Abstract
This study aimed to determine the anti-collagenase, anti-elastase, and anti-hyaluronidase activities of Passiflora quadrangularis fruit extracts (epicarp, mesocarp, endocarp, and seed), develop stable nanoemulsions, and evaluate the efficacy of the nanoemulsions containing extracts in human volunteers. The results indicated that the epicarp and seed extracts exhibited collagenase, elastase, and hyaluronidase inhibition effects. Gallic acid was identified in the extracts, with the highest concentration found in the endocarp extract (1449.35 mg gallic acid/g extract) followed by the seed extract (839.63 mg gallic acid/g extract). The endocarp and seed extracts demonstrated good stability at different temperatures. Consequently, these extracts were selected for incorporation into nanoemulsions due to their high yield, excellent anti-tyrosinase and anti-aging activity, and good stability, making them suitable for cosmetic products. The nanoemulsions were prepared using the ultrasonication method, resulting in a particle size of 133.1 ± 0.8 nm, a polydispersity index of 0.33 ± 0.03, and a zeta potential of −61.8 ± 0.6 mV, indicating good stability. The nano-serum containing extracts was non-irritating and safe for the skin. Skin evaluation among human volunteers after 60 days of application revealed improvements in skin hydration and reduction in wrinkles. In conclusion, nanoemulsions containing P. quadrangularis fruit extracts have the potential to be effective cosmetic products.
1. Introduction
Skin aging is a complex and progressive process resulting from intrinsic aging and environmental factors, particularly photoaging [1]. Reactive oxygen species (ROS) induce the matrix metalloproteinases (MMPs) enzymes such as collagenase, leading to collagen and elastin degradation. Additionally, hyaluronic acid or hyaluronan (HA) in the dermal and epidermal skin layers, which mainly promotes skin rejuvenation, hydration, and elasticity, is naturally decreased during the aging process by the hyaluronidase enzyme.
The giant granadilla (Passiflora quadrangularis L.) belongs to the family Passifloraceae together with the well-known Passiflora edulis Sims and is commonly found in northern Thailand. The Passiflora species has received attention in recent years due to its aromatic flowers and unique and excellent fruit flavor. Plants within the Passiflora genus have been traditionally used in medicine for various health benefits. Evidence from previous studies supports the antioxidant, antibacterial, anti-inflammatory, antifungal, and other activities of Passiflora species [2,3,4,5]. Several studies have highlighted the potential of Passiflora plants in cosmetic applications. For instance, a sun-protective makeup product containing P. edulis seed extract was successfully developed, demonstrating UVA and UVB protection [6]. Moreover, nanoemulsions formulated with different seed oils from wild Passiflora species (such as P. setacea, P. cincinnata, P. tenuifila, and P. alata) induced the proliferation of keratinocytes, indicating a healing effect and potential as natural materials in cosmeceutical applications [7]. The most important components in P. quadrangularis fruit extract are vitamin C and phenolic and flavonoid compounds possessing anti-oxidant properties. Our related study found that the ethanolic P. quadrangularis fruit extract can scavenge DPPH and ABTS free radicals and whiten the skin by reducing melanin production via inhibition of the tyrosinase enzyme [8]. We found that the seed extract has the highest bioactive content including phenolics and flavonoids with the best anti-oxidant activity, while the endocarp extract produced the highest yield with a good tyrosinase inhibitory effect when using L-DOPA as a substrate. Thus, we suppose that P. quadrangularis fruit extract has the potential to be applied in the field of cosmetics and can add value to local crops. However, little scientific research has been conducted on the anti-aging effect of P. quadrangularis extract through clinical study and application in cosmetic products.
Nanotechnology is widely used in various fields, including cosmetics and cosmeceuticals. Further, nanomaterials have greatly contributed to and increased the global market share of pharmaceuticals and cosmetics. The major advantages of using nanotechnology are prolonging the duration of action, increasing dermal penetration and bio-availability due to its small size, and enhancing the aesthetic appeal and stability of the formulation [9]. Nanoemulsions are a fluid colloidal system in the nanoparticle size range containing two immiscible liquids mixed to form a heterogeneous dispersion using an emulsifying agent. The droplet size of nanoemulsions normally ranges from 20 to 200 nm [10]. The small-sized droplets increase surface area, providing greater absorption, improving physical stability, and improving the solubility of lipophilic substances.
P. quadrangularis has not been used as a cosmetic/cosmeceutical ingredient. In addition, the challenges associated with cosmetic/cosmeceutical development of P. quadrangularis extracts include unpleasant physical and organoleptic characteristics, such as low water solubility, poor skin permeability, sensitivity to environmental factors, and unpleasant smell. These challenges often result in ineffective and unattractive products. Nanoemulsions represent potential nanocarriers to enhance skin permeation, stability, and reduce toxicity. They can be prepared in various formulations [11]. Therefore, the utilization of nanotechnology delivery systems presents an interesting approach to deliver the extracts effectively.
This study aimed to determine the anti-aging activity by collagenase, elastase, and hyaluronidase inhibition of the ethanolic P. quadrangularis fruit (epicarp, mesocarp, endocarp, and seed) extracts and to develop stable nanoemulsions containing extracts with a good aesthetic appearance. In addition, the clinical study among human volunteers was evaluated in terms of skin hydration and wrinkles.
2. Materials and Methods
2.1. Plant Materials
The giant granadilla fruit (Passiflora quadrangularis L.) was received from the Chiang Rai Community Enterprise Farmers Group (Chiang Rai, Thailand).
2.2. Preparation of P. quadrangularis Fruit Extracts
The P. quadrangularis fresh fruits were collected and separated into epicarp, mesocarp, endocarp, and seed. They were washed and dried in a hot air oven (BINDER GmbH, Tuttlingen, Germany), then mashed into powder and extracted by maceration using 95% v/v ethanol (RCI Labscan Ltd., Bangkok, Thailand) as the solvent at a ratio of 1:2 for 72 h at 30 ± 2 °C. After that, the sample was filtered, and the ethanol evaporated using a rotary vacuum evaporator (BUCHI (Thailand) Ltd., Bangkok, Thailand). The crude extracts were stored at −4 °C and protected from light before further experiments.
2.3. Collagenase Inhibitory Assay
The collagenase inhibitory assay was performed by measuring FALGPA hydrolysis using the method of Nitthikan et al. [12] with slight modifications. The assay was conducted in 50 mM tricine buffer (pH 7.5) (Sigma Chemical Co, St. Louis, MO, USA) containing 400 mM NaCl (EMSURE®, Darmstadt, Germany) and 10 mM CaCl2 (EMSURE®, Darmstadt, Germany). The collagenase enzyme (Sigma Chemical Co, St. Louis, MO, USA) and the 2 mM N-[3-(2-Furyl)acryloyl]-Leu-Gly-Pro-Ala (FALGPA) substrate (Sigma Chemical Co, St. Louis, MO, USA) were dissolved in the tricine buffer, and the collagenase activity was measured before starting the assay. The reaction mixture was prepared by mixing the collagenase enzyme, the sample solution (0.45 mg/mL), the tricine buffer, and the FALGPA substrate, then measuring the absorbance at 340 nm using a microplate reader (BMG LABTECH, Ortenberg, Germany). Gallic acid (Sigma Chemical Co, St. Louis, MO, USA) and epigallocatechin gallate (EGCG) (Sigma Chemical Co, St. Louis, MO, USA) were used as standards. The inhibition percentage was calculated by using the equation below:
where Acontrol is the absorbance of the reaction of the control solution, and Asample is the absorbance of the reaction of sample solution.
% Collagenase inhibition = (Acontrol − Asample)/(Acontrol) × 100
2.4. Elastase Inhibitory Assay
Elastase inhibition was determined using the method of Nitthikan et al. [12] with slight modifications. Briefly, 4.4 mM N-Succinyl-Ala-Ala-Ala-p-nitroanilide (AAAVPN) substrate (Sigma Chemical Co, St. Louis, MO, USA) solution and elastase enzyme (Sigma Chemical Co, St. Louis, MO, USA) were prepared in 100 mM Tris buffer (pH 8.0) (RCI Labscan Ltd., Bangkok, Thailand). The enzyme activity was measured before starting the assay. Tris buffer, elastase enzyme, and sample solution (1.25 mg/mL) were mixed and incubated for 20 min at 30 ± 2 °C, and then the AAAVPN substrate solution was added. The absorbance was measured at 410 nm using a microplate reader (BMG LABTECH, Ortenberg, Germany), while gallic acid and EGCG were used as standards. The inhibition percentage was calculated using the equation below:
where Acontrol is the absorbance of the reaction of the control solution, and Asample is the absorbance of the reaction of sample solution.
% Elastase inhibition = (Acontrol − Asample)/(Acontrol) × 100
2.5. Hyaluronidase Inhibitory Assay
The hyaluronidase inhibitory assay used the method of Nitthikan et al. [12] with slight modifications. A sample solution (0.2 mg/mL) was pre-incubated with bovine hyaluronidase enzyme (Sigma Chemical Co, St. Louis, MO, USA) in a solution containing sodium phosphate buffer (pH 7.0) (RCI Labscan Ltd., Bangkok, Thailand), 77 mM NaCl, and 0.01% bovine serum albumin (BSA) (Sigma Chemical Co, St. Louis, MO, USA) for 10 min at 37.5 °C. The assay was initiated by adding 0.003% hyaluronic acid (Sigma Chemical Co, St. Louis, MO, USA) in 300 mM sodium phosphate (pH 5.35) (RCI Labscan Ltd., Bangkok, Thailand) to the incubation mixture, followed by incubating for 45 min at 37.5 °C. Undigested hyaluronic acid was precipitated with acetic albumin solution (pH 3.75) composed of 0.1% BSA, 24 mM sodium acetate (RCI Labscan Ltd., Bangkok, Thailand), and 79 mM acetic acid (RCI Labscan Ltd., Bangkok, Thailand) in de-ionized water (RCI Labscan Ltd., Bangkok, Thailand). The solution was maintained at 30 ± 2 °C for 10 min, and the absorbance was measured at 600 nm using a microplate reader (BMG LABTECH, Ortenberg, Germany). Tannic acid (Loba Chemie Pvt. Ltd., Mumbai, India) and gallic acid were used as standards. The inhibition percentage was calculated using the equation below:
where Asample is the absorbance of the reaction of sample solution, and Acontrol is the absorbance of the reaction of the control solution.
% Hyaluronidase inhibition = (Asample/Acontrol) × 100
2.6. Characterization of the Chemical Marker in P. quadrangularis Fruit Extracts
The extracts’ chemical markers were analyzed using high-performance liquid chromatography (HPLC) (Shimadzu Corporation, Kyoto, Japan) with a photodiode array detector. Samples were separated on a reversed-phase column (C18 5 μm, 4.6 × 250 mm) with a solvent system consisting of acetonitrile (RCI Labscan Ltd., Bangkok, Thailand) and phosphoric acid (RCI Labscan Ltd., Bangkok, Thailand) buffer using isocratic elution with a flow rate of 1.0 mL/min. The UV detection wavelength was 254 nm (Shimadzu Corporation, Kyoto, Japan). All solvents and standards were HPLC grade, and gallic acid was used as standard. The chromatogram and retention time of the sample were compared with the standard. The quantitative analysis is based on the peak area compared with the standard curve.
2.7. Stability Study of P. quadrangularis Fruit Extracts
The extracts were stored at low temperature (4 °C) and high temperature (45 °C) conditions as well as at 30 ± 2 °C with direct light and with protection from light for one month. The content of chemical markers was analyzed using HPLC (Shimadzu Corporation, Kyoto, Japan) with the same condition as the characterization of the chemical markers in the extracts.
2.8. Development of Nanoemulsions Containing Extracts
2.8.1. Development and Characterization of Unloaded Nanoemulsions
Unloaded nanoemulsions consisted of propylene glycol (Namsiang Group, Bangkok, Thailand), PEG-40 hydrogenated castor oil (Union Science Co., Ltd., Chiang Mai, Thailand), jojoba oil (Namsiang Group, Bangkok, Thailand), isononyl isononanoate (Stearinerie Dubois, Boulogne-Billancourt, France), phenoxyethanol (Namsiang Group, Bangkok, Thailand), and EDTA (Chanjao Longevity Co., Ltd., Bangkok, Thailand). The formulations were prepared using the method of Divakaran [13] with slight modifications. The coarse o/w emulsion was prepared by mixing the oil and aqueous phases using a high-shear homogenizer (IKA Works (Thailand) Co., Ltd., Bangkok, Thailand) at 10,000 rpm for 5 min. After that, the nanoemulsions were formed using a probe sonicator (Sonics & Materials, Inc., Newtown, CT, USA). The nine formulations were prepared by varying the ultrasonication amplitude and sonication time at 50 to 70% and 5 to 15 min, respectively. All formulations were characterized for physical appearance by observation, while particle size, PDI, and zeta potential were measured using a Zetasizer (Malvern Analytical, Malvern, UK). The best formulation with good physical appearance, small droplet size, low polydispersity index, and good stability was selected to load the extract.
2.8.2. Preparation and Characterization of Nanoemulsions Containing Extracts
Extracts with high active content and biological activities were selected to prepare the nanoemulsions. The nanoemulsions containing extracts consisted of the same compositions as unloaded nanoemulsions. The extracts were dissolved in propylene glycol and then added to the aqueous phase. After that, the oil phase and the aqueous phase were mixed using a high-shear homogenizer (IKA Works (Thailand) Co., Ltd., Bangkok, Thailand) at 10,000 rpm for 5 min, then using a probe sonicator (Sonics & Materials, Inc., Newton, CT, USA) to form the nanoemulsions. The physical appearance, pH value, particle size, PDI, and zeta potential were evaluated by observation, pH meter, and Zetasizer, respectively.
2.8.3. Determination of Entrapment Efficiency
The prepared nanoemulsions were diluted with methanol (RCI Labscan Ltd., Bangkok, Thailand) at a ratio of 1:2 to determine the total polyphenol content in nanoemulsions, while the free polyphenol content in the nanoemulsions was determined by passing 1 mL of sample solutions through an Amicon® Ultra centrifugal filter (Merck Millipore Ltd., Carrigtwohill, Co., Cork, Ireland) of MWCO 50 kDa (pore size 10 to 100 nm). These were then centrifuged at 3500 rpm and 25 °C for 30 min. Then, the permeate was collected to calculate the encapsulation efficiency by measuring the total phenolic content using the Folin–Ciocalteu method.
Percentage of entrapment efficiency (%EE) was performed using the equation below:
where A is the total polyphenol content in nanoemulsions, and B is the total polyphenol content in permeate.
% Entrapment efficiency = (A − B)/(A) × 100
2.9. Stability Study
The unloaded nanoemulsions and nanoemulsions containing extracts were investigated regarding stability under various conditions. The accelerated stability study was conducted by heating–cooling cycling at 45 °C for 48 h and 4 °C for 48 h, and this was repeated for six cycles. To determine the stability under actual storage conditions, the formulations were stored at 30 ± 2 °C for three months. The total phenolic content and physicochemical properties, including appearance, pH, particle size, PDI, and zeta potential, were determined.
2.10. Skin Irritation and Efficacy Tests among Human Volunteers of Nano-Serum Containing Extracts
2.10.1. Preparation of the Nano-Serum Containing P. quadrangularis Fruit Extracts
The sodium polyacrylate starch (Daito Kasei Kogyo, Osaka, Japan) and xanthan gum (Namsiang Group, Bangkok, Thailand) were dispersed in glycerin (Namsiang Group, Bangkok, Thailand) and added to the nanoemulsions containing extracts in an amount of 1.7% w/w. The formulation was mixed using a high-shear homogenizer (IKA Works (Thailand) Co., Ltd., Bangkok, Thailand) at 10,000 rpm for 5 min until homogeneous.
2.10.2. Ethics Consideration
The study protocol was checked and approved by the Human Research Ethics Committee of the Faculty of Pharmacy, Chiang Mai University, Thailand before starting the study (Protocol Number: 018/2565).
2.10.3. Subjects
All twenty healthy female volunteers, ages ranging from 35 to 50 years (43.3 ± 4.1 years), presented normal or dry skin with wrinkles and fine lines and without history of allergy to chemicals or natural substances. They were not volunteers for other research studies and could follow up test results for a specified period. The participants signed a consent form to join the study.
2.10.4. Skin Irritation Study
A patch test was used to evaluate the skin irritation of the participants using the method of Basketter et al. [14] with slight modifications. The investigator applied a Finn chamber (SmartPractice Europe GmbH, Greven, Germany) occlusive patch containing the nano-serum with extracts on the forearm of the participants compared with the positive control (2% w/v sodium lauryl sulfate). After 4 h of application, the Finn chamber was removed, observed, and evaluated concerning skin reactions, and the relevant scores were recorded at the initial time, 24 h, 48 h, and 72 h. The results are presented as the primary dermal irritation index (PII) and calculated using the equation below:
where Σ erythema grade is the sum of the erythema score the first day, after 24 h, 48 h, and 72 h. The Σ edema grade is the sum of the edema score the first day, after 24 h, 48 h, and 72 h. N is the number of participants, and PII values over 0.5 indicate skin irritation.
PII = (∑〖[erythema grade]〗 + ∑〖[edema grade]〗)/(4 × N)
2.10.5. Efficacy Test of the Nano-Serum Containing Extracts
The efficacy test of the formulations was determined using the method of Manosroi et al. [15] with slight modifications. Participants completing the skin irritation study received a nano-serum containing extracts. The nano-serum containing extracts were applied to a 4 × 4 cm2 area on the left volar forearm twice daily for two months. The efficacy of the nano-serum containing extract was evaluated using a Corneometer® (CK electronic GmbH, Cologne, Germany) and Visioscan® (CK Electronic GmbH, Cologne, Germany) to assess skin moisture and skin wrinkles (depth of wrinkles and skin roughness). The results were measured at baseline and monthly. The questionnaire to assess satisfaction was also conducted to confirm product appearance (texture, color, and odor) and performance (such as stickiness, hydration, and tightness). Satisfaction assessment was conducted using a Likert scale divided into five levels: one representing ‘very dissatisfied’ and five representing ‘very satisfied’. The scores were presented as the mean ± standard deviation (SD) from 20 volunteers (n = 20).
2.11. Statistical Analysis
The data were presented as the mean ± SD for three replicate measurements (n = 3). The one-way ANOVA test was utilized to evaluate differences between each part of the extracts and standards in skin aging-related enzyme inhibitory assays. The paired sample t-test was employed to assess the stability results of the extracts and formulations before and after storage. Skin improvement before and after using the product in 20 human volunteers was compared using a paired sample t-test, and data were presented as the mean ± SD for three replicate measurements (n = 3). Statistical analysis was performed using the SPSS Program, Version 17.0. The level of statistical significance was set at a 95% confidence level (p < 0.05).
3. Results
3.1. Extraction of P. quadrangularis Fruit Extracts
The ethanolic extracts of different parts of the P. quadrangularis fruit were obtained as a viscous extract. The endocarp extract showed the highest percentage yield (25.22%), followed by the mesocarp (23.62%), the epicarp (8.67%), and the seed extracts (6.00%), respectively.
3.2. Skin Aging and Related Enzyme Inhibitory Effects of the P. quadrangularis Fruit Extracts
In vitro skin aging and related enzyme inhibitory studies were conducted to estimate the possible anti-aging effects of the extracts. The inhibitory effects of different parts of the fruit extracts are presented in Figure 1.
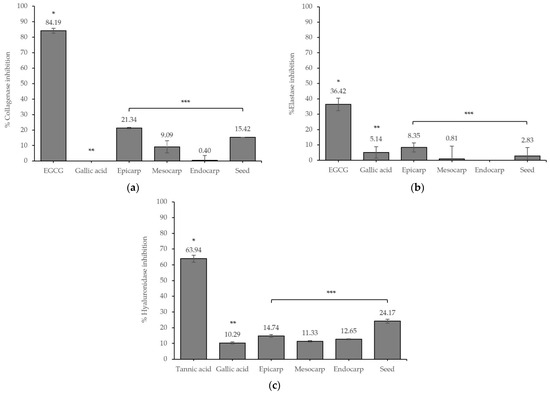
Figure 1.
Inhibition effect of the extracts against (a) collagenase, (b) elastase, and (c) hyaluronidase enzymes. Values are given as mean ± S.D. from triplicate. The asterisk (*) indicates significant differences (p < 0.05) between EGCG/tannic acid and the samples. The double asterisks (**) indicate significant differences (p < 0.05) between gallic acid and the samples. The triple asterisks (***) indicate significant differences (p < 0.05) between each part of the samples.
The epicarp extract showed the highest collagenase and elastase inhibitory effects with inhibition values of 21.34 ± 0.40% and 8.35 ± 2.94%, respectively, followed by the seed. Interestingly, the seed extract presented the highest hyaluronidase inhibitory effect of 24.17 ± 1.27%. However, the effects of the extracts were less than those of the standard.
3.3. Chemical Marker of P. quadrangularis Fruit Extracts
Figure 2 shows the HPLC chromatographic profile of each part of P. quadrangularis fruit extracts compared with those of the standard. The extracts showed a chromatogram at a retention time of about 3.2 min, similar to the chromatogram of gallic acid. The gallic acid content in each extract was analyzed from the standard curve (y = 1610.6x + 37,822, R2 = 1). The endocarp extract exhibited the highest gallic acid content (1449.35 mg gallic acid/g extract), followed by seed extract (839.63 mg gallic acid/g extract).
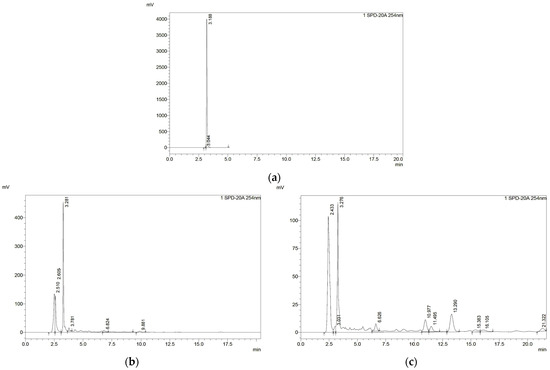
Figure 2.
Chromatographic profiles of (a) gallic acid, (b) endocarp, and (c) seed extracts.
3.4. Stability of the P. quadrangularis Fruit Extracts
Each part of the extracts was analyzed for gallic acid content after being stored in different conditions for one month, and this is presented in Figure 3. The results showed that the gallic acid content in the endocarp extract did not change after being stored under different conditions, except at 30 ± 2 °C with protected light, which significantly decreased and remained at about 83.85%. Additionally, the seed extract significantly increased in gallic acid content at high temperature (45 °C).
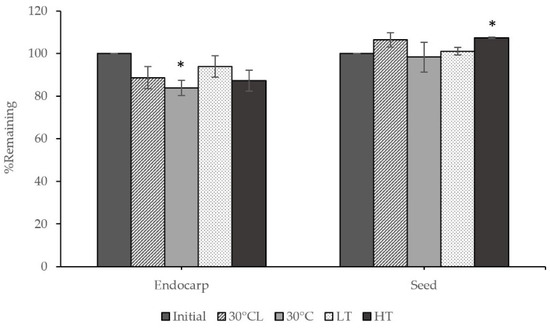
Figure 3.
Comparison of the remaining chemical content in the extracts before and after stability tests included 30 ± 2 °C with light (30 °CL), 30 ± 2 °C with protected light (30 °C), low temperature (4 °C; LT), and high temperature (45 °C; HT). Values are given as mean ± S.D. from triplicate. The asterisk (*) indicates significant differences (p < 0.05) from the initial in the same sample.
3.5. Development and Characterization of Unloaded Nanoemulsions
The nanoemulsions were successfully formulated at different amplitudes and sonication times. The particle size, PDI, and zeta potential of the nanoemulsions are shown in Table 1.

Table 1.
Particle size, PDI, and zeta potential of unloaded nanoemulsions.
No formulations significantly differed regarding particle size, PDI, or zeta potential. The formulations had particle sizes ranging from 95.7 to 107.4 nm, PDI values of 0.19 to 0.22, and zeta potentials ranging from −19.6 to −41.8 mV. Therefore, formulations No. 1 and No. 7, using the least amount of sonication time with the lowest and highest sonication amplitudes, were chosen to load the extracts.
3.6. Nanoemulsions Containing Extracts Preparation and Characterization
According to our related results, the endocarp and seed extracts, exhibiting high yield, excellent bio-activities, and good stability with an appropriate appearance for cosmetic products, were chosen to incorporate in the formulation. The endocarp and seed extracts at a concentration of 1% w/w were added to the formulation. The two formulations were developed under the same conditions as unloaded nanoemulsions No. 1 and No. 7. The characterizations of the formulation are shown in Table 2. The nanoemulsions containing extracts revealed a yellowish color with a pH value of about 5. The particle size, PDI value, and zeta potential of the two formulations were approximately 133 nm, 0.30, and −60 mV, respectively. Formulation No. 1 showed an entrapment efficiency of 51.38 ± 0.03%, while formulation No. 7 indicated 60.30 ± 0.00%.

Table 2.
pH, particle size, PDI, and zeta potential of nanoemulsions containing extracts.
3.7. Stability Study of Unloaded Nanoemulsions and Nanoemulsions Containing Extracts
The selected unloaded nanoemulsions were evaluated for their physicochemical characteristics compared before and after the tests and are presented in Figure 4. The two formulations were stable without creaming or sedimentation. The No. 1 formulation increased in the PDI value after storage at 30 ± 2 °C, whereas other parameters were unchanged. In comparison, the parameters, including particle size and PDI value of the No. 7 formulation, significantly changed. The results showed that the particle size of the No. 7 formulation decreased after stability tests, and the PDI value increased.
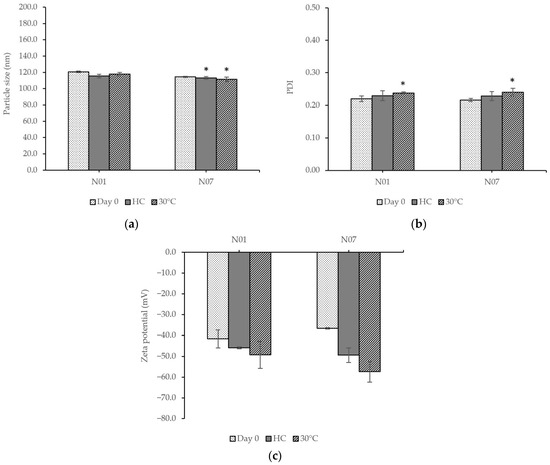
Figure 4.
Comparison of the (a) particle size, (b) PDI value, and (c) zeta potential of the formulations before and after stability tests. Unloaded nanoemulsion No. 1 (N01). Unloaded nanoemulsion No. 7 (N07). Heating–cooling cycling (HC). 30 ± 2 °C (30 °C). Values are given as mean ± S.D. from triplicate. The asterisk (*) indicates significant differences (p < 0.05) from day 0 in the same group.
The nanoemulsions containing extracts (NE) were also stable without creaming or sedimentation. The physicochemical characteristics of all formulations were evaluated before and after the stability tests, and the results are shown in Figure 5. After stability tests, some of the parameters significantly changed. The results showed that the pH values of the nanoemulsions containing the extracts were lower than those of formulations without the extracts and decreased after stability tests. The droplet size measurement revealed that the nanoemulsions had a particle size below 200 nm. We found that the particle size of the nanoemulsions tended to decrease except NE1, which significantly increased in particle size and PDI value after storage at 30 ± 2 °C for three months. On the other hand, the NE7 formulation did not change in particle size or PDI value after the stability test. All formulations had a zeta potential higher than −30 mV.
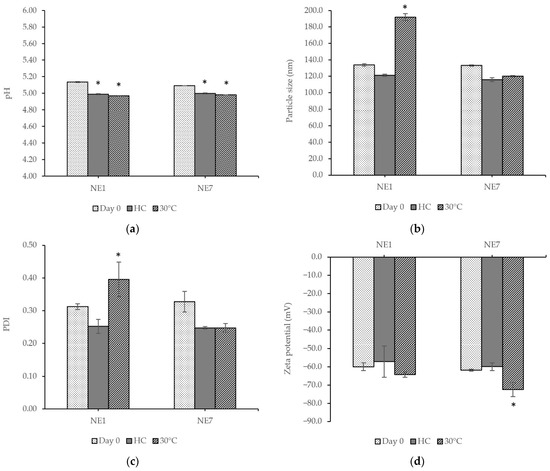
Figure 5.
Comparison of the (a) pH value, (b) particle size, (c) PDI value, and (d) zeta potential of nanoemulsion containing extracts No. 1 (NE1) and nanoemulsion containing extracts No. 7 (NE7) before and after stability tests with heating–cooling cycling (HC) and at 30 ± 2 °C (30 °C) for 3 months. Values are given as mean ± S.D. from triplicate. The asterisk (*) indicates significant differences (p < 0.05) between day 0 and other conditions in the same group.
The total phenolic content was measured to confirm the bioactive compounds in the nanoemulsions after the stability tests. The total phenolic content of NE1 significantly decreased at 2.65% and 0.17% after heating–cooling cycling and 30 ± 2 °C, respectively. A similar trend was also observed for the total phenolic content in NE7, which significantly decreased by about 32.75% and 38.50% after heating–cooling cycling and at 30 ± 2 °C, respectively. From the results above, although the NE1 had more phenolic content in the formulation after stability tests, the NE7 presented better stability of particle size, PDI, and zeta potential. Thereby, NE7 was chosen to study the safety and efficiency tests among human volunteers.
3.8. Safety and Efficacy Tests of Nano-Serum Containing P. quadrangularis Fruit Extracts among Human Volunteers
The nanoemulsions containing extracts in serum were formulated to use for safety and efficacy tests among human volunteers. The skin irritation evaluation was reported as the PII score. The PII score of the nano-serum containing extracts was 0.2, indicating non-irritation, whereas the PII score of the positive control was 0.5, indicating slight irritation. The results of the skin assessment of the nano-serum containing extracts compared before and after application for 30 days and 60 days are shown in Figure 6.
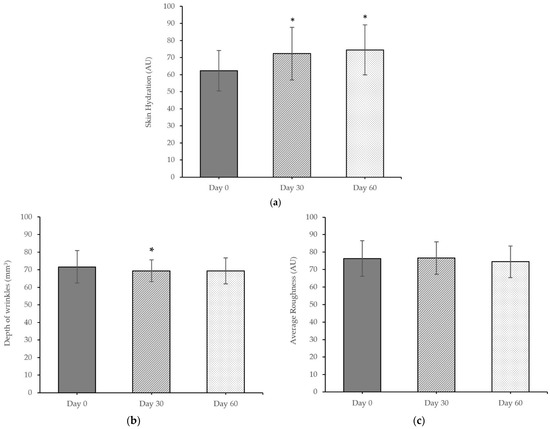
Figure 6.
Efficacy results in (a) skin hydration, (b) depth of wrinkles, and (c) skin roughness of human volunteers before and after applying the nano-serum containing extracts. AU means arbitrary units. Values are given as mean ± S.D. from 20 volunteers. The asterisk (*) indicates significant differences (p < 0.05) between day 0 with day 30 and day 60.
Skin hydration increased by 16.05% and 19.58% after 30 and 60 days of application, respectively, which was significantly higher than baseline (day 0). To determine the anti-aging effect of the formulation on the skin, the depth of wrinkles and the skin roughness after the nano-serum containing P. quadrangularis fruit extracts application were evaluated. The depth of wrinkles and the skin roughness decreased over time after use of the nano-serum containing P. quadrangularis fruit extracts. The depth of wrinkles significantly decreased by 8.56% after 30 days of application. In addition, the skin roughness decreased by 2.42% after 60 days of application.
The satisfaction assessment was conducted through questionnaires regarding product appearance and performance after applying the nano-serum containing P. quadrangularis fruit extracts for two months. Concerning product appearance, more than 50% of volunteers were satisfied with the texture (3.8 ± 0.7), while most were neither satisfied nor dissatisfied with the color (3.2 ± 0.9) and odor (2.9 ± 1.0). Regarding product performance, more than half of the volunteers expressed satisfaction with aspects such as non-stickiness (4.0 ± 0.9), skin hydration (4.2 ± 0.7), skin tightness (4.0 ± 0.7), and smooth skin (4.0 ± 0.7), with no reported irritation (4.8 ± 0.4). Furthermore, 70% of volunteers were overall satisfied (4.0 ± 0.7) with the nano-serum containing P. quadrangularis fruit extracts, with all values being significantly different.
4. Discussion
One of the biggest concerns for women is skin aging, which can be induced by both intrinsic and extrinsic factors. Intrinsic aging is natural aging over time involving genetics and hormones, while extrinsic aging results from exposure to external factors, such as smoking, pollutants, and predominantly ultraviolet (UV) radiation, which is called photoaging [16]. UV radiation is the primary factor inducing the overproduction of reactive oxygen species (ROS), increasing the oxidative stress in the epidermis and causing degradation of the extracellular matrix (ECM). It induces unhealthy conditions and contributes to wrinkling, roughness, dryness, loss of elasticity, and irregular pigmentation. The ECM is the outermost part of the skin containing fibroblasts and proteins, including collagen and elastin. Collagen, elastin, and hyaluronic acid or hyaluronan (HA) are necessary and responsible for conferring integrity and elasticity to the skin to maintain the skin’s youthful and healthy appearance. Collagen and elastin are protein structures providing tensile strength and elastic recoil properties of the skin, while HA is a glucose-based polymer belonging to the ECM molecules. After skin exposure to UV radiation, the ROS accumulates and activates the production of dermal enzymes such as collagenase or matrix metalloproteinase-1 (MMP-1) and elastase, causing malfunction and degradation of collagen and elastin. In conclusion, the synthesis of collagenase, elastase, and hyaluronidase promotes premature skin aging. Inhibiting MMP-1 is a primary target for preventing skin photoaging and wrinkles [17]. This study revealed that certain compounds in P. quadrangularis fruit extracts are potential inhibitors of collagenase, elastase, and hyaluronidase. The extracts of P. quadrangularis fruit contain bioactive compounds, including phenolics and flavonoids. Previous studies have also shown that gallic acid exhibits skin aging-related enzyme inhibitory activity. Synergistic interactions between polyphenols and enzymes may play an essential role in the inhibition mechanism [18,19]. The amount of bioactive compounds in each extract part is associated with enzyme inhibition. Consistent with a study by Wittenauer et al. [18], enzyme inhibition increased with an increasing polyphenol concentration. In addition, the chemical composition of P. quadrangularis fruit was reported in the study of Ramaiya S et al. [20], indicating secondary metabolites such as isopropyl methoxy cinnamic acid in the endocarp and mesocarp parts related to DPPH scavenging activity. Moreover, the related study also found organic acids, including citric acid and malic acid, in the Passiflora fruit [21]. The stability study of the extracts revealed that the gallic acid content remained unchanged under sunlight but proved sensitive to high temperatures. These results were consistent with the findings of Volf et al. [22], who observed that gallic acid, when combined with ascorbic acid, exhibited high stability under UV exposure.
The bioactivity study results revealed that the P. quadrangularis fruit extracts had anti-oxidant, anti-tyrosinase, and anti-aging activities. Although the epicarp extract exhibited great bioactivity, its color and smell could negatively affect the appearance of the finished product. Therefore, the endocarp and seed extracts were chosen for further study because of their high yield as well as their excellent anti-tyrosinase and anti-aging activity.
The preparation of nanoemulsions can be divided into high-energy or low-energy methods. High-energy methods employ mechanical devices to disrupt micro-droplets, forming nanosized droplets. These methods include high-pressure homogenization (HPH), microfluidization, and ultrasonication. In contrast, low-energy methods leverage the physicochemical properties of surfactants and co-surfactants. Nanoemulsions are formed spontaneously under specific system compositions or environmental conditions. Low-energy emulsification can be further classified into phase inversion temperature (PIT), phase inversion composition (PIC), and solvent diffusion [23]. The ultrasonication method was chosen to develop nanoemulsions in this study due to its simplicity, speed, and effectiveness in formulating stable nanoemulsions with small droplet sizes and low size distribution. Furthermore, the study of Nakabayashi et al. [24] reported that this method can produce highly stable nanoemulsions, even without surfactants. Droplet sizes can be controlled by optimizing parameters such as oil or emulsifier concentration, oil and surfactant ratio, continuous phase viscosity, sonication time, and amplitude. This study prepared nine formulations by varying the sonication time and amplitude. The particle size decreased after increasing sonication amplitude. As in the study of Guzmán et al. [25], the droplet size decreased as the ultrasonication amplitude and time increased, providing greater shear force to break the droplets. Due to the decrease in particle size, a sufficient surfactant could cover the surfaces of the extract droplets, stabilizing and preventing the coalescence of nanoemulsion droplets, thereby increasing the entrapment efficiency [26]. The concentration of oil or substance solubility as well as compatibility with the oil phase and excipients in the formulations also significantly impact the entrapment efficiency. A 60% entrapment efficiency was considered high, suitable for the controlled release of bioactive compounds [27,28]. The results indicate that NE7, with a smaller particle size, exhibited higher entrapment efficiency, suggesting that NE7 could more effectively entrap the extract in nanoparticles than the NE1 formulation.
The stability results of the formulation containing the extracts revealed significant differences in some parameters compared to the baseline. Although the pH values showed a significant decrease, they did not affect the stability and remained acceptable for skincare products that should possess a pH in the range of four to six [29], which is usually considered ideal for topical products. NE1 exhibited more changes in particle size and size distribution than NE7 with a tendency to increase after the stability study. After the stability study, both NE1 and NE7 formulations had particle sizes below 200 nm. The study of Su et al. [30] indicated that a particle size smaller than 200 nm showed good transdermal permeation. The PDI value represents the distribution of particle size in the dispersion system, typically ranging from 0.0 to 1.0. A lower or closer-to-zero PDI indicates a narrow droplet size distribution and homogeneity, with values of 0.2 and below generally considered acceptable [31]. Another critical parameter for nanoemulsions is zeta potential. It shows the overall charges and represents the stability of a particulate dispersion. Favorable zeta potential is typically greater than ±30 mV, allowing adequate electrostatic repulsion and inhibiting particle aggregation. All formulations in this study exhibited a PDI value between 0.2–0.4 and a zeta potential greater than −60 mV, considered highly stable.
This study indicated that the nano-serum containing P. quadrangularis fruit extracts could improve skin hydration after 30 days of application and could decrease the depth of wrinkles over time, but it did not significantly affect skin roughness. Several related studies have reported the association between the anti-aging effect and the bioactive compounds present in natural extracts. Compounds such as antioxidants, including ascorbic acid and polyphenols, have been shown to reduce MMP-1 production, enhance fibroblast proliferation, increase procollagen production, and improve skin characteristics such as hydration and firmness [32,33].
However, this study encountered a few limitations, such as a small number of participants and a short testing duration. Longer durations of more than eight weeks with a follow-up period are needed. According to the study by Ji J et al. [34], skin roughness and wrinkles were significantly reduced by 20% at 12 weeks. Additionally, treatment efficacy in the study by Fonseca AP et al. [35] was evidenced by a significant increase in collagen after 56 and 84 days of use compared to baseline.
5. Conclusions
The ethanolic P. quadrangularis fruit extracts contain bioactive compounds, including phenolics and flavonoids, with gallic acid serving as a chemical marker. These extracts exhibited collagenase, elastase, and hyaluronidase inhibitory effects and demonstrated good stability under various conditions. Nanoemulsions containing P. quadrangularis endocarp and seed extracts were successfully formulated with an acceptable appearance, small droplet size, narrow polydispersity index, and good stability under accelerated conditions. The formulation was non-irritating and deemed safe for the skin. The nano-serum containing extracts improved skin hydration and reduced the depth of wrinkles. Additionally, 70% of the volunteers expressed satisfaction with the product. This study reveals that P. quadrangularis fruit extracts could serve as an active ingredient for skin hydration and anti-aging effects, and nanoemulsions containing these extracts have the potential to create effective cosmetic products.
Author Contributions
Conceptualization, S.N., K.K. and W.W.; method, S.N. and K.K.; software, N.Y.; validation, N.Y. and K.K.; formal analysis, N.Y. and K.K.; investigation, N.Y., S.N. and K.K.; resources, W.W., S.N. and K.K.; data curation, S.N. and K.K.; writing—original draft preparation, N.Y. and K.K.; writing—review and editing, S.N. and K.K.; visualization, N.Y.; supervision, S.N. and K.K.; project administration, S.N. and K.K.; funding acquisition, S.N. and K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research project was partially supported by Chiang Mai University, Faculty of Pharmacy, Chiang Mai University, and Teaching Assistant and Research Assistant Scholarships (TA/RA) academic year 2022 from Chiang Mai University.
Institutional Review Board Statement
The study was conducted according to the Declaration of Helsinki and approved by the Ethics Committee of the Faculty of Pharmacy, Chiang Mai University, Thailand (protocol code: 018/2565 on 9 March 2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available within the article.
Acknowledgments
The authors would like to acknowledge Chiang Mai University for financial support. The authors would also like to acknowledge the Faculty of Pharmacy, Chiang Mai University for the research grant and facilities used in the project.
Conflicts of Interest
Author Worrapon Wangkananon was employed by the company TNK Beauty Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The company had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Chiang, H.-M.; Lin, T.-J.; Chiu, C.-Y.; Chang, C.-W.; Hsu, K.-C.; Fan, P.-C.; Wen, K.-C. Coffea arabica extract and its constituents prevent photoaging by suppressing MMPs expression and MAP kinase pathway. Food Chem. Toxicol. 2011, 49, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, K.; Velikova, M.; Gentscheva, G.; Gerasimova, A.; Slavov, P.; Harbaliev, N.; Makedonski, L.; Buhalova, D.; Petkova, N.; Gavrilova, A. Chemical Compositions, Pharmacological Properties and Medicinal Effects of Genus Passiflora L.: A Review. Plants 2024, 13, 228. [Google Scholar] [CrossRef] [PubMed]
- Echeverry Gonzalez, S.; Medina, H.; Costa, G.; Aragón, M. Optimization of flavonoid extraction from Passiflora quadrangularis leaves with sedative activity and evaluation of its stability under stress conditions. Rev. Bras. Farmacogn. 2018, 28, 610–617. [Google Scholar] [CrossRef]
- Yuldasheva, L.N.; Carvalho, E.B.; Catanho, M.T.; Krasilnikov, O.V. Cholesterol-dependent hemolytic activity of Passiflora quadrangularis leaves. Braz. J. Med. Biol. Res. 2005, 38, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Pereira, Z.C.; Cruz, J.; Corrêa, R.F.; Sanches, E.A.; Campelo, P.H.; Bezerra, J.A. Passion fruit (Passiflora spp.) pulp: A review on bioactive properties, health benefits and technological potential. Food Res. Int. 2023, 166, 112626. [Google Scholar] [CrossRef] [PubMed]
- Lourith, N.; Kanlayavattanakul, M.; Chingunpitak, J. Development of sunscreen products containing passion fruit seed extract. Braz. J. Pharm. Sci. 2017, 53, e16116. [Google Scholar] [CrossRef]
- de Souza, M.; Dourado, D.; Lôbo, I.; Pires, V.; Nunes, S.; Rebouças, J.; Costa, A.; Fernandes, C.; Machado Tavares, N.; De Paula Pereira, N.; et al. Wild Passiflora (Passiflora spp.) seed oils and their nanoemulsions induce proliferation in HaCaT keratinocytes cells. J. Drug Deliv. Sci. Technol. 2021, 67, 102803. [Google Scholar] [CrossRef]
- Yanasan, N.; Natakankitkul, S.; Kiattisin, K.; Phupaisan, N.; Inkongngam, S.; Wangkananon, W. Bioactive content, anti-tyrosinase, and antioxidant activities of ethanolic Giant Granadilla (Passiflora quadrangularis L.) fruit extracts for cosmetic applications. In Proceedings of the Cosmetic and Beauty International Conference, Chiang Rai, Thailand, 7–9 December 2022; pp. 15–23. [Google Scholar]
- Gupta, V.; Mohapatra, S.; Mishra, H.; Farooq, U.; Kumar, K.; Ansari, M.J.; Aldawsari, M.F.; Alalaiwe, A.S.; Mirza, M.A.; Iqbal, Z. Nanotechnology in cosmetics and cosmeceuticals—A review of latest advancements. Gels 2022, 8, 173. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, M.; Dudhe, R.; Sharma, P.K. Nanoemulsion: An advanced mode of drug delivery system. 3 Biotech 2015, 5, 123–127. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Potential of Nanoparticles as Permeation Enhancers and Targeted Delivery Options for Skin: Advantages and Disadvantages. Drug Des. Dev. Ther. 2020, 14, 3271–3289. [Google Scholar] [CrossRef]
- Nitthikan, N.; Leelapornpisid, P.; Naksuriya, O.; Intasai, N.; Kiattisin, K. Potential and Alternative Bioactive Compounds from Brown Agaricus bisporus Mushroom Extracts for Xerosis Treatment. Sci. Pharm. 2022, 90, 59. [Google Scholar] [CrossRef]
- Divakaran, D. Preparation and Characterization of Nanoemulsions Encapsulating Polyphenol Extracts. Ph.D. Thesis, National Dairy Research Institute, Karnal, India, 2012. [Google Scholar]
- Basketter, D.A.; York, M.; McFadden, J.P.; Robinson, M.K. Determination of skin irritation potential in the human 4-h patch test. Contact Dermat. 2004, 51, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Manosroi, J.; Chankhampan, C.; Kitdamrongtham, W.; Zhang, J.; Abe, M.; Akihisa, T.; Manosroi, W.; Manosroi, A. In vivo anti-ageing activity of cream containing niosomes loaded with purple glutinous rice (Oryza sativa Linn.) extract. Int. J. Cosmet. Sci. 2020, 42, 622–631. [Google Scholar] [CrossRef]
- Jiratchayamaethasakul, C.; Ding, Y.; Hwang, O.; Im, S.T.; Jang, Y.; Myung, S.W.; Lee, J.M.; Kim, H.S.; Ko, S.C.; Lee, S.H. In vitro screening of elastase, collagenase, hyaluronidase, and tyrosinase inhibitory and antioxidant activities of 22 halophyte plant extracts for novel cosmeceuticals. J. Fish. Aquat. Sci. 2020, 23, 6. [Google Scholar] [CrossRef]
- Bravo, K.; Duque, L.; Ferreres, F.; Moreno, D.A.; Osorio, E. Passiflora tarminiana fruits reduce UVB-induced photoaging in human skin fibroblasts. J. Photochem. Photobiol. B 2017, 168, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Wittenauer, J.; Mäckle, S.; Sußmann, D.; Schweiggert-Weisz, U.; Carle, R. Inhibitory effects of polyphenols from grape pomace extract on collagenase and elastase activity. Fitoterapia 2015, 101, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Barla, F.; Higashijima, H.; Funai, S.; Sugimoto, K.; Harada, N.; Yamaji, R.; Fujita, T.; Nakano, Y.; Inui, H. Inhibitive effects of alkyl gallates on hyaluronidase and collagenase. Biosci. Biotechnol. Biochem. 2009, 73, 2335–2337. [Google Scholar] [CrossRef] [PubMed]
- Ramaiya, S.D.; Lee, H.H.; Xiao, Y.J.; Shahbani, N.S.; Zakaria, M.H.; Bujang, J.S. Organic cultivation practices enhanced antioxidant activities and secondary metabolites in giant granadilla (Passiflora quadrangularis L.). PLoS ONE 2021, 16, e0255059. [Google Scholar] [CrossRef]
- Ramaiya, S.; Bujang, J.B.; Zakaria, M.H.; Saupi, N. Nutritional, mineral and organic acid composition of passion fruit (Passiflora species). Food Res. 2018, 3, 231–240. [Google Scholar] [CrossRef]
- Volf, I.; Ignat, I.; Neamţu, M.; Popa, V. Thermal stability, antioxidant activity, and photo-oxidation of natural polyphenols. Chem. Pap. 2014, 68, 121–129. [Google Scholar] [CrossRef]
- Maali, A.; Mosavian, M.T.H. Preparation and Application of Nanoemulsions in the Last Decade (2000–2010). J. Dispers. Sci. Technol. 2013, 34, 92–105. [Google Scholar] [CrossRef]
- Nakabayashi, K.; Amemiya, F.; Fuchigami, T.; Machida, K.; Takeda, S.; Tamamitsu, K.; Atobe, M. Highly clear and transparent nanoemulsion preparation under surfactant-free conditions using tandem acoustic emulsification. Chem. Commun. 2011, 47, 5765–5767. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, C.; Rojas, M.A.; Aragón, M. Optimization of Ultrasound-Assisted Emulsification of Emollient Nanoemulsions of Seed Oil of Passiflora edulis var. edulis. Cosmetics 2021, 8, 1. [Google Scholar] [CrossRef]
- Chaudhari, P.; Kuchekar, M. Development and evaluation of nanoemulsion as a carrier for topical delivery system by box-behnken design. Asian J. Pharm. Clin. Res. 2018, 11, 286. [Google Scholar] [CrossRef]
- Agnish, S.; Sharma, A.D.; Kaur, I. Nanoemulsions (O/W) containing Cymbopogon pendulus essential oil: Development, characterization, stability study, and evaluation of in vitro anti-bacterial, anti-inflammatory, anti-diabetic activities. BioNanoScience 2022, 12, 540–554. [Google Scholar] [CrossRef] [PubMed]
- Latif, M.S.; Nawaz, A.; Asmari, M.; Uddin, J.; Ullah, H.; Ahmad, S. Formulation Development and In Vitro/In Vivo Characterization of Methotrexate-Loaded Nanoemulsion Gel Formulations for Enhanced Topical Delivery. Gels 2022, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Lukić, M.; Pantelić, I.; Savić, S.D. Towards Optimal pH of the Skin and Topical Formulations: From the Current State of the Art to Tailored Products. Cosmetics 2021, 8, 69. [Google Scholar] [CrossRef]
- Su, R.; Fan, W.; Yu, Q.; Dong, X.; Qi, J.; Zhu, Q.; Zhao, W.; Wu, W.; Chen, Z.; Li, Y.; et al. Size-dependent penetration of nanoemulsions into epidermis and hair follicles: Implications for transdermal delivery and immunization. Oncotarget 2017, 8, 38214–38226. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.P. Development of Hierarchical Magnetic Nanocomposite Materials for Biomedical Applications. Ph.D. Thesis, Dublin City University, Dublin, Ireland, 2013. [Google Scholar]
- Fujii, T.; Wakaizumi, M.; Ikami, T.; Saito, M. Amla (Emblica officinalis Gaertn.) extract promotes procollagen production and inhibits matrix metalloproteinase-1 in human skin fibroblasts. J. Ethnopharmacol. 2008, 119, 53–57. [Google Scholar] [CrossRef]
- Hernandez, D.F.; Cervantes, E.L.; Luna-Vital, D.A.; Mojica, L. Food-derived bioactive compounds with anti-aging potential for nutricosmetic and cosmeceutical products. Crit. Rev. Food Sci. Nutr. 2021, 61, 3740–3755. [Google Scholar] [CrossRef]
- Ji, J.; Yang, X.; Flavel, M.; Shields, Z.P.; Neoh, J.; Bowen, M.L.; Kitchen, B. Age-deterring and skin care function of a polyphenol rich sugarcane concentrate. Cosmetics 2020, 7, 30. [Google Scholar] [CrossRef]
- Fonseca, A.P.; Pizzol, C.D.; Vanzo, A.C.; da Silva, G.H.; Facchini, G.; Pinheiro, A.; Eberlin, S.; Maia Campos, P. Antiaging effects of a skin care formulation containing nanoencapsulated antioxidants: A clinical, in vitro, and ex vivo study. J. Cosmet. Dermatol. 2023, 23, 510–524. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).