Abstract
The quest for youthful, healthy skin and full, vibrant hair has long been a driving force in the dermocosmetics field. However, traditional approaches often struggle to address the underlying causes of aging, damage, and hair loss. Regenerative cosmetics powered by skin tissue engineering offer a transformative alternative. This review explores the emerging field of using engineered skin tissues for cosmetic purposes, focusing specifically on their potential for anti-aging, repair, and hair restoration applications. We discuss how these technologies aim to rejuvenate aging skin by promoting collagen production, reducing wrinkles, and improving overall skin function. Additionally, the use of engineered skin for wound healing and scar reduction is examined, highlighting their potential to improve the appearance and functionality of damaged skin. Finally, we advance the exciting prospects of utilizing skin tissue engineering techniques to regenerate hair follicles, potentially offering solutions for hair loss and promoting denser hair growth.
1. Introduction
The pursuit of rejuvenating skin and hair to achieve a fresh, younger look has been a longstanding motivation for the cosmetics industry. However, conventional treatment strategies frequently target only the most apparent manifestations and not the core causes of deterioration and aging. This review delves into an exciting new wave of cosmetic solutions based on tissue regeneration. Regenerative cosmetics, powered by the cutting-edge field of skin tissue engineering (TE) and all related fields, offer a transformative alternative with the potential to revolutionize the way we care for our skin and hair.
1.1. Importance of Healthy Skin and Hair in Society
The skin, a complex organ with multiple layers, plays a crucial role as the body’s first line of defense, protecting us from harmful environmental factors like UV radiation, pathogens, and allergens. It also plays a vital role in regulating body temperature and maintaining hydration [1]. It is a key target for dermocosmetic products aimed at enhancing skin health and appearance [2]. Additionally, healthy hair is a protective barrier for the scalp and helps regulate heat loss [3].
The condition of our skin and hair significantly impacts our psychological well-being and social interactions [4,5]. Radiant and healthy skin and hair can boost our confidence and self-esteem, contributing to a positive self-image [6]. Regardless of its cause or severity, hair loss is a distressing condition often underestimated. It encompasses various pathogeneses, including androgenetic alopecia (AGA, the most widespread type of hair loss), alopecia areata, diffuse alopecia, and therapy-induced hair loss. Despite treatment advancements, affected individuals commonly face stigmatization, which may exacerbate psychological conditions and diminish overall contentedness [7]. Concerns about blemishes, wrinkles, or hair loss can lead to insecurity and social anxiety, impacting our quality of life and interactions with others [8]. Therefore, maintaining healthy skin and hair is not only essential for physical health but also contributes significantly to our overall well-being and social integration.
1.2. Limitations of Traditional Cosmetic Approaches
Traditional cosmetic approaches primarily focus on superficial effects. They operate on the surface of the skin or hair, offering temporary improvements like hydration, masking imperfections, or providing color. These products often lack the ability to address the underlying biological processes that contribute to aging, damage, or hair loss [9,10].
Furthermore, the effectiveness of traditional cosmetics can vary significantly depending on individual factors like age, skin type, and the severity of the concern. Additionally, some individuals may experience adverse reactions to certain ingredients, limiting their suitability [11] or requiring continuous use to maintain the desired effects, leading to inconvenience and cost burdens for consumers [12].
Finally, traditional testing methods for these products, such as animal testing and two-dimensional (2D) cell cultures, have significant drawbacks. Animal testing raises ethical concerns and often fails to represent human skin physiology accurately. Similarly, 2D cell cultures lack the complexity of three-dimensional (3D) tissues, hindering the evaluation of product safety and efficacy [13,14].
1.3. The Promise of TE-Based Dermocosmetics
Dermocosmetics, formulated with ingredients that target specific biological pathways or are based on TE principles, offer a more directed approach than traditional cosmetics. TE is a rapidly evolving field that combines the three well-known key components (cells, scaffolds and signals). Some of them are included in Table 1, summarizing relevant research data from the literature for each of these key components.

Table 1.
Key TE components that have an important role in dermocosmetics applications.
By incorporating these components, TE-based dermocosmetics offer several advantages over traditional approaches:
- Addressing the root cause: TE-based dermocosmetics aim to address the underlying biological processes responsible for various skin and hair concerns. This can involve stimulating collagen production for anti-aging effects, promoting wound healing through the delivery of growth factors, or even facilitating hair follicle (HF) regeneration through the use of bioengineered scaffolds [18].
- Enhanced efficacy: By targeting specifically the dermal compartment, dermocosmetics derived from TE, including new delivery methods, improve the efficacy of the bioactive compounds or key proteins such as collagen. This can significantly improve areas like scar regeneration and wound healing [19,20].
- Long-lasting results: Some TE techniques, like the application of stem cells or their exosomes, show promise for promoting long-lasting results by stimulating cell proliferation and collagen production. This can significantly reduce the need for frequent product application and improve patient compliance [21,22].
- Better testers: Ex vivo skin models, such as 3D “skin-on-a-chip” (SoC) systems combined with microfluidics, offer a promising alternative to traditional testing methods. These models provide a more realistic recreation of human skin architecture and function, enabling more accurate dermocosmetic product testing [23,24].
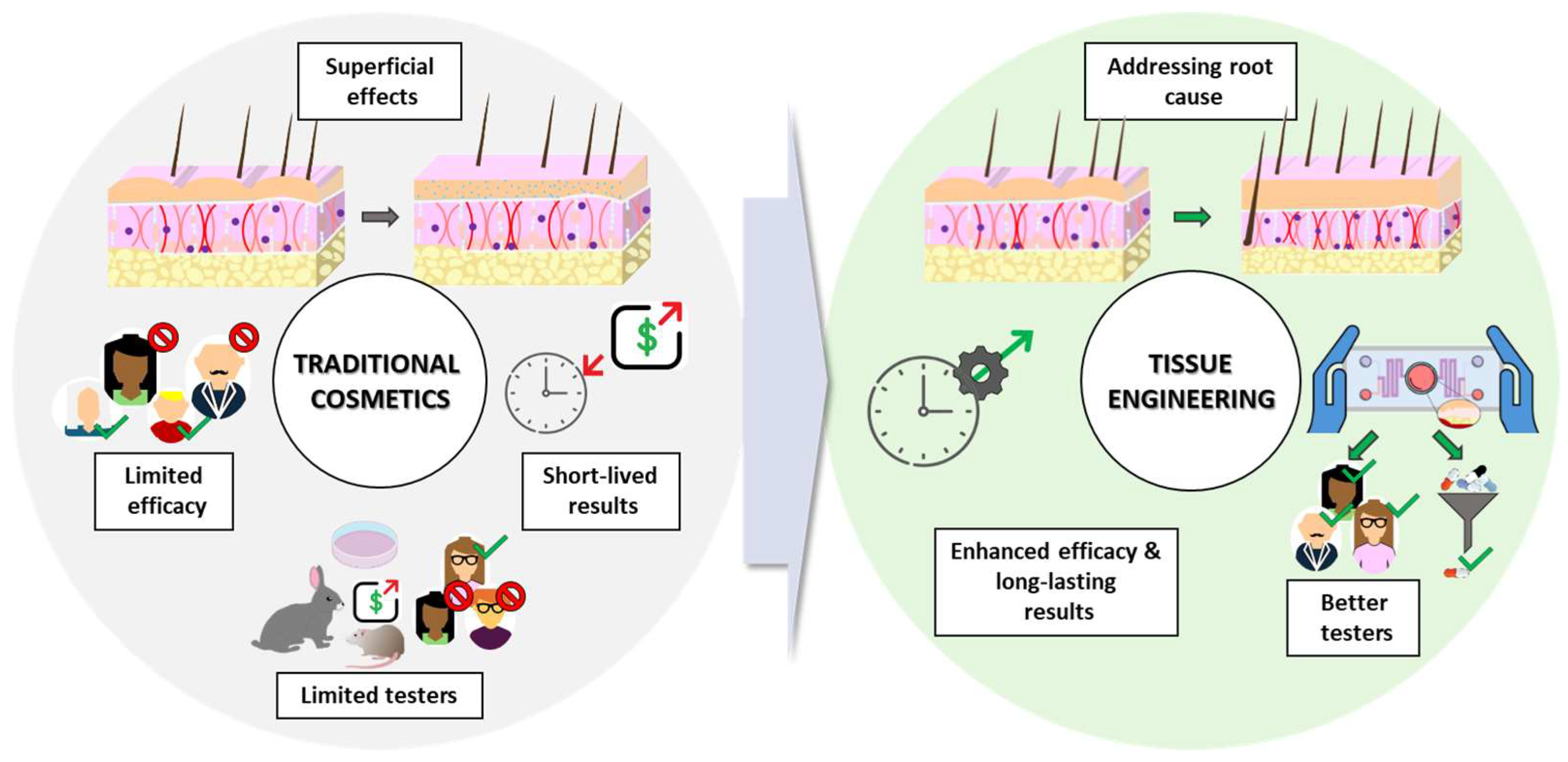
While traditional cosmetics play a role in aesthetics, they often have limitations. In contrast, advancements in TE-based dermocosmetics hold significant promise for the future of personalized and effective cosmetic interventions (Figure 1).
Figure 1.
Limitations of traditional cosmetic approaches and the promise of tissue engineering. Source of some vectors: https://scidraw.io/ (accessed on 17 June 2024).
2. Mechanisms Involved in Regenerative Cosmetics
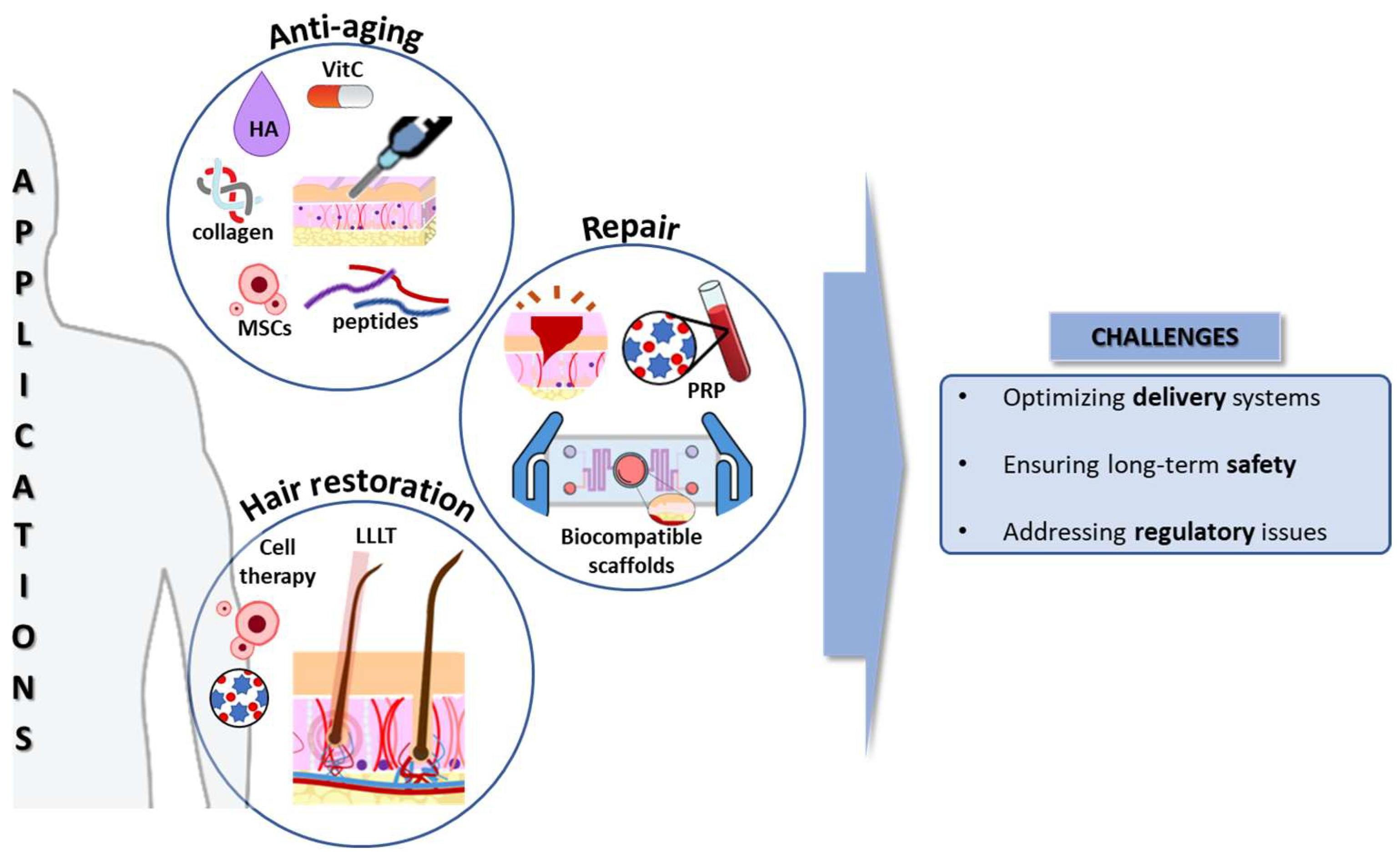
Regenerative cosmetics, situated at the intersection of dermatological science and innovative biotechnology, focus on mechanisms underlying skin aging. This multifactorial process involves oxidative stress, cellular damage, and senescence. Additionally, fibrosis affects skin architecture, reducing elasticity and resilience [25]. Moreover, the HF regeneration approach explores stem cell dynamics, signaling pathways, and microenvironmental cues, offering the potential for advanced anti-aging interventions (Figure 2).
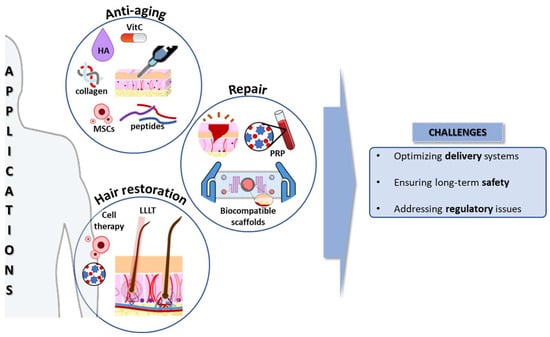
Figure 2.
Applications and challenges of regenerative cosmetics. HA: Hyaluronic acid; LLLT: Low-level laser therapy; MSCs: mesenchymal stem/stromal cells; PRP: platelet-rich plasma; VitC: vitamin C. Source of some vectors: https://scidraw.io/ (accessed on 17 June 2024).
2.1. Skin Aging
- Skin aging is characterized by a decline in collagen production and a reduction of cell proliferation, together with a decrease in stemness from each tissue, among other factors [25]. Regenerative cosmetics offer solutions to combat these age-related changes, here is a summary of the key approaches in regenerative medicine applied to address age-related skin changes (Table 2).
 Table 2. Summary of the key approaches to addressing age-related skin changes using regenerative cosmetics.
Table 2. Summary of the key approaches to addressing age-related skin changes using regenerative cosmetics.
2.2. Oxidative Stress
Life in an oxygen-rich atmosphere comes with a toll to pay. While oxygen’s reactivity supports cellular energetics, its unique properties also lead to partial reduction into potentially damaging molecular species known as reactive oxygen species (ROS).
Being the most exposed area of the body, environmental-derived ROS activity affects skin health and appearance. Oxidative stress, an imbalance between free radicals and endogenous antioxidants, plays a central role in aging [32] and plays a major role in the etiopathogenesis or aggravation of atopic [33], acne [34], rosacea [35], melasma [36] and alopecia areata [37], and rare skin disorders [38]. Thus, it comes as no surprise that the dermocosmetics industry has paid great attention to combating its effects.
Modern skincare routines frequently aim to mitigate oxidative stress, with vitamin C being a popular ingredient. In its natural form or modified in more stable derivatives, ascorbic acid acts as a potent hydrosoluble electron donor, neutralizing free radicals [39]. In cosmetology, this molecule possesses several relevant properties. It regenerates the endogenous antioxidant α-tocopherol, protecting against lipid peroxidation [40]. Additionally, it inhibits the tyrosinase enzyme responsible for hyperpigmentation collagen synthesis and processing [41]. However, the extensive list of prophylactic or therapeutic antioxidant agents in dermocosmetics includes both synthetic and naturally obtained bioactives [42].
Notably, some of these compounds may impact stem cell pluripotency and differentiation [43,44,45], warranting cautious application. While reactive oxygen species (ROS), particularly H2O2, play physiological roles at low concentrations, excessive antioxidant doses could disrupt endogenous regenerative processes, potentially worsening skin conditions [46]. In the context of skin, these include cell survival proliferation and tissue repair pathways [47,48,49]. Thus, excessive antioxidant doses could disrupt endogenous regenerative processes, worsening skin conditions. Indeed, many reactive oxygen species (ROS) mechanisms remain unclear and may be cell-specific. Interestingly, preconditioning with low H2O2 doses in regenerative medicine shows promise [50,51,52]. Guided by these efforts, in the near future, oxidants and antioxidants will be carefully and timely dosed to rescue pathology without challenging the function of the other. To this end, defining their respective action windows is of utmost importance and an extremely relevant objective to pursue the development of novel dermocosmeticals.
2.3. Repair vs. Regeneration
The shift from repair to regeneration in dermocosmetics is crucial for sustainable skin health. Regeneration aligns with the skin’s natural healing, offering restoration of its structure and function. This approach not only preserves the skin’s youthful properties but also ensures compatibility with its biological processes, minimizing long-term damage. Emphasizing regeneration represents a significant leap in skin care, promising lasting benefits and alignment with natural dermal recovery mechanisms. Skin damage caused by wounds, burns, or scars can significantly impact both appearance and functionality. Regenerative cosmetics offer several aspects:
- Promoting wound healing: Engineered skin substitutes like biocompatible scaffolds provide a structure for cell migration and tissue regeneration, accelerating wound healing and minimizing scar formation [53]. Current skin substitutes have been tested for addressing regeneration in burn patients, chronic ulcers (diabetes), and rare genodermatoses (Epidermolysis bullosa) [54]. Novel technologies, including injectable cell suspensions and 3D scaffolds, are promising for improving wound healing and skin regeneration [55].
- Scar reduction: Microneedling and fractional laser therapy, combined with regenerative ingredients like growth factors, can stimulate collagen production and improve the appearance of existing scars [56,57]. Additionally, platelet-rich plasma (PRP) therapy is gaining traction as a potential scar reduction technique. Studies suggest that PRP injections may improve scar quality and reduce scar tissue formation [58], which is particularly interesting in relation to acne scars.
2.4. Fibrosis and Connective Tissue in Skin Rejuvenation
Fibrosis, the excessive deposition of collagen and other extracellular matrix (ECM) components, can occur following wound healing or in response to chronic inflammation. Proper management of connective tissue is essential for maintaining skin elasticity and preventing the stiffening associated with aging. While essential for wound closure, excessive fibrosis can lead to scar formation and impair skin function [59], which is particularly interesting in a pathological context in some genodermatoses such as Epidermolysis bullosa [60]. By understanding and controlling fibrotic pathways, we can enhance skin rejuvenation and promote a more youthful appearance. Regenerative cosmetics aim to:
- Modulate the fibrotic process: by understanding the molecular mechanisms underlying fibrosis, researchers can develop strategies to control collagen deposition and promote scarless wound healing, and also highlight the role of macrophages in the inflammatory phase [59].
- Enhance the functionality of the connective tissue: supporting the health and organization of the connective tissue, which provides structural support and elasticity to the skin, is crucial for maintaining a youthful appearance and function, and this is particularly interesting when the role of MSCs is studied in UV-associated skin aging [61].
Wound healing involves many cell types working together in a specific order. While some interactions are essential, others have redundancies to ensure successful repair [62].
2.5. Hair Follicle Regeneration
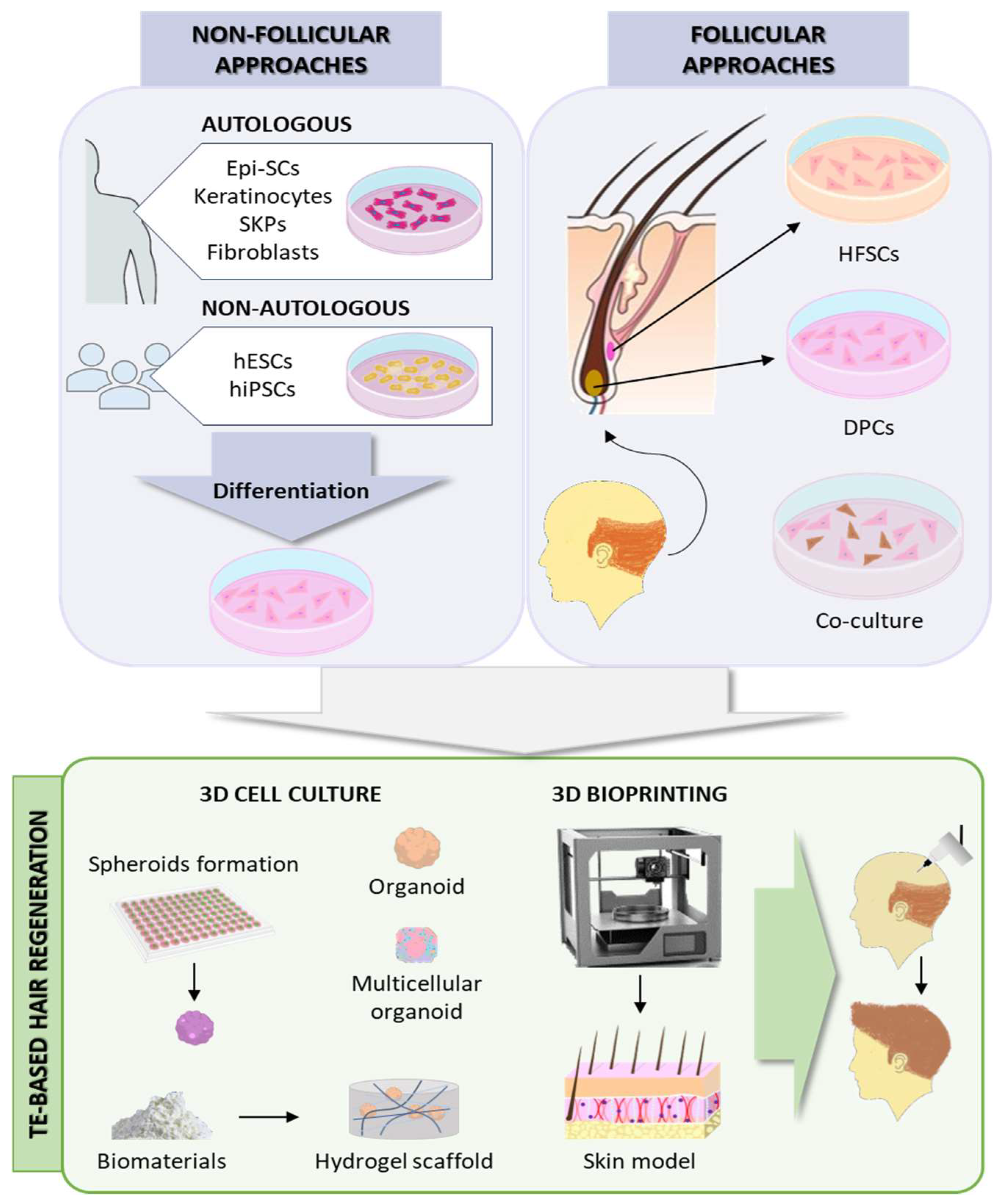
The HF is composed of epithelial and mesenchymal components, whose interaction regulates HF morphogenesis and regeneration. The epithelial elements comprise three concentric layers: Outer root sheath (ORS), inner root sheath (IRS), and hair shaft (HS). The bulge, an engrossed part of the ORS, is considered the largest reservoir of hair follicle stem cells (HFSCs). The HF mesenchyme includes the dermal papilla (DP), composed of dermal papilla cells (DPCs), and the dermal sheath (DS). During the anagen phase of the hair cycle, the quiescent HFSCs in the bulge are stimulated by the DP through signaling molecules and transcription factors to proliferate and initiate the hair growth process [63,64]. Therefore, the TE strategies to form bioengineered constructs capable of regenerating HFs when transplanted to the patient should focus on the epithelial–mesenchymal interactions (EMIs) [65].
Non-follicular cell sources for HF regeneration include either autologous mesenchymal (adult dermal fibroblasts, skin-derived precursors [SKPs]) and epithelial (epidermal stem cells [Epi-SCs], adult epidermal keratinocytes) precursors obtained from outside the HF; or non-autologous reprogrammed pluripotent stem cells, such as human embryonic stem cells (hESCs) or human-induced pluripotent stem cells (hiPSCs) [66]. Conversely, follicular-based approaches are based on the combination of epithelial and mesenchymal cells from the HF. For that, DPCs and HFSCs need to be isolated from the patient and expanded in culture, but DPCs lose their innate trichogenic capacity in 2D in vitro culture [64]. To avoid this and better mimic the in vivo HF microenvironment, the current approaches are mainly focused on 3D cultures to preserve intercellular interactions. DPCs in spheroid and organoid cultures have been shown to preserve their characteristics even in high passages [64].
Furthermore, the cell–ECM interactions are also crucial in the HF cycle [67]. A variety of biomaterials have been used to create spheroids or hydrogel scaffolds to enhance HF regeneration by simulating the cell–ECM interactions, such as collagen [68], gelatin [69,70], PRP [70,71], chitosan [70,72], HA [73], Matrigel [74], or human placenta decellularized ECM [75,76].
Despite the advances, these in vitro TE approaches often lack the intricate physiological, structural organization and microenvironment of the native HF niche, which is essential for HF regeneration. To better mimic the native tissue, 3D bioprinting enables the automated, reproducible, and high-throughput biofabrication of advanced skin models incorporating several cell types and adnexal structures [77]. Regeneration of HF-like structures has been achieved by 3D bioprinting complex skin constructs incorporating DPCs, as well as Epi-SCs and SKPs [77,78,79] (Figure 3).
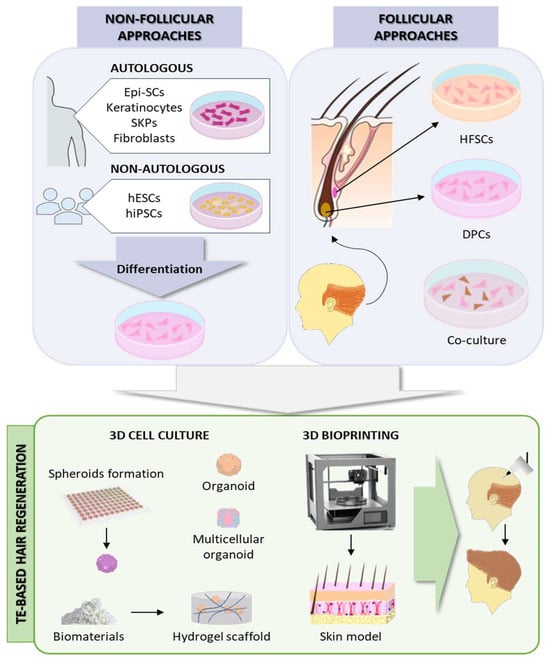
Figure 3.
Hair follicle regeneration approaches. Epi-SCs: epidermal stem cells; hESCs: human embryonic stem cells; hiPSCs: human-induced pluripotent stem cells; SKPs: skin-derived precursors; HFSCs: hair follicle stem cells; DPCs: dermal papilla cells. Source of some vectors: https://scidraw.io/ (accessed on 17 June 2024).
3. Regenerative Cosmetics: A Transformative Alternative
OMIC technologies and tissue engineering have revolutionized regenerative cosmetics, marking a paradigm shift from symptomatic treatment to root-level correction. This revolution is driven by deep insights into the molecular mechanisms of skin aging, damage, and regeneration facilitated by OMIC approaches. This section explores three pivotal areas: the application of OMIC approaches in understanding and enhancing skin regeneration, the use of advanced skin modeling techniques to accelerate drug development, and innovative solutions for hair loss and promoting healthier hair growth. Together, these advancements offer promising avenues for the development of effective, safe, and personalized cosmetic treatments.
3.1. OMIC Approaches: A Key Tool for Regenerative Cosmetics
All kinds of OMIC approaches have been widely used to understand the intricate molecular landscape in a myriad of applications, including the development and characterization of novel and effective regenerative cosmetic approaches.
3.1.1. Proteomics
Proteomics, the comprehensive study of proteins, has emerged as a powerful tool to identify biomarkers in diseased skin [80], which could be potentially useful in developing targeted cosmetic therapies. Other applications in regenerative cosmetics include elucidating cellular processes involved in skin aging, regeneration, and damage [81] and characterizing biocompatible materials used in engineered skin substitutes [82]. In this regard, a recent publication highlighted the importance of the MSCs secretome in the improvement of anti-aging, whitening, and wound healing processes and its potential use as novel biomaterials for skin regeneration [83].
3.1.2. Metabolomics
Other OMIC technologies, such as metabolomics, have also been implemented in cosmetic applications and safety studies [84]. Specifically, the skin lipid structure and composition are known to be affected in many dermal diseases, impairing the barrier function [85]. Therefore, studying the lipidomic profile of known bioactive products provides insights into the molecular mechanisms behind their therapeutic effects [86]. It is well known that skin functionality is maintained by a complex system formed by the relationships between microorganisms and host cells [87]. In this regard, the use of metabolomics to elucidate these interactions is key for the development of innovative microbiome-based approaches to deal with skin conditions [88].
3.1.3. Multi-OMICs Integration
Integrating multiple OMIC approaches (multi-OMICs) plays a crucial role in understanding the mechanistic processes behind the use of cosmetics for skin regeneration. For example, a recent study integrated metagenomics and transcriptomics to study the role of eugenol in skin photoaging [89]. Additionally, advances in gene editing technologies, such as CRISPR-Cas9, enable targeting specific genetic factors underlying skin and hair conditions, paving the way for more precise and effective therapeutic interventions [90]. Moreover, the integration of regenerative medicine with Artificial Intelligence (AI) and machine learning algorithms holds promise for optimizing treatment protocols, predicting treatment outcomes, and enhancing patient satisfaction.
3.2. Skin Modeling Accelerates Drug Development
TE-based humanized animal models are crucial for the preclinical testing of novel therapeutic agents for skin conditions conditions [54]. By mimicking human skin physiology and pathology, including cellular composition, architecture, and immune responses, they offer a valuable platform for assessing the safety and efficacy of potential treatments before clinical trials in human patients [91], providing more clinically relevant data compared to traditional animal models [92] and bridging the gap between preclinical and clinical research, enhancing dermatological care outcomes.
3D Skin-on-Chip Models and Microfluidics for Dermocosmetics
The integration of microfluidic devices in skin-on-chip (SoC) models enhances drug testing accuracy by closely mimicking the dynamic human skin microenvironment. These devices allow precise control of fluid flow, nutrient delivery, and waste removal, maintaining vascularized skin models for long-term studies. Microfluidic systems in skin chips replicate immune responses, inflammation, edema, and drug effects while also facilitating permeation and skin irritation tests. Additionally, they enable real-time monitoring of key parameters like oxygen levels, pH, and transepithelial/transendothelial electrical resistance, providing continuous feedback on cell viability and metabolic responses. Overall, incorporating microfluidic technology in skin models offers a more realistic and comprehensive platform for drug testing, advancing pharmaceutical research and skincare product development [93].
Current human skin models consist of epidermal keratinocytes and dermal fibroblasts integrated into a biocompatible scaffold, nourished by a culture medium. The 3D microfluidic systems, such as organ-on-a-chip (OoC) and skin-on-chip (SoC), revolutionize organ function simulation, offering realistic disease models and treatment testing [94,95]. They are faster and more cost-effective than traditional animal testing and can be used to investigate various skin phenomena, including wound healing, barrier function, and the effects of cosmetic products [96].
However, despite significant strides in creating an in vitro skin model, challenges persist, primarily because, until now, there are no SoC devices that fully incorporate all skin layers (the epidermal, dermal, and hypodermal layers) [97].
- Materials: The 3D SoC models require balancing cost and biomimicry through material selection. Synthetic polymers (PDMS, PCL, PLA) offer affordability and biocompatibility but lack the intricate structure of natural tissues. Hydrogels (alginate, collagen) mimic tissues but struggle with maintaining precise mechanical properties. Animal-derived materials (decellularized ECM, silk) provide the most biomimetic environment, closely resembling natural skin, but are expensive and raise ethical concerns [95,98,99,100,101,102]. Recent innovations like decellularized ECM and silk offer promising solutions, aiming to bridge the gap between affordability and biomimicry [15,103].
- Challenges: SoC models face challenges in controlling chemical gradients, technical sampling, and analysis. Integrating vasculature and microbiomes is crucial for physiological accuracy. Despite these, models like EpiDerm from MatTek Corporation or SkinEthic from L’Oréal show promise for dermocosmetics, offering safety and efficacy benefits over traditional methods [93,104,105].
3.3. Potential Solutions for Hair Loss and Promotion of Thicker, Healthier Hair Growth
Current treatments for alopecia typically involve oral or topical pharmacological treatments with minoxidil and finasteride, the only drugs approved by the Food and Drug Administration (FDA). However, these medications can cause side effects and do not always yield satisfactory results [10]. While surgical treatment by autologous hair transplant (by follicular unit extraction [FUE]) is considered the most effective method, it may be limited by the lack of enough follicles in the donor area in patients with advanced AGA [65,106].
Furthermore, to enhance the outcomes achieved with these therapies and promote better hair growth, some minimally invasive techniques have been used as complementary treatments (Figure 3), but a lack of standardization for these techniques is frequent:
- Low-level laser therapy (LLLT) stimulates hair growth with minimal side effects by exposing tissues to low-level light energy, showing a synergistic effect on promoting hair regrowth [107].
- Mesotherapy, involving the intradermal infusion of a mixture of factors, has demonstrated improvements in hair growth [93,108,109].
- Carboxytherapy, which entails the intradermic or subcutaneous insufflation of medical-grade sterile CO2, enhances blood flow and nutrient delivery to the HF, potentially aiding in cases of alopecia [93,110,111].
- Microneedling promotes hair growth by inducing percutaneous wounds with sterile microneedles, stimulating the activation of HFSCs and the release of growth factors that promote wound healing and angiogenesis [112,113].
- Autologous PRP treatment, derived from the patient’s blood, stimulates hair growth through the release of growth factors, cytokines, and chemokines, promoting cell proliferation, differentiation, and angiogenesis [114].
- Nanoparticles have been studied for drug delivery directly into the HF, minimizing the systemic adverse effects [107].
Different intricate intercellular signaling pathways regulate HF morphogenesis and cycle, including Bone Morphogenetic Protein (BMP), Ectodysplasin A Receptor (EDAR), Sonic hedgehog (Shh), Notch, or Transforming Growth Factor β (TGF-β). Among them, WNT/β-catenin is considered to be the main regulator of the HF cycle. Some HF regenerative medicine approaches aim to activate these signaling pathways [63].
Therapy with stem cells (derived from the HF, bone marrow, adipose tissue, or umbilical cord blood) has been demonstrated to improve hair growth, as they regulate hair cycles and regeneration through the secretion of proteins, extracellular vesicles, and nucleic acids, that play a role in paracrine factor signaling [115]. Similarly, exosome therapy could be a potential approach for hair loss, as they transport Wnt proteins [116]. Exosomes derived from DPC spheroids have been reported to stimulate proliferation, increase their trichogenic properties, and induce and prolong anagen in mice, promoting hair growth and regeneration [117]. Additionally, gene therapy emerges as a tool to introduce growth-promoting genes, replace defective ones, or reprogram surrounding cells for hair restoration [118].
4. Revolutionizing Beauty: The Convergence of Regenerative Medicine and Cosmetic Science
Regenerative medicine plays a pivotal role in the cosmetics industry, offering innovative solutions for skin and hair rejuvenation. Techniques such as stem cell therapy, TE, and growth factor application have shown promising results in promoting skin regeneration, reducing signs of aging, and treating hair loss conditions. For instance, stem cell-based therapies can stimulate collagen production, improve skin texture, and enhance elasticity, leading to a more youthful appearance [21,22]. Similarly, TE approaches enable the development of advanced skin substitutes and HF regeneration techniques, providing effective solutions for cosmetic enhancement. Moreover, the application of growth factors, such as PRP, accelerates tissue repair, promotes hair growth, and enhances the overall quality of skin and hair [58,114].
Looking ahead, the field of regenerative medicine presents exciting opportunities for further advancements in cosmetic applications. Future research efforts may focus on harnessing the regenerative potential of stem cells and TE to develop personalized cosmetic treatments tailored to individual needs and preferences.
Regenerative cosmetics hold promise for addressing skin and hair concerns through targeted biological interventions. They can stimulate collagen production, improve skin function, and mitigate oxidative stress for skin rejuvenation [26,27,31]. Additionally, engineered skin substitutes accelerate wound closure, minimize scar formation, and enhance scar tissue functionality [50]. Furthermore, advancements in TE offer potential solutions for hair loss and the promotion of thicker, healthier hair growth [63,115,116,117,118]. Lastly, developments in proteomics enable personalized cosmetic solutions tailored to individual needs and characteristics [81,82].
Overall, the intersection of regenerative medicine and cosmetics represents a dynamic and evolving field with vast potential to revolutionize the beauty industry and empower individuals to achieve healthier, more radiant skin and hair.
5. Challenges, Future Opportunities, and the Role of AI in this Field
As the field of regenerative cosmetics continues to evolve, there are several materials and areas that are likely to garner more attention from researchers in the coming years. However, the integration of tissue engineering (TE) in dermocosmetics presents a transformative approach to skin and hair care, yet it is not without its challenges.
The complexity of skin and hair physiology requires a multidisciplinary effort to replicate these structures accurately. One of the primary challenges lies in the scalability of TE products, ensuring they are accessible and affordable for widespread use. Additionally, the heterogeneity of human skin and hair types calls for personalized solutions that can adapt to individual variations. Standardization of tissue engineering-based substitutes could significantly reduce overall research and development costs by eliminating the need for animal testing.
Regulatory hurdles also pose a significant challenge, as the approval process for new biotechnological products can be lengthy and rigorous. Ensuring safety and efficacy through clinical trials is paramount, but it can delay the availability of innovative treatments to the market.
Looking to the future, the field holds immense potential. Advancements in 3D bioprinting could revolutionize the creation of skin and hair grafts, allowing for more precise and customized applications.
The exploration of novel biomaterials and growth factors promises to enhance the regenerative capabilities of cosmetic products. Furthermore, the integration of AI could streamline the designing and testing of new therapies, predicting outcomes, and personalizing treatments.
Artificial Intelligence in Regenerative Cosmetics
The integration of Artificial Intelligence (AI) can significantly enhance various aspects of regenerative cosmetics. Artificial intelligence (AI) is on the verge of becoming a standard tool in cosmetic dermatology. Presently, AI is being utilized to enable patients to participate more actively in their treatment choices through personalized skincare, augmented reality features, and home-based skin analysis instruments. Many dermatology practices have incorporated AI-powered skin analysis tools, which include the creation of three-dimensional facial reconstructions and predictive models for clinical results. This article underscores the existing and evolving uses of AI in cosmetic dermatology and offers a glimpse into prospective methods in this area. It is crucial for dermatologists to stay updated on these emerging technologies to effectively educate their patients and enhance their clinical practice [119]. AI can play a crucial role also in data analysis and drug discovery:
Data Analysis
AI algorithms can analyze large datasets from clinical trials and patient information to identify patterns and predict optimal treatment strategies for skin regeneration. This includes personalizing treatments based on an individual’s unique genetic makeup and skin characteristics [16].
AI-Powered Drug Discovery
AI can accelerate the development of new drugs and therapies for skin regeneration. By analyzing vast chemical libraries, AI can identify potential drug candidates with targeted effects on specific cell types or signaling pathways involved in skin rejuvenation [120].
As research progresses, we may see a shift towards holistic regenerative solutions that not only address cosmetic concerns but also promote overall health and well-being. The ultimate goal is to harness the full potential of TE and AI to create dermocosmetic products that are not only effective but also sustainable and ethical.
6. Conclusions
The field of regenerative cosmetics, particularly skin TE, has made significant strides in anti-aging, repair, and hair restoration. Innovations like SoC models have revolutionized testing and understanding of skin responses. Oxidative stress and fibrosis, critical factors in skin aging, are now better managed through advanced application methods. OMIC technologies have provided deeper insights into skin biology. The application of stem cells, either directly or indirectly through their secreted factors, holds great potential for regenerative cosmetics. However, further research is needed to optimize stem cell delivery and differentiation, as well as to address safety concerns. As the field of regenerative cosmetics continues to advance, it is poised to revolutionize the way we care for our skin and hair, offering personalized and effective solutions for a wide range of skin and hair concerns.
Author Contributions
Conceptualization, S.G.-A. formal analysis, S.H.-G. and S.G.-A.; data curation, S.G.-A. and P.P.-B.; writing—original draft preparation, P.P.-B., D.M.-M., C.L. and I.M.-F. writing—review and editing, S.H.-G., C.L., I.M.-F., P.P.-B., D.M.-M. and S.G.-A.; visualization, S.H.-G., P.P.-B. and S.G.-A.; supervision, S.G.-A.; funding acquisition, I.M.-F., C.L. and P.P.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported in part by grants from the Spanish Ministry of Science and Innovation and the European Regional Development fund (PID2020-119792RB-I00), Institute of Health Carlos III (RD21/0001/0022, Spanish Network of Advanced Therapies, TERAV-ISCIII). SHG is supported by a UC3M–PhD research training scholarship (PIPF). IMF was supported by the Madrid Government (Comunidad de Madrid) under the Multiannual Agreement with UC3M in the line of “Research Funds for Beatriz Galindo Fellowships’‘ (REDOXSKIN-CM-UC3M) and in the context of the V PRICIT (Regional Programme of Research and Technological Innovation”, and by “Proyectos de I + D + I” (PID2020-114230GA-I00) funded by MCIN/AEI/10.13039/501100011033/. PPB thanks the Listen2Future project, funded by 101096884 in HORIZON-CHIPS-JU-2021-2-RIA and by PCI2022-135048-2 by Ministerio de Ciencia e Innovación.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable.
Acknowledgments
This paper is dedicated to the Memory of Claudio J. Conti, whose groundbreaking research in the field of Cancer has inspired generations of scientists and whose unforgettable personality has also inspired generations of mentees.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bouwstra, J.A.; Nadaban, A.; Bras, W.; McCabe, C.; Bunge, A.; Gooris, G.S. The skin barrier: An extraordinary interface with an exceptional lipid organization. Prog. Lipid Res. 2023, 92, 101252. [Google Scholar] [CrossRef]
- Mansbridge, J. Skin tissue engineering. J. Biomater. Sci. Polym. Ed. 2008, 19, 955–968. [Google Scholar] [CrossRef] [PubMed]
- Lasisi, T.; Smallcombe, J.W.; Kenney, W.L.; Shriver, M.D.; Zydney, B.; Jablonski, N.G.; Havenith, G. Human scalp hair as a thermoregulatory adaptation. Proc. Natl. Acad. Sci. USA 2023, 120, e2301760120. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, J.I. Comorbidities and the impact of atopic dermatitis. Ann. Allergy Asthma Immunol. 2019, 123, 144–151. [Google Scholar] [CrossRef]
- Hwang, H.W.; Ryou, S.; Jeong, J.H.; Lee, J.W.; Lee, K.J.; Lee, S.B.; Shin, H.T.; Byun, J.W.; Shin, J.; Choi, G.S. The Quality of Life and Psychosocial Impact on Female Pattern Hair Loss. Ann. Dermatol. 2024, 36, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, J.; Fabi, S.G. The psychological and aesthetic impact of age-related hair changes in females. J. Cosmet. Dermatol. 2019, 18, 1161–1169. [Google Scholar] [CrossRef]
- Schielein, M.C.; Tizek, L.; Ziehfreund, S.; Sommer, R.; Biedermann, T.; Zink, A. Stigmatization caused by hair loss—A systematic literature review. JDDG J. Der Dtsch. Dermatol. Ges. 2020, 18, 1357–1368. [Google Scholar] [CrossRef]
- Nandy, P.; Shrivastava, T. Exploring the Multifaceted Impact of Acne on Quality of Life and Well-Being. Cureus 2024, 16, e52727. [Google Scholar] [CrossRef]
- Bertoli, M.J.; Sadoughifar, R.; Schwartz, R.A.; Lotti, T.M.; Janniger, C.K. Female pattern hair loss: A comprehensive review. Dermatol. Ther. 2020, 33, e14055. [Google Scholar] [CrossRef]
- York, K.; Meah, N.; Bhoyrul, B.; Sinclair, R. A review of the treatment of male pattern hair loss. Expert Opin. Pharmacother. 2020, 21, 603–612. [Google Scholar] [CrossRef]
- Dhapte-Pawar, V.; Kadam, S.; Saptarsi, S.; Kenjale, P.P. Nanocosmeceuticals: Facets and aspects. Future Sci. OA 2020, 6, FSO613. [Google Scholar] [CrossRef] [PubMed]
- Vyas, K.S.; Kaufman, J.; Munavalli, G.S.; Robertson, K.; Behfar, A.; Wyles, S.P. Exosomes: The latest in regenerative aesthetics. Regen. Med. 2023, 18, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Mathes, S.H.; Ruffner, H.; Graf-Hausner, U. The use of skin models in drug development. Adv. Drug Deliv. Rev. 2014, 69–70, 81–102. [Google Scholar] [CrossRef] [PubMed]
- Sarkiri, M.; Fox, S.C.; Fratila-Apachitei, L.E.; Zadpoor, A.A. Bioengineered Skin Intended for Skin Disease Modeling. Int. J. Mol. Sci. 2019, 20, 1407. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Yonezawa, T.; Hubbell, K.; Dai, G.; Cui, X. Inkjet-bioprinted acrylated peptides and PEG hydrogel with human mesenchymal stem cells promote robust bone and cartilage formation with minimal printhead clogging. Biotechnol. J. 2015, 10, 1568–1577. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Yan, M.; Wang, Y.; Fu, J.; Suo, H. 3D Bioprinting of Low-Concentration Cell-Laden Gelatin Methacrylate (GelMA) Bioinks with a Two-Step Cross-linking Strategy. ACS Appl. Mater. Interfaces 2018, 10, 6849–6857. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Li, Y.; Yu, K.; Liu, L.; Fu, J.; Yao, X.; Zhang, A.; He, Y. 3D Printing of Physical Organ Models: Recent Developments and Challenges. Adv. Sci. 2021, 8, e2101394. [Google Scholar] [CrossRef] [PubMed]
- Khalili, M.H.; Zhang, R.; Wilson, S.; Goel, S.; Impey, S.A.; Aria, A.I. Additive Manufacturing and Physicomechanical Characteristics of PEGDA Hydrogels: Recent Advances and Perspective for Tissue Engineering. Polymers 2023, 15, 2341. [Google Scholar] [CrossRef] [PubMed]
- Matama, T.; Costa, C.; Fernandes, B.; Araujo, R.; Cruz, C.F.; Tortosa, F.; Sheeba, C.J.; Becker, J.D.; Gomes, A.; Cavaco-Paulo, A. Changing human hair fibre colour and shape from the follicle. J. Adv. Res. 2023. [Google Scholar] [CrossRef]
- Monavarian, M.; Kader, S.; Moeinzadeh, S.; Jabbari, E. Regenerative Scar-Free Skin Wound Healing. Tissue Eng. Part B Rev. 2019, 25, 294–311. [Google Scholar] [CrossRef]
- Dehkordi, A.N.; Babaheydari, F.M.; Chehelgerdi, M.; Dehkordi, S.R. Skin tissue engineering: Wound healing based on stem-cell-based therapeutic strategies. Stem Cell Res. Ther. 2019, 10, 111. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.; Brito, S.; Kwak, B.M.; Park, S.; Lee, M.G.; Bin, B.H. Applications of Mesenchymal Stem Cells in Skin Regeneration and Rejuvenation. Int. J. Mol. Sci. 2021, 22, 2410. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhang, B.; Yang, Y.; Jiang, Q.; Li, T.; Gong, J.; Tang, H.; Zhang, Q. Stem cell-derived exosomes: Emerging therapeutic opportunities for wound healing. Stem Cell Res. Ther. 2023, 14, 107. [Google Scholar] [CrossRef] [PubMed]
- Eberlin, S.; Silva, M.S.D.; Facchini, G.; Silva, G.H.D.; Pinheiro, A.; Eberlin, S.; Pinheiro, A.D.S. The Ex Vivo Skin Model as an Alternative Tool for the Efficacy and Safety Evaluation of Topical Products. Altern. Lab. Anim. 2020, 48, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Bataillon, M.; Lelievre, D.; Chapuis, A.; Thillou, F.; Autourde, J.B.; Durand, S.; Boyera, N.; Rigaudeau, A.S.; Besne, I.; Pellevoisin, C. Characterization of a New Reconstructed Full Thickness Skin Model, T-Skin, and its Application for Investigations of Anti-Aging Compounds. Int. J. Mol. Sci. 2019, 20, 2240. [Google Scholar] [CrossRef]
- Shin, S.H.; Lee, Y.H.; Rho, N.K.; Park, K.Y. Skin aging from mechanisms to interventions: Focusing on dermal aging. Front. Physiol. 2023, 14, 1195272. [Google Scholar] [CrossRef]
- Gref, R.; Delomenie, C.; Maksimenko, A.; Gouadon, E.; Percoco, G.; Lati, E.; Desmaele, D.; Zouhiri, F.; Couvreur, P. Vitamin C-squalene bioconjugate promotes epidermal thickening and collagen production in human skin. Sci. Rep. 2020, 10, 16883. [Google Scholar] [CrossRef] [PubMed]
- Skibska, A.; Perlikowska, R. Signal Peptides—Promising Ingredients in Cosmetics. Curr. Protein Pept. Sci. 2021, 22, 716–728. [Google Scholar] [CrossRef]
- Bukhari, S.N.A.; Roswandi, N.L.; Waqas, M.; Habib, H.; Hussain, F.; Khan, S.; Sohail, M.; Ramli, N.A.; Thu, H.E.; Hussain, Z. Hyaluronic acid, a promising skin rejuvenating biomedicine: A review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects. Int. J. Biol. Macromol. 2018, 120, 1682–1695. [Google Scholar] [CrossRef]
- Spataro, E.A.; Dierks, K.; Carniol, P.J. Microneedling-Associated Procedures to Enhance Facial Rejuvenation. Facial Plast. Surg. Clin. N. Am. 2022, 30, 389–397. [Google Scholar] [CrossRef]
- Kim, Y.J.; Yoo, S.M.; Park, H.H.; Lim, H.J.; Kim, Y.L.; Lee, S.; Seo, K.W.; Kang, K.S. Exosomes derived from human umbilical cord blood mesenchymal stem cells stimulates rejuvenation of human skin. Biochem. Biophys. Res. Commun. 2017, 493, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Sanchez, A.; Kim, K.H.; Blasco-Morente, G.; Arias-Santiago, S. Cellular human tissue-engineered skin substitutes investigated for deep and difficult to heal injuries. NPJ Regen. Med. 2021, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.; Lee, M.G. Oxidative stress and antioxidant strategies in dermatology. Redox Rep. 2016, 21, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Trouba, K.J.; Hamadeh, H.K.; Amin, R.P.; Germolec, D.R. Oxidative stress and its role in skin disease. Antioxid. Redox Signal. 2002, 4, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Popa, G.L.; Mitran, C.I.; Mitran, M.I.; Tampa, M.; Matei, C.; Popa, M.I.; Georgescu, S.R. Markers of Oxidative Stress in Patients with Acne: A Literature Review. Life 2023, 13, 1433. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.A. Rosacea, reactive oxygen species, and azelaic Acid. J. Clin. Aesthet. Dermatol. 2009, 2, 26–30. [Google Scholar] [PubMed]
- Rahimi, H.; Mirnezami, M.; Yazdabadi, A.; Hajihashemi, A. Evaluation of systemic oxidative stress in patients with melasma. J. Cosmet. Dermatol. 2024, 23, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Prie, B.E.; Voiculescu, V.M.; Ionescu-Bozdog, O.B.; Petrutescu, B.; Iosif, L.; Gaman, L.E.; Clatici, V.G.; Stoian, I.; Giurcaneanu, C. Oxidative stress and alopecia areata. J. Med. Life 2015, 8, 43–46. [Google Scholar]
- Zapatero-Solana, E.; Garcia-Gimenez, J.L.; Guerrero-Aspizua, S.; Garcia, M.; Toll, A.; Baselga, E.; Duran-Moreno, M.; Markovic, J.; Garcia-Verdugo, J.M.; Conti, C.J.; et al. Oxidative stress and mitochondrial dysfunction in Kindler syndrome. Orphanet J. Rare Dis. 2014, 9, 211. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.H.; Chen, S.; Corpe, C.; Dutta, A.; Dutta, S.K.; et al. Vitamin C as an antioxidant: Evaluation of its role in disease prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef]
- Niki, E. Interaction of ascorbate and alpha-tocopherol. Ann. N. Y. Acad. Sci. 1987, 498, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Farris, P.K. Topical vitamin C: A useful agent for treating photoaging and other dermatologic conditions. Dermatol. Surg. 2005, 31, 814–817; discussion 818. [Google Scholar] [CrossRef] [PubMed]
- Turcov, D.; Zbranca-Toporas, A.; Suteu, D. Bioactive Compounds for Combating Oxidative Stress in Dermatology. Int. J. Mol. Sci. 2023, 24, 17517. [Google Scholar] [CrossRef] [PubMed]
- Shaban, S.; El-Husseny, M.W.A.; Abushouk, A.I.; Salem, A.M.A.; Mamdouh, M.; Abdel-Daim, M.M. Effects of Antioxidant Supplements on the Survival and Differentiation of Stem Cells. Oxidative Med. Cell. Longev. 2017, 2017, 5032102. [Google Scholar] [CrossRef]
- Luo, L.; Kawakatsu, M.; Guo, C.W.; Urata, Y.; Huang, W.J.; Ali, H.; Doi, H.; Kitajima, Y.; Tanaka, T.; Goto, S.; et al. Effects of antioxidants on the quality and genomic stability of induced pluripotent stem cells. Sci. Rep. 2014, 4, 3779. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.M.; Seo, Y.K.; Yoon, H.H.; Song, K.Y.; Kwon, S.Y.; Lee, H.S.; Park, J.K. Effect of ascorbic acid on bone marrow-derived mesenchymal stem cell proliferation and differentiation. J. Biosci. Bioeng. 2008, 105, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Loo, A.E.; Wong, Y.T.; Ho, R.; Wasser, M.; Du, T.; Ng, W.T.; Halliwell, B. Effects of hydrogen peroxide on wound healing in mice in relation to oxidative damage. PLoS ONE 2012, 7, e49215. [Google Scholar] [CrossRef]
- Lyublinskaya, O.G.; Borisov, Y.G.; Pugovkina, N.A.; Smirnova, I.S.; Obidina, J.V.; Ivanova, J.S.; Zenin, V.V.; Shatrova, A.N.; Borodkina, A.V.; Aksenov, N.D.; et al. Reactive Oxygen Species Are Required for Human Mesenchymal Stem Cells to Initiate Proliferation after the Quiescence Exit. Oxidative Med. Cell. Longev. 2015, 2015, 502105. [Google Scholar] [CrossRef]
- De Deken, X.; Corvilain, B.; Dumont, J.E.; Miot, F. Roles of DUOX-mediated hydrogen peroxide in metabolism, host defense, and signaling. Antioxid. Redox Signal. 2014, 20, 2776–2793. [Google Scholar] [CrossRef]
- Guo, L.; Du, J.; Yuan, D.F.; Zhang, Y.; Zhang, S.; Zhang, H.C.; Mi, J.W.; Ning, Y.L.; Chen, M.J.; Wen, D.L.; et al. Optimal H2O2 preconditioning to improve bone marrow mesenchymal stem cells’ engraftment in wound healing. Stem Cell Res. Ther. 2020, 11, 434. [Google Scholar] [CrossRef]
- Buron, M.; Palomares, T.; Garrido-Pascual, P.; Herrero de la Parte, B.; Garcia-Alonso, I.; Alonso-Varona, A. Conditioned Medium from H2O2—Preconditioned Human Adipose-Derived Stem Cells Ameliorates UVB-Induced Damage to Human Dermal Fibroblasts. Antioxidants 2022, 11, 2011. [Google Scholar] [CrossRef]
- Lee, Y.; Son, J.Y.; Kang, J.I.; Park, K.M.; Park, K.D. Hydrogen Peroxide-Releasing Hydrogels for Enhanced Endothelial Cell Activities and Neovascularization. ACS Appl. Mater. Interfaces 2018, 10, 18372–18379. [Google Scholar] [CrossRef]
- Pleguezuelos-Beltran, P.; Galvez-Martin, P.; Nieto-Garcia, D.; Marchal, J.A.; Lopez-Ruiz, E. Advances in spray products for skin regeneration. Bioact. Mater. 2022, 16, 187–203. [Google Scholar] [CrossRef]
- Martinez-Santamaria, L.; Guerrero-Aspizua, S.; Del Rio, M. Skin bioengineering: Preclinical and clinical applications. Actas Dermosifiliogr. 2012, 103, 5–11. [Google Scholar] [CrossRef][Green Version]
- Chocarro-Wrona, C.; Lopez-Ruiz, E.; Peran, M.; Galvez-Martin, P.; Marchal, J.A. Therapeutic strategies for skin regeneration based on biomedical substitutes. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 484–496. [Google Scholar] [CrossRef]
- Disphanurat, W.; Sivapornpan, N.; Srisantithum, B.; Leelawattanachai, J. Efficacy of a triamcinolone acetonide-loaded dissolving microneedle patch for the treatment of hypertrophic scars and keloids: A randomized, double-blinded, placebo-controlled split-scar study. Arch. Dermatol. Res. 2023, 315, 989–997. [Google Scholar] [CrossRef]
- Waghmare, K.B.; Sequeira, J.; Rao, B.H.S. An objective assessment of microneedling therapy in atrophic facial acne scars. Natl. J. Maxillofac. Surg. 2022, 13, S103–S107. [Google Scholar] [CrossRef]
- Long, T.; Gupta, A.; Ma, S.; Hsu, S. Platelet-rich plasma in noninvasive procedures for atrophic acne scars: A systematic review and meta-analysis. J. Cosmet. Dermatol. 2020, 19, 836–844. [Google Scholar] [CrossRef]
- Mony, M.P.; Harmon, K.A.; Hess, R.; Dorafshar, A.H.; Shafikhani, S.H. An Updated Review of Hypertrophic Scarring. Cells 2023, 12, 678. [Google Scholar] [CrossRef]
- Tartaglia, G.; Cao, Q.; Padron, Z.M.; South, A.P. Impaired Wound Healing, Fibrosis, and Cancer: The Paradigm of Recessive Dystrophic Epidermolysis Bullosa. Int. J. Mol. Sci. 2021, 22, 5104. [Google Scholar] [CrossRef]
- Cao, Z.; Jin, S.; Wang, P.; He, Q.; Yang, Y.; Gao, Z.; Wang, X. Microneedle based adipose derived stem cells-derived extracellular vesicles therapy ameliorates UV-induced photoaging in SKH-1 mice. J. Biomed. Mater. Res. A 2021, 109, 1849–1857. [Google Scholar] [CrossRef]
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin Wound Healing: An Update on the Current Knowledge and Concepts. Eur. Surg. Res. 2017, 58, 81–94. [Google Scholar] [CrossRef]
- Lin, X.; Zhu, L.; He, J. Morphogenesis, Growth Cycle and Molecular Regulation of Hair Follicles. Front. Cell Dev. Biol. 2022, 10, 899095. [Google Scholar] [CrossRef]
- Vandishi, A.K.; Esmaeili, A.; Taghipour, N. The promising prospect of human hair follicle regeneration in the shadow of new tissue engineering strategies. Tissue Cell 2024, 87, 102338. [Google Scholar] [CrossRef]
- Llamas-Molina, J.M.; Carrero-Castano, A.; Ruiz-Villaverde, R.; Campos, A. Tissue Engineering and Regeneration of the Human Hair Follicle in Androgenetic Alopecia: Literature Review. Life 2022, 12, 117. [Google Scholar] [CrossRef]
- Castro, A.R.; Logarinho, E. Tissue engineering strategies for human hair follicle regeneration: How far from a hairy goal? Stem Cells Transl. Med. 2020, 9, 342–350. [Google Scholar] [CrossRef]
- Xu, K.; Yu, E.; Wu, M.; Wei, P.; Yin, J. Cells, growth factors and biomaterials used in tissue engineering for hair follicles regeneration. Regen. Ther. 2022, 21, 596–610. [Google Scholar] [CrossRef]
- Kageyama, T.; Yan, L.; Shimizu, A.; Maruo, S.; Fukuda, J. Preparation of hair beads and hair follicle germs for regenerative medicine. Biomaterials 2019, 212, 55–63. [Google Scholar] [CrossRef]
- Wang, J.; Miao, Y.; Huang, Y.; Lin, B.; Liu, X.; Xiao, S.; Du, L.; Hu, Z.; Xing, M. Bottom-up Nanoencapsulation from Single Cells to Tunable and Scalable Cellular Spheroids for Hair Follicle Regeneration. Adv. Healthc. Mater. 2018, 7, 1700447. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, P.; Huang, J.; Yang, L.; Liu, Z.; Fu, D.; Hu, Z.; Huang, W.; Miao, Y. Scalable and high-throughput production of an injectable platelet-rich plasma (PRP)/cell-laden microcarrier/hydrogel composite system for hair follicle tissue engineering. J. Nanobiotechnol. 2022, 20, 465. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, T.; Nanmo, A.; Yan, L.; Nittami, T.; Fukuda, J. Effects of platelet-rich plasma on in vitro hair follicle germ preparation for hair regenerative medicine. J. Biosci. Bioeng. 2020, 130, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Bai, X.; Yuan, Z.; Cao, X.; Jiao, X.; Qin, Y.; Wen, Y.; Zhang, X. Cellular Nanofiber Structure with Secretory Activity-Promoting Characteristics for Multicellular Spheroid Formation and Hair Follicle Regeneration. ACS Appl. Mater. Interfaces 2020, 12, 7931–7941. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Martos, S.; Calvo-Sanchez, M.; Garcia-Alonso, K.; Castro, B.; Hashtroody, B.; Espada, J. Sustained Human Hair Follicle Growth Ex Vivo in a Glycosaminoglycan Hydrogel Matrix. Int. J. Mol. Sci. 2019, 20, 1741. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Chen, L.; Zhang, M.; Zhang, C.; Li, H. Self-assembled complete hair follicle organoids by coculture of neonatal mouse epidermal cells and dermal cells in Matrigel. Ann. Transl. Med. 2022, 10, 767. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xiao, S.; Liu, B.; Miao, Y.; Hu, Z. Use of extracellular matrix hydrogel from human placenta to restore hair-inductive potential of dermal papilla cells. Regen. Med. 2019, 14, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Barat, T.; Abdollahimajd, F.; Dadkhahfar, S.; Moravvej, H. Evaluation of the efficacy and safety of cow placenta extract lotion versus minoxidil 2% in the treatment of female pattern androgenetic alopecia. Int. J. Women’s Dermatol. 2020, 6, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Motter Catarino, C.; Cigaran Schuck, D.; Dechiario, L.; Karande, P. Incorporation of hair follicles in 3D bioprinted models of human skin. Sci. Adv. 2023, 9, eadg0297. [Google Scholar] [CrossRef]
- Kang, D.; Liu, Z.; Qian, C.; Huang, J.; Zhou, Y.; Mao, X.; Qu, Q.; Liu, B.; Wang, J.; Hu, Z.; et al. 3D bioprinting of a gelatin-alginate hydrogel for tissue-engineered hair follicle regeneration. Acta Biomater. 2023, 165, 19–30. [Google Scholar] [CrossRef]
- Kang, M.S.; Kwon, M.; Lee, S.H.; Kim, W.H.; Lee, G.W.; Jo, H.J.; Kim, B.; Yang, S.Y.; Kim, K.S.; Han, D.W. 3D Printing of Skin Equivalents with Hair Follicle Structures and Epidermal-Papillary-Dermal Layers Using Gelatin/Hyaluronic Acid Hydrogels. Chem. Asian J. 2022, 17, e202200620. [Google Scholar] [CrossRef]
- Zheng, S.Y.; Hu, X.M.; Huang, K.; Li, Z.H.; Chen, Q.N.; Yang, R.H.; Xiong, K. Proteomics as a tool to improve novel insights into skin diseases: What we know and where we should be going. Front. Surg. 2022, 9, 1025557. [Google Scholar] [CrossRef] [PubMed]
- Benoit, I.; Burty-Valin, E.; Radman, M. A Proteome-Centric View of Ageing, including that of the Skin and Age-Related Diseases: Considerations of a Common Cause and Common Preventative and Curative Interventions. Clin. Cosmet. Investig. Dermatol. 2023, 16, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Pien, N.; Bray, F.; Gheysens, T.; Tytgat, L.; Rolando, C.; Mantovani, D.; Dubruel, P.; Vlierberghe, S.V. Proteomics as a tool to gain next level insights into photo-crosslinkable biopolymer modifications. Bioact. Mater. 2022, 17, 204–220. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.X.; Chang, T.; Lin, X. Secretomes as an emerging class of bioactive ingredients for enhanced cosmeceutical applications. Exp. Dermatol. 2022, 31, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Masutin, V.; Kersch, C.; Schmitz-Spanke, S. A systematic review: Metabolomics-based identification of altered metabolites and pathways in the skin caused by internal and external factors. Exp. Dermatol. 2022, 31, 700–714. [Google Scholar] [CrossRef]
- Knox, S.; O’Boyle, N.M. Skin lipids in health and disease: A review. Chem. Phys. Lipids 2021, 236, 105055. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhu, Z.; Du, Q.; Wang, Z.; Wu, W.; Xue, Y.; Wang, Y.; Wu, Y.; Zeng, Q.; Jiang, C.; et al. A Skin Lipidomics Study Reveals the Therapeutic Effects of Tanshinones in a Rat Model of Acne. Front. Pharmacol. 2021, 12, 675659. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhao, Q.; Zhong, Q.; Duan, C.; Krutmann, J.; Wang, J.; Xia, J. Skin Microbiome, Metabolome and Skin Phenome, from the Perspectives of Skin as an Ecosystem. Phenomics 2022, 2, 363–382. [Google Scholar] [CrossRef] [PubMed]
- Gueniche, A.; Perin, O.; Bouslimani, A.; Landemaine, L.; Misra, N.; Cupferman, S.; Aguilar, L.; Clavaud, C.; Chopra, T.; Khodr, A. Advances in Microbiome-Derived Solutions and Methodologies Are Founding a New Era in Skin Health and Care. Pathogens 2022, 11, 121. [Google Scholar] [CrossRef]
- Tong, T.; Geng, R.; Kang, S.G.; Li, X.; Huang, K. Revitalizing Photoaging Skin through Eugenol in UVB-Exposed Hairless Mice: Mechanistic Insights from Integrated Multi-Omics. Antioxidants 2024, 13, 168. [Google Scholar] [CrossRef]
- Bonafont, J.; Mencia, A.; Garcia, M.; Torres, R.; Rodriguez, S.; Carretero, M.; Chacon-Solano, E.; Modamio-Hoybjor, S.; Marinas, L.; Leon, C.; et al. Clinically Relevant Correction of Recessive Dystrophic Epidermolysis Bullosa by Dual sgRNA CRISPR/Cas9-Mediated Gene Editing. Mol. Ther. 2019, 27, 986–998. [Google Scholar] [CrossRef]
- Carretero, M.; Guerrero-Aspizua, S.; Del Rio, M. Bioengineered skin humanized model of psoriasis. Methods Mol. Biol. 2013, 961, 305–323. [Google Scholar] [CrossRef]
- Guerrero-Aspizua, S.; Carretero, M.; Conti, C.J.; Del Rio, M. The importance of immunity in the development of reliable animal models for psoriasis and atopic dermatitis. Immunol. Cell Biol. 2020, 98, 626–638. [Google Scholar] [CrossRef]
- Fernandez-Carro, E.; Angenent, M.; Gracia-Cazana, T.; Gilaberte, Y.; Alcaine, C.; Ciriza, J. Modeling an Optimal 3D Skin-on-Chip within Microfluidic Devices for Pharmacological Studies. Pharmaceutics 2022, 14, 1417. [Google Scholar] [CrossRef]
- Rodrigues, R.O.; Sousa, P.C.; Gaspar, J.; Banobre-Lopez, M.; Lima, R.; Minas, G. Organ-on-a-Chip: A Preclinical Microfluidic Platform for the Progress of Nanomedicine. Small 2020, 16, e2003517. [Google Scholar] [CrossRef]
- Ponmozhi, J.; Dhinakaran, S.; Varga-Medveczky, Z.; Fonagy, K.; Bors, L.A.; Ivan, K.; Erdo, F. Development of Skin-On-A-Chip Platforms for Different Utilizations: Factors to Be Considered. Micromachines 2021, 12, 294. [Google Scholar] [CrossRef]
- Mohamadali, M.; Ghiaseddin, A.; Irani, S.; Amirkhani, M.A.; Dahmardehei, M. Design and evaluation of a skin-on-a-chip pumpless microfluidic device. Sci. Rep. 2023, 13, 8861. [Google Scholar] [CrossRef]
- Wufuer, M.; Lee, G.; Hur, W.; Jeon, B.; Kim, B.J.; Choi, T.H.; Lee, S. Skin-on-a-chip model simulating inflammation, edema and drug-based treatment. Sci. Rep. 2016, 6, 37471. [Google Scholar] [CrossRef]
- Randall, M.J.; Jungel, A.; Rimann, M.; Wuertz-Kozak, K. Advances in the Biofabrication of 3D Skin in vitro: Healthy and Pathological Models. Front. Bioeng. Biotechnol. 2018, 6, 154. [Google Scholar] [CrossRef]
- Bellas, E.; Seiberg, M.; Garlick, J.; Kaplan, D.L. In vitro 3D full-thickness skin-equivalent tissue model using silk and collagen biomaterials. Macromol. Biosci. 2012, 12, 1627–1636. [Google Scholar] [CrossRef]
- Parenteau-Bareil, R.; Gauvin, R.; Cliche, S.; Gariepy, C.; Germain, L.; Berthod, F. Comparative study of bovine, porcine and avian collagens for the production of a tissue engineered dermis. Acta Biomater. 2011, 7, 3757–3765. [Google Scholar] [CrossRef]
- Rosa, E.; Diaferia, C.; Gianolio, E.; Sibillano, T.; Gallo, E.; Smaldone, G.; Stornaiuolo, M.; Giannini, C.; Morelli, G.; Accardo, A. Multicomponent Hydrogel Matrices of Fmoc-FF and Cationic Peptides for Application in Tissue Engineering. Macromol. Biosci. 2022, 22, e2200128. [Google Scholar] [CrossRef]
- Arab, W.T.; Susapto, H.H.; Alhattab, D.; Hauser, C.A.E. Peptide nanogels as a scaffold for fabricating dermal grafts and 3D vascularized skin models. J. Tissue Eng. 2022, 13, 20417314221111868. [Google Scholar] [CrossRef]
- Bal-Ozturk, A.; Miccoli, B.; Avci-Adali, M.; Mogtader, F.; Sharifi, F.; Cecen, B.; Yasayan, G.; Braeken, D.; Alarcin, E. Current Strategies and Future Perspectives of Skin-on-a-Chip Platforms: Innovations, Technical Challenges and Commercial Outlook. Curr. Pharm. Des. 2018, 24, 5437–5457. [Google Scholar] [CrossRef]
- Vurat, M.T.; Ergun, C.; Elcin, A.E.; Elcin, Y.M. 3D Bioprinting of Tissue Models with Customized Bioinks. Adv. Exp. Med. Biol. 2020, 1249, 67–84. [Google Scholar] [CrossRef]
- Jimenez, F.; Alam, M.; Vogel, J.E.; Avram, M. Hair transplantation: Basic overview. J. Am. Acad. Dermatol. 2021, 85, 803–814. [Google Scholar] [CrossRef]
- Katzer, T.; Leite Junior, A.; Beck, R.; da Silva, C. Physiopathology and current treatments of androgenetic alopecia: Going beyond androgens and anti-androgens. Dermatol. Ther. 2019, 32, e13059. [Google Scholar] [CrossRef]
- Gupta, A.K.; Polla Ravi, S.; Wang, T.; Talukder, M.; Starace, M.; Piraccini, B.M. Systematic review of mesotherapy: A novel avenue for the treatment of hair loss. J. Dermatol. Treat. 2023, 34, 2245084. [Google Scholar] [CrossRef]
- Tang, Z.; Hu, Y.; Wang, J.; Fan, Z.; Qu, Q.; Miao, Y. Current application of mesotherapy in pattern hair loss: A systematic review. J. Cosmet. Dermatol. 2022, 21, 4184–4193. [Google Scholar] [CrossRef]
- Bagherani, N.; Smoller, B.R.; Tavoosidana, G.; Ghanadan, A.; Wollina, U.; Lotti, T. An overview of the role of carboxytherapy in dermatology. J. Cosmet. Dermatol. 2023, 22, 2399–2407. [Google Scholar] [CrossRef]
- Kroumpouzos, G.; Arora, G.; Kassir, M.; Galadari, H.; Wollina, U.; Lotti, T.; Grabbe, S.; Goldust, M. Carboxytherapy in dermatology. Clin. Dermatol. 2022, 40, 305–309. [Google Scholar] [CrossRef]
- English, R.S., Jr.; Ruiz, S.; DoAmaral, P. Microneedling and Its Use in Hair Loss Disorders: A Systematic Review. Dermatol. Ther (Heidelb) 2022, 12, 41–60. [Google Scholar] [CrossRef]
- Ocampo-Garza, S.S.; Fabbrocini, G.; Ocampo-Candiani, J.; Cinelli, E.; Villani, A. Micro needling: A novel therapeutic approach for androgenetic alopecia, A Review of Literature. Dermatol. Ther. 2020, 33, e14267. [Google Scholar] [CrossRef]
- Paichitrojjana, A.; Paichitrojjana, A. Platelet Rich Plasma and Its Use in Hair Regrowth: A Review. Drug Des. Dev. Ther. 2022, 16, 635–645. [Google Scholar] [CrossRef]
- Yuan, A.R.; Bian, Q.; Gao, J.Q. Current advances in stem cell-based therapies for hair regeneration. Eur. J. Pharmacol. 2020, 881, 173197. [Google Scholar] [CrossRef]
- Rose, P.T. Advances in Hair Restoration. Dermatol. Clin. 2018, 36, 57–62. [Google Scholar] [CrossRef]
- Kwack, M.H.; Seo, C.H.; Gangadaran, P.; Ahn, B.C.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Exosomes derived from human dermal papilla cells promote hair growth in cultured human hair follicles and augment the hair-inductive capacity of cultured dermal papilla spheres. Exp. Dermatol. 2019, 28, 854–857. [Google Scholar] [CrossRef]
- Mahmoudian-Sani, M.R.; Jamshidi, M.; Asgharzade, S. Combined Growth Factor and Gene Therapy: An Approach for Hair Cell Regeneration and Hearing Recovery. ORL J. Otorhinolaryngol. Relat. Spec. 2018, 80, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Elder, A.; Cappelli, M.O.; Ring, C.; Saedi, N. Artificial intelligence in cosmetic dermatology: An update on current trends. Clin. Dermatol. 2024, 42, 216–220. [Google Scholar] [CrossRef]
- Waddell, S.J.; de Andres, M.C.; Tsimbouri, P.M.; Alakpa, E.V.; Cusack, M.; Dalby, M.J.; Oreffo, R.O. Biomimetic oyster shell-replicated topography alters the behaviour of human skeletal stem cells. J. Tissue Eng. 2018, 9, 2041731418794007. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).