Anti-Aging Properties of Cannabis sativa Leaf Extract against UVA Irradiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Extract Solution of Cannabis sativa Leaves (CLES)
2.2. Determination of Cannabidiol (CBD) and Tetrahydrocannabinol (THC) Contents in the CLES
2.3. Determination of Total Phenolic Content (TPC) of the CLES
2.4. Determination of Antioxidant Activity of the CLES by Using DPPH Assay
2.5. Determination of Cytotoxicity of the CLES on Human Skin Fibroblasts
2.6. Determination of Preventive Effect of the CLES on UVA-Induced NHDFs Alterations
2.6.1. UVA Irradiation and Cell Treatment
2.6.2. Determination of the Secreted Type I Pro-Collagen, Matrix Metalloproteinase-1 (MMP-1), and Interleukin-6 (IL-6) Levels
2.7. Determination of Restoration of Aged NHDFs by the CLES
3. Results
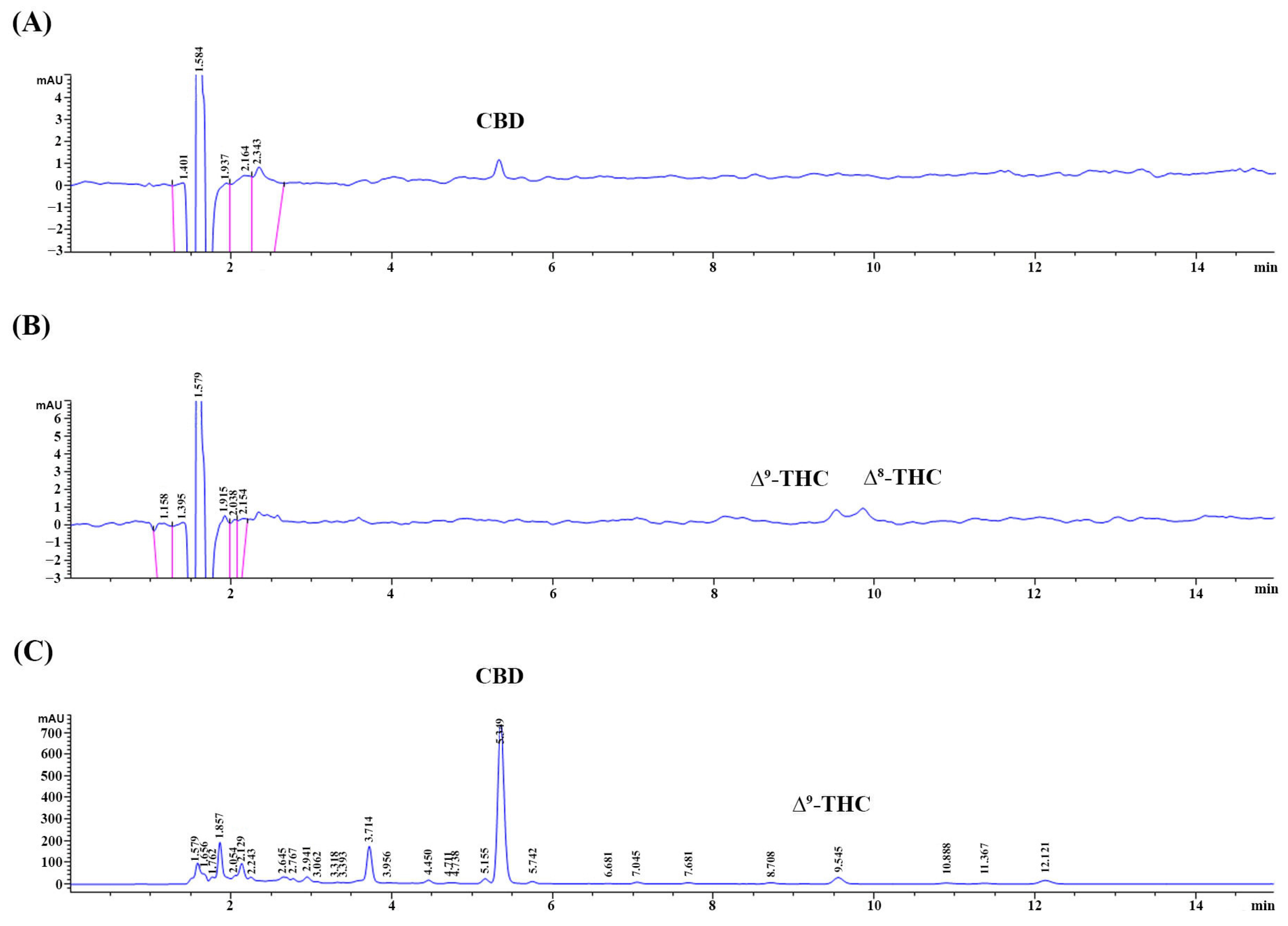
3.1. The Appearance of the CLES and Its CBD, THC, and TPC
3.2. Free Radical Scavenging Activity of the CLES
3.3. Effect of the CLES on Viability of NHDFs
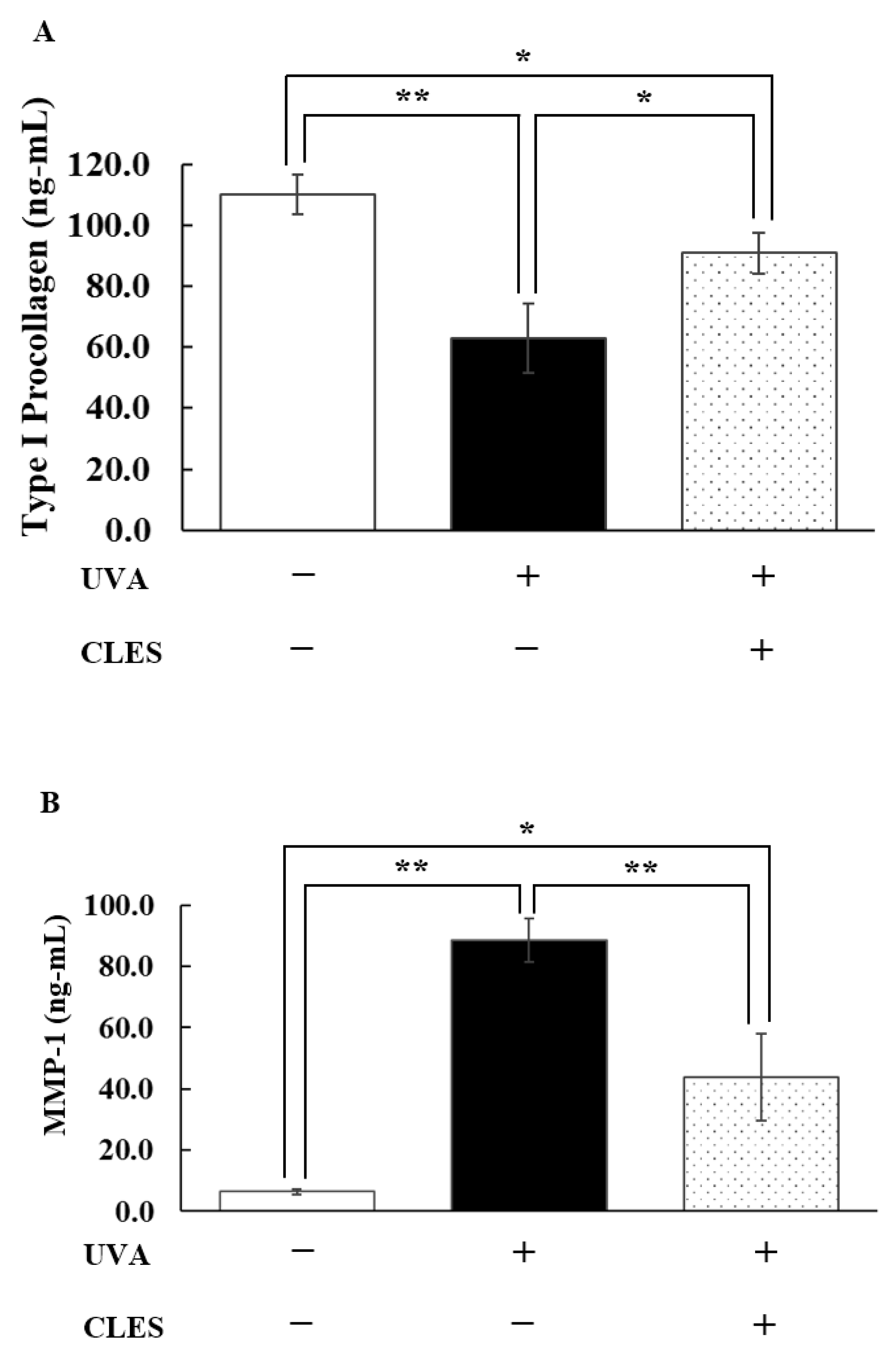
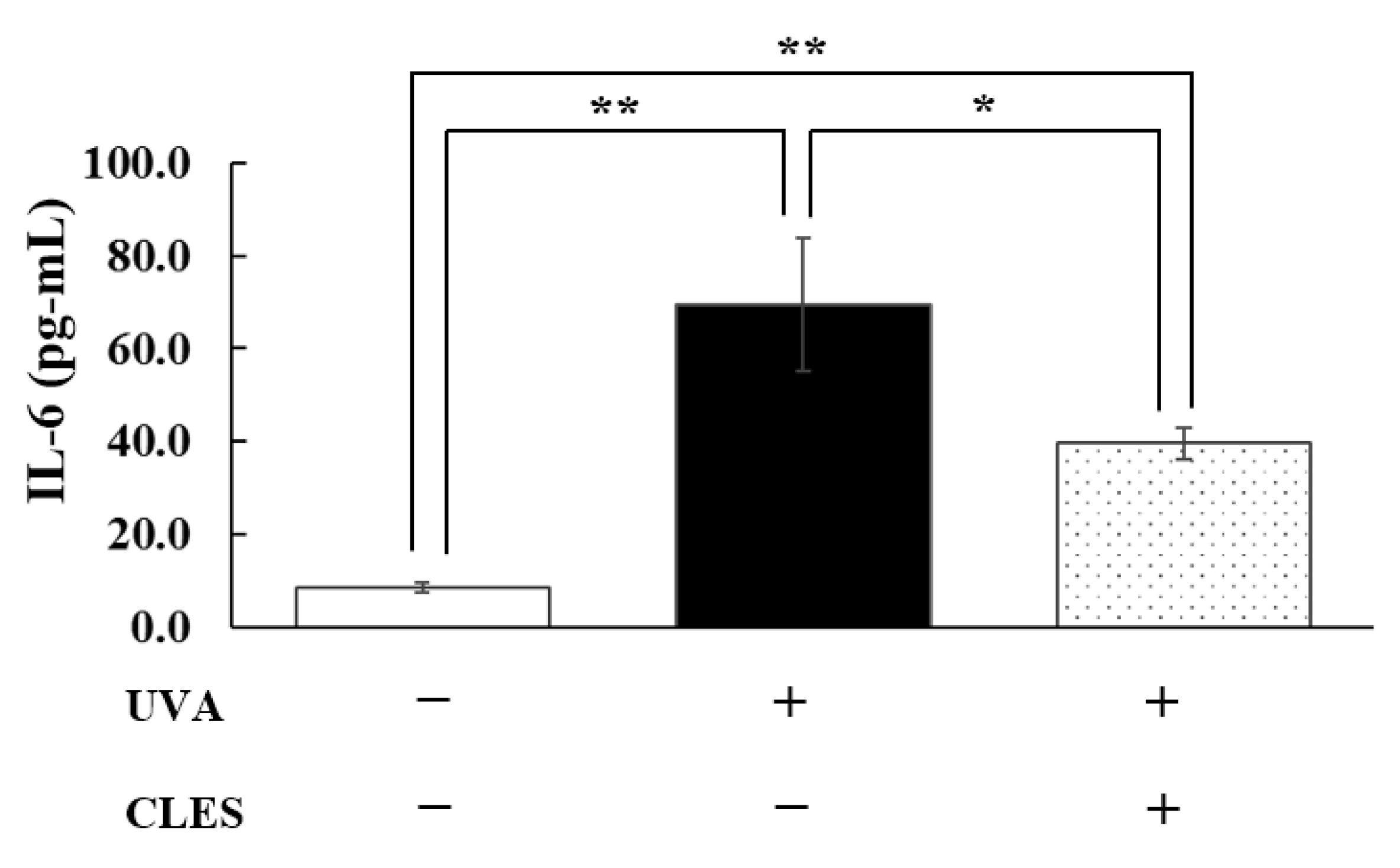
3.4. Preventive Effect of the CLES on UVA-Induced Alterations of NHDFs
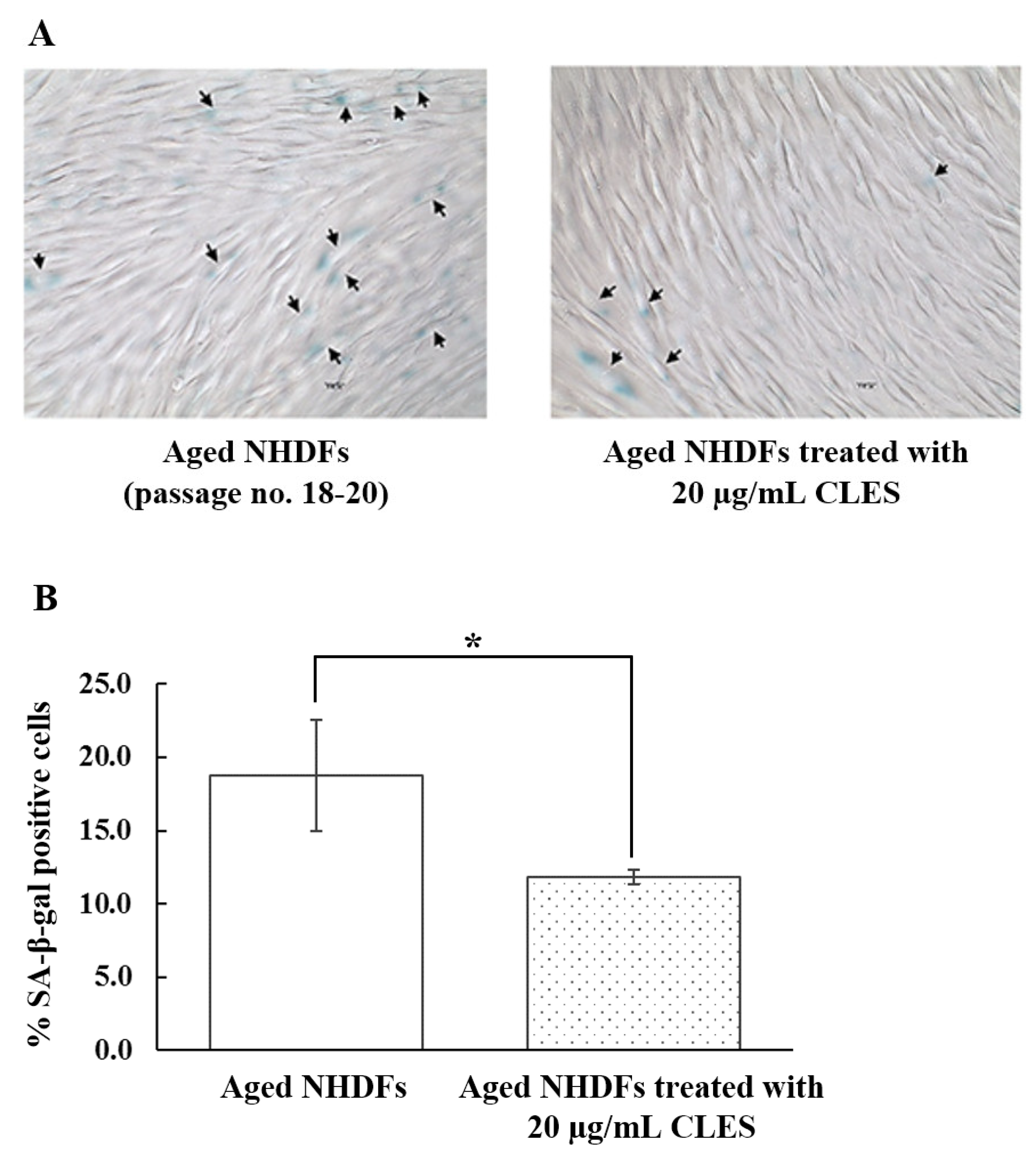
3.5. Restoration of Aged NHDFs by the CLES
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, S.; Mohri, S.; Manabe, Y.; Ejima, A.; Sato, K.; Sugawara, T. Gly-Pro protects normal human dermal fibroblasts from UVA-induced damages via MAPK-NF-κB signaling pathway. J. Photochem. Photobiol. B 2022, 237, 112601. [Google Scholar] [CrossRef]
- Karthikeyan, R.; Kanimozhi, G.; Prasad, N.R.; Agilan, B.; Ganesan, M.; Mohana, S.; Srithar, G. 7-Hydroxycoumarin prevents UVB-induced activation of NF-κB and subsequent overexpression of matrix metalloproteinases and inflammatory markers in human dermal fibroblast cells. J. Photochem. Photobiol. B 2016, 161, 170–176. [Google Scholar] [CrossRef]
- Reelfs, O.; Tyrrell, R.M.; Pourzand, C. Ultraviolet A radiation-induced immediate iron release is a key modulator of the activation of NF-ĸB in human skin fibroblasts. J. Investig. Dermatol. 2004, 122, 1440–1447. [Google Scholar] [CrossRef]
- Yang, B.; Ji, C.; Chen, X.; Cui, L.; Bi, Z.; Wan, Y.; Xu, J. Protective effect of astragaloside IV against matrix metalloproteinase-1 expression in ultraviolet-irradiated human dermal fibroblasts. Arch. Pharm. Res. 2011, 34, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; He, T.; Voorhees, J.J.; Fisher, G.J. Ultraviolet irradiation induces Smad7 via induction of transcription factor AP-1 in human skin fibroblasts. J. Biol. Chem. 2005, 280, 8079–8085. [Google Scholar] [CrossRef] [PubMed]
- Wlaschek, M.; Bolsen, K.; Herrmann, G.; Schwarz, A.; Wilmroth, F.; Heinrich, P.C.; Goerz, G.; Scharffetter-Kochanek, K. UVA-induced autocrine stimulation of fibroblasts-derived-collagenase by IL-6: A possible mechanism in dermal photodamage? J. Investig. Dermatol. 1993, 101, 164–168. [Google Scholar] [CrossRef]
- Lämmermann, I.; Terlecki-Zaniewicz, L.; Weinmüllner, R.; Schosserer, M.; Dellago, H.; de Matos Branco, A.D.; Autheried, D.; Sevcnikar, B.; Kleissl, L.; Berlin, I.; et al. Blocking negative effects of senescence in human skin fibroblasts with a plant extract. N.P.J. Aging Mech. Dis. 2018, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.R.; Cho, K.A.; Kang, H.T.; Lee, J.B.; Kaeberlein, M.; Suh, Y.; Chung, I.K.; Park, S.C. Restoration of senescent human diploid fibroblasts by modulation of the extracellular matrix. Aging Cell 2011, 10, 148–157. [Google Scholar] [CrossRef]
- Miyachi, Y.; Imamura, S.; Niwa, Y. Decreased Skin Superoxide Dismutase Activity by a Single Exposure of Ultraviolet Radiation Is Reduced by Liposomal Superoxide Dismutase Pretreatment. J. Investig. Dermatol. 1987, 89, 111–112. [Google Scholar] [CrossRef]
- Fuchs, J.; Huflejt, M.E.; Rothfuss, L.M.; Wilson, D.S.; Carcamo, G.; Packer, L. Impairment of Enzymic and Nonenzymic Antioxidants in Skin by UVB Irradiation. J. Investig. Dermatol. 1989, 93, 769–773. [Google Scholar] [CrossRef]
- Steenvoorden, D.P.T.; Beijersbergen van Henegouwen, G.M.J. The use of endogenous antioxidants to improve photoprotection. J. Photochem. Photobiol. B Biol. 1997, 41, 1–10. [Google Scholar] [CrossRef]
- Miyachi, Y. Photoaging from an oxidative standpoint. J. Dermatol. Sci. 1995, 9, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, Y.; Zhao, Z.; Qiu, J. Oxidative stress in the skin: Impact and related protection. Int. J. Cosmet. Sci. 2021, 43, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Varesi, A.; Chirumbolo, S.; Campagnoli, L.I.M.; Pierella, E.; Piccini, G.B.; Carrara, A.; Ricevuti, G.; Scassellati, C.; Bonvicini, C.; Pascale, A. The Role of Antioxidants in the Interplay between Oxidative Stress and Senescence. Antioxidants 2022, 11, 1224. [Google Scholar] [CrossRef]
- Liu, X.; Xing, Y.; Yuen, M.; Yuen, T.; Yuen, H.; Peng, Q. Anti-Aging Effect and Mechanism of Proanthocyanidins Extracted from Sea buckthorn on Hydrogen Peroxide-Induced Aging Human Skin Fibroblasts. Antioxidants 2022, 11, 1900. [Google Scholar] [CrossRef] [PubMed]
- Dunaway, S.; Odin, R.; Zhou, L.; Ji, L.; Zhang, Y.; Kadekaro, A.L. Natural Antioxidants: Multiple Mechanisms to Protect Skin from Solar Radiation. Front. Pharmacol. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zagórska-Dziok, M.; Bujak, T.; Ziemlewska, A.; Nizioł-Łukaszewska, Z. Positive effect of Cannabis sativa L. herb extracts on skin cells and assessment of cannabinoid-based hydrogels properties. Molecules 2021, 26, 802. [Google Scholar] [CrossRef]
- Di Giacomo, V.; Recinella, L.; Chiavaroli, A.; Orlando, G.; Cataldi, A.; Rapino, M.; Di Valerio, V.; Politi, M.; Antolini, M.D.; Acquaviva, A.; et al. Metabolomic profile and antioxidant/anti-Inflammatory effects of industrial hemp water extract in fibroblasts, keratinocytes and isolated mouse skin specimens. Antioxidants 2021, 10, 44. [Google Scholar] [CrossRef]
- Manosroi, A.; Chankhampan, C.; Kietthanakorn, B.; Ruksiriwanich, W.; Chaikul, C.; Boonpisuttinant, K.; Sainakham, M.; Manosroi, W.; Tangjai, T.; Manosroi, J. Pharmaceutical and cosmeceutical biological activities of hemp (Cannabis sativa L var. sativa) leaf and seed extracts. Chiang Mai J. Sci. 2019, 46, 180–195. [Google Scholar]
- Jaidee, W.; Siridechakorn, I.; Nessopa, S.; Wisuitiprot, V.; Chaiwangrach, N.; Ingkaninan, K.; Waranuch, N. Kinetics of CBD, ∆9-THC degradation and cannabinol formation in cannabis Resin at various temperature and pH conditions. Cannabis Cannbinoid Res. 2022, 7, 537–547. [Google Scholar] [CrossRef]
- Singleton, L.V.; Orthofer, R.; Lamuela-Raventó, M.R. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Chana, N.; Muengpoon, A.; Pethkaew, S.; Kulsin, N.; Mahasuk, A.; Srirat, S. Screening for phenolic compounds and oxidative capacity of fruit peels, agricultural waste, and traditional herbal medicine for use as biodiesel fuel additive. Songklanakarin J. Sci. Technol. 2022, 44, 1067–1074. [Google Scholar]
- Luangpraditkun, K.; Charoensit, P.; Grandmottet, F.; Viennet, C.; Viyoch, J. Photoprotective potential of the natural artocarpin against in vitro UVB-induced apoptosis. Oxid. Med. Cell Longev. 2020, 2020, 1042451. [Google Scholar] [CrossRef]
- Booz, G.W. Cannabidiol as an Emergent Therapeutic Strategy for Lessening the Impact of Inflammation on Oxidative Stress. Free Radic. Biol. Med. 2011, 51, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Gould, J. The Cannabis Crop. Nature 2015, 525, S2–S3. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Masteikova, R.; Lazauskas, R.; Bernatoniene, J. Cannabis Sativa L. Bioactive Compounds and Their Protective Role in Oxidative Stress and Inflammation. Antioxidants 2022, 11, 660. [Google Scholar] [CrossRef] [PubMed]
- Mounessa, J.S.; Siegel, J.A.; Dunnick, C.A.; Dellavalle, R.P. The role of cannabinoids in dermatology. J. Am. Acad. Dermatol. 2017, 77, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Stinchcomb, A.L.; Valiveti, S.; Hammell, D.C.; Ramsey, D.R. Human skin permeation of Delta8-tetrahydrocannabinol, cannabidiol and cannabinol. J. Pharm. Pharmacol. 2004, 56, 291–297. [Google Scholar] [CrossRef]
- Jin, D.; Dai, K.; Xie, Z.; Chen, J. Secondary metabolites profiled in cannabis inflorescences, leaves, stem barks, and roots for medicinal purposes. Sci. Rep. 2020, 10, 3309. [Google Scholar] [CrossRef]
- Casiraghi, A.; Musazzi, U.M.; Centin, G.; Franzè, S.; Minghetti, P. Topical administration of cannabidiol: Influence of vehicle-related aspects on skin permeation process. Pharmaceuticals 2020, 13, 337. [Google Scholar] [CrossRef]
- Lapteva, M.; Barros, J.F.; Kalia, Y.N. Cutaneous Delivery and Biodistribution of Cannabidiol in Human Skin after Topical Application of Colloidal Formulations. Pharmaceutics 2024, 16, 202. [Google Scholar] [CrossRef]
- Demisli, S.; Galani, E.; Goulielmaki, M.; Kyrilis, F.L.; Ilić, T.; Hamdi, F.; Crevar, M.; Kastritis, P.L.; Pletsa, V.; Nallet, F.; et al. Encapsulation of cannabidiol in oil-in-water nanoemulsions and nanoemulsion-filled hydrogels: A structure and biological assessment study. J. Colloid Interface Sci. 2023, 634, 300–313. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, F.; Nikolai, A.; Merchart, R.; Sosa, S.; Tubaro, A.; Novak, J. Residues of herbal hemp leaf teas—How much of the cannabinoids remain? Food Control. 2021, 127, 108146. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food Agric. 1999, 76, 270–276. [Google Scholar] [CrossRef]
- Herrmann, G.; Wlaschek, M.; Lange, T.S.; Prenzel, K.; Goerz, G.; Scharffetter-Kochanek, K. UVA irradiation stimulates the synthesis of various matrix-metalloproteinases (MMPs) in cultured human fibroblasts. Exp. Dermatol. 1993, 2, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Philips, N.; Chalensouk-Khaosaat, J.; Gonzalez, S. Simulation of the Elastin and Fibrillin in Non-Irradiated or UVA Radiated Fibroblasts, and Direct Inhibition of Elastase or Matrix Metalloptoteinases Activity by Nicotinamide or Its Derivatives. J. Cosmet. Sci. 2018, 69, 47–56. [Google Scholar]
- Uitto, J. Connective tissue biochemistry of the aging dermis. Age-related alterations in collagen and elastin. Dermatol. Clin. 1986, 4, 433–446. [Google Scholar] [CrossRef]
- Scharffetter-Kochanek, K.; Brenneisen, P.; Wenk, J.; Herrmann, G.; Ma, W.; Kuhr, L.; Meewes, C.; Wlaschek, M. Photoaging of the skin from phenotype to mechanisms. Exp. Gerontol. 2000, 35, 307–316. [Google Scholar] [CrossRef]
- Buechner, N.; Schroeder, P.; Jakob, S.; Kunze, K.; Maresch, T.; Calles, C.; Krutmann, J.; Haendeler, J. Changes of MMP-1 and collagen type Ialpha1 by UVA, UVB and IRA are differentially regulated by Trx-1. Exp. Gerontol. 2008, 43, 633–637. [Google Scholar] [CrossRef]
- Ding, Y.; Jiratchayamaethasakul, C.; Lee, S.H. Protocatechuic aldehyde attenuates UVA-induced photoaging in human dermal fibroblast cells by suppressing MAPKs/AP-1 and NF-κB signaling pathways. Int. J. Mol. Sci. 2020, 21, 4619. [Google Scholar] [CrossRef]
- Ryšavá, A.; Čížková, K.; Franková, J.; Roubalová, L.; Ulrichová, J.; Vostálová, J.; Vrba, J.; Zálešák, B.; Svobodová, A.R. Effect of UVA radiation on the Nrf2 signalling pathway in human skin cells. J. Photochem. Photobiol. B 2020, 209, 111948. [Google Scholar] [CrossRef] [PubMed]
- Malar, D.S.; Suryanarayanan, V.; Prasanth, M.I.; Singh, S.K.; Balamurugan, K.; Devi, K.P. Vitexin inhibits Aβ25-35 induced toxicity in Neuro-2a cells by augmenting Nrf-2/HO-1 dependent antioxidant pathway and regulating lipid homeo-stasis by the activation of LXR-α. Toxicol. In Vitro 2018, 50, 160–171. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luangpraditkun, K.; Pimjuk, P.; Phimnuan, P.; Wisanwattana, W.; Wisespongpand, C.; Waranuch, N.; Viyoch, J. Anti-Aging Properties of Cannabis sativa Leaf Extract against UVA Irradiation. Cosmetics 2024, 11, 45. https://doi.org/10.3390/cosmetics11020045
Luangpraditkun K, Pimjuk P, Phimnuan P, Wisanwattana W, Wisespongpand C, Waranuch N, Viyoch J. Anti-Aging Properties of Cannabis sativa Leaf Extract against UVA Irradiation. Cosmetics. 2024; 11(2):45. https://doi.org/10.3390/cosmetics11020045
Chicago/Turabian StyleLuangpraditkun, Kunlathida, Preeyanuch Pimjuk, Preeyawass Phimnuan, Wisanee Wisanwattana, Chothip Wisespongpand, Neti Waranuch, and Jarupa Viyoch. 2024. "Anti-Aging Properties of Cannabis sativa Leaf Extract against UVA Irradiation" Cosmetics 11, no. 2: 45. https://doi.org/10.3390/cosmetics11020045
APA StyleLuangpraditkun, K., Pimjuk, P., Phimnuan, P., Wisanwattana, W., Wisespongpand, C., Waranuch, N., & Viyoch, J. (2024). Anti-Aging Properties of Cannabis sativa Leaf Extract against UVA Irradiation. Cosmetics, 11(2), 45. https://doi.org/10.3390/cosmetics11020045






