Artichoke Leaf Extract Effectiveness on the Skin Aging Exposome: Efficacy and Safety Results of a Split-Face Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Design
2.2. Participants
2.3. Interventions and Randomization
2.4. Outcomes
2.4.1. Skin Profilometry
2.4.2. Skin Radiance
2.4.3. Biochemical Parameters
2.4.4. Skin Tolerability
2.4.5. Self-Assessment Questionnaire
2.5. Statistical Methods
3. Results
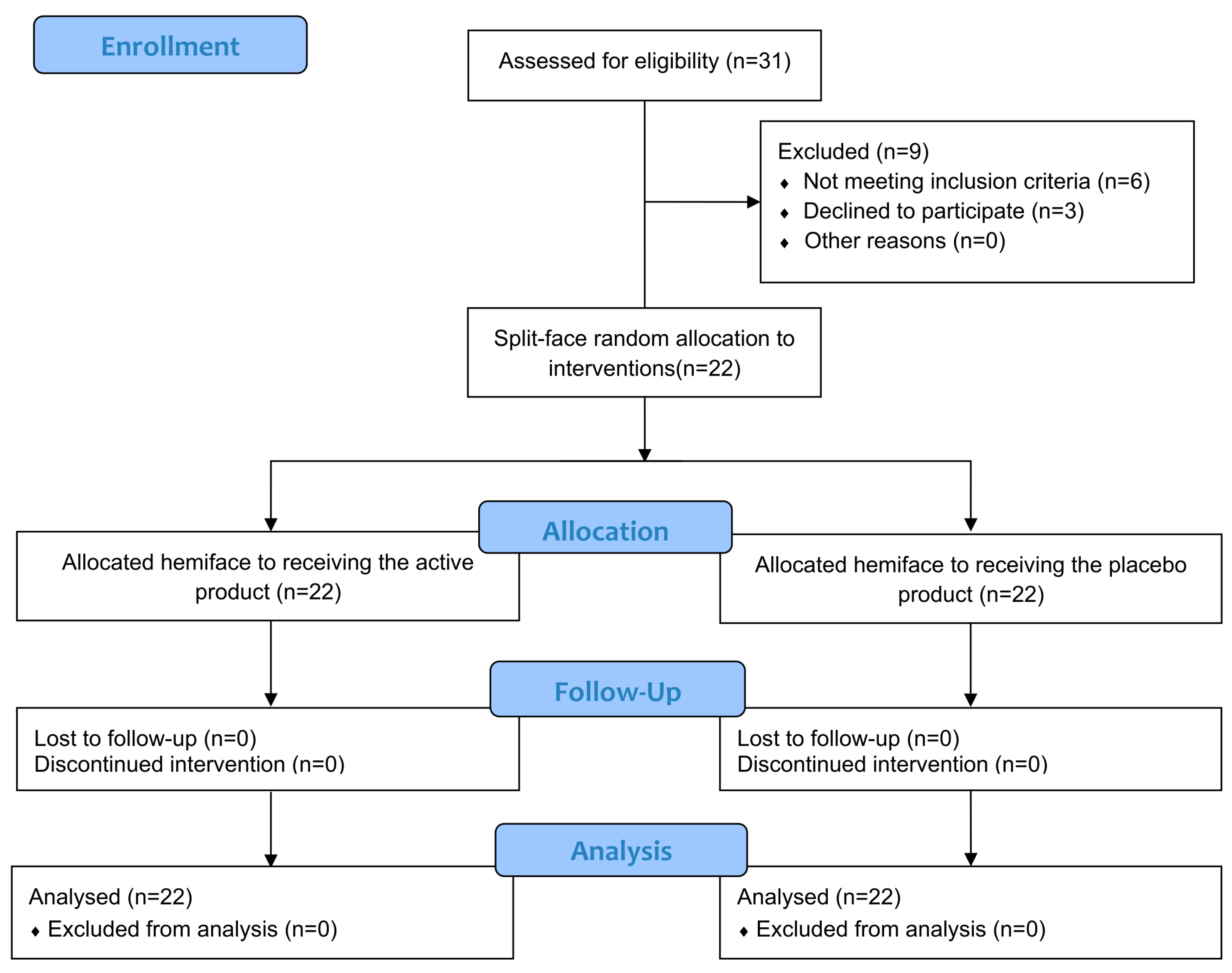
3.1. Study Population
3.2. Skin Tolerability
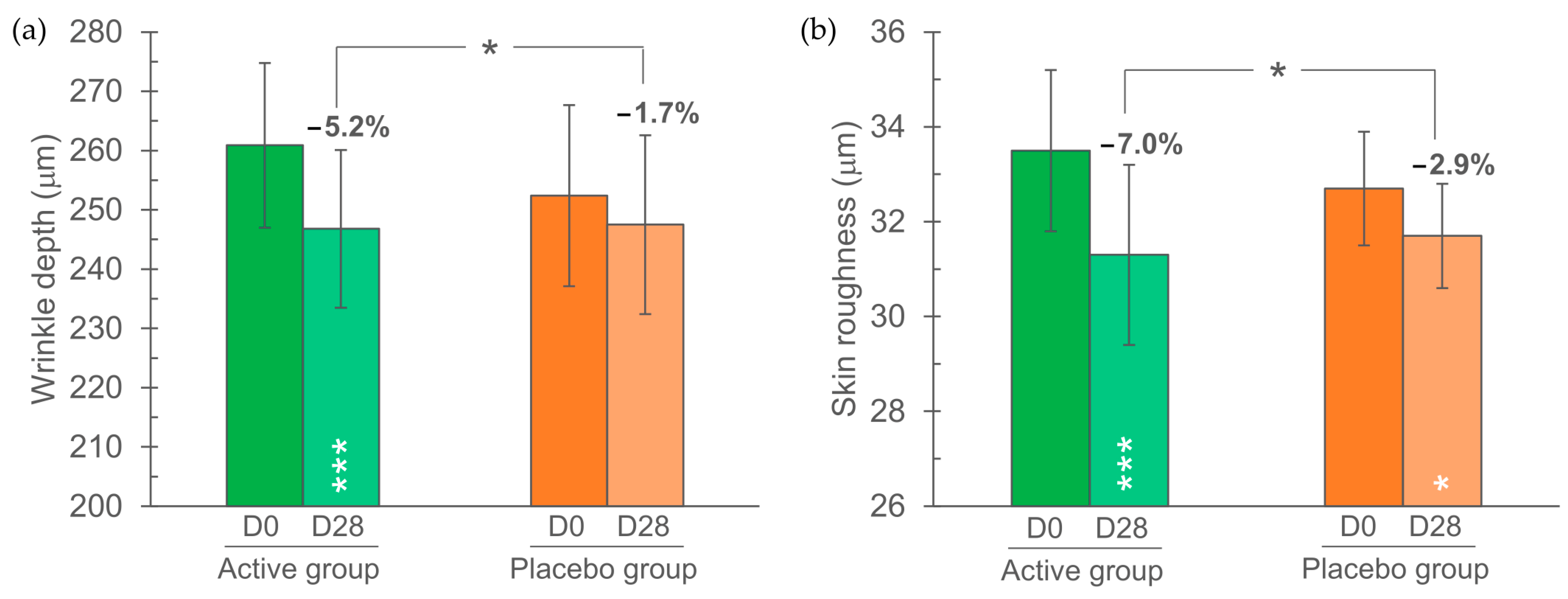
3.3. Skin Profilometry
3.4. Skin Radiance
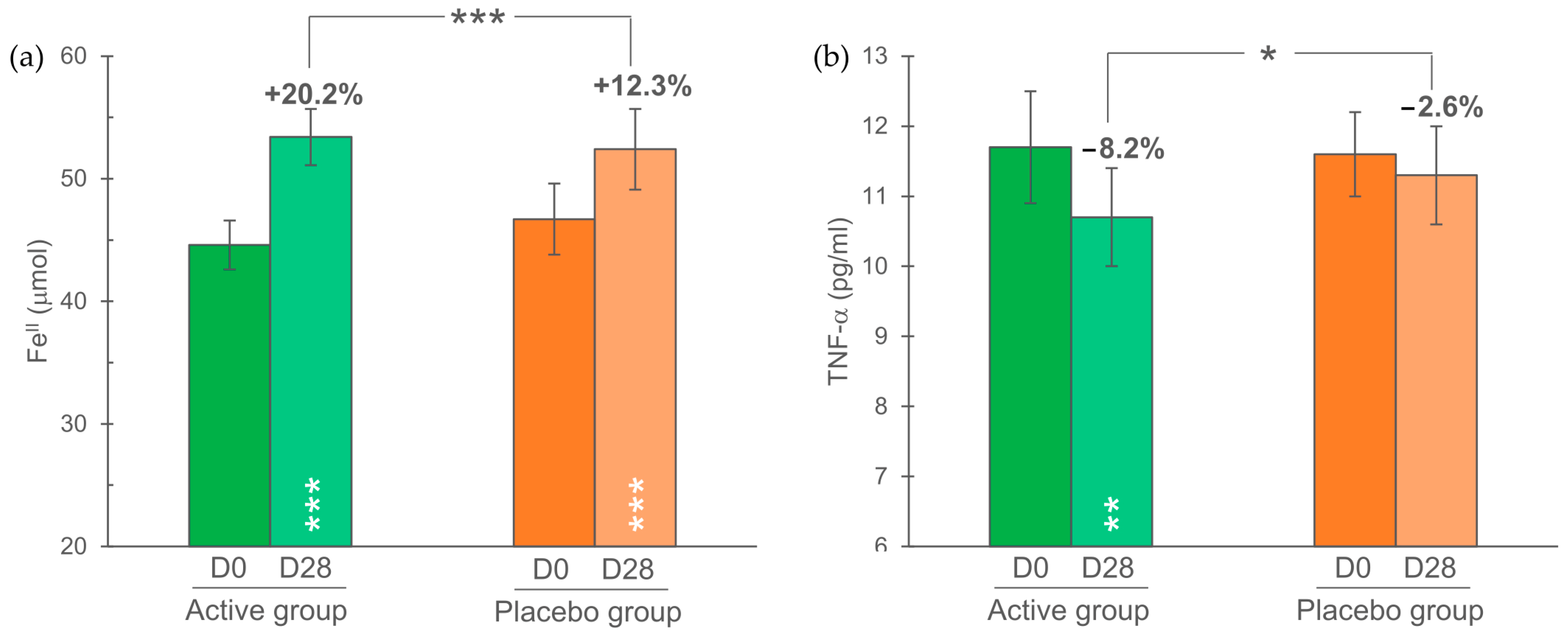
3.5. Biochemical Parameters
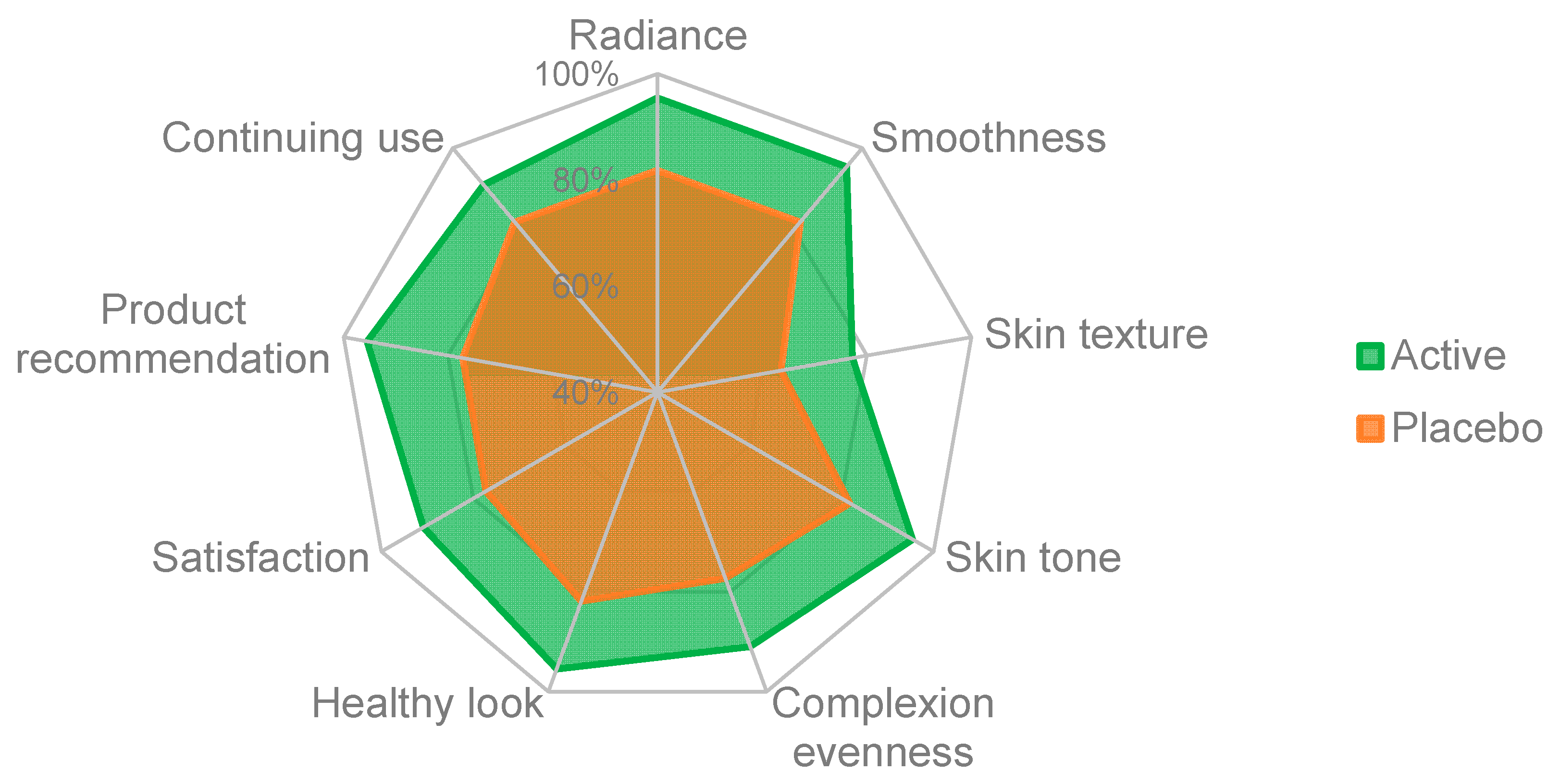
3.6. Self-Assessment Questionnaire
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pat, Y.; Yazici, D.; D’Avino, P.; Li, M.; Ardicli, S.; Ardicli, O.; Mitamura, Y.; Akdis, M.; Dhir, R.; Nadeau, K.; et al. Recent Advances in the Epithelial Barrier Theory. Int. Immunol. 2024, 36, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Akdis, C.A. Does the Epithelial Barrier Hypothesis Explain the Increase in Allergy, Autoimmunity and Other Chronic Conditions? Nat. Rev. Immunol. 2021, 21, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Bouloc, A.; Sore, G.; Bernard, B.A.; Passeron, T. The Skin Aging Exposome. J. Dermatol. Sci. 2017, 85, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Buendía-Eisman, A.; Prieto, L.; Abarquero, M.; Arias-Santiago, S. Study of the Exposome Ageing-Related Factors in the Spanish Population. Acta Derm.-Venereol. 2020, 100, 5755. [Google Scholar] [CrossRef] [PubMed]
- Khmaladze, I.; Leonardi, M.; Fabre, S.; Messaraa, C.; Mavon, A. The Skin Interactome: A Holistic “Genome-Microbiome-Exposome” Approach to Understand and Modulate Skin Health and Aging. Clin. Cosmet. Investig. Dermatol. 2020, 13, 1021–1040. [Google Scholar] [CrossRef] [PubMed]
- Rappaport, S.M. Genetic Factors Are Not the Major Causes of Chronic Diseases. PLoS ONE 2016, 11, e0154387. [Google Scholar] [CrossRef] [PubMed]
- Leffers, H.C.B.; Lange, T.; Collins, C.; Ulff-Møller, C.J.; Jacobsen, S. The Study of Interactions between Genome and Exposome in the Development of Systemic Lupus Erythematosus. Autoimmun. Rev. 2019, 18, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Battie, C.; Jitsukawa, S.; Bernerd, F.; Del Bino, S.; Marionnet, C.; Verschoore, M. New Insights in Photoaging, UVA Induced Damage and Skin Types. Exp. Dermatol. 2014, 23, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Bernerd, F.; Asselineau, D. UVA Exposure of Human Skin Reconstructed In Vitro Induces Apoptosis of Dermal Fibroblasts: Subsequent Connective Tissue Repair and Implications in Photoaging. Cell Death Differ. 1998, 5, 792–802. [Google Scholar] [CrossRef]
- Wei, M.; He, X.; Liu, N.; Deng, H. Role of Reactive Oxygen Species in Ultraviolet-Induced Photodamage of the Skin. Cell Div 2024, 19, 1. [Google Scholar] [CrossRef]
- Marionnet, C.; Grether-Beck, S.; Seité, S.; Marini, A.; Jaenicke, T.; Lejeune, F.; Bastien, P.; Rougier, A.; Bernerd, F.; Krutmann, J. A Broad-Spectrum Sunscreen Prevents UVA Radiation-Induced Gene Expression in Reconstructed Skin In Vitro and in Human Skin In Vivo. Exp. Dermatol. 2011, 20, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Marionnet, C.; Lejeune, F.; Pierrard, C.; Vioux-Chagnoleau, C.; Bernerd, F. Biological Contribution of UVA Wavelengths in Non Extreme Daily UV Exposure. J. Dermatol. Sci. 2012, 66, 238–240. [Google Scholar] [CrossRef] [PubMed]
- Dijkhoff, I.M.; Drasler, B.; Karakocak, B.B.; Petri-Fink, A.; Valacchi, G.; Eeman, M.; Rothen-Rutishauser, B. Impact of Airborne Particulate Matter on Skin: A Systematic Review from Epidemiology to In Vitro Studies. Part. Fibre Toxicol. 2020, 17, 35. [Google Scholar] [CrossRef] [PubMed]
- Martic, I.; Jansen-Dürr, P.; Cavinato, M. Effects of Air Pollution on Cellular Senescence and Skin Aging. Cells 2022, 11, 2220. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Navarro, L.; Jansen-Dürr, P.; Cavinato, M. Synergistic Interplay of UV Radiation and Urban Particulate Matter Induces Impairment of Autophagy and Alters Cellular Fate in Senescence-Prone Human Dermal Fibroblasts. Aging Cell 2024, 23, e14086. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-M.; Cheng, M.-Y.; Xun, M.-H.; Zhao, Z.-W.; Zhang, Y.; Tang, W.; Cheng, J.; Ni, J.; Wang, W. Possible Mechanisms of Oxidative Stress-Induced Skin Cellular Senescence, Inflammation, and Cancer and the Therapeutic Potential of Plant Polyphenols. Int. J. Mol. Sci. 2023, 24, 3755. [Google Scholar] [CrossRef] [PubMed]
- Papaccio, F.; D’Arino, A.; Caputo, S.; Bellei, B. Focus on the Contribution of Oxidative Stress in Skin Aging. Antioxidants 2022, 11, 1121. [Google Scholar] [CrossRef] [PubMed]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative Stress in Aging Human Skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, P.; Gęgotek, A.; Žarković, N.; Skrzydlewska, E. Oxidative Stress and Lipid Mediators Modulate Immune Cell Functions in Autoimmune Diseases. Int. J. Mol. Sci. 2021, 22, 723. [Google Scholar] [CrossRef]
- Agrawal, R.; Hu, A.; Bollag, W.B. The Skin and Inflamm-Aging. Biology 2023, 12, 1396. [Google Scholar] [CrossRef]
- Gonçalves, A.; Sampaio, C.I.; Ševčovičová, A.; Dias, A.M.; Oliveira, R. Cynaropicrin- and Chlorogenic Acid-Rich Extracts Easily Prepared from Cynara cardunculus Var. scolymus: Antioxidant and Antigenotoxic Properties. Biocatal. Agric. Biotechnol. 2023, 52, 102808. [Google Scholar] [CrossRef]
- Girsang, E.; Ginting, C.N.; Lister, I.N.E.; Gunawan, K.Y.; Widowati, W. Anti-Inflammatory and Antiaging Properties of Chlorogenic Acid on UV-Induced Fibroblast Cell. PeerJ 2021, 9, e11419. [Google Scholar] [CrossRef] [PubMed]
- Elsebai, M.F.; Mocan, A.; Atanasov, A.G. Cynaropicrin: A Comprehensive Research Review and Therapeutic Potential as an Anti-Hepatitis C Virus Agent. Front. Pharmacol. 2016, 7, 472. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.T.; Tanaka, K.; Kojima, H.; Hamada, T.; Masutani, T.; Tsuboi, M.; Akao, Y. Cynaropicrin from Cynara scolymus L. Suppresses Photoaging of Skin by Inhibiting the Transcription Activity of Nuclear Factor-Kappa B. Bioorg. Med. Chem. Lett. 2013, 23, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Passeron, T.; Krutmann, J.; Andersen, M.L.; Katta, R.; Zouboulis, C.C. Clinical and Biological Impact of the Exposome on the Skin. J. Eur. Acad. Dermatol. Venereol. 2020, 34 (Suppl. S4), 4–25. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Lee, Y.H. Skin Aging from Mechanisms to Interventions: Focusing on Dermal Aging. Front. Physiol. 2023, 14, 1195272. [Google Scholar] [CrossRef]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, Oxidative Stress and Autophagy in Skin Aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef]
- Pilkington, S.M.; Bulfone-Paus, S.; Griffiths, C.E.M.; Watson, R.E.B. Inflammaging and the Skin. J. Investig. Dermatol. 2021, 141, 1087–1095. [Google Scholar] [CrossRef]
- Nobile, V.; Schiano, I.; Germani, L.; Cestone, E.; Navarro, P.; Jones, J.; Caturla, N. Skin Anti-Aging Efficacy of a Four-Botanical Blend Dietary Ingredient: A Randomized, Double Blind, Clinical Study. Cosmetics 2023, 10, 16. [Google Scholar] [CrossRef]
- De Tollenaere, M.; Meunier, M.; Scandolera, A.; Sandre, J.; Lambert, C.; Chapuis, E.; Auriol, D.; Reynaud, R. Well-Aging: A New Strategy for Skin Homeostasis under Multi-Stressed Conditions. J. Cosmet. Dermatol. 2020, 19, 444–455. [Google Scholar] [CrossRef]
- Chung, C.L.; Lawrence, I.; Hoffman, M.; Elgindi, D.; Nadhan, K.; Potnis, M.; Jin, A.; Sershon, C.; Binnebose, R.; Lorenzini, A.; et al. Topical Rapamycin Reduces Markers of Senescence and Aging in Human Skin: An Exploratory, Prospective, Randomized Trial. GeroScience 2019, 41, 861. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Li, K.; Zong, X.; Eun, S.; Morimoto, N.; Guo, S. Hallmarks of Skin Aging: Update. Aging Dis. 2023, 14, 2167–2176. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, K.; Tamatsu, Y.; Sugawara, Y.; Shimada, K. The Relationship between Wrinkle Depth and Dermal Thickness in the Forehead and Lateral Canthal Region. Arch. Dermatol. 2011, 147, 822–828. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, Y.; Feng, B.; Lee, J.; Lu, N.; Pierce, D.M. A Multi-Layered Model of Human Skin Elucidates Mechanisms of Wrinkling in the Forehead. J. Mech. Behav. Biomed. Mater. 2020, 105, 103694. [Google Scholar] [CrossRef] [PubMed]
- Haydont, V.; Bernard, B.A.; Fortunel, N.O. Age-Related Evolutions of the Dermis: Clinical Signs, Fibroblast and Extracellular Matrix Dynamics. Mech. Ageing Dev. 2019, 177, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Labat-Robert, J.; Fourtanier, A.; Boyer-Lafargue, B.; Robert, L. Age Dependent Increase of Elastase Type Protease Activity in Mouse Skin: Effect of UV-Irradiation. J. Photochem. Photobiol. B Biol. 2000, 57, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Lavker, R.M.; Veres, D.A.; Irwin, C.J.; Kaidbey, K.H. Quantitative Assessment of Cumulative Damage from Repetitive Exposures to Suberythemogenic Doses of UVA in Human Skin. Photochem. Photobiol. 1995, 62, 348–352. [Google Scholar] [CrossRef]
- D’Antuono, I.; Carola, A.; Sena, L.M.; Linsalata, V.; Cardinali, A.; Logrieco, A.F.; Colucci, M.G.; Apone, F. Artichoke Polyphenols Produce Skin Anti-Age Effects by Improving Endothelial Cell Integrity and Functionality. Molecules 2018, 23, 2729. [Google Scholar] [CrossRef]
| Active | Placebo | Units | |
|---|---|---|---|
| Sex | |||
| Male | 0% (0) | 0% (0) | % (no.) |
| Female | 100% (22) | 100% (22) | % (no.) |
| Age | 53.3 ± 1.4 | 53.3 ± 1.4 | Years |
| Skin type | |||
| Normal | 31.8% (7) | 31.8% (7) | % (no.) |
| Mixed/Oily | 27.3% (6) | 27.3% (6) | % (no.) |
| Dry | 40.9% (9) | 40.9% (9) | % (no.) |
| Wrinkle depth | 260.9 ± 13.9 | 252.4 ± 15.3 | μm |
| Skin roughness | 33.5 ± 1.7 | 32.7 ± 1.2 | μm |
| 8° gloss (skin radiance) | 12.0 ± 0.6 | 12.7 ± 0.7 | a.u. |
| FRAP (antioxidant capacity) | 44.6 ± 2.0 | 46.7 ± 2.9 | μmol FeII |
| TNF-α | 11.7 ± 0.8 | 11.6 ± 0.6 | pg/mL |
| Radiance (8° Gloss) | Active | Placebo |
|---|---|---|
| D0 | 12.0 ± 0.6 | 12.7 ± 0.7 |
| D28 | 13.8 ± 0.4 *** (+19.0%) ‡ | 13.9 ± 0.5 ** (+14.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roveda, G.; Cestone, E.; De Gennaro, F.; Poggi, A.; Insolia, V.; Zaccaria, V.; Nobile, V. Artichoke Leaf Extract Effectiveness on the Skin Aging Exposome: Efficacy and Safety Results of a Split-Face Study. Cosmetics 2024, 11, 69. https://doi.org/10.3390/cosmetics11030069
Roveda G, Cestone E, De Gennaro F, Poggi A, Insolia V, Zaccaria V, Nobile V. Artichoke Leaf Extract Effectiveness on the Skin Aging Exposome: Efficacy and Safety Results of a Split-Face Study. Cosmetics. 2024; 11(3):69. https://doi.org/10.3390/cosmetics11030069
Chicago/Turabian StyleRoveda, Gloria, Enza Cestone, Francesca De Gennaro, Andrea Poggi, Violetta Insolia, Vincenzo Zaccaria, and Vincenzo Nobile. 2024. "Artichoke Leaf Extract Effectiveness on the Skin Aging Exposome: Efficacy and Safety Results of a Split-Face Study" Cosmetics 11, no. 3: 69. https://doi.org/10.3390/cosmetics11030069
APA StyleRoveda, G., Cestone, E., De Gennaro, F., Poggi, A., Insolia, V., Zaccaria, V., & Nobile, V. (2024). Artichoke Leaf Extract Effectiveness on the Skin Aging Exposome: Efficacy and Safety Results of a Split-Face Study. Cosmetics, 11(3), 69. https://doi.org/10.3390/cosmetics11030069






