Preliminary Experience with a Cleansing Mousse and a Non-Steroidal Emulsion for the Prevention and Treatment of Acute Radiation Dermatitis in Breast Cancer Patients Undergoing Adjuvant Radiotherapy
Abstract
1. Introduction
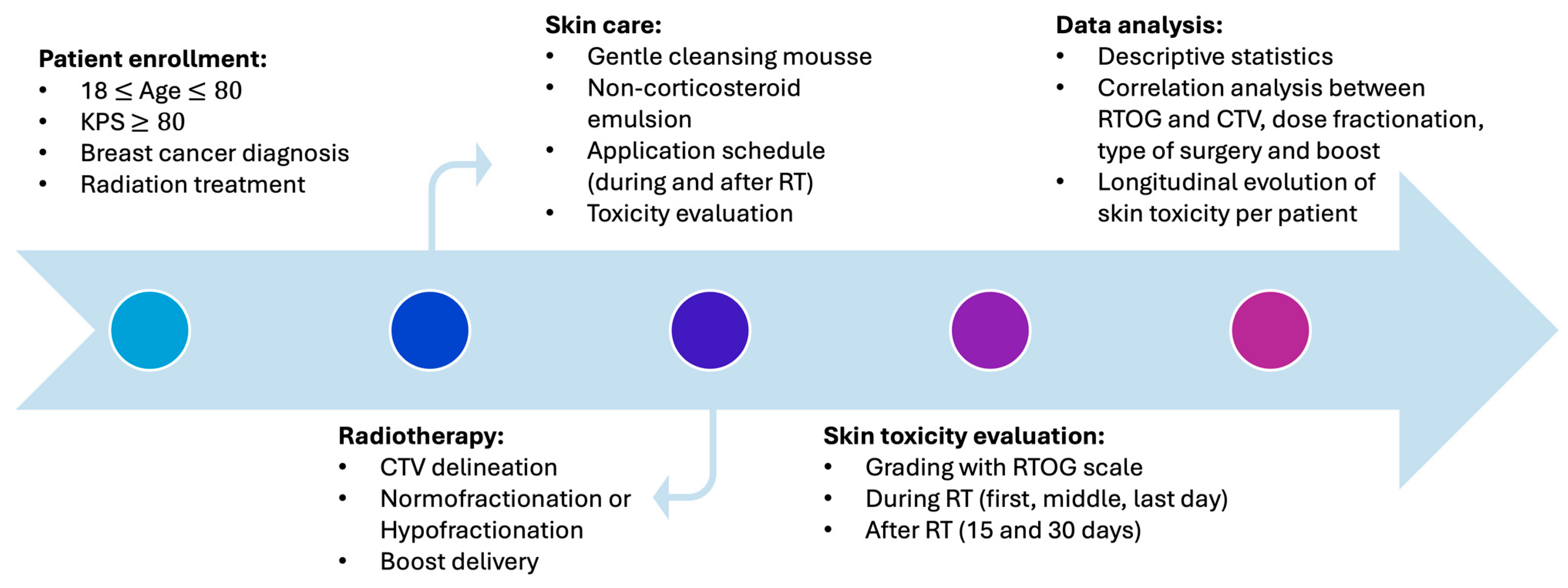
2. Materials and Methods
2.1. Radiotherapy
2.2. Skin Care
- -
- Vegetable glycerin (12%): a natural compound that retains moisture and spread along the stratum corneum of the epidermis, thereby exhibiting moisturizing properties [25];
- -
- Phytoextract of chamomile (0.5%): a medicinal plant with antioxidant and antibacterial activity [26];
- -
- Yarrow phytoextract (0.5%): a flowering plant with powerful anti-inflammatory activity [27].
- -
- Sweet almond (0.1%): an oil widely used for its moisturizing and anti-inflammatory properties [28];
- -
- Oenothera oil (0.1%): it contains polyphenols, aliphatic alcohols, and fatty acids, which exhibit anti-inflammatory activity [29].
- -
- Rice protein hydrolyzate (0.1%): known for its ability to hydrate and protect the skin. It works by increasing ceramide production, stimulating collagen production, and repairing cell damage [30].
- -
- Micronized zinc oxide (3.7%): known as a topical protector against UV rays [31];
- -
- Rapeseed phytosterols (1.7%): a plant with antioxidant and anti-inflammatory activity [32];
- -
- Aloe (0.5%): known as a topical antibiotic with healing abilities [33].
- -
- 18-beta glycyrrhetinic acid (0.5%): an anti-inflammatory, antibacterial, antioxidant herbal compound [34];
- -
- Alpha bisabolol (0.5%): a herbal formulation with antimicrobial, antifungal, and analgesic activity [35];
- -
- Zanthalene (0.5%): an anti-itching ingredient that works by inhibiting synaptic transmissions [36].
2.3. Aim of the Study and Toxicity Assessment
2.4. Statistical Analysis
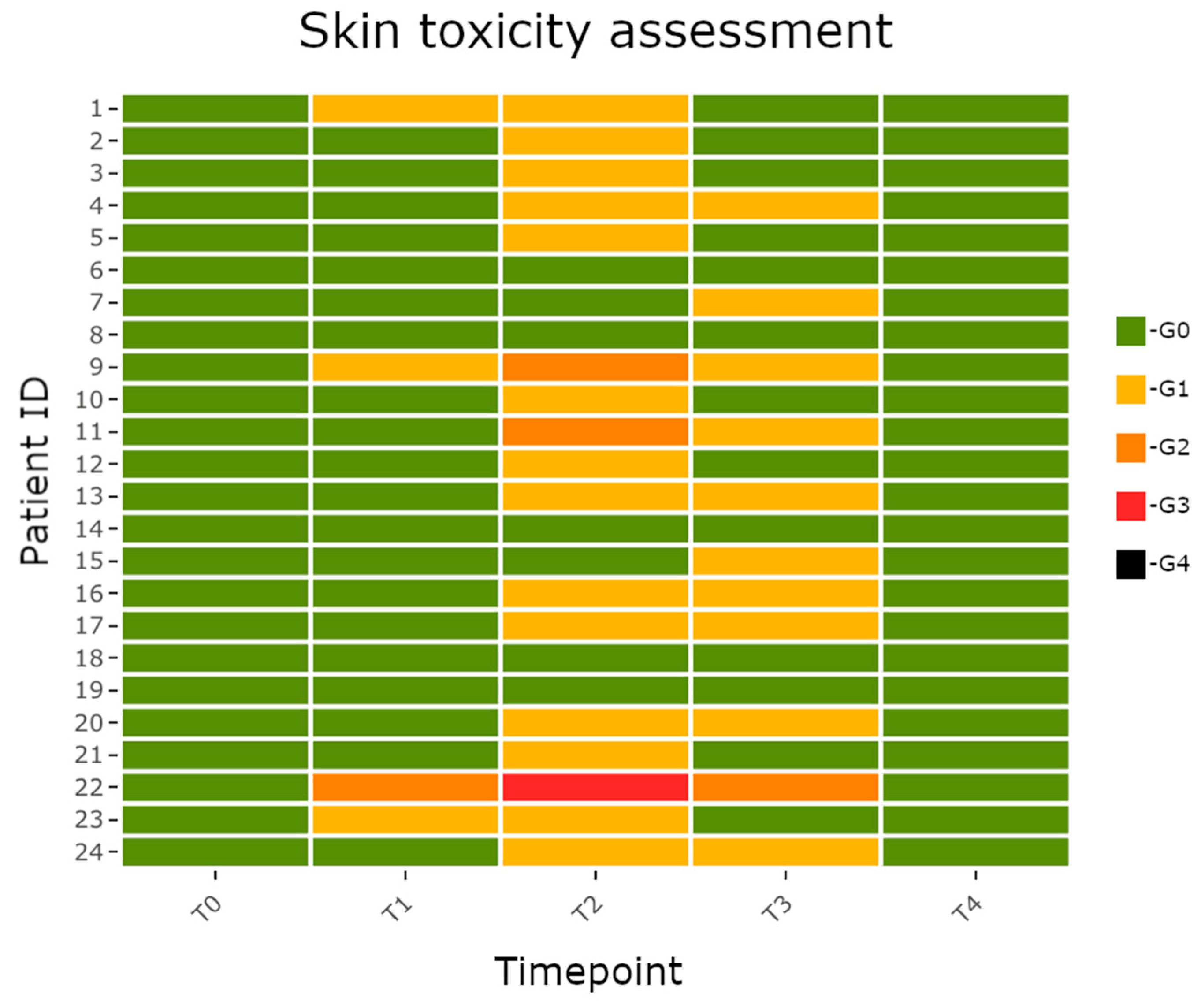
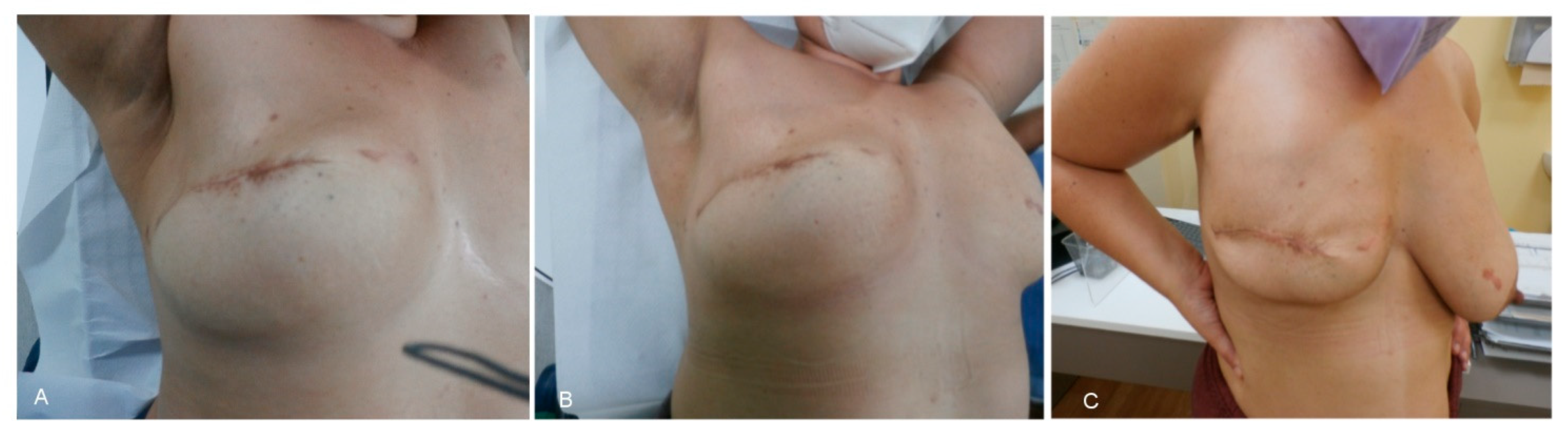
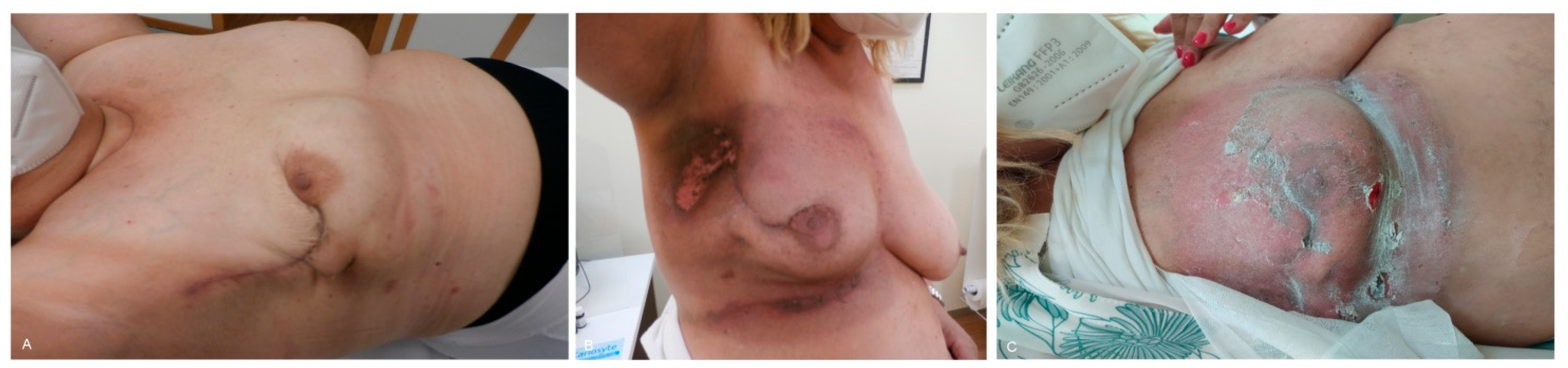
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loibl, S.; André, F.; Bachelot, T.; Barrios, C.H.; Bergh, J.; Burstein, H.J.; Cardoso, M.J.; Carey, L.A.; Dawood, S.; Del Mastro, L.; et al. Early breast cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2024, 35, 159–182. [Google Scholar] [CrossRef] [PubMed]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG); Darby, S.; McGale, P.; Correa, C.; Taylor, C.; Arriagada, R.; Clarke, M.; Cutter, D.; Davies, C.; Ewertz, M.; et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011, 378, 1707–1716. [Google Scholar] [CrossRef] [PubMed]
- EBCTCG (Early Breast Cancer Trialists’ Collaborative Group); McGale, P.; Taylor, C.; Correa, C.; Cutter, D.; Duane, F.; Ewertz, M.; Gray, R.; Mannu, G.; Peto, R.; et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: Meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 2014, 383, 2127–2135, Erratum in Lancet 2014, 384, 1848. [Google Scholar] [CrossRef] [PubMed]
- Darby, S.C.; Ewertz, M.; McGale, P.; Bennet, A.M.; Blom-Goldman, U.; Brønnum, D.; Correa, C.; Cutter, D.; Gagliardi, G.; Gigante, B.; et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N. Engl. J. Med. 2013, 368, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Ferini, G.; Valenti, V.; Viola, A.; Umana, G.E.; Martorana, E. A Critical Overview of Predictors of Heart Sparing by Deep-Inspiration-Breath-Hold Irradiation in Left-Sided Breast Cancer Patients. Cancers 2022, 14, 3477. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, C.; Liang, Z.; Ma, S.; Liu, X. Radiotherapy and Cytokine Storm: Risk and Mechanism. Front. Oncol. 2021, 11, 670464. [Google Scholar] [CrossRef]
- Wei, J.; Meng, L.; Hou, X.; Qu, C.; Wang, B.; Xin, Y.; Jiang, X. Radiation-induced skin reactions: Mechanism and treatment. Cancer Manag. Res. 2018, 11, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Vieira, L.A.C.; Menêses, A.G.; Bontempo, P.S.M.; Simino, G.P.R.; Ferreira, E.B.; Guerra, E.N.D.S.; Reis, P.E.D.D. Incidence of radiodermatitis in breast cancer patients during hypofractionated radiotherapy. Rev. Esc. Enferm. USP 2022, 56, e20220173. [Google Scholar] [CrossRef]
- Kry, S.F.; Smith, S.A.; Weathers, R.; Stovall, M. Skin dose during radiotherapy: A summary and general estimation technique. J. Appl. Clin. Med. Phys. 2012, 13, 20–34. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, M.; Alavi, A.; Wong, R.; Akita, S. Radiodermatitis: A Review of Our Current Understanding. Am. J. Clin. Dermatol. 2016, 17, 277–292. [Google Scholar] [CrossRef] [PubMed]
- Sindoni, A.; Severo, C.; Vadala’, R.E.; Ferini, G.; Mazzei, M.M.; Vaccaro, M.; IatÌ, G.; Pontoriero, A.; Pergolizzi, S. Levetiracetam-induced radiation recall dermatitis in a patient undergoing stereotactic radiotherapy. J. Dermatol. 2016, 43, 1440–1441. [Google Scholar] [CrossRef] [PubMed]
- Gagliano, A.; Prestifilippo, A.; Cantale, O.; Ferini, G.; Fisichella, G.; Fontana, P.; Sciacca, D.; Giuffrida, D. Role of the Combination of Cyclin-Dependent Kinase Inhibitors (CDKI) and Radiotherapy (RT) in the Treatment of Metastatic Breast Cancer (MBC): Advantages and Risks in Clinical Practice. Front. Oncol. 2021, 11, 643155. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferini, G.; Zagardo, V.; Strazzanti, A. Acute generalized exanthematous pustulosis induced by exemestane during adjuvant radiotherapy for breast cancer. Med. Clin. 2023, 161, 502–503, (In English, Spanish). [Google Scholar] [CrossRef] [PubMed]
- Schnur, J.B.; Ouellette, S.C.; Dilorenzo, T.A.; Green, S.; Montgomery, G.H. A qualitative analysis of acute skin toxicity among breast cancer radiotherapy patients. Psychooncology 2011, 20, 260–268. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martella, S.; Rietjens, M.; Lohsiriwat, V.; Lazzari, R.; Vavassori, A.; Jereczek, B.; Lazzati, V.; Leonardi, M.; Petit, J. Acute radiation dermatitis in breast cancer: Topical therapy with vitamin E acetate in lipophilic gel base. Ecancermedicalscience 2010, 4, 190. [Google Scholar] [CrossRef] [PubMed]
- Bese, N.S.; Hendry, J.; Jeremic, B. Effects of prolongation of overall treatment time due to unplanned interruptions during radiotherapy of different tumor sites and practical methods for compensation. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.S.; Wu, Y.C.; Wu, M.Y.; Wu, P.L.; Lu, L.Y.; Hung, C.H. Topical Prevention of Radiation Dermatitis in Breast Cancer Patients: A Network Meta-analysis of Randomized Controlled Trials. In Vivo 2023, 37, 1346–1357. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.F.; Shariati, S.; Caini, S.; Wong, H.; Chan, A.W.; Gojsevic, M.; Ogita, M.; Ye, J.C.; Chia, D.; Chao, M.; et al. StrataXRT for the prevention of acute radiation dermatitis in breast cancer: A systematic review and meta-analysis of randomized controlled trials. Support. Care Cancer 2023, 31, 515. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, S.; Kanee, L.; Behroozian, T.; Wolf, J.R.; van den Hurk, C.; Chow, E.; Bonomo, P. Comparison of clinical practice guidelines on radiation dermatitis: A narrative review. Support. Care Cancer 2022, 30, 4663–4674. [Google Scholar] [CrossRef] [PubMed]
- Robijns, J.; Becherini, C.; Caini, S.; Wolf, J.R.; van den Hurk, C.; Beveridge, M.; Lam, H.; Bonomo, P.; Chow, E.; Behroozian, T. Natural and miscellaneous agents for the prevention of acute radiation dermatitis: A systematic review and meta-analysis. Support. Care Cancer 2023, 31, 195. [Google Scholar] [CrossRef] [PubMed]
- Ferini, G.; Molino, L.; Tripoli, A.; Valenti, V.; Illari, S.I.; Marchese, V.A.; Cravagno, I.R.; Borzi, G.R. Anatomical Predictors of Dosimetric Advantages for Deep-inspiration-breath-hold 3D-conformal Radiotherapy among Women with Left Breast Cancer. Anticancer Res. 2021, 41, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Offersen, B.V.; Boersma, L.J.; Kirkove, C.; Hol, S.; Aznar, M.C.; Biete Sola, A.; Kirova, Y.M.; Pignol, J.P.; Remouchamps, V.; Verhoeven, K.; et al. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer. Radiother. Oncol. 2015, 114, 3–10. [Google Scholar] [CrossRef] [PubMed]
- International Comission on Radiation Unit and Measurment (ICRU). Prescribing, Recording, and Reporting Photon Beam Therapy; ICRU Report No.50; International Comission on Radiation Unit and Measurment (ICRU): Bethesda, MD, USA, 1993; pp. 1–72. [Google Scholar]
- Keenan, L.G.; Lavan, N.; Dunne, M.; McArdle, O. Modifiable risk factors for acute skin toxicity in adjuvant breast radiotherapy: Dosimetric analysis and review of the literature. Med. Dosim. 2019, 44, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Milani, M.; Sparavigna, A. The 24-h skin hydration and barrier function effects of a hyaluronic 1%, glycerin 5%, and Centella asiatica stem cells extract moisturizing fluid: An intra-subject, randomized, assessor-blinded study. Clin. Cosmet. Investig. Dermatol. 2017, 10, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Mailänder, L.K.; Lorenz, P.; Bitterling, H.; Stintzing, F.C.; Daniels, R.; Kammerer, D.R. Phytochemical Characterization of Chamomile (Matricaria recutita L.) Roots and Evaluation of Their Antioxidant and Antibacterial Potential. Molecules 2022, 27, 8508. [Google Scholar] [CrossRef]
- Tadić, V.; Arsić, I.; Zvezdanović, J.; Zugić, A.; Cvetković, D.; Pavkov, S. The estimation of the traditionally used yarrow (Achillea millefolium L. Asteraceae) oil extracts with anti-inflamatory potential in topical application. J. Ethnopharmacol. 2017, 199, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Zeichner, J.A.; Berson, D.; Donald, A. The Use of an Over-the-Counter Hand Cream with Sweet Almond Oil for the Treatment of Hand Dermatitis. J. Drugs Dermatol. 2018, 17, 78–82. [Google Scholar] [PubMed]
- Timoszuk, M.; Bielawska, K.; Skrzydlewska, E. Evening Primrose (Oenothera biennis) Biological Activity Dependent on Chemical Composition. Antioxidants 2018, 7, 108. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Dai, F.J.; Chen, C.Y.; Fan, S.L.; Zheng, J.H.; Huang, Y.C.; Chau, C.F.; Lin, Y.S.; Chen, C.S. Evaluating the Antioxidants, Whitening and Antiaging Properties of Rice Protein Hydrolysates. Molecules 2021, 26, 3605. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.M.; Song, Z.; Moghimi, H.R.; Roberts, M.S. Relative Penetration of Zinc Oxide and Zinc Ions into Human Skin after Application of Different Zinc Oxide Formulations. ACS Nano 2016, 10, 1810–1819. [Google Scholar] [CrossRef]
- Tileuberdi, N.; Turgumbayeva, A.; Yeskaliyeva, B.; Sarsenova, L.; Issayeva, R. Extraction, Isolation of Bioactive Compounds and Therapeutic Potential of Rapeseed (Brassica napus L.). Molecules 2022, 27, 8824. [Google Scholar] [CrossRef] [PubMed]
- Hekmatpou, D.; Mehrabi, F.; Rahzani, K.; Aminiyan, A. The Effect of Aloe Vera Clinical Trials on Prevention and Healing of Skin Wound: A Systematic Review. Iran. J. Med. Sci. 2019, 44, 1–9. [Google Scholar] [PubMed]
- Kowalska, A.; Kalinowska-Lis, U. 18β-Glycyrrhetinic acid: Its core biological properties and dermatological applications. Int. J. Cosmet. Sci. 2019, 41, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Eddin, L.B.; Jha, N.K.; Goyal, S.N.; Agrawal, Y.O.; Subramanya, S.B.; Bastaki, S.M.A.; Ojha, S. Health Benefits, Pharmacological Effects, Molecular Mechanisms, and Therapeutic Potential of α-Bisabolol. Nutrients 2022, 14, 1370. [Google Scholar] [CrossRef] [PubMed]
- Artaria, C.; Maramaldi, G.; Bonfigli, A.; Rigano, L.; Appendino, G. Lifting properties of the alkamide fraction from the fruit husks of Zanthoxylum bungeanum. Int. J. Cosmet. Sci. 2011, 33, 328–333. [Google Scholar] [CrossRef]
- Cox, J.D.; Stetz, J.; Pajak, T.F. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- R Studio Team. Integrated Development Environment for R; RStudio, PBC: Boston, MA, USA, 2021; Available online: http://www.rstudio.com/ (accessed on 29 May 2024).
- Galili, T.; O’Callaghan, A.; Sidi, J.; Sievert, C. heatmaply: An R package for creating interactive cluster heatmaps for online publishing. Bioinformatics 2018, 34, 1600–1602. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; Available online: https://doi.org/10.1007/978-3-319-24277-4./ (accessed on 9 August 2023).
- Behroozian, T.; Bonomo, P.; Patel, P.; Kanee, L.; Finkelstein, S.; van den Hurk, C.; Chow, E.; Wolf, J.R.; Multinational Association of Supportive Care in Cancer (MASCC) Oncodermatology Study Group Radiation Dermatitis Guidelines Working Group. Multinational Association of Supportive Care in Cancer (MASCC) clinical practice guidelines for the prevention and management of acute radiation dermatitis: International Delphi consensus-based recommendations. Lancet Oncol. 2023, 24, e172–e185. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.O.; Boin, M.F.; Crocco, E.I. Striae after topical corticosteroid: Treatment with nonablative fractional laser 1540 nm. J. Cosmet. Laser Ther. 2015, 17, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Ulff, E.; Maroti, M.; Serup, J.; Nilsson, M.; Falkmer, U. Late cutaneous effects of a local potent steroid during adjuvant radiotherapy for breast cancer. Clin. Transl. Radiat. Oncol. 2017, 7, 9–12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, C.J.; Fang, H.F.; Wang, C.Y.; Chou, K.R.; Huang, T.W. Effect of hyaluronic acid on radiodermatitis in patients with breast cancer: A meta-analysis of randomized controlled trials. Support. Care Cancer 2022, 30, 3965–3975. [Google Scholar] [CrossRef] [PubMed]
- Tai, R.Z.; Loh, E.W.; Tsai, J.T.; Tam, K.W. Effect of hyaluronic acid on radiotherapy-induced mucocutaneous side effects: A meta-analysis of randomized controlled trials. Support. Care Cancer 2022, 30, 4845–4855. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Lu, G.L.; Shen, Z.J. Comparison of intravesical hyaluronic acid instillation and hyperbaric oxygen in the treatment of radiation-induced hemorrhagic cystitis. BJU Int. 2012, 109, 691–694. [Google Scholar] [CrossRef] [PubMed]
- Ferini, G.; Tripoli, A.; Umina, V.; Borzì, G.R.; Marchese, V.A.; Illari, S.I.; Cacciola, A.; Lillo, S.; Parisi, S.; Valenti, V. Radiation Proctitis: The Potential Role of Hyaluronic Acid in the Prevention and Restoration of Any Damage to the Rectal Mucosa among Prostate Cancer Patients Submitted to Curative External Beam Radiotherapy. Gastroenterol. Insights 2021, 12, 446–455. [Google Scholar] [CrossRef]
- Brunault, P.; Suzanne, I.; Trzepidur-Edom, M.; Garaud, P.; Calais, G.; Toledano, A.; Camus, V. Depression is associated with some patient-perceived cosmetic changes, but not with radiotherapy-induced late toxicity, in long-term breast cancer survivors. Psychooncology 2013, 22, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Rose, P. Radiation-induced skin toxicity: Prophylaxis or management? J. Med. Radiat. Sci. 2020, 67, 168–169. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rzepecki, A.; Birnbaum, M.; Ohri, N.; Daily, J.; Fox, J.; Bodner, W.; Kabarriti, R.; Garg, M.; Mehta, K.; Kalnicki, S.; et al. Characterizing the effects of radiation dermatitis on quality of life: A prospective survey-based study. J. Am. Acad. Dermatol. 2022, 86, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.C.W.; Wong, H.C.Y.; Riad, M.A.; Caini, S.; Wolf, J.R.; van den Hurk, C.; Beveridge, M.; Lam, H.; Bonomo, P.; Chow, E.; et al. Prevention of radiation dermatitis with skin hygiene and washing: A systematic review and meta-analysis. Support. Care Cancer 2023, 31, 294, Erratum in Support. Care Cancer 2024, 32, 107. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wang, Q.; Hu, T.; Chen, R.; Wang, J.; Chang, H.; Cheng, J. Risk Factors Related to Acute Radiation Dermatitis in Breast Cancer Patients after Radiotherapy: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 738851. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rübe, C.E.; Freyter, B.M.; Tewary, G.; Roemer, K.; Hecht, M.; Rübe, C. Radiation Dermatitis: Radiation-Induced Effects on the Structural and Immunological Barrier Function of the Epidermis. Int. J. Mol. Sci. 2024, 25, 3320. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bruand, M.; Salleron, J.; Guihard, S.; Crety, C.M.; Liem, X.; Pasquier, D.; Lamrani-Ghaouti, A.; Charra-Brunaud, C.; Peiffert, D.; Clavier, J.B.; et al. Acute skin toxicity of conventional fractionated versus hypofractionated radiotherapy in breast cancer patients receiving regional node irradiation: The real-life prospective multicenter HYPOBREAST cohort. BMC Cancer 2022, 22, 1318. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Freedman, G.M.; Anderson, P.R.; Li, J.; Eisenberg, D.F.; Hanlon, A.L.; Wang, L.; Nicolaou, N. Intensity modulated radiation therapy (IMRT) decreases acute skin toxicity for women receiving radiation for breast cancer. Am. J. Clin. Oncol. 2006, 29, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Joseph, K.; Vos, L.J.; Gabos, Z.; Pervez, N.; Chafe, S.; Tankel, K.; Warkentin, H.; Ghosh, S.; Amanie, J.; Powell, K.; et al. Skin Toxicity in Early Breast Cancer Patients Treated with Field-In-Field Breast Intensity-Modulated Radiotherapy versus Helical Inverse Breast Intensity-Modulated Radiotherapy: Results of a Phase III Randomised Controlled Trial. Clin. Oncol. 2021, 33, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Ferini, G.; Tripoli, A.; Molino, L.; Cacciola, A.; Lillo, S.; Parisi, S.; Umina, V.; Illari, S.I.; Marchese, V.A.; Cravagno, I.R.; et al. How Much Daily Image-guided Volumetric Modulated Arc Therapy Is Useful for Proctitis Prevention with Respect to Static Intensity Modulated Radiotherapy Supported by Topical Medications among Localized Prostate Cancer Patients? Anticancer Res. 2021, 41, 2101–2110, Erratum in Anticancer Res. 2022, 42, 5165. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.C.; Tsai, J.T.; Chou, Y.C.; Li, M.H.; Liu, W.H. Compared with intensity-modulated radiotherapy, image-guided radiotherapy reduces severity of acute radiation-induced skin toxicity during radiotherapy in patients with breast cancer. Cancer Med. 2018, 7, 3622–3629. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, S.N.; Ramachandran, P.; Deb, P. Dosimetric comparative study of 3DCRT, IMRT, VMAT, Ecomp, and Hybrid techniques for breast radiation therapy. Radiat. Oncol. J. 2020, 38, 270–281. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aznar, M.C.; Duane, F.K.; Darby, S.C.; Wang, Z.; Taylor, C.W. Exposure of the lungs in breast cancer radiotherapy: A systematic review of lung doses published 2010–2015. Radiother. Oncol. 2018, 126, 148–154. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hall, E.J.; Wuu, C.S. Radiation-induced second cancers: The impact of 3D-CRT and IMRT. Int. J. Radiat. Oncol. Biol. Phys. 2003, 56, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.D.; Bellon, J.R.; Blitzblau, R.; Freedman, G.; Haffty, B.; Hahn, C.; Halberg, F.; Hoffman, K.; Horst, K.; Moran, J.; et al. Radiation therapy for the whole breast: Executive summary of an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Pract. Radiat. Oncol. 2018, 8, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Petillion, S.; Verhoeven, K.; Weltens, C.; Van den Heuvel, F. Accuracy of a new paired imaging technique for position correction in whole breast radiotherapy. J. Appl. Clin. Med. Phys. 2015, 16, 4796. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cláudia Paiva-Santos, A.; Gama, M.; Peixoto, D.; Sousa-Oliveira, I.; Ferreira-Faria, I.; Zeinali, M.; Abbaspour-Ravasjani, S.; Mascarenhas-Melo, F.; Hamishehkar, H.; Veiga, F. Nanocarrier-based dermopharmaceutical formulations for the topical management of atopic dermatitis. Int. J. Pharm. 2022, 618, 121656, Erratum in Int. J. Pharm. 2022, 627, 122146. [Google Scholar] [CrossRef] [PubMed]
| RTOG Score | Skin Changes |
|---|---|
| 0 | No change over baseline |
| 1 | Follicular, faint or dull erythema/epilation/dry desquamation/decreased sweating |
| 2 | Tender or bright erythema, patchy moist desquamation/moderate edema |
| 3 | Confluent, moist desquamation other than skin folds, pitting edema |
| 4 | Ulceration, hemorrhage, necrosis |
| Patients % (n) | |
|---|---|
| Age, median (range) years | 59 (42–75) |
| Sex | |
| Male | 0 |
| Female | 100 (24) |
| Surgery treatment before radiotherapy | |
| Breast-conserving surgery | 87.5 (21) |
| Mastectomy | 12.5 (3) |
| Involved side | |
| Right breast | 50 (12) |
| Left breast | 50 (12) |
| CTV_T size, median (range) cc | 541 (406.1–763.5) |
| Radiotherapy scheme | |
| Normofractionation | 75 (18) |
| Hypofractionation | 25 (6) |
| Boost delivery | 75 (18) |
| 10 Gy in 5 fractions | 83.3 (15) |
| 9 Gy in 3 frations | 16.7 (3) |
| Correlation Analysis | Toxicity T1 | Toxicity T2 | Toxicity T3 | |
|---|---|---|---|---|
| Kendall’s correlation T (p-value) | CTV_T (cc) | 0.26 (0.13) | 0.29 (0.07) | 0.36 (0.03) |
| Fisher’s test p-value | RT target | 0.15 | 0.17 | 0.06 |
| Dose fractionation | 0.37 | 0.43 | 0.19 | |
| Boost delivery | 0.22 | 0.18 | 0.38 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viola, A.; Martorana, E.; Zagardo, V.; Ferini, G. Preliminary Experience with a Cleansing Mousse and a Non-Steroidal Emulsion for the Prevention and Treatment of Acute Radiation Dermatitis in Breast Cancer Patients Undergoing Adjuvant Radiotherapy. Cosmetics 2024, 11, 117. https://doi.org/10.3390/cosmetics11040117
Viola A, Martorana E, Zagardo V, Ferini G. Preliminary Experience with a Cleansing Mousse and a Non-Steroidal Emulsion for the Prevention and Treatment of Acute Radiation Dermatitis in Breast Cancer Patients Undergoing Adjuvant Radiotherapy. Cosmetics. 2024; 11(4):117. https://doi.org/10.3390/cosmetics11040117
Chicago/Turabian StyleViola, Anna, Emanuele Martorana, Valentina Zagardo, and Gianluca Ferini. 2024. "Preliminary Experience with a Cleansing Mousse and a Non-Steroidal Emulsion for the Prevention and Treatment of Acute Radiation Dermatitis in Breast Cancer Patients Undergoing Adjuvant Radiotherapy" Cosmetics 11, no. 4: 117. https://doi.org/10.3390/cosmetics11040117
APA StyleViola, A., Martorana, E., Zagardo, V., & Ferini, G. (2024). Preliminary Experience with a Cleansing Mousse and a Non-Steroidal Emulsion for the Prevention and Treatment of Acute Radiation Dermatitis in Breast Cancer Patients Undergoing Adjuvant Radiotherapy. Cosmetics, 11(4), 117. https://doi.org/10.3390/cosmetics11040117








