Abstract
Photoallergy, a unique form of skin sensitization induced by specific compounds under ultraviolet irradiation, has traditionally been investigated using animals. However, the prohibition of animal testing for the assessment of cosmetic ingredients in Europe and other countries underscores the necessity for in vitro or in silico alternative methods. Currently, there are no validated methods for assessing photoallergy or photosensitization, presenting a significant challenge in the development of new cosmetic ingredients. This review examines the landscape of alternative methods for detecting photosensitization, emphasizing recent publications, and considering the underlying principles of the different proposed assays.
1. Introduction
The exposure of the skin to sunlight can lead to adverse effects that are not specifically limited to the development of malignant alterations (cancer) or aging, but also involve photosensitivity reactions. The latter, though less studied, are also important and constitute a growing public health problem. Thus, using a patch test, ref. [1] revealed that almost 27% of the European population develops allergic reactions. Furthermore, this highlights a rise in allergic dermatitis in children [2], alongside instances of exogenous photodermatosis affecting the elderly and polypharmacy patients. Additionally, it underscores the exacerbation of skin conditions due to sunlight exposure, including transient photosensitivity [3,4].
Photosensitivity refers to a skin response to light, particularly ultraviolet light (UV), triggered by the presence of intrinsic or extrinsic chromophores in the epidermis or dermis. Common culprits for photosensitivity include photoactive molecules found in skin-applied products or systemic medications. Phototoxicity is defined as an acute reaction that develops after the application of a chemical, topically or systemically, along with exposure to ultraviolet light [5]. When it comes to topical administration, instead of phototoxicity, we can also refer to photoirritation, as the clinical manifestations are similar to contact dermatitis [6].
However, there is also the potential for the development of hypersensitivity reactions to the chemicals or their photoproducts, specifically mediated by T-cells, leading to what is termed photoallergy. Frequently, both phototoxicity and photoallergy mechanisms coexist, where non-specific phototoxic inflammation can contribute to sensitization to the photoactive product. This process may have implications such as the recognition of chemically related compounds, establishment of lifelong immunologic memory, and the potential progression to persistent light reactivity [7]. Photosensitivity arises when a chromophore, whether exogenous or endogenous, accumulates in the skin and undergoes selective activation upon exposure to radiation. This activation primarily occurs with UVA (320–400 nm) but can also involve UVB (290–320 nm), visible, or infrared light. The resultant photoactivation of the chromophore can lead to various outcomes: the induction of non-specific inflammation and aggressiveness within skin cells, referred to as photoirritation; the initiation of a specific immune response known as photoallergy; the development of enduring sensitivity to UV light, recognized as chronic actinic dermatitis; or the induction of persistent DNA damage with delayed repercussions such as photocarcinogenesis and photoaging [8].
The pathophysiological response differs between photoirritation or photoallergy. Clinically, activation of a photosensitizer leads to an eczematous eruption, characterized by symptoms such as erythema (redness), papules/vesicles (bumps/blister-like lesions), and occasionally bullae (large fluid-filled blisters) [5]. In opposition to the photoirritant reactions, the presence of keratinocyte necrosis and hyperpigmentation are not observed in skin histological samples of photoallergic patients [9].
On the other hand, photoallergy is not common in the entire population; it is a delayed-type hypersensitivity reaction of type IV that manifests as allergic contact photodermatitis. This type of dermatitis occurs when a photoantigen is applied to the skin of individuals previously sensitized to that substance. In this adverse reaction, UV radiation, typically UVA, is necessary to form a complete antigen, and therefore, the inducing substance must be in contact with the skin while exposed to UVA rays [10,11]. Hence, dermal photosensitization reactions represent a special type of allergic contact dermatitis wherein the allergen is activated by electromagnetic radiation, such as ultraviolet light. Historically, photodermatitis reactions were reported to be linked to plant components or extracts [7], but in 1961, cases of photodermatitis were identified among factory workers in England who used tetrachlorosalicylanilide as a bactericidal agent in soaps [12,13].
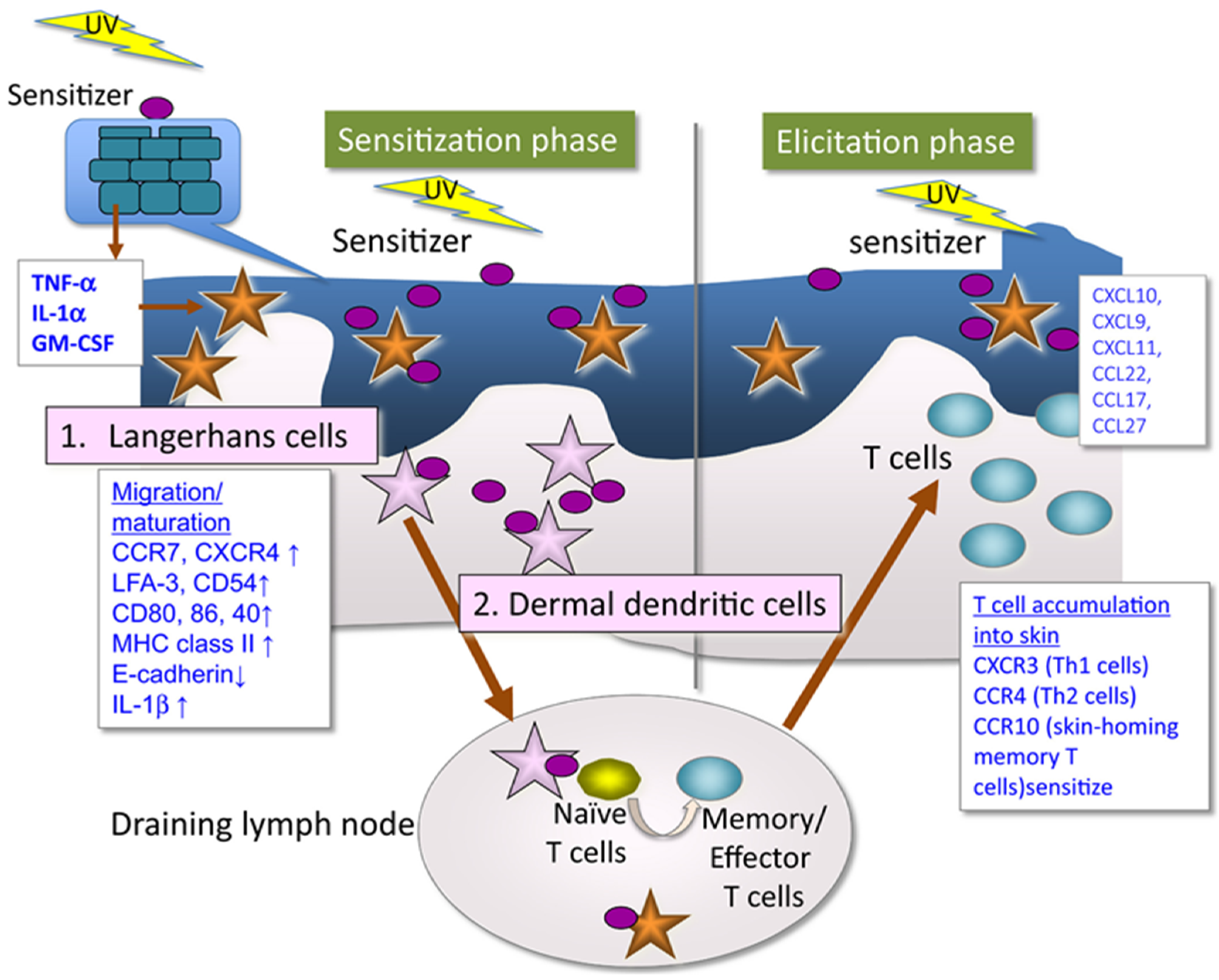
In any case, the immune system becomes involved, leading to an allergic reaction upon re-exposure to the substance and sunlight. In a first exposure or sensitization phase (Figure 1), the photoallergen will form the complete hapten by binding to skin proteins. These events will trigger cellular responses at various levels involving the activation, maturation and release of humoral mediators that finally will promote naïve T cells into memory/effector T cells [9,13]. During later exposures, sensitized T cells elicit the clinical manifestations of photoallergic contact dermatitis.
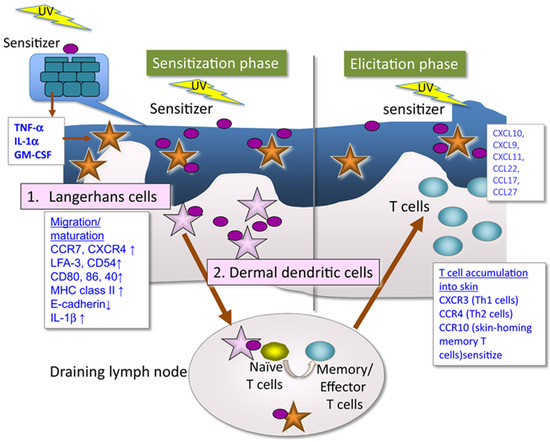
Figure 1.
Mechanism of photoallergic contact dermatitis [13]. In a first exposure or sensitization phase, the photoallergen is converted to a complete hapten by UV irradiation that can bind to skin proteins, leading to production proinflammatory cytokines that induce maturation of epidermal Langerhans cells and/or dermal dendritic cells that migrate to the draining lymph nodes where they sensitize naïve T cells to be memory/effector T cells. Subsequent exposure induces sensitized T cells elicit the clinical manifestations of photoallergic contact dermatitis (elicitation phase).
Currently, the growing prevalence of photoallergy is due to a combination of factors, including the continual introduction of new drugs, cosmetics, and chemicals each year, along with shifts in behaviors due to heightened exposure to natural sunlight or artificial lamps. These trends have contributed to greater concern about this type of response, both among patients and within dermatologists and the scientific community [6,14]. Dermatologists use the photo patch test, employing a battery of photoallergenic products, for diagnostic purposes. Over the years, changes in population habits have altered the products that cause photoallergic contact dermatitis. The decrease in the use of certain products and the introduction of new products have a significant impact on the prevalence of specific photoallergens that trigger photoallergic contact dermatitis. While in the 1960s, antimicrobial agents were mainly responsible for photoallergies, in the 1970s, fragrances such as 6-methylcoumarin were the culprits, and from the 1980s onwards, topical sunscreens and non-steroidal anti-inflammatories emerged as the leading causes [15]. At present, ketoprofen is recognized as an important photoallergen with high cross-reactivity with UV filters, especially benzophenones [16,17]. Etofenamate, which is particularly common in the Mediterranean area, and octocrylene are among the other compounds that often elicit positive reactions. However, the photoallergic reaction is attributed to photosensitization induced by ketoprofen [18]. Therefore, the potential phototoxic or photoallergic effects must be evaluated through appropriate and validated safety assays before they are launched into the market to avoid potential adverse effects on consumers [19]. These assays serve as preventive tools to mitigate adverse effects and prevent the development of a pathological process.
Traditionally, the phototoxic properties of chemicals are generally assessed using experimental animals, typically guinea pigs, rabbits, rats, and mice [13,20,21,22]. For pharmaceutical products, the European Medicines Agency mandates these assays, as well as photoallergy tests, to ensure their safety [23]. Nevertheless, the constraints of animal models, combined with recent regulations created by various international regulatory bodies [24,25,26] banning animal use in cosmetic safety assessments, and the tightening of ethical considerations demanding the reduction and refinement of such practices, highlight the urgent necessity for the development of in vitro assays as preventive tools to identify potential hazards for both safety and human health [6]. Our aim is to offer additional perspectives on the gradual incorporation of New-Approach Methodologies (NAMs) into chemical risk assessment protocols, with the goal of safeguarding human health. This transition is envisioned to culminate in the adoption of an animal-free approach termed “Next-Generation Risk Assessment” [27]. For this reason, there is an urgent demand for the development of feasible, reliable, and reproducible alternative methods.
Concerning mandatory phototoxicity studies for regulatory purposes, currently, the pharmaceutical and cosmetic industries must rely on the in vitro 3T3 Neutral Red Uptake phototoxicity assay (3T3 NRU PT) [28], alongside newer methods such as the ROS assay for photoreactivity [29], and the reconstructed human epidermis phototoxicity test method [30]. However, none of these methods discriminate between photoirritants and photoallergens, and therefore, novel animal-free proposals must be further explored. In this regard, the objective of this work is to investigate the latest advances in the development of methodological proposals as alternatives to traditional assays for identifying products with photoallergic potential (hazard identification). Specifically, we focus on assays that allow for the reduction or replacement of animal models by addressing the question: “What are the most recent developments in alternative methods to animal experimentation for identifying photoallergens?”.
The beauty industry continually adapts to meet the diverse needs of consumers, evolving through innovation and a quest for perfection. Despite this drive, prioritizing the safety of cosmetic products remains essential. Notably, the European Union and several other nations have made significant advancements in discontinuing animal testing for cosmetics. This progressive shift underscores a dedication to ethical standards, animal welfare, and the endorsement of alternative testing methods that are both humane and efficacious.
2. Materials and Methods
A search was conducted across different databases, following the recommendations of PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) statement [31] including Pubmed Central (National Institutes of Health’s National Library of Medicine, Pubmed), Web of Science (Clarivate, WoS), Scopus (Elsevier), and Toxcenter (Toxicology Center Database). The search was performed by combining representative keywords for the adverse effect, namely photoallergy, photoallergen/ic, photoallergenicity, photoassay, photosafety, with those representation evaluation methods, alternative, in vitro, in chemico and in silico, and limited to the title, abstract or indexing words. The search was refined by including the documents published between 2013 and 2023.
The found documents have been classified in an Excel file where the following fields have been recorded: authors, title of the work, journal, year of publication, type of document (article, review, book chapter, conference abstract, etc.), database where it was found, and language. Each document has been identified by a reference number, and their appearance in the same or more than one database has been assessed to eliminate repetitions. Then, those records not written in English as well as those that were not original articles or reviews (conference abstracts, clinical studies, letters, book chapters, etc.) were discarded. Finally, the registers were assessed for eligibility by screening the title and abstract. Eligible documents were based on the mechanism of photoallergy.
3. Results
3.1. Study Selection
We found a total of 876 registers in the different databases selected (Figure 2): 201 in Pubmed, 249 in WoS, 213 in Scopus, and 215 in Toxcenter. According to the words used, photoassay provided very few results (5 documents), while photosafety, with 298 reviews, and photoallergy, with 213, retrieved the maximal number of registers. Regarding the words related to methods, in chemico (51) is the phrase that generated the fewest results and in vitro the one that generated the most (595).
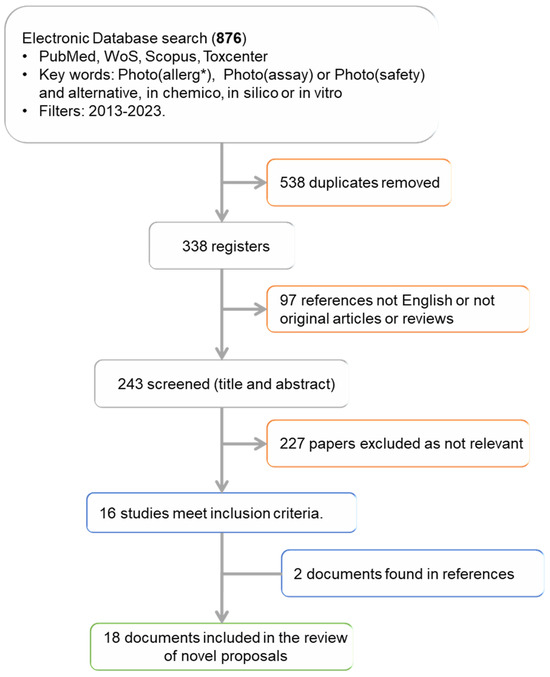
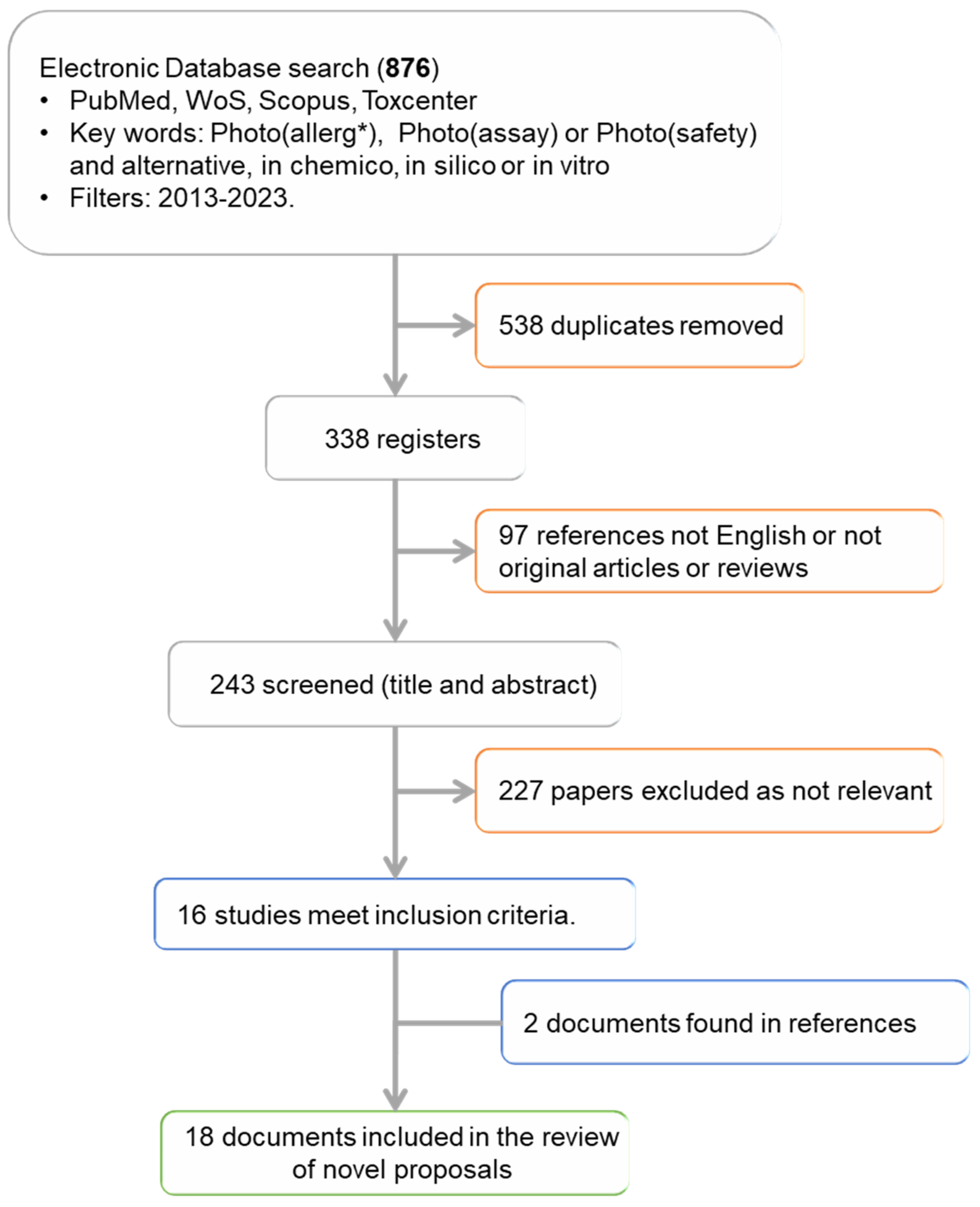
Figure 2.
Study flowchart. * Photoallergy, photoallergen/ic or photoallergenicity.
Once the documents found in more than one database and different searches were eliminated, the number of studies was reduced to 338 (Figure 3), which represents 38% of the initial documents. An initial analysis of these 338 documents was performed to find out in which years they were published (Figure 3a), the type of document (Figure 3b), in which database they were found, as well as the language in which they were written. This previous analysis has allowed us to apply a pre-eligibility criterion to include or exclude the studies based on the type of document and language used.
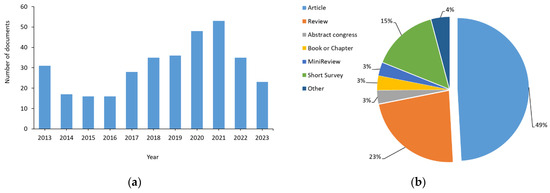
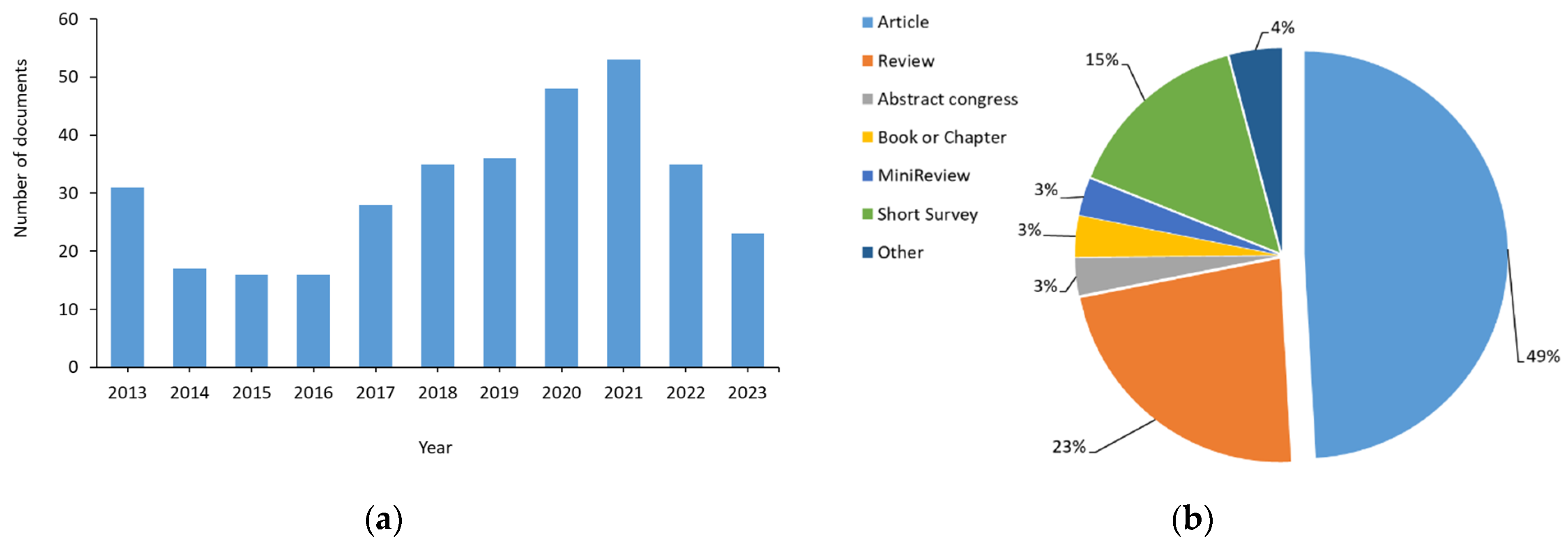
Figure 3.
Analysis of the registers found: (a) Distribution of the number of documents according to the year of publication; (b) distribution of the number of documents according to type. Other document types include letters, conferences, short communications, editorials, short surveys, clinical trials, retrospective and comparatives studies, case reports, protocols, and guidelines.
The distribution of documents based on the year of publication (Figure 3a) indicates that 30% of the articles were published during 2020 and 2021, when most laboratories were closed due to COVID-19. However, the maximum number of reviews was found in 2013 (22), followed by 2021 (14), and 2020 (10).
Regarding the type of document found (Figure 3b), almost 50% were research articles. However, reviews, books, and book chapters, and even editorials and letters (included in other) have also been found.
Regarding the database, 117 of the 338 documents were found in PubMed, 146 in WoS, 149 in Scopus, and 76 in Toxcenter. Only 19 documents (6%) were found in the four databases, which represents the 35% of the documents found in Toxcenter, and only 13% of the registers were found in WoS and Scopus. Based on this, we could assume at first sight that these 19 documents are the ones of greatest interest for answering our question. If this is corroborated, the best database for our search would be Toxcenter, while WoS and Scopus would be the least accurate.
Finally, in terms of language, less than 4% (13) of the documents were written in a language other than English. Thus, seven documents were written in Chinese, three in Japanese, and of the remaining three, one was written in German, another in Polish, and the last one in Turkish.
Based on this previous analysis, we excluded from subsequent analysis documents that did not fit into the categories of articles and reviews. Likewise, articles written in a language other than English were not considered either, although we may have lost some information as a result. With the application of these criteria, we evaluated the main objective of the remaining 243 registers (Figure 2), via analysis of the title and abstract. This analysis allowed us to classify the works in a very general way according to Table 1, allowing us to identify the works related to our final objective.

Table 1.
Main objective of the 231 articles analyzed.
As seen in Table 1, studies that contain the prefix “photo” within the title or description objectives deal with physiological changes or pathophysiological entities (photoaging, photoallergy, phototoxicity); protection against sunlight (photoprotection); therapeutic strategy (phototherapy) but also analytical and diagnostic techniques (photostability, photodegradation, photoreactivity) or safety studies of ingredients and formulations (photosafety). Therefore, there are a considerable number of studies that do not correspond to the objective of this review.
Finally, only 16 articles had the study of photoallergenic capacity or activity as their main objective (Table 2). Most of these studies were found in Scopus (13 of 16) and Pubmed (12 of 16), with 3 documents not identified in these databases but rather in WoS. This suggests that to obtain reliable results, at least two databases must be consulted in parallel [32]. In this scenario, Pubmed or Scopus together with WoS, are the preferred choices, as they enable the identification of all 16 registers. However, despite initially appearing as the top database choice, Toxcenter yielded the least accurate results, with less than 50% of records being localized.

Table 2.
Documents included in our study. References are listed according to the year of publication.
After analyzing these 16 documents, 2 additional studies were identified in the list of references of de Ávila et al. [48] and consequently were included in the final list of selected studies as depicted in Table 2. Our search strategy failed to identify the work of de Ávila et al. 2017 [41] and Nguyen et al. [47] because these two studies included photosensitization in the title, abstract or indexing keywords instead the search terms used here (photoallergy, photoallergen/ic, photoallergenicity, photoassay, photosafety).
Out of the 18 articles selected, 3 were reviews, 3 mechanistic studies, and 12 were innovative in vitro or in chemico proposals aimed at categorizing the assessed compounds as photoallergen, photoirritant, or non-phototoxic. Five of the eighteen studies were published in the last five years, suggesting the need for further investigation and exploration of this safety aspect.
3.2. Alternative Assays Proposed
Like the approach taken in the case of ACD, an adverse outcome pathway (AOP) has been proposed to guide the development of novel alternative methods [48,49]. This AOP includes four key events that will guide both the development of alternative methods to animal experimentation as well as the integrated test strategies (ITS) to assess the skin photosensitization hazard of chemicals.
The different assays proposed to identify photoallergens are briefly described in the following sections. A summary of the proposed methods according to adverse outcome pathway and key events described in [48] is shown in Table 3.

Table 3.
Summary of the existent and novel assays proposed to identify photoallergens based on the adverse outcome pathway and key events described in [48].
3.2.1. Galbiati et al., 2013 and 2014 [33,35]
The research team proposed the use of keratinocytes (NCTC 2455) and the production of Interleukin-18 (IL-18) as a tool to discriminate among photoirritants and photoallergens. Using the NCTC 2455 cell line, the authors have determined the conditions of the assay [33] by using chlorpromazine (CPZ) as a well-known photoirritant and photoallergen chemical. Then, a panel of other 16 chemicals including photoirritants, photallergens, and negative compounds (Table 4) allowed the authors to propose a prediction model (SI) based on the relationship between IL-18 production when cells are exposed to subtoxic concentrations of chemicals and UVA light (I) respect to the non-irradiated conditions (NI). Thus, a potential photoallergen was identified when SI ≥ 1.3 and statistical differences were found in the amount of IL-18 with respect to untreated cells. The in vitro assay was considered a promising tool in photosafety assessment according to the reproducibility of data and accuracy of chemical classification. Key aspects of the assay were discussed in a subsequent article [35].

Table 4.
Summary of the compounds assessed with the proposed photoassays reported in 13 of the selected articles. Classification among phototoxic, photoallergen or non-phototoxic agents was performed according to assumption provided by the authors. Activity of the compounds and relationship to cosmetic (C) industry is also presented. Only EU accepted ingredients are considered.
3.2.2. Martínez et al., 2013 [34]
Based on the capacity of the THP-1 cell line to secrete IL-8 when exposed to contact sensitizers [50], the authors explored the suitability of this model to identify photoallergens. They calculated a stimulation index based on the ratio of IL-8 measured in non-irradiated and irradiated conditions and established a cut-off of two to identify photoallergens. The chemical panel studied includes seven photoallergens, three photoirritants, and five non-phototoxic chemicals (three allergens and two irritants) as summarized in Table 4. The assays showed an accuracy of 93%.
3.2.3. Stiefel and Schwack, 2014 [36]
Protein adducts formation is pivotal in skin sensitization, and the authors have previously reported that some UV filters can react with primary amines under UV radiation [51]. Based on this observation, in this article, the reactivity of these UV filters towards skin proteins was explored by using more intricate models such as Boc-protected lysine, the tetrapeptide Boc-Gly-Phe-Gly-Lys-OH, bovine serum albumin, and porcine gelatin. A high reactivity was reported for the UV absorbers 4-t-Butyl-40-methoxydibenzoyl methane, octocrylene and benzophenone-3, which is attributed to their capacity to develop allergic or photoallergic reactions (Table 4). Although no prediction model was established, the authors determined a gradation of UV filter reactivity.
3.2.4. Oeda et al., 2016 [37]
The authors developed a cell-based in vitro photosensitization assay, examining the changes in cell-surface thiols and amines on human monocytic cell line THP-1 (photo-SH/NH2 test) in a similar way as previously reported for contact sensitizers [52]. In this case, Ketoprofen (KET) was selected as a paradigm of photoallergic chemical. The final panel consists of 18 photoallergens, 8 photoirritants, and 7 non-photosensitizers (Table 4). The seven known non-photosensitizers, along with three photoallergens and one photoirritant, did not alter cell-surface thiols or amines. This overall result achieved an accuracy and sensitivity of 87.9% and 84.6%, respectively. However, the assay was much less precise when predicting photoallergy because accuracy was lower, at 69.7%. Photosafety information of the chemicals was obtained from in vivo assays and clinical data [53,54]. The discussion regarding false-negative prediction suggests that the exclusive use of UVA, and not a solar simulator that includes UVB, could explain this phenomenon in the cases of furosemide, piroxicam, sulfanilamide, and tenoxicam [55,56].
3.2.5. Onoue et al., 2016 and 2017 [9,38]
In 2016, the research team evaluated the suitability of different assays to identify photoallergens. These assays included the ultraviolet/visible (UV/VIS) spectral analysis [57,58]; the reactive oxygen species (ROS)/micellar ROS (mROS) assays [29]; and the 3T3 NRU PT [28]. A battery of different phototoxins that included 23 photoallergens and 7 non-phototoxic/non-photoallergenic chemicals (Table 4) was employed to establish the ability of the strategy to predict the photoallergenicity. Finally, the ROS assay was found to be useful for predicting photoallergenic potential, although it presented some limitations regarding false-positive classification.
In 2017, the authors reviewed the mechanism of photoallergic reactions, including the clinical manifestations, the physicochemical characteristics of photoallergens, and the pathophysiology of photoallergic reactions. Descriptions of some known photoallergens previously used in pharmaceutic and cosmetic formulations and products are also included. Finally, a discussion of the potential use of current predictive tools of phototoxicity and the novel in vitro proposals regarding the identification of photoallergens was included. However, no specific integrated strategy or sequential test battery was suggested.
3.2.6. Tsujita-Inoue et al., 2016 [39]
The authors examined whether activation of the Keap1 (Kelch-like ECH-associated protein 1)–Nrf2 (nu-clear factor-erythroid 2-related factor 2)–ARE (antioxidant response element) pathway could be used to assess the photoallergenic potential of chemicals, using the reporter cell line AREc32 or KeratinoSens™ and concluded that activation of the Keap1-Nrf2-ARE pathway is an effective biomarker for evaluating both photoallergenic and phototoxic potentials. Twelve out of seventeen photoallergens (Table 4) were judged as positive.
3.2.7. Vayá et al., 2016 [40]
The research team explored the photoreactivity mechanism of fenofibric acid, a pharmacologically active metabolite of fenofibrate, by using human and bovine albumins and different light sources of photolysis. Fenobric acid was identified by the FDA as responsible for photosensitivity reactions [59]. The authors concluded that the primary photochemical process underlying photoallergy involved formal hydrogen atom transfer from an amino acid residue present in the binding site to the excited benzophenone chromophore. The authors’ intentions did not extend to identifying photoallergens.
3.2.8. Pérez-Ruíz et al., 2017 [41]
The authors reported that the covalent binding of β-lactams to proteins upon photochemical activation leads to an amide adduct by a different protein haptenation pathway than that previously described [60]. The research team has proven that by an integrated approach that combines photochemical, proteomic, and computational studies, human serum albumin (HSA) was selected as a target protein and ezetimibe as a probe.
These results could allow the development of new in vitro methods, although further research in this area is still required.
3.2.9. de Àvila et al., 2017 and 2023 [42,48]
A modified Direct Peptide Reactivity Assay (DPRA) assay to minimize the reaction volume was explored alongside the introduction of an irradiation step (Photo-mDPRA) to assess the potential skin sensitization and photosensitization capacity of agricultural formulations and ingredients. The authors propose the assay as a first step of a battery of photosafety tests, emphasizing that further research is needed to determine the applicability domain to other products such as cosmetics and medicines. Moreover, they highlight the importance of the environmentally friendly assay by reducing the organic waste and the economic cost. The OECD 442C guideline was followed to predict the photosensitive ability [61]. Finally, the authors reported a greater peptide depletion in the case of photosensitizers after irradiation, particularly in the case of cysteine.
Recently, de Ávila et al. [48] reviewed the mechanisms involved in the photosensitization process and discussed the different methodologies. While this review is compelling, there are several important papers that have not been analyzed. Other potential photoallergens are natural products which are more difficult to evaluate using in vitro methodologies. There are some studies involving volunteers [62], but this kind of study presents ethical concerns, as pointed by the Scientific Committee of Consumer Safety of the European Union [63]. Photopatch tests should be performed only for diagnostic purposes not for the safety evaluation of cosmetic ingredients.
3.2.10. Toyoda and Itagaki, 2018 [43]
The authors determined the intracellular ROS production of THP-1 A31 cells exposed to UV light as an endpoint of phototoxicity. The assay seemed to be suitable for identify photoreactivity but failed to discriminate between photoirritants and photoallergens, as no differences could be found in the production of ROS. Moreover, the number of chemicals was very poor (Table 4) and further studies are needed. The authors used 95% confidence intervals to calculate the maximal ROS production when the cell viability (CV) was ≥80% in both UVA- and non-UVA-exposed cells. The use of chemical quenchers demonstrated the participation of ROS in photoallergenic reactions. Interestingly, the assay identified methyl salicylate (MS) as a non-phototoxic chemical whereas it failed to identify sulfanilamide (SA) as a photoallergic one, probably because this chemical is activated by UVB [43]. Despite the limited number of chemicals used, the authors proposed this method due to the lack of in vitro methods.
3.2.11. Patel et al., 2019 [44]
The authors modified the DPRA (photo-DPRA) as well as the Amino acid Derivative Reactivity Assay (ADRA) (photo-ADRA) by introduction of a photoirradiation parameter. Analysis using photo-DPRA and photo-ADRA correctly distinguished known photoallergens from non-photoallergens. The authors suggested that photoallergens selectively showed higher depletion of model peptides or modified amino acids. Although no threshold was defined, assayed photoallergens showed an average depletion higher than 40 in both assays, while for non-photosensitizers, this percentage was nearly zero.
Thus, photo-DPRA and/or photo-ADRA can serve as non-animal in chemico methods for the identification and assessment of photoallergens/photosensitizers. The principal limitation of the method is that only nine chemicals were used (Table 4).
3.2.12. Yamamoto et al., 2020 [45]
In this study, the authors performed a more detailed analysis of the predictive capacity of the photo-ADRA assay. The information of the photoallergenic activity of the different chemicals has been obtained from in vivo animal experiments and from patch test studies in volunteers [64]. Criteria to classify the 60 chemicals and mixtures analyzed (Table 4) as contact photosensitizers or non-photosensitizers was based on the difference of percentage of N-(2-(1-naphthyl)acetyl)-l-cysteine (NAC) and α-N-(2-(1-naphthyl)acetyl)-l-lysine (NAL) depletion after reaction in non-irradiated and irradiated conditions. When the threshold was set at a value ≥ 15% for each peptide or an average value of ≥10%, the authors correctly identified 48 of 60 or 48 of 59 chemicals, depending on the high-performance liquid chromatography analysis used.
3.2.13. Nishida et al., 2021 [46]
The authors developed the photo-DPRA based on the OECD guideline 442C [51] to be used in an in chemico sequential testing strategy that includes spectral UV-VIS analysis and ROS/mROS assay [29]. A battery of products consisting of 34 photoallergens and 16 non-photoallergens was studied (Table 4). The authors proposed a sequential testing strategy (STS) starting with the determination of the molar extinction coefficient (MEC), followed by the ROS/mROS assay, and finally the photo-DPRA test. Guideline criteria were followed to classify chemicals as potential photoallergens [29,58].
3.2.14. Nguyen et al., 2021 [47]
The authors proposed the method PhotoSENSIL to identify photoallergens. In a similar way as in the work of Galbiati et al. [33,35], here, IL-18 was explored as an in vitro biomarker on the reconstructed human epidermis model EpiCS™ RHE. The model was assessed with sixteen chemicals (Table 4) with different photosensitizing capacities. The authors concluded that the best assay conditions were 1 h of preincubation followed by a recovery period of 23 h after the exposition to the potential photoallergen and UV light. The ratio of IL-18 production in non-irradiated and irradiated conditions was set at 1.5 because eight to nine photosensitizers were identified.
In total, 96 chemicals and compounds were tested, not including the six glyphosate-based herbicides assessed in [42]. Among them, about 50% were ingredients or actives in cosmetics formulations, with UV filters being the main group (14), followed by preservatives. The other 51 compounds were mainly drugs with medicinal uses (79%) as NSAIDS, diuretics, antibiotics, and others. The primary classification of products for establishing the predictive ability of assays was mainly based on in vivo and human reports, as summarized in the review of de Àvila et al., 2023 [48].
The main objective of the photoassays proposed was to identify photoallergens. In this sense, ten articles have previously classified the chemicals and compounds used to validate their potential predictive capacity into photoirritants, photoallergens (or photosensitizers) and non-phototoxic agents, and in three of them, the non-phototoxic agents were identified as irritants or allergens. In two cases, the authors only refer to phototoxicity because they used the information obtained by the NRU 3T3 PT that only allows classification among phototoxic and non-phototoxic agents [44] or because the phototoxic chemicals used are considered both phototoxic and photoallergenic [42]. Finally, Stieffel and Schwack [36] did not provide any classification at all.
BNZ, CPZ, and KET were used in thirteen studies and thus are referred to the most often. However, the authors classified BNZ and KET as only photoallergens in eight studies, while Nguyen [47] considered both phototoxic and photoallergic agents and finally, Nishida et al. [46] indicated that KET was both a photoirritant and a photoallergen (Table 4). Similarly, 6-MC, BIT, Enoxacin, Fenticlor, Furosemide, Hidrochlortiazide, Hexachlorophene, PABA, Piroxicam, and Pyridoxine were classified as phototoxic and photoallergenic or only photoallergenic. Other discrepancies were detected for Acridine considered either as phototoxic or only photoallergen or even completely non-phototoxic; PPD was either classified as photoallergenic and allergenic, while 5-MOP and Musk ketone were identified as phototoxic and, surprisingly, as non-phototoxic. These disagreements make it difficult either to establish adequately novel assays and to further validate them.
4. Discussion
The primary objective of the cosmetic industry is to develop, produce, and market products that enhance or ameliorate the appearance of the human body. The cosmetic industry encompasses a wide range of products, including skincare, hair care, makeup, perfumes, and personal care items. However, beauty products are formulated not just for aesthetic purposes but also to optimize the natural functions of the skin as enhancing hydration [65]. On the other hand, ensuring the safety of cosmetics involves navigating numerous challenges, including the complexity of formulations, potential adverse reactions, and long-term health effects. The last regulation of EU on cosmetic products banning the traditional animal testing [24] along with the REACH regulation [25] and other regulatory norms has served as a catalyst for the emerge of innovative NAMs and underscores the necessity for new integrated approaches in testing and assessment besides using more human-relevant data [27]. In this sense, the International Cooperation on Cosmetics Regulation (ICCR) has studied the fundamentals that should be observed to incorporate such NAMs into an integrated strategy for risk assessment of cosmetics ingredients [66] and the Scientific Committee of Consumer Safety (SCCS) has included the requirements for evaluation of cosmetic ingredients [63].
Among the potential adverse effects that cosmetic ingredients or products can cause, photoinduced toxicity is described for different ingredients [4,67]. As explained previously, phototoxic reactions are caused when a compound or substance triggers harmful effects when exposed to light. UVA radiation is primarily responsible for such reactions, but adverse responses to certain drugs have also been documented upon exposure to UVB, visible light, or a combination of various wavelengths [68,69]. Phototoxic reactions encompass photoirritation and photoallergy, which differ from each other by their inherent pathophysiological mechanisms. Photoallergic reactions involve an immune response triggered by exposure to sunlight, as described elsewhere (Figure 3), and are regarded as T cell-mediated delayed type hypersensitivity [5,9,13,70]. In contrast, photoirritation induces cellular cytotoxicity, is a more common affection that can happen after the first and single contact with the photosensitizer, and is dose dependent [7,9,71,72,73].
There is extensive literature that explores the chemical and biological basis of drug-induced photosensitivity [72,74,75,76,77,78] but not much in the case of cosmetics. In this context, a recent examination by Hofmann et al. [69] identified a total of 393 distinct drugs or drug compounds reported as photosensitizers. However, the level of evidence varies across cases and may not be sufficient in all instances.
Personal care and cosmetic products contain a variety of compounds, including fragrances, plant extracts, polymers, detergents, and emulsifiers that sometimes make it difficult to use NAMs for safety assessment [79]. It has been reported that exposure to some of the constituents in personal care and cosmetic products could result in skin sensitization [5,80,81], but also other immune responses such as photoallergy. This is the case for fragrance mix I, a well-known contact sensitizer that contains a mixture of eight individual fragrances (cinnamic alcohol, cinnamic aldehyde, hydroxycitronellal, amylcinnamaldehyde, geraniol, euginol, isoeuginol, and oakmosse absolute) that also can cause photoallergy, as reported in a photopatch study [82].
UV filters and fragrances are the cosmetic ingredients considered to be the main culprits of photoallergic reactions [83,84,85,86,87,88]. In the case of sunscreens, allergic and photoallergic contact dermatitis were first reported over thirty years ago [89], and these adverse reactions were mainly attributed to organic filters, with oxybenzone (benzophenone-3) being the most frequently reported contact and photocontact allergen [88]. It is reported that patients photosensitized to KET develop photoallergy to octocrylene, a NSAID that contains a benzophenone moiety in its chemical structure. Synthesis of octocrylene implies the use of benzophenone that can remain as a residue, explaining such cross-reactivity [90]. Additionally, photoallergy from fragrances is observed in patients with drug allergies, such as ketoprofen [91].
Other cosmetic components that frequently hold photoallergenic capacity are preservatives like methylisothiazolinone, as demonstrated through photopatch tests [92]. Certain derivatives of cinnamaldehyde, cinnamyl alcohol, dihydrocinnamyl alcohol, and cinnamic acid, commonly used in cosmetics, face restrictions due to their photoallergenic effects [93].
Based on all of these considerations, to assay the potential photoallergy induced by UV filters, as well as other ingredients such as fragrances, presents a challenge for innovation in discovering and synthesizing novel components without adverse effects, optimizing sun-protection effects [94].
Assaying photoallergic reactions in the case of cosmetics ingredients and formulations is thus paramount for comprehensive safety assessments, requiring methods that accurately replicate human responses without relying on animal testing. Despite the rapid progress in the development of skincare products, there is a scarcity of suitable models for assessing the efficacy and safety of new active ingredients and formulations.
The studies collected here propose different assays based on the mechanism of photoallergy according to the strategy proposed by OECD for contact sensitization [49] and in keeping with the key events (KE) described by de Ávila et al. [48]. Moreover, the methods described in the articles try to adapt the validated assays for contact sensitization by including the UV irradiation, and some of them propose a sequential test strategy combining the novel adopted proposals with the first steps described for phototoxicity assessment, in particular UV-VIS spectral analysis and ROS assay [29,58].
Regarding the KE1 or molecular initiating event, seven studies have explored the capacity to form the complete hapten–protein complex formation. However, three of them were more mechanistic and focused on a specific class of compounds as UV filters [36], fenofibrates [40], or β-lactams [41] rather than in hazard identification. These studies can be useful for understanding certain aspects of photobinding steps that will help researchers to design future potential in silico models, but do not provide real progress in the field of photosafety assessment. The other four adapt ADRA [44,45] or DPRA [42,44,46] assays to identify contact sensitizers by coupling UVA lamps. The assays proved to be transferable among laboratories experienced in conducting high-performance liquid chromatography analysis [61]. Taking into consideration that among the four studies, almost 70 different chemicals besides allergen mixtures and herbicide formulations had been assessed, it has been proven that the volume of reaction [42], irradiation and time of incubation [44] can be lowered, and therefore these photoassays are promising tools for photosafety assessment.
In the case of KE2, two endpoints were selected as a strategy to identify photoallergens. The first one is based on the production of IL-18 using a keratinocyte cell line [33,35] or reconstructed human epidermis (RhE) [47]. It is well known that the development of allergic contact dermatitis (ACD) relies significantly on keratinocytes produced by the secretion of different cytokines as IL-18, which is necessary for the maturation and migration of dendritic cells activated by hapten, from the skin to the draining lymph nodules where they will present the antigens to naïve T cells [95]. Moreover, this interleukin has shown its utility to identify allergens even with weak capacity using cell lines [96], evaluating hair-coloring products [97] and could be a useful tool for classifying photoallergens according to their potency, as described for allergens using RhE [98]. The second one is an adaptation of Keratinosens™ [99] that also explores the use of the AREc32 cell line, but the number of chemicals assessed is still very low and the accuracy needs to be improved.
The third key event in the process of photoallergy implies phenotypic and functional changes in dendritic cells (DCs) as well as the induction of cytokines to facilitate the antigen-presenting capacity of DCs. Related to ACD, changes in the expression of surface molecules CD54 and CD86 were described in OECD 442E [100] to identify allergens in addition to the changes in IL-8 expression. Thus, Martínez et al. [34] demonstrated that using IL-8 to discriminate photoirritants from photoallergens could be an interesting tool for hazard identification. However, more studies need to be performed regarding the applicability domain. In the case of the other two proposals, one centered in changes in cell-surface thiols [37] and the other in intracellular ROS production [43], only the photo-SH/NH2 presented encouraging outcomes with an acceptable predictivity to identify photosensitizers but a less satisfactory performance regarding its ability to discriminate among photoirritants and photoallergens. More recently, Forreryd et al. [101] and Lindberg et al. [102] presented adaptations of the GARD™skin [100] using the SenzaCell cell line by analyzing changes in the genomic signature after exposure to chemicals. However, further research is still ongoing to improve their use for evaluating fragrances in the cosmetics industry.
Important aspects for the validation of any novel assay are the prediction model, as well as which chemicals and compounds with well-known toxicity patterns are used as positive controls. In this sense, some chemicals present different classifications among the studies, although most of them are recognized as phototoxic.
Moreover, to establish a reliable predictive capacity, the previous classification must be reliable and based on human data. Epidemiological studies indicate the difficulties of evaluating the prevalence of photoallergy, attributing such difficulty to different patch test protocols, among factors. However, it was reported that between 18 and 37% of the patients with a positive reaction were diagnosed with photoallergy [82,103,104], indicating that photoallergic reactions seem more frequent than previously believed. Moreover, it has been found that a narrow relationship exists among chronic actinic dermatitis (CAD), allergic contact dermatitis, and photoallergic contact dermatitis, although it is not clear whether CAD predisposes people to developing skin allergy or photoallergy or vice versa [7,82]. Similarly, an exacerbation of ACD manifestations with light has been described in a low percentage of patients [92,104]. In a recent case report, photoaggravation of ACD has been attributed to the UVB absorber isoamyl p-methoxycinnamate [105], which previous reports had identified as a photoallergic agent [106].
Identifying photoallergy presents challenges due to complex clinical presentations, broad differentials, and a scarcity of specialists conducting photo-testing [103]. Therefore, standardizing the panel agents as well as the patch-test procedure is crucial not only to facilitate comparisons among studies, but also to swiftly incorporate new agents when necessary [107,108]. Moreover, is important that physicians can recognize and manage drug-induced photosensitivity early by reviewing constantly renewed information [109].
Cosmetic ingredients in Europe are subject to two different regulations: the cosmetic regulation, which bans in vivo testing [24], and the REACH regulation [25], which allows animal testing for chemical safety assessment. However, there are still cosmetic ingredients registered under the REACH regulation that involve animal testing [110]. This situation requires more investment to accelerate the improvement of existing photoassays and the development of new NAMs. In this sense, the recent progress in microfluid technology as well as bioprinting offers us the opportunity to improve and refine all the photoassays described here. Examples of such improvements might include constructing three-dimensional skin models that include immunocompetent cells and melanocytes, or utilizing skin-on-a-chip platforms that mimic tissue microenvironments [111]. For these reasons, it is imperative to coordinate and collaborate among the industry, regulatory bodies, research institutions, academia, dermatologists, and administrative entities.
5. Conclusions
To address the complexities of photoallergic reactions and the challenges in discriminating them from phototoxic reactions, it is crucial to adopt a comprehensive approach to testing strategies. In silico, in chemico, and in vitro tests, including reconstructed human epidermis models, present promising alternatives. By integrating these methods into a sequential battery of tests, a thorough evaluation of cosmetic safety can be achieved, ensuring that products meet stringent standards without compromising ethical principles.
Author Contributions
Conceptualization, M.M. and M.P.V.; formal analysis, A.S.M. and M.M.; data curation, M.M.; writing—original draft preparation, M.M.; writing—review and editing, A.S.M., M.M. and M.P.V. All authors have read and agreed to the published version of the manuscript.
Funding
Grant PID2020-113186RB-I00 funded by MCIN/AEI/10.13039/501100011033.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
This study was performed thanks to grant PID2020-113186RB-I00, from Ministerio de Ciencia e inovación (España).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Diepgen, T.L.; Ofenloch, R.F.; Bruze, M.; Bertuccio, P.; Cazzaniga, S.; Coenraads, P.J.; Elsner, P.; Goncalo, M.; Svensson, Å.; Naldi, L. Prevalence of contact allergy in the general population in different European regions. Br. J. Dermatol. 2016, 174, 319–329. [Google Scholar] [CrossRef]
- Lagrelius, M.; Wahlgren, C.F.; Matura, M.; Kull, I.; Lidén, C. High prevalence of contact allergy in adolescence: Results from the population-based BAMSE birth cohort. Contact Dermat. 2016, 74, 44–51. [Google Scholar] [CrossRef]
- Alrashidi, A.; Rhodes, L.E.; Sharif, J.C.H.; Kreeshan, F.C.; Farrar, M.D.; Ahad, T. Systemic drug photosensitivity—Culprits, impact and investigation in 122 patients. Photodermatol. Photoimmunol. Photomed. 2020, 36, 441–451. [Google Scholar] [CrossRef]
- Korzeniowska, K.; Cieślewicz, A.; Chmara, E.; Jabłecka, A.; Pawlaczyk, M. Photosensitivity reactions in the elderly population: Questionnaire-based survey and literature review. Ther. Clin. Risk Manag. 2019, 15, 1111–1119. [Google Scholar] [CrossRef]
- Towle, K.M.; Fung, E.S.; Monnot, A.D. Phototoxicity Evaluation of Hair Cleansing Conditioners. Cosmetics 2019, 6, 53. [Google Scholar] [CrossRef]
- Elkeeb, D.; Elkeeb, L.; Maibach, H. Photosensitivity: A current biological overview. Cutan. Ocul. Toxicol. 2012, 31, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Gonçalo, M. Phototoxic and Photoallergic Contact Reactions. In Contact Dermatitis, 6th ed.; Johansen, J.D., Mahler, V., Lepoittevin, J.P., Frosch, P.J., Eds.; Springer: Cham, Switzerland, 2021; pp. 365–389. [Google Scholar] [CrossRef]
- Glatz, M.; Hofbauer, G.F.L. Phototoxic and photoallergic cutaneous drug reactions. Chem. Immunol. Allergy 2012, 97, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Onoue, S.; Seto, Y.; Sato, H.; Nishida, H.; Hirota, M.; Ashikaga, T.; Api, A.M.; Basketter, D.; Tokura, Y. Chemical photoallergy: Photobiochemical mechanisms, classification, and risk assessments. J. Dermatol. Sci. 2017, 85, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Deleo, V.A. Photocontact dermatitis. Dermatol. Ther. 2004, 17, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.F.; Rato, M.; Martins, C. Drug-induced photosensitivity: Photoallergic and phototoxic reactions. Clin. Dermatol. 2016, 34, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.S. Photodermatitis due to tetrachlorsalicylanilide. Brit J. Dermatol. 1961, 73, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Tokura, Y. Drug photoallergy. J. Cutan. Immunol. Allergy 2018, 1, 48–57. [Google Scholar] [CrossRef]
- Snyder, M.; Turrentine, J.E.; Cruz, P.D., Jr. Photocontact Dermatitis and Its Clinical Mimics: An Overview for the Allergist. Clin. Rev. Allergy Immunol. 2019, 56, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Victor, F.C.; Cohen, D.E.; Soter, N.A. A 20-year analysis of previous and emerging allergens that elicit photoallergic contact dermatitis. J. Am. Acad. Dermatol. 2010, 62, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ferriols, A.; de la Cuadra, O. La nueva batería europea de fotoalérgenos. Piel 2013, 28, 66–68. [Google Scholar] [CrossRef]
- Subiabre-Ferrer, D.; Esteve-Martínez, A.; Blasco-Encinas, R.; Sierra-Talamantes, C.; Pérez-Ferriols, A.; Zaragoza-Ninet, V. European photopatch test baseline series: A 3-year experience. Contact Dermat. 2019, 80, 5–8. [Google Scholar] [CrossRef]
- SCCS (Scientific Committee on Consumer Safety). Opinion on Octocrylene (CAS No 6197-30-4, EC No 228-250-8), Preliminary Version of 15 January 2021, Final Version of 30–31 March 2021, SCCS/1627/21. Available online: https://health.ec.europa.eu/system/files/2022-08/sccs_o_249.pdf (accessed on 14 February 2024).
- Aguiar, B.; Carmo, H.; Garrido, J.; Sousa Lobo, J.M.; Almeida, I.F. In Vitro Evaluation of the Photoreactivity and Phototoxicity of Natural Polyphenol Antioxidants. Molecules 2022, 27, 189. [Google Scholar] [CrossRef]
- Lovell, W.W.; Sanders, D.J. Phototoxicity testing in guinea-pigs. Food Chem. Toxicol. 1992, 30, 155–160. [Google Scholar] [CrossRef]
- Gerberick, G.F.; Ryan, C. A predictive mouse ear-swelling model for investigating topical phototoxicity. Food Chem. Toxicol. 1989, 27, 813–819. [Google Scholar] [CrossRef]
- Marzulli, F.N.; Maibach, H.I. Contact allergy: Predictive testing in man. Contact Dermat. 1976, 2, 1–17. [Google Scholar] [CrossRef]
- European Medicines Agency. ICH Guidance S10 on Photosafety Evaluation of Pharmaceuticals. 2015. Available online: https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/ich-guideline-s10-guidance-photosafety-evaluation-pharmaceuticals-step-3_en.pdf (accessed on 31 January 2024).
- European Parliament. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products. Off. J. Eur Union. 2009, L342, 59–209. Available online: http://data.europa.eu/eli/reg/2009/1223/oj (accessed on 31 January 2024).
- European Commission. Regulation (EC) No. 1907/2006 of the European Parliament and of the Council of 18 December 2006 Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACh), Establishing a European Chemicals Agency, Amending Directive 1999/45/EC and Repealing Council Regulation (EEC) No. 793/93 and Commission Regulation (EC) No. 1488/94 as Well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC. 2006. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02006R1907-20140410 (accessed on 31 January 2024).
- European Medicines Agency. ICH Guideline M3 (R2) on Non-Clinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorisation for Pharmaceuticals. 2009. Available online: https://www.ema.europa.eu/en/ich-m3-r2-non-clinical-safety-studies-conduct-human-clinical-trials-pharmaceuticals-scientific-guideline (accessed on 31 January 2024).
- Schmeisser, S.; Miccoli, A.; von Bergen, M.; Berggren, E.; Braeuning, A.; Busch, W.; Desaintes, C.; Gourmelon, A.; Grafström, R.; Harrill, J.; et al. New approach methodologies in human regulatory toxicology—Not if, but how and when! Environ. Int. 2023, 178, 108082. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 432: In Vitro 3T3 NRU Phototoxicity Test, OECD Guidelines for the Testing of Chemicals; Section 4; OECD Publishing: Paris, France, 2019. [Google Scholar] [CrossRef]
- OECD. Test No. 495: Ros (Reactive Oxygen Species) Assay for Photoreactivity, OECD Guidelines for the Testing of Chemicals; Section 4; OECD Publishing: Paris, France, 2019. [Google Scholar] [CrossRef]
- OECD. Test No. 498: In Vitro Phototoxicity—Reconstructed Human Epidermis Phototoxicity Test Method, OECD Guidelines for the Testing of Chemicals; Section 4; OECD Publishing: Paris, France, 2023. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Ak, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Sys. Rev. 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Bramer, W.M.; de Jonge, G.B.; Rethlefsen, M.L.; Mast, F.; Kleijnen, J. A systematic approach to searching: An efficient and complete method to develop literature searches. J. Med. Libr. Assoc. 2018, 106, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Galbiati, V.; Martínez, V.; Bianchi, S.; Mitjans, M.; Corsini, E. Establishment of an in vitro photoallergy test using NCTC2544 cells and IL-18 production. Toxicol. Vitr. 2013, 27, 103–110. [Google Scholar] [CrossRef]
- Martínez, V.; Galbiati, V.; Corsini, E.; Martín-Venegas, R.; Vinardell, M.P.; Mitjans, M. Establishment of an in vitro photoassay using THP-1 cells and IL-8 to discriminate photoirritants from photoallergens. Toxicol. Vitr. 2013, 27, 1920–1927. [Google Scholar] [CrossRef] [PubMed]
- Galbiati, V.; Bianchi, S.; Martínez, V.; Mitjans, M.; Corsini, E. NCTC 2544 and IL-18 production: A tool for the in vitro identification of photoallergens. Toxicol. Vitr. 2014, 28, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Stiefel, C.; Schwack, W. Reactivity of cosmetic UV filters towards skin proteins: Model studies with Boc-lysine, Boc-Gly-Phe-Gly-Lys-OH, BSA and gelatin. Int. J. Cosmet. Sci. 2014, 36, 561–570. [Google Scholar] [CrossRef]
- Oeda, S.; Hirota, M.; Nishida, H.; Ashikaga, T.; Sasa, H.; Aiba, S.; Tokura, Y.; Kouzuki, H. Development of an in vitro photosensitization test based on changes of cell-surface thiols and amines as biomarkers: The photo-SH/NH2 test. J. Toxicol. Sci. 2016, 41, 129–142. [Google Scholar] [CrossRef]
- Onoue, S.; Ohtake, H.; Suzuki, G.; Seto, Y.; Nishida, H.; Hirota, M.; Ashikaga, T.; Kouzuki, H. Comparative study on prediction performance of photosafety testing tools on photoallergens. Toxicol. Vitr. 2016, 33, 147–152. [Google Scholar] [CrossRef]
- Tsujita-Inoue, K.; Hirota, M.; Atobe, T.; Ashikaga, T.; Tokura, Y.; Kouzuki, H. Development of novel in vitro photosafety assays focused on the Keap1-Nrf2-ARE pathway. J. Appl. Toxicol. 2016, 36, 956–968. [Google Scholar] [CrossRef]
- Vayá, I.; Andreu, I.; Monje, V.T.; Jiménez, M.C.; Miranda, M.A. Mechanistic Studies on the Photoallergy Mediated by Fenofibric Acid: Photoreactivity with SerumAlbumins. Chem. Res Toxicol. 2016, 29, 40–46. [Google Scholar] [CrossRef]
- Pérez-Ruíz, R.; Lence, E.; Andreu, I.; Limones-Herrero, D.; González-Bello, C.; Miranda, M.A.; Jiménez, M.C. A New Pathway for Protein Haptenation by β-Lactams. Chemistry 2017, 23, 13986–13994. [Google Scholar] [CrossRef]
- de Ávila, R.I.; Teixeira, G.C.; Veloso, D.F.M.C.; Moreira, L.C.; Lima, E.M.; Valadares, M.C. In vitro assessment of skin sensitization, photosensitization and phototoxicity potential of commercial glyphosate-containing formulations. Toxicol. Vitr. 2017, 45, 386–392. [Google Scholar] [CrossRef]
- Toyoda, A.; Itagaki, H. Development of an in vitro photosafety evaluation method utilizing intracellular ROS production in THP-1 cells. J. Toxicol. Sci. 2018, 43, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.H.; Mishra, P.K.; Nagane, R.; Deshpande, A.; Tamboli, I.Y.; Date, R. Comparison of in chemico skin sensitization methods and development of an in chemico skin photosensitization assay. ALTEX 2019, 36, 373–387. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Fujita, M.; Wanibuchi, S.; Sato, A.; Katsuoka, Y.; Kasahara, T. Development of photo-amino acid derivative reactivity assay: A novel in chemico alternative method for predicting photoallergy. J. Appl. Toxicol. 2020, 40, 655–678. [Google Scholar] [CrossRef] [PubMed]
- Nishida, H.; Ohtake, T.; Ashikaga, T.; Hirota, M.; Onoue, S.; Seto, Y.; Tokura, Y.; Kouzuki, H. In chemico sequential testing strategy for assessing the photoallegic potential. Toxicol. Vitr. 2021, 77, 105245. [Google Scholar] [CrossRef]
- Nguyen, R.; Barry, M.; Azevedo Loiola, R.; Ferret, P.-J.; Andres, E. PhotoSENSIL-18 assay development: Enhancing the safety testing of cosmetic raw materials and finished products to support the in vitro photosensitization assessment? Toxicology 2023, 495, 153613. [Google Scholar] [CrossRef]
- de Ávila, R.I.; Aleksic, M.; Zhu, B.; Li, J.; Pendlington, R.; Valadares, M.C. Non-animal approaches for photoallergenicity safety assessment: Needs and perspectives for the toxicology for the 21st century. Regul. Toxicol. Pharmacol. 2023, 145, 105499. [Google Scholar] [CrossRef] [PubMed]
- OECD. The Adverse Outcome Pathway for Skin Sensitisation Initiated by Covalent Binding to Proteins, OECD Series on Testing and Assessment; No. 168; OECD Publishing: Paris, France, 2014. [Google Scholar] [CrossRef]
- Mitjans, M.; Galbiati, V.; Lucchi, L.; Viviani, B.; Marinovich, M.; Galli, C.L.; Corsini, E. Use of IL-8 release and p38 MAPK activation in THP-1 cells to identify allergens and to assess their potency in vitro. Toxicol. Vitr. 2010, 24, 1803–1809. [Google Scholar] [CrossRef]
- Stiefel, C.; Schwack, W. Rapid screening method to study the reactivity of UV filter substances towards skin proteins by high-performance thin-layer chromatography. Int. J. Cosmet. Sci. 2013, 35, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Hirota, M.; Hagino, S.; Itagaki, H.; Aiba, S. Evaluation of changes of cell-surface thiols as a new biomarker for in vitro sensitization test. Toxicol. Vitr. 2009, 23, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Lovell, W.W.; Jones, P.A. An Evaluation of Mechanistic In Vitro Tests for the Discrimination of Photoallergic and Photoirritant Potential. Altern. Lab. Anim. 2000, 28, 707–724. [Google Scholar] [CrossRef]
- Onoue, S.; Suzuki, G.; Kato, M.; Hirota, M.; Nishida, H.; Kitagaki, M.; Kouzuki, H.; Yamada, S. Non-animal photosafety assessment approaches for cosmetics based on the photochemical and photobiochemical properties. Toxicol. Vitr. 2013, 27, 2316–2324. [Google Scholar] [CrossRef]
- Moore, D.E. Drug-induced cutaneous photosensitivity: Incidence, mechanism, prevention and management. Drug Saf. 2002, 25, 345–372. [Google Scholar] [CrossRef]
- Vargas, F.; Martinez Volkmar, I.; Sequera, J.; Mendez, H.; Rojas, J.; Fraile, G.; Velasquez, M.; Medina, R. Photodegradation and phototoxicity studies of furosemide. Involvement of singlet oxygen in the photoinduced hemolysis and lipid peroxidation. J. Photochem. Photobiol. B. 1998, 42, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.; Foti, C.; Alsante, K. Can light absorption and photostability data be used to assess the photosafety risks in patients for a new drug molecule? Photochem. Photobiol. B. 2009, 96, 57–62. [Google Scholar] [CrossRef]
- OECD. Test No. 101: UV-VIS Absorption Spectra, OECD Guidelines for the Testing of Chemicals; Section 1; OECD Publishing: Paris, France, 1981. [Google Scholar] [CrossRef]
- FDA. Available online: https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/july-september-2017-potential-signals-serious-risksnew-safety-information-identified-fda-adverse (accessed on 1 February 2024).
- Naisbitt, D.J.; Nattrass, R.G.; Ogese, M.O. In Vitro Diagnosis of Delayed-type Drug Hypersensitivity: Mechanistic Aspects and Unmet Needs. Immunol. Allergy Clin. N. Am. 2014, 34, 691–705. [Google Scholar] [CrossRef]
- OECD. Test No. 442C: In Chemico Skin Sensitisation: Assays Addressing the Adverse Outcome Pathway Key Event on Covalent Binding to Proteins, OECD Guidelines for the Testing of Chemicals; Section 4; OECD Publishing: Paris, France, 2023. [Google Scholar] [CrossRef]
- Pan, N.; Xia, Y.; Hou, W.; Zhu, G.; Zhang, J.; Lai, W.; Zheng, Y. Assessment of Skin Photoallergy Risk in Cosmetics Containing Herbal Extract Ingredients. Ski. Pharmacol. Physiol. 2021, 34, 253–261. [Google Scholar] [CrossRef]
- SCCS (Scientific Committee on Consumer Safety). SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety Evaluation 12th Revision, 15 May 2023, Corrigendum 1 on 26 October 2023, Corrigendum 2 on 21 December 2023, SCCS/1647/22. Available online: https://health.ec.europa.eu/latest-updates/sccs-notes-guidance-testing-cosmetic-ingredients-and-their-safety-evaluation-12th-revision-2023-05-16_en (accessed on 20 February 2024).
- Rato, M.; Gil, F.; Monteiro, A.F.; Parente, J. Fenofibrate photoallergy—Relevance of patch and photopatch testing. Contact Dermat. 2018, 78, 413–414. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Ji, W.; Dicolandrea, T.; Finlay, D.; Supp, D.; Boyce, S.; Wei, K.; Kadekaro, A.L.; Zhang, Y. An improved human skin explant culture method for testing and assessing personal care products. J. Cosmet. Dermatol. 2023, 22, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- Dent, M.; Amaral, R.; Amores Da Silva, P.; Ansell, J.; Boisleve, F.; Hatao, M.; Hirose, A.; Kasai, Y.; Kern, P.; Kreiling, R.; et al. Principles underpinning the use of new methodologies in the risk assessment of cosmetic ingredients. Comput. Toxicol. 2018, 7, 20–26. [Google Scholar] [CrossRef]
- Ritacco, G.; Hilberer, A.; Lavelle, M.; Api, A.M. Use of alternative test methods in a tiered testing approach to address photoirritation potential of fragrance materials. Regul. Toxicol. Pharm. 2022, 129, 105098. [Google Scholar] [CrossRef] [PubMed]
- Blakely, K.M.; Drucker, A.M.; Rosen, C.F. Drug-Induced Photosensitivity—An Update: Culprit Drugs, Prevention and Management. Drug Saf. 2019, 42, 827–847. [Google Scholar] [CrossRef]
- Hofmann, G.A.; Weber, B. Drug-induced photosensitivity: Culprit drugs, potential mechanisms and clinical consequences. J. Dtsch Dermatol. Ges. 2021, 19, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, S.; Worswick, S. Photosensitizing drug reactions. Clin. Dermatol. 2022, 40, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Beani, J.C. Phototoxicity, Photoirritation, and Photoallergy Detection and Assessment. In Agache’s Measuring the Skin, 2nd ed.; Humbert, P., Fanian, F., Maibach, H., Agache, P., Eds.; Springer: Cham, Switzerland, 2017; pp. 1061–1069. [Google Scholar] [CrossRef]
- Kowalska, J.; Rok, J.; Rzepka, Z.; Wrześniok, D. Drug-Induced Photosensitivity—From Light and Chemistry to Biological Reactions and Clinical Symptoms. Pharmaceuticals 2021, 14, 723. [Google Scholar] [CrossRef]
- Davis, A.E.; Kennelley, G.E.; Amaye-Obu, T.; Jowdy, P.F.; Ghadersohi, S.; Nasir-Moin, M.; Paragh, G.; Berman, H.A.; Huss, W.J. The phenomenon of phototoxicity and long-term risks of commonly prescribed and structurally diverse drugs. J. Photochem. Photobiol. 2024, 19, 100221. [Google Scholar] [CrossRef]
- Al-Jarrah, R.; Blasini, A.; Kurgyis, Z.; Brockow, K.; Eberlein, B. Severe photoallergy to systemic dronedarone (Multaq). Contact Dermat. 2020, 83, 241–242. [Google Scholar] [CrossRef]
- Lozzi, F.; Di Raimondo, C.; Lanna, C.; Diluvio, L.; Mazzilli, S.; Garofalo, V.; Dika, E.; Dellambra, E.; Coniglione, F.; Bianchi, L.; et al. Latest Evidence Regarding the Effects of Photosensitive Drugs on the Skin: Pathogenetic Mechanisms and Clinical Manifestations. Pharmaceutics 2020, 12, 1104. [Google Scholar] [CrossRef]
- Rojas Perez-Ezquerra, P.; Torrado-Español, I.; Tejero-Alcalde, M.; Cuevas-Bravo, C.; Noguerado-Mellado, B. Photoallergy to Naproxen. Cureus 2021, 13, e18961. [Google Scholar] [CrossRef] [PubMed]
- Kelemen, H.; Hancu, G.; Kacsó, E.; Papp, L.A. Photosensitivity Reactions Induced by Photochemical Degradation of Drugs. Adv. Pharm. Bull. 2022, 12, 77–85. [Google Scholar] [CrossRef]
- Romita, P.; Foti, C.; Mennuni, B.G.; Ambrogio, F.; Poli, M.A.; Tramontana, M.; Hansel, K.; Stingeni, L. Perioral photoallergic dermatitis to promazine hydrochloride. Contact Dermat. 2022, 86, 561–562. [Google Scholar] [CrossRef]
- Nishida, H.; Hirota, M.; Seto, Y.; Suzuki, G.; Kato, M.; Kitagaki, M.; Sugiyama, M.; Kouzuki, H.; Onoue, S. Non-animal photosafety screening for complex cosmetic ingredients with photochemical and photobiochemical assessment tools. Regul. Toxicol. Pharm. 2015, 72, 578–585. [Google Scholar] [CrossRef]
- Drechsel, D.A.; Towle, K.M.; Fung, E.S.; Novick, R.M.; Paustenbach, D.J.; Monnot, A.D. Skin sensitization induction potential from daily exposure to fragrances in personal care products. Dermatitis 2018, 29, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Monnot, A.D.; Towle, K.M.; Ahmed, S.S.; Dickinson, A.M.; Fung, E.S. An in vitro human assay for evaluating immunogenic and sensitization potential of a personal care and cosmetic product. Toxicol. Mech. Method 2021, 31, 205–211. [Google Scholar] [CrossRef]
- Shao, Y.; Hu, Y.; Wang, D.; Zhu, Y.; Shen, Y.; Xu, J.; Tang, H. Photopatch testing in Chinese patients: A 5-year experience. Contact Dermat. 2021, 85, 78–84. [Google Scholar] [CrossRef]
- Nash, J.F.; Tanner, P.R. Relevance of UV filter/sunscreen product photostability to human safety. Photodermatol. Photoimmunol. Photomed. 2014, 30, 88–95. [Google Scholar] [CrossRef]
- Berardesca, E.; Zuberbier, T.; Sanchez Viera, M.; Marinovich, M. Review of the safety of octocrylene used as an ultraviolet filter in cosmetics. J. Eur. Acad. Dermatol. Venereol. 2019, 33 (Suppl. S7), 25–33. [Google Scholar] [CrossRef]
- Ludriksone, L.; Elsner, P. Adverse Reactions to Sunscreens. Curr. Probl. Dermatol. 2021, 55, 223–235. [Google Scholar] [CrossRef]
- Pastor-Nieto, M.A.; Gatica-Ortega, M.E. Ubiquity, Hazardous Effects, and Risk Assessment of Fragrances in Consumer Products. Curr. Treat Options Allergy 2021, 8, 21–41. [Google Scholar] [CrossRef]
- Roh, J.; Cheng, H. Ultraviolet filter, fragrance and preservative allergens in New Zealand sunscreens. Australas. J. Dermatol. 2022, 63, e21–e25. [Google Scholar] [CrossRef]
- Ekstein, S.F.; Hylwa, S. Sunscreens: A Review of UV Filters and Their Allergic Potential. Dermatitis 2023, 34, 176–190. [Google Scholar] [CrossRef]
- English, J.S.; White, I.R.; Cronin, E. Sensitivity to sunscreens. Contact Dermat. 1987, 17, 159–162. [Google Scholar] [CrossRef]
- Foubert, K.; Dendooven, E.; Theunis, M.; Naessens, T.; Ivanova, B.; Pieters, L.; Gilissen, L.; Huygens, S.; De Borggraeve, W.; Lambert, J.; et al. The presence of benzophenone in sunscreens and cosmetics containing the organic UV filter octocrylene: A laboratory study. Contact Dermat. 2021, 85, 69–77. [Google Scholar] [CrossRef]
- Bruze, M.; Marmgren, V.; Antelmi, A.; Hindsén-Stenström, M.; Svedman, C.; Zimersson, E.; Mowitz, M. Contact Allergy to Oxidized Linalool and Oxidized Limonene is Over-represented in Individuals with Photocontact Allergy to Ketoprofen. Acta Derm. Venereol. 2021, 101, adv00454. [Google Scholar] [CrossRef] [PubMed]
- Aerts, O.; Goossens, A.; Marguery, M.C.; Castelain, M.; Boursault, L.; Giordano-Labadie, F.; Lambert, J.; Milpied, B. Photoaggravated allergic contact dermatitis and transient photosensitivity caused by methylisothiazolinone. Contact Dermat. 2018, 78, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Gunia-Krzyżak, A.; Słoczyńska, K.; Popiół, J.; Koczurkiewicz, P.; Marona, H.; Pękala, E. Cinnamic acid derivatives in cosmetics: Current use and future prospects. Int. J. Cosmet. Sci. 2018, 40, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Ngoc, L.T.N.; Tran, V.V.; Moon, J.-Y.; Chae, M.; Park, D.; Lee, Y.-C. Recent Trends of Sunscreen Cosmetic: An Update Review. Cosmetics 2019, 6, 64. [Google Scholar] [CrossRef]
- Yamaguchi, H.L.; Yamaguchi, Y.; Peeva, E. Role of Innate Immunity in Allergic Contact Dermatitis: An Update. Int. J. Mol. Sci. 2023, 24, 12975. [Google Scholar] [CrossRef] [PubMed]
- Galbiati, V.; Maddalon, A.; Iulini, M.; Marina Marinovich, M.; Corsini, E. Human keratinocytes and monocytes co-culture cell system: An important contribution for the study of moderate and weak sensitizers. Toxicol. Vitr. 2020, 68, 104929. [Google Scholar] [CrossRef] [PubMed]
- de Avila, R.I.; Veloso, D.F.M.C.; Teixeira, G.C.; Rodrigues, T.L.; Lindberg, T.; Lindstedt, M.; Fonseca, S.G.; Lima, E.M.; Valadares, M.C. Evaluation of in vitro testing strategies for hazard assessment of the skin sensitization potential of “real-life” mixtures: The case of henna-based hair-colouring products containing p-phenylenediamine. Contact Dermat. 2019, 81, 194–209. [Google Scholar] [CrossRef] [PubMed]
- Corsini, E.; Gibbs, S.; Roggen, E.; Kimber, I.; Basketter, D.A. Skin Sensitization Tests: The LLNA and the RhE IL-18 Potency Assay. In Toxicity Assessement. Methods and Protocols, 1st ed.; Palmeira, C.M.M., de Oliveira, D.P., Dorta, D.J., Eds.; Humana New York: New York, NY, USA, 2021; pp. 13–29. [Google Scholar] [CrossRef]
- OECD. Test No. 442D: In Vitro Skin Sensitisation: ARE-Nrf2 Luciferase Test Method, OECD Guidelines for the Testing of Chemicals; Section 4; OECD Publishing: Paris, France, 2022. [Google Scholar] [CrossRef]
- OECD. Test No. 442E: In Vitro Skin Sensitisation: In Vitro Skin Sensitisation Assays Addressing the Key Event on Activation of Dendritic Cells on the Adverse Outcome Pathway for Skin Sensitisation, OECD Guidelines for the Testing of Chemicals; Section 4; OECD Publishing: Paris, France, 2023. [Google Scholar] [CrossRef]
- Forreryd, A.; Johansson, A.; Ritacco, G.; Api, A.M.; Henrik Johansson, H. Hazard Assessment of Photoallergens Using GARD™skin. ALTEX Proc. 2021, 9, 251. [Google Scholar]
- Lindberg, T.; Ritacco, G.; Jerre, A.; Gradin, R.; Forreryd, A.; Johansson, H.; Api, A.M. GARD®skin Dose-Response for Photosensitization: Assessment of Reference Photoirritants and Photoallergens. The 2023 Society of Toxicology (SOT) Annual Meeting. 2023. Available online: https://senzagen.com/2023/03/19/gardskin-dose-response-for-photosensitization-assessment-of-reference-photoirritants-and-photoallergens/ (accessed on 1 February 2024).
- Hinton, A.N.; Goldminz, A.M. Feeling the Burn: Phototoxicity and Photoallergy. Dermatol. Clin. 2020, 38, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.D.; Goon, A.T.; Leow, Y.H.; Chong, T.Y.R.; Tan, E.S.T.; Cheng, W.N.S. Photopatch testing in Singapore: A 10-year retrospective study. Photodermatol. Photoimmunol. Photomed. 2023, 39, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Linares-Navarro, R.; Ruiz González, I.; Olmos Nieva, C.C.; Rodríguez Prieto, M.Á. Photoaggravated Allergic Contact Dermatitis Due to Isoamyl p-Methoxycinnamate in a Pediatric Patient. Actas Dermo-Sifiliográficas 2023, 114, T833–T834. Available online: https://www.actasdermo.org/es-translated-article-photoaggravated-allergic-contact-articulo-S000173102300621X (accessed on 9 February 2024). [CrossRef]
- Ghazavi, M.K.; Johnston, G.A. Photo-allergic contact dermatitis caused by isoamyl p-methoxycinnamate in an ‘organic’ sunscreen. Contact Dermat. 2011, 64, 115–116. [Google Scholar] [CrossRef]
- Ferguson, J.; Kerr, A.C. Photoallergic Contact Dermatitis. In Kanerva’s Occupational Dermatology, 3rd ed.; John, S., Johansen, J., Rustemeyer, T., Elsner, P., Maibach, H., Eds.; Springer: Cham, Switzerland, 2020; pp. 211–227. [Google Scholar] [CrossRef]
- García-Castro, R.; Velasco-Tirado, V.; Alonso-Sardón, M.; González-de Arriba, M. Standard photopatch test battery? Proposal based on current epidemiology and experience in our Skin Allergy Unit. Photodermatol. Photoimmunol. Photomed. 2021, 37, 449–453. [Google Scholar] [CrossRef]
- Di Bartolomeo, L.; Irrera, N.; Campo, G.M.; Borgia, F.; Motolese, A.; Vaccaro, F.; Squadrito, F.; Altavilla, D.; Condorelli, A.G.; Motolese, A.; et al. Drug-Induced Photosensitivity: Clinical Types of Phototoxicity and Photoallergy and Pathogenetic Mechanisms. Front. Allergy 2022, 3, 876695. [Google Scholar] [CrossRef]
- Knight, J.; Rovida, C.; Kreiling, R.; Zhu, C.; Knudsen, M.; Hartung, T. Continuing animal tests on cosmetic ingredients for REACH in the EU. ALTEX 2021, 38, 653–668. [Google Scholar] [CrossRef] [PubMed]
- Filaire, E.; Nachat-Kappes, R.; Laporte, C.; Harmand, M.F.; Marina Simon, M.; Christian Poinsot, C. Alternative in vitro models used in the main safety tests of cosmetic products and new challenges. Int. J. Cosmet. Sci. 2022, 44, 604–613. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).