Advances in the Pathogenesis and Treatment of Rosacea: A Phenotype-Based Therapeutic Approach
Abstract
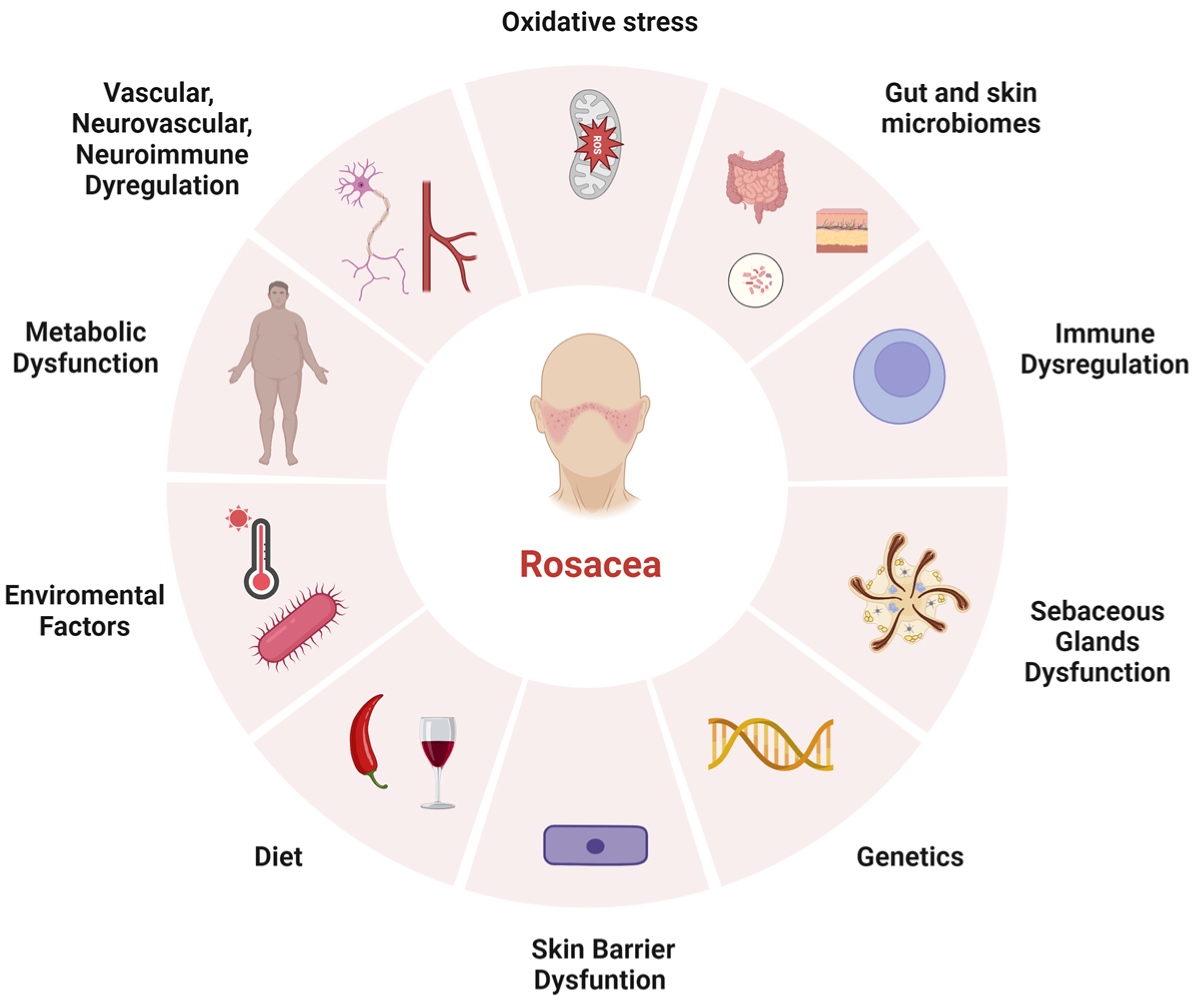
:1. Introduction
2. Pathophysiology
2.1. Environmental Factors
2.2. Genetic Predisposition
2.3. Immune Dysregulation
2.4. Vascular, Neurovascular, and Neuroinflammatory Dysregulation
2.5. Other Contributors (Sebaceous Glands, Skin Barrier Dysfunction, and Microbial Dysbiosis)
3. Classification
4. Treatments
4.1. Transient/Persistent Centrofacial Erythema and Telangiectasia
4.2. Papulopustular Rosacea
4.3. Phymatous Changes
4.4. Ocular Symptoms
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Zuuren, E.J. Rosacea. N. Engl. J. Med. 2017, 377, 1754–1764. [Google Scholar] [CrossRef]
- Tavassoli, S.; Wong, N.; Chan, E. Ocular manifestations of rosacea: A clinical review. Clin. Exp. Ophthalmol. 2021, 49, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.C.; Huang, Y.C.; Lien, Y.J.; Chang, Y.S. Association of rosacea with depression and anxiety: A systematic review and meta-analysis. J. Affect. Disord. 2022, 299, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.T.; Le, P.H.; Kuo, C.J.; Tang, Y.C.; Chiou, M.J.; Chiu, C.T.; Kuo, C.F.; Huang, Y.H. The Temporal Relationships and Associations between Cutaneous Manifestations and Inflammatory Bowel Disease: A Nationwide Population-Based Cohort Study. J. Clin. Med. 2021, 10, 1311. [Google Scholar] [CrossRef]
- Oussedik, E.; Bourcier, M.; Tan, J. Psychosocial Burden and Other Impacts of Rosacea on Patients’ Quality of Life. Dermatol. Clin. 2018, 36, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Romanowicz, M.; Stephenson, J.J.; Del Rosso, J.Q.; Lenhart, G. Healthcare utilization and costs of patients with rosacea in an insured population. J. Drugs Dermatol. 2008, 7, 41–49. [Google Scholar]
- Gether, L.; Overgaard, L.K.; Egeberg, A.; Thyssen, J.P. Incidence and prevalence of rosacea: A systematic review and meta-analysis. Br. J. Dermatol. 2018, 179, 282–289. [Google Scholar] [CrossRef]
- Chen, C.; Wang, P.; Zhang, L.; Liu, X.; Zhang, H.; Cao, Y.; Wang, X.; Zeng, Q. Exploring the Pathogenesis and Mechanism-Targeted Treatments of Rosacea: Previous Understanding and Updates. Biomedicines 2023, 11, 2153. [Google Scholar] [CrossRef]
- Ahn, C.S.; Huang, W.W. Rosacea Pathogenesis. Dermatol. Clin. 2018, 36, 81–86. [Google Scholar] [CrossRef]
- Wilkin, J.; Dahl, M.; Detmar, M.; Drake, L.; Feinstein, A.; Odom, R.; Powell, F. Standard classification of rosacea: Report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J. Am. Acad. Dermatol. 2002, 46, 584–587. [Google Scholar] [CrossRef]
- Tan, J.; Almeida, L.M.; Bewley, A.; Cribier, B.; Dlova, N.C.; Gallo, R.; Kautz, G.; Mannis, M.; Oon, H.H.; Rajagopalan, M.; et al. Updating the diagnosis, classification and assessment of rosacea: Recommendations from the global ROSacea COnsensus (ROSCO) panel. Br. J. Dermatol. 2017, 176, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Barakji, Y.A.; Ronnstad, A.T.M.; Christensen, M.O.; Zachariae, C.; Wienholtz, N.K.F.; Halling, A.S.; Maul, J.T.; Thomsen, S.F.; Egeberg, A.; Thyssen, J.P. Assessment of Frequency of Rosacea Subtypes in Patients with Rosacea: A Systematic Review and Meta-analysis. JAMA Dermatol. 2022, 158, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kroumpouzos, G.; Kassir, M.; Galadari, H.; Goren, A.; Grabbe, S.; Goldust, M. Rosacea management: A comprehensive review. J. Cosmet. Dermatol. 2022, 21, 1895–1904. [Google Scholar] [CrossRef]
- Aktas Karabay, E.; Aksu Cerman, A. Demodex folliculorum infestations in common facial dermatoses: Acne vulgaris, rosacea, seborrheic dermatitis. An. Bras. Dermatol. 2020, 95, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Forton, F.; Seys, B. Density of Demodex folliculorum in rosacea: A case-control study using standardized skin-surface biopsy. Br. J. Dermatol. 1993, 128, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Forton, F.M. Papulopustular rosacea, skin immunity and Demodex: Pityriasis folliculorum as a missing link. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 19–28. [Google Scholar] [CrossRef]
- Forton, F.M.N. Rosacea, an infectious disease: Why rosacea with papulopustules should be considered a demodicosis. A narrative review. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 987–1002. [Google Scholar] [CrossRef] [PubMed]
- Jarmuda, S.; O’Reilly, N.; Zaba, R.; Jakubowicz, O.; Szkaradkiewicz, A.; Kavanagh, K. Potential role of Demodex mites and bacteria in the induction of rosacea. J. Med. Microbiol. 2012, 61 Pt 11, 1504–1510. [Google Scholar] [CrossRef]
- Alia, E.; Feng, H. Rosacea pathogenesis, common triggers, and dietary role: The cause, the trigger, and the positive effects of different foods. Clin. Dermatol. 2022, 40, 122–127. [Google Scholar] [CrossRef]
- Reich, A.; Wojcik-Maciejewicz, A.; Slominski, A.T. Stress and the skin. G Ital. Dermatol. Venereol. 2010, 145, 213–219. [Google Scholar]
- Awosika, O.; Oussedik, E. Genetic Predisposition to Rosacea. Dermatol. Clin. 2018, 36, 87–92. [Google Scholar] [CrossRef]
- Yazici, A.C.; Tamer, L.; Ikizoglu, G.; Kaya, T.I.; Api, H.; Yildirim, H.; Adiguzel, A. GSTM1 and GSTT1 null genotypes as possible heritable factors of rosacea. Photodermatol. Photoimmunol. Photomed. 2006, 22, 208–210. [Google Scholar] [CrossRef]
- Chang, A.L.S.; Raber, I.; Xu, J.; Li, R.; Spitale, R.; Chen, J.; Kiefer, A.K.; Tian, C.; Eriksson, N.K.; Hinds, D.A.; et al. Assessment of the genetic basis of rosacea by genome-wide association study. J. Investig. Dermatol. 2015, 135, 1548–1555. [Google Scholar] [CrossRef]
- Karpouzis, A.; Avgeridis, P.; Tripsianis, G.; Gatzidou, E.; Kourmouli, N.; Veletza, S. Assessment of Tachykinin Receptor 3′ Gene Polymorphism rs3733631 in Rosacea. Int. Sch. Res. Not. 2015, 2015, 469402. [Google Scholar] [CrossRef] [PubMed]
- Fukui, K.; Kariyama, H.; Kashiba, A.; Kato, N.; Kimura, H. Further confirmation of heterogeneity of the rat striatum: Different mosaic patterns of dopamine fibers after administration of methamphetamine or reserpine. Brain Res. 1986, 382, 81–86. [Google Scholar] [CrossRef]
- Hayran, Y.; Lay, I.; Mocan, M.C.; Bozduman, T.; Ersoy-Evans, S. Vascular endothelial growth factor gene polymorphisms in patients with rosacea: A case-control study. J. Am. Acad. Dermatol. 2019, 81, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Chen, M.; Zhao, Z.; Xiao, W.; Liu, T.; Peng, Q.; Wu, Z.; Xu, S.; Shi, W.; Jian, D.; et al. Whole genome sequencing identifies genetic variants associated with neurogenic inflammation in rosacea. Nat. Commun. 2023, 14, 3958. [Google Scholar] [CrossRef]
- van Zuuren, E.J.; Arents, B.W.M.; van der Linden, M.M.D.; Vermeulen, S.; Fedorowicz, Z.; Tan, J. Rosacea: New Concepts in Classification and Treatment. Am. J. Clin. Dermatol. 2021, 22, 457–465. [Google Scholar] [CrossRef]
- Aluri, J.; Cooper, M.A.; Schuettpelz, L.G. Toll-Like Receptor Signaling in the Establishment and Function of the Immune System. Cells 2021, 10, 1374. [Google Scholar] [CrossRef]
- Rodrigues-Braz, D.; Zhao, M.; Yesilirmak, N.; Aractingi, S.; Behar-Cohen, F.; Bourges, J.L. Cutaneous and ocular rosacea: Common and specific physiopathogenic mechanisms and study models. Mol. Vis. 2021, 27, 323–353. [Google Scholar] [PubMed]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Nascimento, L.; Massari, P.; Wetzler, L.M. The Role of TLR2 in Infection and Immunity. Front. Immunol. 2012, 3, 79. [Google Scholar] [CrossRef]
- Yamasaki, K.; Di Nardo, A.; Bardan, A.; Murakami, M.; Ohtake, T.; Coda, A.; Dorschner, R.A.; Bonnart, C.; Descargues, P.; Hovnanian, A.; et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat. Med. 2007, 13, 975–980. [Google Scholar] [CrossRef]
- Yamasaki, K.; Kanada, K.; Macleod, D.T.; Borkowski, A.W.; Morizane, S.; Nakatsuji, T.; Cogen, A.L.; Gallo, R.L. TLR2 expression is increased in rosacea and stimulates enhanced serine protease production by keratinocytes. J. Investig. Dermatol. 2011, 131, 688–697. [Google Scholar] [CrossRef]
- Zhu, Y.; Underwood, J.; Macmillan, D.; Shariff, L.; O’Shaughnessy, R.; Harper, J.I.; Pickard, C.; Friedmann, P.S.; Healy, E.; Di, W.L. Persistent kallikrein 5 activation induces atopic dermatitis-like skin architecture independent of PAR2 activity. J. Allergy Clin. Immunol. 2017, 140, 1310–1322.e5. [Google Scholar] [CrossRef]
- Wang, G.; Narayana, J.L.; Mishra, B.; Zhang, Y.; Wang, F.; Wang, C.; Zarena, D.; Lushnikova, T.; Wang, X. Design of Antimicrobial Peptides: Progress Made with Human Cathelicidin LL-37. Adv. Exp. Med. Biol. 2019, 1117, 215–240. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Pena, F.J.; Romero-Tlalolini, M.L.A.; Torres-Aguilar, H.; Cruz-Hernandez, D.S.; Baltierrez-Hoyos, R.; Sanchez-Aparicio, S.R.; Aquino-Dominguez, A.S.; Aguilar-Ruiz, S.R. LL-37 Triggers Antimicrobial Activity in Human Platelets. Int. J. Mol. Sci. 2023, 24, 2816. [Google Scholar] [CrossRef] [PubMed]
- Marson, J.W.; Baldwin, H.E. Rosacea: A wholistic review and update from pathogenesis to diagnosis and therapy. Int. J. Dermatol. 2020, 59, e175–e182. [Google Scholar] [CrossRef]
- Muto, Y.; Wang, Z.; Vanderberghe, M.; Two, A.; Gallo, R.L.; Di Nardo, A. Mast cells are key mediators of cathelicidin-initiated skin inflammation in rosacea. J. Investig. Dermatol. 2014, 134, 2728–2736. [Google Scholar] [CrossRef]
- Sorensen, O.E.; Follin, P.; Johnsen, A.H.; Calafat, J.; Tjabringa, G.S.; Hiemstra, P.S.; Borregaard, N. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood 2001, 97, 3951–3959. [Google Scholar] [CrossRef]
- Li, T.; Zeng, Q.; Chen, X.; Wang, G.; Zhang, H.; Yu, A.; Wang, H.; Hu, Y. The therapeutic effect of artesunate on rosacea through the inhibition of the JAK/STAT signaling pathway. Mol. Med. Rep. 2018, 17, 8385–8390. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Chen, M.; Liu, Y.; Xu, S.; Ouyang, Y.; Shi, W.; Jian, D.; Wang, B.; Liu, F.; Li, J.; et al. A positive feedback loop between mTORC1 and cathelicidin promotes skin inflammation in rosacea. EMBO Mol. Med. 2021, 13, e13560. [Google Scholar] [CrossRef] [PubMed]
- Vallabhapurapu, S.; Karin, M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu. Rev. Immunol. 2009, 27, 693–733. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Calabrese, L.; Fiocco, Z.; Satoh, T.K.; Peris, K.; French, L.E. Therapeutic potential of targeting interleukin-1 family cytokines in chronic inflammatory skin diseases. Br. J. Dermatol. 2022, 186, 925–941. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.D.; Steinhoff, M. Integrative concepts of rosacea pathophysiology, clinical presentation and new therapeutics. Exp. Dermatol. 2017, 26, 659–667. [Google Scholar] [CrossRef]
- Buhl, T.; Sulk, M.; Nowak, P.; Buddenkotte, J.; McDonald, I.; Aubert, J.; Carlavan, I.; Deret, S.; Reiniche, P.; Rivier, M.; et al. Molecular and Morphological Characterization of Inflammatory Infiltrate in Rosacea Reveals Activation of Th1/Th17 Pathways. J. Investig. Dermatol. 2015, 135, 2198–2208. [Google Scholar] [CrossRef]
- Lee, H.J.; Hong, Y.J.; Kim, M. Angiogenesis in Chronic Inflammatory Skin Disorders. Int. J. Mol. Sci. 2021, 22, 12035. [Google Scholar] [CrossRef]
- Kulkarni, N.N.; Takahashi, T.; Sanford, J.A.; Tong, Y.; Gombart, A.F.; Hinds, B.; Cheng, J.Y.; Gallo, R.L. Innate Immune Dysfunction in Rosacea Promotes Photosensitivity and Vascular Adhesion Molecule Expression. J. Investig. Dermatol. 2020, 140, 645–655.e6. [Google Scholar] [CrossRef]
- Melincovici, C.S.; Bosca, A.B.; Susman, S.; Marginean, M.; Mihu, C.; Istrate, M.; Moldovan, I.M.; Roman, A.L.; Mihu, C.M. Vascular endothelial growth factor (VEGF)—Key factor in normal and pathological angiogenesis. Rom. J. Morphol. Embryol. 2018, 59, 455–467. [Google Scholar]
- Schwab, V.D.; Sulk, M.; Seeliger, S.; Nowak, P.; Aubert, J.; Mess, C.; Rivier, M.; Carlavan, I.; Rossio, P.; Metze, D.; et al. Neurovascular and neuroimmune aspects in the pathophysiology of rosacea. J. Investig. Dermatol. Symp. Proc. 2011, 15, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Sanchez, D.A.; Ishiuji, Y.; Patel, T.; Fountain, J.; Chan, Y.H.; Yosipovitch, G. Enhanced skin blood flow and sensitivity to noxious heat stimuli in papulopustular rosacea. J. Am. Acad. Dermatol. 2007, 57, 800–805. [Google Scholar] [CrossRef]
- Wang, H.; Siemens, J. TRP ion channels in thermosensation, thermoregulation and metabolism. Temperature 2015, 2, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, A. Transient Receptor Potential (TRP) Channels in Health and Disease. Cells 2019, 8, 413. [Google Scholar] [CrossRef] [PubMed]
- Silverman, H.A.; Chen, A.; Kravatz, N.L.; Chavan, S.S.; Chang, E.H. Involvement of Neural Transient Receptor Potential Channels in Peripheral Inflammation. Front. Immunol. 2020, 11, 590261. [Google Scholar] [CrossRef]
- Zhou, X.; Su, Y.; Wu, S.; Wang, H.; Jiang, R.; Jiang, X. The temperature-sensitive receptors TRPV4 and TRPM8 have important roles in the pruritus of rosacea. J. Dermatol. Sci. 2022, 108, 68–76. [Google Scholar] [CrossRef]
- Szolcsanyi, J. A pharmacological approach to elucidation of the role of different nerve fibres and receptor endings in mediation of pain. J. Physiol. 1977, 73, 251–259. [Google Scholar]
- Shi, V.Y.; Leo, M.; Hassoun, L.; Chahal, D.S.; Maibach, H.I.; Sivamani, R.K. Role of sebaceous glands in inflammatory dermatoses. J. Am. Acad. Dermatol. 2015, 73, 856–863. [Google Scholar] [CrossRef]
- Clayton, R.W.; Langan, E.A.; Ansell, D.M.; de Vos, I.; Gobel, K.; Schneider, M.R.; Picardo, M.; Lim, X.; van Steensel, M.A.M.; Paus, R. Neuroendocrinology and neurobiology of sebaceous glands. Biol. Rev. Camb. Philos. Soc. 2020, 95, 592–624. [Google Scholar] [CrossRef]
- Khalil, N.Y.; Darwish, I.A.; Al-Qahtani, A.A. Isotretinoin. Profiles Drug Subst. Excip. Relat. Methodol. 2020, 45, 119–157. [Google Scholar] [CrossRef]
- Ni Raghallaigh, S.; Bender, K.; Lacey, N.; Brennan, L.; Powell, F.C. The fatty acid profile of the skin surface lipid layer in papulopustular rosacea. Br. J. Dermatol. 2012, 166, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Medgyesi, B.; Dajnoki, Z.; Beke, G.; Gaspar, K.; Szabo, I.L.; Janka, E.A.; Poliska, S.; Hendrik, Z.; Mehes, G.; Torocsik, D.; et al. Rosacea Is Characterized by a Profoundly Diminished Skin Barrier. J. Investig. Dermatol. 2020, 140, 1938–1950.e5. [Google Scholar] [CrossRef]
- Jensen, J.M.; Proksch, E. The skin’s barrier. G. Ital. Dermatol. Venereol. 2009, 144, 689–700. [Google Scholar] [PubMed]
- Gunzel, D.; Yu, A.S. Claudins and the modulation of tight junction permeability. Physiol. Rev. 2013, 93, 525–569. [Google Scholar] [CrossRef]
- Daou, H.; Paradiso, M.; Hennessy, K.; Seminario-Vidal, L. Rosacea and the Microbiome: A Systematic Review. Dermatol. Ther. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Condro, G.; Guerini, M.; Castello, M.; Perugini, P. Acne Vulgaris, Atopic Dermatitis and Rosacea: The Role of the Skin Microbiota-A Review. Biomedicines 2022, 10, 2523. [Google Scholar] [CrossRef]
- Mahmud, M.R.; Akter, S.; Tamanna, S.K.; Mazumder, L.; Esti, I.Z.; Banerjee, S.; Akter, S.; Hasan, M.R.; Acharjee, M.; Hossain, M.S.; et al. Impact of gut microbiome on skin health: Gut-skin axis observed through the lenses of therapeutics and skin diseases. Gut Microbes 2022, 14, 2096995. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Hamblin, M.R.; Wen, X. Role of the skin microbiota and intestinal microbiome in rosacea. Front. Microbiol. 2023, 14, 1108661. [Google Scholar] [CrossRef]
- Akin Belli, A.; Ozbas Gok, S.; Akbaba, G.; Etgu, F.; Dogan, G. The relationship between rosacea and insulin resistance and metabolic syndrome. Eur. J. Dermatol. 2016, 26, 260–264. [Google Scholar] [CrossRef]
- Gonulal, M.; Teker, K.; Ozturk, A.; Yasar, F.Y. Investigation of thyroid blood tests and thyroid ultrasound findings of patients with rosacea. Dermatol. Ther. 2021, 34, e14632. [Google Scholar] [CrossRef]
- Haber, R.; El Gemayel, M. Comorbidities in rosacea: A systematic review and update. J. Am. Acad. Dermatol. 2018, 78, 786–792.e8. [Google Scholar] [CrossRef]
- Seremet, S.; Gurel, M.S. Miscellaneous skin disease and the metabolic syndrome. Clin. Dermatol. 2018, 36, 94–100. [Google Scholar] [CrossRef]
- Schaller, M.; Almeida, L.M.C.; Bewley, A.; Cribier, B.; Del Rosso, J.; Dlova, N.C.; Gallo, R.L.; Granstein, R.D.; Kautz, G.; Mannis, M.J.; et al. Recommendations for rosacea diagnosis, classification and management: Update from the global ROSacea COnsensus 2019 panel. Br. J. Dermatol. 2020, 182, 1269–1276. [Google Scholar] [CrossRef]
- Anzengruber, F.; Czernielewski, J.; Conrad, C.; Feldmeyer, L.; Yawalkar, N.; Hausermann, P.; Cozzio, A.; Mainetti, C.; Goldblum, D.; Lauchli, S.; et al. Swiss S1 guideline for the treatment of rosacea. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1775–1791. [Google Scholar] [CrossRef] [PubMed]
- Hampton, P.J.; Berth-Jones, J.; Duarte Williamson, C.E.; Hay, R.; Leslie, T.A.; Porter, I.; Rauz, S.; Seukeran, D.; Winn, R.T.; Hashme, M.; et al. British Association of Dermatologists guidelines for the management of people with rosacea 2021. Br. J. Dermatol. 2021, 185, 725–735. [Google Scholar] [CrossRef]
- Paiva-Santos, A.C.; Goncalves, T.; Peixoto, D.; Pires, P.C.; Velsankar, K.; Jha, N.K.; Chavda, V.P.; Mohammad, I.S.; Cefali, L.C.; Mazzola, P.G.; et al. Rosacea Topical Treatment and Care: From Traditional to New Drug Delivery Systems. Mol. Pharm. 2023, 20, 3804–3828. [Google Scholar] [CrossRef] [PubMed]
- Shim, T.N.; Abdullah, A. The effect of pulsed dye laser on the dermatology life quality index in erythematotelangiectatic rosacea patients: An assessment. J. Clin. Aesthet. Dermatol. 2013, 6, 30–32. [Google Scholar] [PubMed]
- Anderson, M.S.; Nadkarni, A.; Cardwell, L.A.; Alinia, H.; Feldman, S.R. Spotlight on brimonidine topical gel 0.33% for facial erythema of rosacea: Safety, efficacy, and patient acceptability. Patient Prefer. Adherence 2017, 11, 1143–1150. [Google Scholar] [CrossRef]
- Fowler, J.J.; Jackson, M.; Moore, A.; Jarratt, M.; Jones, T.; Meadows, K.; Steinhoff, M.; Rudisill, D.; Leoni, M. Efficacy and safety of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: Results of two randomized, double-blind, and vehicle-controlled pivotal studies. J. Drugs Dermatol. 2013, 12, 650–656. [Google Scholar]
- Moore, A.; Kempers, S.; Murakawa, G.; Weiss, J.; Tauscher, A.; Swinyer, L.; Liu, H.; Leoni, M. Long-term safety and efficacy of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: Results of a 1-year open-label study. J. Drugs Dermatol. 2014, 13, 56–61. [Google Scholar]
- Patel, N.U.; Shukla, S.; Zaki, J.; Feldman, S.R. Oxymetazoline hydrochloride cream for facial erythema associated with rosacea. Expert Rev. Clin. Pharmacol. 2017, 10, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z.D.; Gold, M.H.; Weiss, R.A.; Baumann, L.; Grekin, S.K.; Robinson, D.M.; Kempers, S.E.; Alvandi, N.; Weng, E.; Berk, D.R.; et al. Efficacy and safety of oxymetazoline cream 1.0% for treatment of persistent facial erythema associated with rosacea: Findings from the 52-week open label REVEAL trial. J. Am. Acad. Dermatol. 2018, 78, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Koca, R.; Altinyazar, H.C.; Ankarali, H.; Muhtar, S.; Tekin, N.S.; Cinar, S. A comparison of metronidazole 1% cream and pimecrolimus 1% cream in the treatment of patients with papulopustular rosacea: A randomized open-label clinical trial. Clin. Exp. Dermatol. 2010, 35, 251–256. [Google Scholar] [CrossRef]
- Kim, M.B.; Kim, G.W.; Park, H.J.; Kim, H.S.; Chin, H.W.; Kim, S.H.; Kim, B.S.; Ko, H.C. Pimecrolimus 1% cream for the treatment of rosacea. J. Dermatol. 2011, 38, 1135–1139. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Li, K.; Suh, D.H. Pimecrolimus 1% cream for the treatment of steroid-induced rosacea: An 8-week split-face clinical trial. Br. J. Dermatol. 2008, 158, 1069–1076. [Google Scholar] [CrossRef]
- Meykadeh, N.; Meiss, F.; Marsch, W.C.; Fischer, M. Steroid-aggravated rosacea: Successful therapy with pimecrolimus. Hautarzt 2007, 58, 338–342. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, L.; Wang, Y.; Zhang, D.; Tang, K.; Fang, R.; Sun, Q. Topical calcineurin inhibitors as a double-edged sword in rosacea: A systematic review. J. Cosmet. Dermatol. 2022, 21, 1695–1704. [Google Scholar] [CrossRef]
- Breneman, D.L.; Stewart, D.; Hevia, O.; Hino, P.D.; Drake, L.A. A double-blind, multicenter clinical trial comparing efficacy of once-daily metronidazole 1 percent cream to vehicle in patients with rosacea. Cutis 1998, 61, 44–47. [Google Scholar]
- Kocak, M.; Yagli, S.; Vahapoglu, G.; Eksioglu, M. Permethrin 5% cream versus metronidazole 0.75% gel for the treatment of papulopustular rosacea. A randomized double-blind placebo-controlled study. Dermatology 2002, 205, 265–270. [Google Scholar] [CrossRef]
- Bjerke, R.; Fyrand, O.; Graupe, K. Double-blind comparison of azelaic acid 20% cream and its vehicle in treatment of papulo-pustular rosacea. Acta Derm. Venereol. 1999, 79, 456–459. [Google Scholar] [CrossRef]
- Hsu, C.C.; Lee, J.Y. Carvedilol for the treatment of refractory facial flushing and persistent erythema of rosacea. Arch. Dermatol. 2011, 147, 1258–1260. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.H.; Kim, D.H.; Suh, H.S.; Choi, Y.S. Facial flushing and erythema of rosacea improved by carvedilol. Dermatol. Ther. 2020, 33, e14520. [Google Scholar] [CrossRef]
- Hofmann, M.A.; Lehmann, P. Physical modalities for the treatment of rosacea. J. Dtsch. Dermatol. Ges. 2016, 14 (Suppl. S6), 38–43. [Google Scholar] [CrossRef] [PubMed]
- Kawana, S.; Ochiai, H.; Tachihara, R. Objective evaluation of the effect of intense pulsed light on rosacea and solar lentigines by spectrophotometric analysis of skin color. Dermatol. Surg. 2007, 33, 449–454. [Google Scholar] [CrossRef]
- Sadick, N.S.; Weiss, R. Intense pulsed-light photorejuvenation. Semin. Cutan. Med. Surg. 2002, 21, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, Y.; Goldenberg, G. Devices and topical agents for rosacea management. Cutis 2014, 94, 21–25. [Google Scholar]
- Taub, A.F.; Devita, E.C. Successful treatment of erythematotelangiectatic rosacea with pulsed light and radiofrequency. J. Clin. Aesthet. Dermatol. 2008, 1, 37–40. [Google Scholar]
- Papageorgiou, P.; Clayton, W.; Norwood, S.; Chopra, S.; Rustin, M. Treatment of rosacea with intense pulsed light: Significant improvement and long-lasting results. Br. J. Dermatol. 2008, 159, 628–632. [Google Scholar] [CrossRef]
- Tan, S.R.; Tope, W.D. Pulsed dye laser treatment of rosacea improves erythema, symptomatology, and quality of life. J. Am. Acad. Dermatol. 2004, 51, 592–599. [Google Scholar] [CrossRef]
- Alam, M.; Dover, J.S.; Arndt, K.A. Treatment of facial telangiectasia with variable-pulse high-fluence pulsed-dye laser: Comparison of efficacy with fluences immediately above and below the purpura threshold. Dermatol. Surg. 2003, 29, 681–684; discussion 685. [Google Scholar] [CrossRef]
- Bernstein, E.F.; Kligman, A. Rosacea treatment using the new-generation, high-energy, 595 nm, long pulse-duration pulsed-dye laser. Lasers Surg. Med. 2008, 40, 233–239. [Google Scholar] [CrossRef] [PubMed]
- West, T.B.; Alster, T.S. Comparison of the long-pulse dye (590-595 nm) and KTP (532 nm) lasers in the treatment of facial and leg telangiectasias. Dermatol. Surg. 1998, 24, 221–226. [Google Scholar] [CrossRef]
- Alam, M.; Voravutinon, N.; Warycha, M.; Whiting, D.; Nodzenski, M.; Yoo, S.; West, D.P.; Veledar, E.; Poon, E. Comparative effectiveness of nonpurpuragenic 595-nm pulsed dye laser and microsecond 1064-nm neodymium:yttrium-aluminum-garnet laser for treatment of diffuse facial erythema: A double-blind randomized controlled trial. J. Am. Acad. Dermatol. 2013, 69, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Karsai, S.; Roos, S.; Raulin, C. Treatment of facial telangiectasia using a dual-wavelength laser system (595 and 1064 nm): A randomized controlled trial with blinded response evaluation. Dermatol. Surg. 2008, 34, 702–708. [Google Scholar] [CrossRef]
- Micali, G.; Dall’Oglio, F.; Verzi, A.E.; Luppino, I.; Bhatt, K.; Lacarrubba, F. Treatment of erythemato-telangiectatic rosacea with brimonidine alone or combined with vascular laser based on preliminary instrumental evaluation of the vascular component. Lasers Med. Sci. 2018, 33, 1397–1400. [Google Scholar] [CrossRef]
- Vissing, A.E.; Dierickx, C.; Karmisholt, K.E.; Haedersdal, M. Topical brimonidine reduces IPL-induced erythema without affecting efficacy: A randomized controlled trial in patients with facial telangiectasias. Lasers Surg. Med. 2018, 50, 1002–1009. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, L.; Zhang, Y.; Gao, X.; Wu, Y.; Chen, H. Topical photodynamic therapy with 5-aminolevulinic acid in Chinese patients with Rosacea. J. Cosmet. Laser Ther. 2019, 21, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Bao, N.; Gu, T.; Zeng, J.; Wu, Y.; Sun, Y.; Gao, X.; Chen, H. Combined therapy of 5-aminolevulinic acid photodynamic therapy and intense pulsed light for rosacea. Lasers Med. Sci. 2022, 38, 17. [Google Scholar] [CrossRef]
- Park, S.Y.; Kwon, H.H.; Yoon, J.Y.; Min, S.; Suh, D.H. Clinical and Histologic Effects of Fractional Microneedling Radiofrequency Treatment on Rosacea. Dermatol. Surg. 2016, 42, 1362–1369. [Google Scholar] [CrossRef]
- Kang, C.N.; Shah, M.; Tan, J. Rosacea: An Update in Diagnosis, Classification and Management. Skin. Ther. Lett. 2021, 26, 1–8. [Google Scholar]
- van Zuuren, E.J.; Fedorowicz, Z.; Tan, J.; van der Linden, M.M.D.; Arents, B.W.M.; Carter, B.; Charland, L. Interventions for rosacea based on the phenotype approach: An updated systematic review including GRADE assessments. Br. J. Dermatol. 2019, 181, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Passi, S.; Picardo, M.; Mingrone, G.; Breathnach, A.S.; Nazzaro-Porro, M. Azelaic acid—biochemistry and metabolism. Acta Derm. Venereol. Suppl. 1989, 143, 8–13. [Google Scholar] [CrossRef]
- Searle, T.; Ali, F.R.; Al-Niaimi, F. The versatility of azelaic acid in dermatology. J. Dermatolog. Treat. 2022, 33, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Gollnick, H.; Layton, A. Azelaic acid 15% gel in the treatment of rosacea. Expert Opin. Pharmacother. 2008, 9, 2699–2706. [Google Scholar] [CrossRef]
- Coda, A.B.; Hata, T.; Miller, J.; Audish, D.; Kotol, P.; Two, A.; Shafiq, F.; Yamasaki, K.; Harper, J.C.; Del Rosso, J.Q.; et al. Cathelicidin, kallikrein 5, and serine protease activity is inhibited during treatment of rosacea with azelaic acid 15% gel. J. Am. Acad. Dermatol. 2013, 69, 570–577. [Google Scholar] [CrossRef]
- King, S.; Campbell, J.; Rowe, R.; Daly, M.L.; Moncrieff, G.; Maybury, C. A systematic review to evaluate the efficacy of azelaic acid in the management of acne, rosacea, melasma and skin aging. J. Cosmet. Dermatol. 2023, 22, 2650–2662. [Google Scholar] [CrossRef]
- Elewski, B.E.; Fleischer, A.B.J.; Pariser, D.M. A comparison of 15% azelaic acid gel and 0.75% metronidazole gel in the topical treatment of papulopustular rosacea: Results of a randomized trial. Arch. Dermatol. 2003, 139, 1444–1450. [Google Scholar] [CrossRef]
- Liu, R.H.; Smith, M.K.; Basta, S.A.; Farmer, E.R. Azelaic acid in the treatment of papulopustular rosacea: A systematic review of randomized controlled trials. Arch. Dermatol. 2006, 142, 1047–1052. [Google Scholar] [CrossRef]
- Draelos, Z.D.; Elewski, B.; Staedtler, G.; Havlickova, B. Azelaic acid foam 15% in the treatment of papulopustular rosacea: A randomized, double-blind, vehicle-controlled study. Cutis 2013, 92, 306–317. [Google Scholar]
- Dall’Oglio, F.; Nasca, M.R.; Gerbino, C.; Micali, G. Advances in pharmacotherapy for rosacea: What is the current state of the art? Expert Opin. Pharmacother. 2022, 23, 1845–1854. [Google Scholar] [CrossRef]
- Dahl, M.V.; Jarratt, M.; Kaplan, D.; Tuley, M.R.; Baker, M.D. Once-daily topical metronidazole cream formulations in the treatment of the papules and pustules of rosacea. J. Am. Acad. Dermatol. 2001, 45, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Dahl, M.V.; Katz, H.I.; Krueger, G.G.; Millikan, L.E.; Odom, R.B.; Parker, F.; Wolf, J.E.J.; Aly, R.; Bayles, C.; Reusser, B.; et al. Topical metronidazole maintains remissions of rosacea. Arch. Dermatol. 1998, 134, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Yucel, A.; Yilmaz, M. Investigation of the prevalance of Demodex folliculorum and Demodex brevis in rosacea patients. Turk. Parazitol. Derg. 2013, 37, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Rios-Yuil, J.M.; Mercadillo-Perez, P. Evaluation of Demodex folliculorum as a Risk Factor for the Diagnosis of Rosacea In Skin Biopsies. Mexico’s General Hospital (1975–2010). Indian J. Dermatol. 2013, 58, 157. [Google Scholar] [CrossRef]
- Stein, L.; Kircik, L.; Fowler, J.; Tan, J.; Draelos, Z.; Fleischer, A.; Appell, M.; Steinhoff, M.; Lynde, C.; Liu, H.; et al. Efficacy and safety of ivermectin 1% cream in treatment of papulopustular rosacea: Results of two randomized, double-blind, vehicle-controlled pivotal studies. J. Drugs Dermatol. 2014, 13, 316–323. [Google Scholar]
- Taieb, A.; Ortonne, J.P.; Ruzicka, T.; Roszkiewicz, J.; Berth-Jones, J.; Peirone, M.H.; Jacovella, J.; Ivermectin Phase, I.I.I.s.g. Superiority of ivermectin 1% cream over metronidazole 0.75% cream in treating inflammatory lesions of rosacea: A randomized, investigator-blinded trial. Br. J. Dermatol. 2015, 172, 1103–1110. [Google Scholar] [CrossRef]
- Green, L.J.; Bhatia, N.D.; Toledano, O.; Erlich, M.; Spizuoco, A.; Goodyear, B.C.; York, J.P.; Jakus, J. Silica-based microencapsulation used in topical dermatologic applications. Arch. Dermatol. Res. 2023, 315, 2787–2793. [Google Scholar] [CrossRef]
- Garrido-Mesa, N.; Zarzuelo, A.; Galvez, J. Minocycline: Far beyond an antibiotic. Br. J. Pharmacol. 2013, 169, 337–352. [Google Scholar] [CrossRef]
- Webster, G.; Del Rosso, J.Q. Anti-inflammatory activity of tetracyclines. Dermatol. Clin. 2007, 25, 133–135. [Google Scholar] [CrossRef]
- Parenti, A.; Indorato, B.; Paccosi, S. Minocycline affects human neutrophil respiratory burst and transendothelial migration. Inflamm. Res. 2017, 66, 107–109. [Google Scholar] [CrossRef]
- Monk, E.; Shalita, A.; Siegel, D.M. Clinical applications of non-antimicrobial tetracyclines in dermatology. Pharmacol. Res. 2011, 63, 130–145. [Google Scholar] [CrossRef]
- Del Rosso, J.Q.; Webster, G.; Weiss, J.S.; Bhatia, N.D.; Gold, L.S.; Kircik, L. Nonantibiotic Properties of Tetracyclines in Rosacea and Their Clinical Implications. J. Clin. Aesthet. Dermatol. 2021, 14, 14–21. [Google Scholar]
- Del Rosso, J.Q.; Thiboutot, D.; Gallo, R.; Webster, G.; Tanghetti, E.; Eichenfield, L.F.; Stein-Gold, L.; Berson, D.; Zaenglein, A. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 3: A status report on systemic therapies. Cutis 2014, 93, 18–28. [Google Scholar] [PubMed]
- Del Rosso, J.Q.; Schlessinger, J.; Werschler, P. Comparison of anti-inflammatory dose doxycycline versus doxycycline 100 mg in the treatment of rosacea. J. Drugs Dermatol. 2008, 7, 573–576. [Google Scholar] [PubMed]
- Del Rosso, J.Q. Anti-inflamatory dose doxycycline in the treatment of rosacea. J. Drugs Dermatol. 2009, 8, 664–668. [Google Scholar]
- Del Rosso, J.Q. Effectiveness and safety of doxycycline 40 mg (30-mg immediate-release and 10-mg delayed-release beads) once daily as add-on therapy to existing topical regimens for the treatment of papulopustular rosacea: Results from a community-based trial. Cutis 2010, 86, 16–25. [Google Scholar] [PubMed]
- van der Linden, M.M.D.; van Ratingen, A.R.; van Rappard, D.C.; Nieuwenburg, S.A.; Spuls, P.I. DOMINO, doxycycline 40 mg vs. minocycline 100 mg in the treatment of rosacea: A randomized, single-blinded, noninferiority trial, comparing efficacy and safety. Br. J. Dermatol. 2017, 176, 1465–1474. [Google Scholar] [CrossRef]
- James, K.A.; Burkhart, C.N.; Morrell, D.S. Emerging drugs for acne. Expert Opin. Emerg. Drugs 2009, 14, 649–659. [Google Scholar] [CrossRef]
- El-Heis, S.; Buckley, D.A. Rosacea-like eruption due to topical pimecrolimus. Dermatol. Online J. 2015, 21. [Google Scholar] [CrossRef]
- Gomolin, T.; Cline, A.; Pereira, F. Treatment of rosacea during pregnancy. Dermatol. Online J. 2021, 27, 7. [Google Scholar] [CrossRef]
- Lova Navarro, M.; Sanchez-Pedreno Guillen, P.; Victoria Martinez, A.M.; Martinez Menchon, T.; Corbalan Velez, R.; Frias Iniesta, J. Papulopustular Rosacea: Response to Treatment with Oral Azithromycin. Actas Dermosifiliogr. 2018, 109, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Torresani, C.; Pavesi, A.; Manara, G.C. Clarithromycin versus doxycycline in the treatment of rosacea. Int. J. Dermatol. 1997, 36, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Del Rosso, J.Q.; Kim, G. Optimizing use of oral antibiotics in acne vulgaris. Dermatol. Clin. 2009, 27, 33–42. [Google Scholar] [CrossRef]
- Sbidian, E.; Vicaut, E.; Chidiack, H.; Anselin, E.; Cribier, B.; Dreno, B.; Chosidow, O. A Randomized-Controlled Trial of Oral Low-Dose Isotretinoin for Difficult-To-Treat Papulopustular Rosacea. J. Investig. Dermatol. 2016, 136, 1124–1129. [Google Scholar] [CrossRef]
- Gollnick, H.; Blume-Peytavi, U.; Szabo, E.L.; Meyer, K.G.; Hauptmann, P.; Popp, G.; Sebastian, M.; Zwingers, T.; Willers, C.; von der Weth, R. Systemic isotretinoin in the treatment of rosacea—Doxycycline- and placebo-controlled, randomized clinical study. J. Dtsch. Dermatol. Ges. 2010, 8, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Spring, L.K.; Krakowski, A.C.; Alam, M.; Bhatia, A.; Brauer, J.; Cohen, J.; Del Rosso, J.Q.; Diaz, L.; Dover, J.; Eichenfield, L.F.; et al. Isotretinoin and Timing of Procedural Interventions: A Systematic Review with Consensus Recommendations. JAMA Dermatol. 2017, 153, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.H.; Jung, J.Y.; Lee, W.Y.; Bae, Y.; Park, G.H. Combined treatment of recalcitrant papulopustular rosacea involving pulsed dye laser and fractional microneedling radiofrequency with low-dose isotretinoin. J. Cosmet. Dermatol. 2020, 19, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.M.; Chiou, A.S.; Shih, Y.H.; Li, S.; Chang, A.L.S. An exploratory, open-label, investigator-initiated study of interleukin-17 blockade in patients with moderate-to-severe papulopustular rosacea. Br. J. Dermatol. 2020, 183, 942–943. [Google Scholar] [CrossRef]
- Saal, R.C.; Borda, L.J.; Hoffman, M.L.; Roberts, A.A.; Van Voorhees, A.S. Treatment of granulomatous rosacea with adalimumab. JAAD Case Rep. 2023, 40, 89–91. [Google Scholar] [CrossRef]
- Sun, Y.H.; Man, X.Y.; Xuan, X.Y.; Huang, C.Z.; Shen, Y.; Lao, L.M. Tofacitinib for the treatment of erythematotelangiectatic and papulopustular rosacea: A retrospective case series. Dermatol. Ther. 2022, 35, e15848. [Google Scholar] [CrossRef]
- Searle, T.; Al-Niaimi, F.; Ali, F.R. Rosacea. Br. J. Hosp. Med. 2021, 82, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.F.; Herrera, H.; Motta, A. Electrosurgery for the Treatment of Moderate or Severe Rhinophyma. Actas Dermosifiliogr. 2018, 109, e23–e26. [Google Scholar] [CrossRef] [PubMed]
- Rohrich, R.J.; Griffin, J.R.; Adams, W.P.J. Rhinophyma: Review and update. Plast. Reconstr. Surg. 2002, 110, 860–869; quiz 870. [Google Scholar] [CrossRef]
- Borhani-Khomani, K.; Moller, M.P.; Thomsen, M.V.; Karmisholt, K.; Haedersdal, M.; Bonde, C.T. Treatmentof rhinophyma with laser and surgery. Ugeskr. Laeger 2020, 182, V07190378. [Google Scholar] [PubMed]
- Ismail, D.; Asfour, L.; Madan, V. Rhinophyma in women: A case series. Lasers Med. Sci. 2021, 36, 1283–1287. [Google Scholar] [CrossRef]
- Baldwin, H.E. Systemic therapy for rosacea. Skin. Ther. Lett. 2007, 12, 9. [Google Scholar]
- Malagon-Liceaga, A.; Recillas-Gispert, C.; Ruiz-Quintero, N.C.; Ruelas-Villavicencio, A.L. Treatment of ocular rosacea: A practical review from an interdisciplinary approach. Arch. Soc. Esp. Oftalmol. 2023, 98, 577–585. [Google Scholar] [CrossRef]
- Pfeffer, I.; Borelli, C.; Zierhut, M.; Schaller, M. Treatment of ocular rosacea with 40 mg doxycycline in a slow release form. J. Dtsch. Dermatol. Ges. 2011, 9, 904–907. [Google Scholar] [CrossRef]
- McGhee, C.N.; Dean, S.; Danesh-Meyer, H. Locally administered ocular corticosteroids: Benefits and risks. Drug Saf. 2002, 25, 33–55. [Google Scholar] [CrossRef]
- Vora, G.K.; Gupta, P.K. Intense pulsed light therapy for the treatment of evaporative dry eye disease. Curr. Opin. Ophthalmol. 2015, 26, 314–318. [Google Scholar] [CrossRef]
- Calabrese, L.; Malvaso, D.; Chiricozzi, A.; Tambone, S.; D’Urso, D.F.; Guerriero, C.; Peris, K. Baricitinib: Therapeutic potential for moderate to severe atopic dermatitis. Expert Opin. Investig. Drugs 2020, 29, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, L.; Chiricozzi, A.; De Simone, C.; Fossati, B.; D’Amore, A.; Peris, K. Pharmacodynamics of Janus kinase inhibitors for the treatment of atopic dermatitis. Expert Opin. Drug Metab. Toxicol. 2022, 18, 347–355. [Google Scholar] [CrossRef] [PubMed]
- D’Urso, D.F.; Chiricozzi, A.; Pirro, F.; Calabrese, L.; Caldarola, G.; Fossati, B.; De Simone, C.; Peris, K. New JAK inhibitors for the treatment of psoriasis and psoriatic arthritis. G Ital. Dermatol. Venereol. 2020, 155, 411–420. [Google Scholar] [CrossRef] [PubMed]

| Rosacea Subtype | First Line Therapies | Alternatives |
| Erythemato-telangectatic |
|
|
| Papulopustular |
|
|
| Phymatous |
|
|
| Ocular |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galluccio, G.; D’Onghia, M.; Malvaso, D.; Lazzeri, L.; Cinotti, E.; Rubegni, G.; Rubegni, P.; Calabrese, L. Advances in the Pathogenesis and Treatment of Rosacea: A Phenotype-Based Therapeutic Approach. Cosmetics 2024, 11, 11. https://doi.org/10.3390/cosmetics11010011
Galluccio G, D’Onghia M, Malvaso D, Lazzeri L, Cinotti E, Rubegni G, Rubegni P, Calabrese L. Advances in the Pathogenesis and Treatment of Rosacea: A Phenotype-Based Therapeutic Approach. Cosmetics. 2024; 11(1):11. https://doi.org/10.3390/cosmetics11010011
Chicago/Turabian StyleGalluccio, Giulia, Martina D’Onghia, Dalma Malvaso, Laura Lazzeri, Elisa Cinotti, Giovanni Rubegni, Pietro Rubegni, and Laura Calabrese. 2024. "Advances in the Pathogenesis and Treatment of Rosacea: A Phenotype-Based Therapeutic Approach" Cosmetics 11, no. 1: 11. https://doi.org/10.3390/cosmetics11010011
APA StyleGalluccio, G., D’Onghia, M., Malvaso, D., Lazzeri, L., Cinotti, E., Rubegni, G., Rubegni, P., & Calabrese, L. (2024). Advances in the Pathogenesis and Treatment of Rosacea: A Phenotype-Based Therapeutic Approach. Cosmetics, 11(1), 11. https://doi.org/10.3390/cosmetics11010011






