; Almeida, M.A.-O.; Hackbart, H.A.-O.X.; Rezende, R.A.-O.; Albeny-Simões, D.A.-O.X.; Oliveira, J.A.-O.; et al. Anti-Inflammatory and Antimicrobial Effects of Eucalyptus spp. Essential Oils: A Potential Valuable Use for an Industry Byproduct. Evid. Based Complement. Altern. Med. 2023, 2023, 2582698. [Google Scholar] [CrossRef]
- Sembiring, N.; Napitupulu, H.; Sembiring, M.T.; Ishak, A.; Gunawan, H. Fulfilling Eucalyptus Raw Materials for Pulp and Paper Production Plants. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Yogyakarta, Indonesia, 28–29 July 2021; IOP Publishing: Bristol, UK, 2021; p. 012008. [Google Scholar]
- de Amorim, V.d.S.S.; Monteiro, K.M.S.; Sousa, G.O.; Damascena, J.F.; Pereira, J.A.; dos Santos Moraes, W. Os benefícios ambientais do plantio de eucalipto: Revisão de literatura. Res. Soc. Dev. 2021, 10, e318101119604. [Google Scholar] [CrossRef]
- Dhakad, A.K.; Pandey, V.V.; Beg, S.; Rawat, J.M.; Singh, A. Biological, medicinal and toxicological significance of Eucalyptus leaf essential oil: A review. J. Sci. Food Agric. 2018, 98, 833–848. [Google Scholar] [CrossRef]
- Bachir, R.G.; Benali, M. Antibacterial activity of the essential oils from the leaves of Eucalyptus globulus against Escherichia coli and Staphylococcus aureus. Asian Pac. J. Trop. Biomed. 2012, 2, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Manful, M.E.; Ahmed, L.; Barry-Ryan, C. Cosmetic Formulations from Natural Sources: Safety Considerations and Legislative Frameworks in the European Union. Cosmetics 2024, 11, 72. [Google Scholar] [CrossRef]
- Rathee, P.; Sehrawat, R.; Rathee, P.; Khatkar, A.; Akkol, E.K.; Khatkar, S.; Redhu, N.; Türkcanoğlu, G.; Sobarzo-Sánchez, E. Polyphenols: Natural Preservatives with Promising Applications in Food, Cosmetics and Pharma Industries; Problems and Toxicity Associated with Synthetic Preservatives; Impact of Misleading Advertisements—Recent Trends in Preservation and Legislation. Materials 2023, 16, 4793. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.S.; Estanqueiro, M.; Oliveira, M.B.; Sousa Lobo, J.M. Main Benefits and Applicability of Plant Extracts in Skin Care Products. Cosmetics 2015, 2, 48–65. [Google Scholar] [CrossRef]
- Crupi, P.; De Santis, S.; Bavaro, S.L.; Fracassetti, D. New insights and perspectives of polyphenols in nutrition. Front. Nutr. 2023, 10, 1145135. [Google Scholar] [CrossRef] [PubMed]
- Ciubuca, B.-M.; Saviuc, C.-M.; Chifiriuc, M.-C.; Lazar, V. Microbial Resistance to Natural Compounds: Challenges for Developing Novel Alternatives to Antibiotics. Curr. Org. Chem. 2016, 20, 2983–2988. [Google Scholar] [CrossRef][Green Version]
| Source | Specific Microorganism | Effect | Tested for Cosmetic Use | Reference |
|---|---|---|---|---|
| Pomegranate (Punica granatum L.) peels and seeds | S. aureus, E. coli, P. aeruginosa, Trichophyton rubrum, T. mentagrophytes, Microsporum canis, M. gypseum, and C. albicans | Antibacterial and Antifungal | √ | [9,10,11,50,51] |
| Karkadè (Hibiscus sabdariffa L.) calyx | E. coli, S. aureus, S. epidermidis, L. monocytogenes P. aeruginosa, E. faecalis, Salmonella typhimurium, Bacillus cereus, Vibrio parahaemolyticus, Aspergillus niger, Fusarium oxysporum, Penicillium expansum, P. citrinum, P. simplicissimum, and C. albicans | - | [12,52,53] | |
| Lavender (Lavandula angustifolia) total waste | E. coli, P. aeruginosa, S. aureus, Proteus vulgaris, Enterococcus faecalis, L. monocytogenes, Bacillus subtilis, Aspergillus niger, Penicillium chrysogenum, and C. albicans | - | [17,54] | |
| Melissa (Melissa Officinalis) total waste | P. aeruginosa, S. aureus, Bacillus subtilis, Enterococcus faecalis, Candida utilis, and Penicillium chrysogenum | - | [17,54,55] | |
| Saffron (Crocus sativus L.) petals | B. subtilis, M. luteus, B. cereus, P. aeruginosa, S. aureus, S. mutans, P. gingivalis, E. coli, and C. albicans | Bacteriostatic, Antifungal, and Antibiofilm | - | [13,56,57,58,59] |
| Garlic (Allium sativum L.) peels | Methicillin-resistant Staphylococcus aureus, Acinetobacter baumannii, E. coli, and P. aeruginosa | Antibacterial | - | [14,15,60] |
| Mango (Mangifera indica L.) seeds | S. aureus, E. coli, and C. albicans | Bacteriostatic and Antifungal | √ | [16,61,62,63] |
| Phenolic Compound | Source | Specific Microorganism | Effects | Reference |
|---|---|---|---|---|
| Protocatechuic acid | Red pepper waste | E. coli, S. aureus, S. typhimurium, E. coli, K. pneumoniae, and B. cereus | Antibacterial and bactericidal | [91,92] |
| Quercetin and its glucosides (quercetin aglycone, quercetin-4′-O-monoglucoside, quercetin-3,4′-O-diglucoside, anthocyanin) | Skinned onions | S. aureus, P. aeruginosa, P. vulgaris, E. coli, S. flexneri, and L. casei var. Shirota, drug-resistant E. coli, or carbapenem-resistant P. aeruginosa | Antibacterial and antibiofilm | [93,94] |
| Caffeic acid | Apple, lime, grape, pomegranate, and papaya wastes | E. coli and K. pneumoniae, A. flavus and A. parasiticus | Antibacterial and reduced aflatoxin production | [91,95] |
| Vanillic acid | Unripe tomatoes | Carbapenem-resistant E. hormaechei, A. flavus, and A. parasiticus | Antibacterial, antibiofilm and reduced aflatoxin production | [96,97] |
| Gallic acid | Broccoli leaves and flowers | S. aureus | Enhancement of antibiotic activity | [98,99] |
| Pyrogallol | Cactus pear peels | C. albicans population and S. aureus | Reduction in required concentrations of antibiotics necessary to kill | [99,100] |
| Kaempferol | Grape pomace and peels | S. aureus | Antibacterial and antibiofilm | [101,102,103] |
| p-coumaric acid | Yellow passion fruit pulp and seeds | B. cereus | Antibacterial | [91,104] |
| Oleuropein | Olive leaves | B. cereus | Antibacterial | [42,91] |
| Apigenin and luteolin | Pineapple peels | E. coli and P. aeruginosa | Antibacterial | [105,106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silletta, A.; Mancuso, A.; d’Avanzo, N.; Cristiano, M.C.; Paolino, D. Antimicrobial Compounds from Food Waste in Cosmetics. Cosmetics 2024, 11, 151. https://doi.org/10.3390/cosmetics11050151
Silletta A, Mancuso A, d’Avanzo N, Cristiano MC, Paolino D. Antimicrobial Compounds from Food Waste in Cosmetics. Cosmetics. 2024; 11(5):151. https://doi.org/10.3390/cosmetics11050151
Chicago/Turabian StyleSilletta, Antonio, Antonia Mancuso, Nicola d’Avanzo, Maria Chiara Cristiano, and Donatella Paolino. 2024. "Antimicrobial Compounds from Food Waste in Cosmetics" Cosmetics 11, no. 5: 151. https://doi.org/10.3390/cosmetics11050151
APA StyleSilletta, A., Mancuso, A., d’Avanzo, N., Cristiano, M. C., & Paolino, D. (2024). Antimicrobial Compounds from Food Waste in Cosmetics. Cosmetics, 11(5), 151. https://doi.org/10.3390/cosmetics11050151









