Skin Anti-Aging Potentials of Phytochemicals from Peperomia pellucida against Selected Metalloproteinase Targets: An In Silico Approach
Abstract
:1. Introduction
2. Methods
2.1. Virtual Screening and Docking Platform
2.2. Ligand Library Generation and Preparation
2.3. Target Retrieval and Preparation
2.4. Receptor Grid Generation
2.5. Molecular Docking
2.6. The Molecular Mechanics/Generalized Born Surface Area (MM/GBSA)
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aburjai, T.; Natsheh, F.M. Plants used in cosmetics. Phytother. Res. PTR 2003, 17, 987–1000. [Google Scholar] [CrossRef]
- Agbebi, E.A.; Alabi, O.S.; Nkrumah, A.O.; Ogbole, O.O. Evaluation of the antibacterial and antifungal potentials of peptide-rich extracts from selected Nigerian Plants. Eur. J. Integr. Med. 2022, 54, 102163. [Google Scholar] [CrossRef]
- Altyar, A.E.; Ashour, M.L.; Youssef, F.S. Premna odorata: Seasonal Metabolic Variation in the Essential Oil Composition of Its Leaf and Verification of Its Anti-Ageing Potential via In Vitro Assays and Molecular Modelling. Biomolecules 2020, 10, 879. [Google Scholar] [CrossRef] [PubMed]
- Alves, N.S.F.; Setzer, W.N.; da Silva, J.K.R. The chemistry and biological activities of Peperomia pellucida (Piperaceae): A critical review. J. Ethnopharmacol. 2019, 232, 90–102. [Google Scholar] [CrossRef]
- Amirah, S.; Zain, H.H.M.; Husni, I.; Kassim, N.K.; Amin, I. In vitro Antioxidant Capacity of Peperomia pellucida (L.) Kunth Plant from two different locations in Malaysia using different Solvents Extraction. Res. J. Pharm. Technol. 2020, 13, 1767. [Google Scholar] [CrossRef]
- Arrigoni-Blank, M.D.F.; Dmitrieva, E.G.; Franzotti, E.M.; Antoniolli, A.R.; Andrade, M.R.; Marchioro, M. Anti-inflammatory and analgesic activity of Peperomia pellucida (L.) HBK (Piperaceae). J. Ethnopharmacol. 2004, 91, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Bae-Harboe, Y.-S.C.; Park, H.-Y. Tyrosinase: A Central Regulatory Protein for Cutaneous Pigmentation. J. Investig. Dermatol. 2012, 132, 2678–2680. [Google Scholar] [CrossRef] [PubMed]
- Bertini, I.; Calderone, V.; Cosenza, M.; Fragai, M.; Lee, Y.-M.; Luchinat, C.; Mangani, S.; Terni, B.; Turano, P. Conformational variability of matrix metalloproteinases: Beyond a single 3D structure. Proc. Natl. Acad. Sci. USA 2005, 102, 5334–5339. [Google Scholar] [CrossRef]
- Buonocore, D.; Lazzeretti, A.; Tocabens, P.; Nobile, V.; Cestone, E.; Santin, G.; Bottone, M.G.; Marzatico, F. Resveratrol-procyanidin blend: Nutraceutical and antiageing efficacy evaluated in a placebo-controlled, double-blind study. Clin. Cosmet. Investig. Dermatol. 2012, 159, 159–165. [Google Scholar] [CrossRef]
- Burdock, G.A.; Soni, M.G.; Carabin, I.G. Evaluation of Health Aspects of Kojic Acid in Food. Regul. Toxicol. Pharmacol. 2001, 33, 80–101. [Google Scholar] [CrossRef]
- Elgamal, A.M.; El Raey, M.A.; Gaara, A.; Abdelfattah, M.A.O.; Sobeh, M. Phytochemical profiling and anti-ageing activities of Euphorbia retusa extract: In silico and in vitro studies. Arab. J. Chem. 2021, 14, 103159. [Google Scholar] [CrossRef]
- Eun Lee, K.; Bharadwaj, S.; Yadava, U.; Gu Kang, S. Evaluation of caffeine as inhibitor against collagenase, elastase and tyrosinase using in silico and in vitro approach. J. Enzym. Inhib. Med. Chem. 2019, 34, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, A.; Takahashi, T.; Watanabe, Y. Investigation of topical application of procyanidin B-2 from apple to identify its potential use as a hair growing agent. Phytomedicine 2000, 7, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Karamanos, N.K.; Theocharis, A.D.; Piperigkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E.H.; et al. A guide to the composition and functions of the extracellular matrix. FEBS J. 2021, 288, 6850–6912. [Google Scholar] [CrossRef]
- Kartika, I.G.A.A.; Insanu, M.; Safitri, D.; Putri, C.A.; Adnyana, I.K. New update: Traditional uses, phytochemical, pharmacological and toxicity review of Peperomia pellucida (L.) kunth. Pharmacologyonline 2016, 2016, 30–43. [Google Scholar]
- Kohno, T.; Hochigai, H.; Yamashita, E.; Tsukihara, T.; Kanaoka, M. Crystal structures of the catalytic domain of human stromelysin-1 (MMP-3) and collagenase-3 (MMP-13) with a hydroxamic acid inhibitor SM-25453. Biochem. Biophys. Res. Commun. 2006, 344, 315–322. [Google Scholar] [CrossRef]
- Amid Koparde, A.; Chandrashekar Doijad, R.; Shripal Magdum, C. Natural products in drug discovery. In Pharmacognosy—Medicinal Plants; IntechOpen: London, UK, 2019; Available online: http://dx.doi.org/10.5772/intechopen.82860 (accessed on 14 August 2023).
- Kosasih, S.; Ginting, N.; Chiuman, L.; Nyoman, I.; Lister, E. The Effectiveness of Peperomia Pellucida Extract Against Acne Bacteria. Am. Sci. Res. J. Eng. Technol. Sci. 2019, 59, 149–153. [Google Scholar]
- Lai, X.; Wichers, H.J.; Soler-Lopez, M.; Dijkstra, B.W. Structure of Human Tyrosinase Related Protein 1 Reveals a Binuclear Zinc Active Site Important for Melanogenesis. Angew. Chem. Int. Ed. 2017, 56, 9812–9815. [Google Scholar] [CrossRef]
- Majumder, P. Phytochemical, pharmacognostical and physicochemical standardization of Peperomia pellucida (L.) HBK. STEM. Pharm. Glob. 2011, 2, 1–4. [Google Scholar]
- Majumder, P.; Abraham, P.; Satya, V. Ethno-medicinal, phytochemical and pharmacological review of an amazing medicinal herb Peperomia pellucida (L.) HBK. Res. J. Pharm. Biol. Chem. Sci. 2011, 2, 358–364. [Google Scholar]
- Matos, M.S.; Romero-Díez, R.; Álvarez, A.; Bronze, M.R.; Rodríguez-Rojo, S.; Mato, R.B.; Cocero, M.J.; Matias, A.A. Polyphenol-Rich Extracts Obtained from Winemaking Waste Streams as Natural Ingredients with Cosmeceutical Potential. Antioxidants 2019, 8, 355. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Olson, A.J. Using AutoDock for Ligand-Receptor Docking. Curr. Protoc. Bioinform. 2008, 24, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Ohno, R.; Moroishi, N.; Sugawa, H.; Maejima, K.; Saigusa, M.; Yamanaka, M.; Nagai, M.; Yoshimura, M.; Amakura, Y.; Nagai, R. Mangosteen pericarp extract inhibits the formation of pentosidine and ameliorates skin elasticity. J. Clin. Biochem. Nutr. 2015, 57, 27–32. [Google Scholar] [CrossRef]
- Oloyede, G.; Onocha, P. Phytochemical, toxicity, antimicrobial and antioxidant screening of leaf extracts of Peperomia pellucida from Nigeria. Adv. Environ. Biol. 2011, 5, 3700–3709. [Google Scholar]
- Olugbogi, E.A.; Bodun, D.S.; Omoseeye, S.D.; Onoriode, A.O.; Oluwamoroti, F.O.; Adedara, J.F.; Oriyomi, I.A.; Bello, F.O.; Olowoyeye, F.O.; Laoye, O.G.; et al. Quassia amara bioactive compounds as a Novel DPP-IV inhibitor: An in-silico study. Bull. Natl. Res. Cent. 2022, 46, 217. [Google Scholar] [CrossRef]
- Ooi, D.J.; Iqbal, S.; Ismail, M. Proximate Composition, Nutritional Attributes and Mineral Composition of Peperomia pellucida L. (Ketumpangan Air) Grown in Malaysia. Molecules 2012, 17, 11139–11145. [Google Scholar] [CrossRef] [PubMed]
- Papaccio, F.; D′Arino, A.; Caputo, S.; Bellei, B. Focus on the Contribution of Oxidative Stress in Skin Ageing. Antioxidants 2022, 11, 1121. [Google Scholar] [CrossRef]
- Parrado, C.; Mercado-Saenz, S.; Perez-Davo, A.; Gilaberte, Y.; Gonzalez, S.; Juarranz, A. Environmental Stressors on Skin Ageing. Mechanistic Insights. Front. Pharmacol. 2019, 10, 461144. [Google Scholar] [CrossRef]
- Puizina-Ivić, N.; Mirić, L.; Čarija, A.; Karlica, D.; Marasović, D. Modern approach to topical treatment of ageing skin. Coll. Antropol. 2010, 34, 1145–1153. [Google Scholar]
- Quan, T.; Wang, F.; Shao, Y.; Rittié, L.; Xia, W.; Orringer, J.S.; Voorhees, J.J.; Fisher, G.J. Enhancing Structural Support of the Dermal Microenvironment Activates Fibroblasts, Endothelial Cells, and Keratinocytes in Aged Human Skin In Vivo. J. Investig. Dermatol. 2013, 133, 658–667. [Google Scholar] [CrossRef]
- Rue, E.A.; Rush, M.D.; van Breemen, R.B. Procyanidins: A comprehensive review encompassing structure elucidation via mass spectrometry. Phytochem. Rev. Proc. Phytochem. Soc. Eur. 2018, 17, 1–16. [Google Scholar] [CrossRef]
- Saeedi, M.; Eslamifar, M.; Khezri, K. Kojic acid applications in cosmetic and pharmaceutical preparations. Biomed. Pharmacother. 2019, 110, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Saputri, F.C.; Hutahaean, I.; Mun’im, A. Peperomia pellucida (L.) Kunth as an angiotensin-converting enzyme inhibitor in two-kidney, one-clip Goldblatt hypertensive rats. Saudi J. Biol. Sci. 2021, 28, 6191–6197. [Google Scholar] [CrossRef] [PubMed]
- Senol Deniz, F.S.; Orhan, I.E.; Duman, H. Profiling cosmeceutical effects of various herbal extracts through elastase, collagenase, tyrosinase inhibitory and antioxidant assays. Phytochem. Lett. 2021, 45, 171–183. [Google Scholar] [CrossRef]
- Shoko, T.; Maharaj, V.J.; Naidoo, D.; Tselanyane, M.; Nthambeleni, R.; Khorombi, E.; Apostolides, Z. Anti-ageing potential of extracts from Sclerocarya birrea, (A. Rich.) Hochst and its chemical profiling by UPLC-Q-TOF-MS. BMC Complement. Altern. Med. 2018, 18, 54. [Google Scholar] [CrossRef] [PubMed]
- Sonibare, M.A.; Moody, J.O.; Adesanya, E.O. Use of medicinal plants for the treatment of measles in Nigeria. J. Ethnopharmacol. 2009, 122, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Tanigawa, T.; Kanazawa, S.; Ichibori, R.; Fujiwara, T.; Magome, T.; Shingaki, K.; Miyata, S.; Hata, Y.; Tomita, K.; Matsuda, K.; et al. (+)-Catechin protects dermal fibroblasts against oxidative stress-induced apoptosis. BMC Complement. Altern. Med. 2014, 14, 133. [Google Scholar] [CrossRef] [PubMed]
- Tobin, D.J. Introduction to skin ageing. J. Tissue Viability 2017, 26, 37–46. [Google Scholar] [CrossRef]
- Venkatesh, S.; Maymone, M.B.C.; Vashi, N.A. Ageing in skin of color. Clin. Dermatol. 2019, 37, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Vierkötter, A.; Krutmann, J. Environmental influences on skin ageing and ethnic-specific manifestations. Derm. Endocrinol. 2012, 4, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Widowati, W.; Ginting, C.N.; Lister, I.N.E.; Girsang, E.; Amalia, A.; Wibowo, S.H.B.; Kusuma, H.; Rizal, R. Anti-ageing Effects of Mangosteen Peel Extract and Its Phytochemical Compounds: Antioxidant Activity, Enzyme Inhibition and Molecular Docking Simulation. Trop. Life Sci. Res. 2020, 31, 127–144. [Google Scholar] [CrossRef] [PubMed]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef] [PubMed]


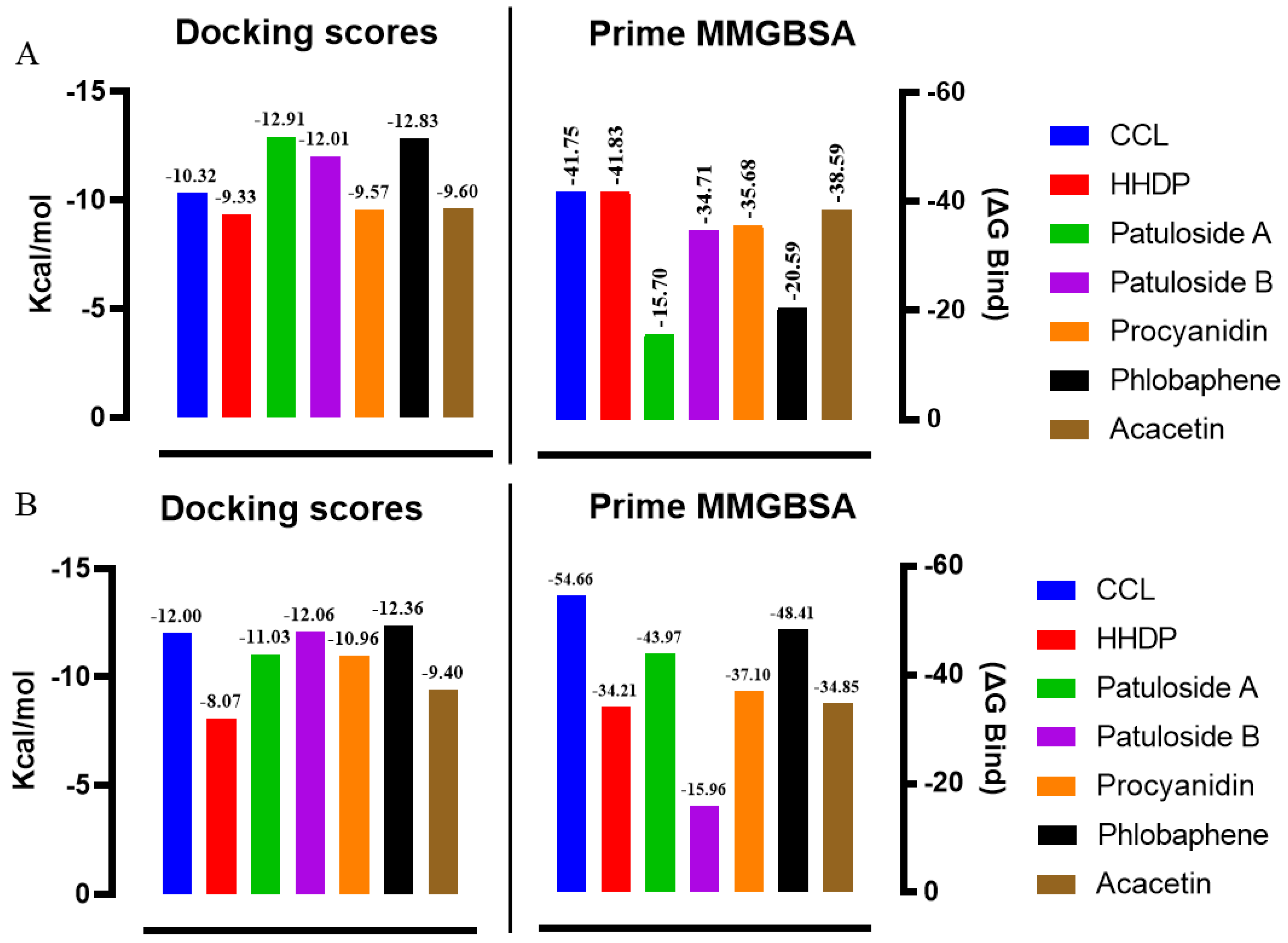
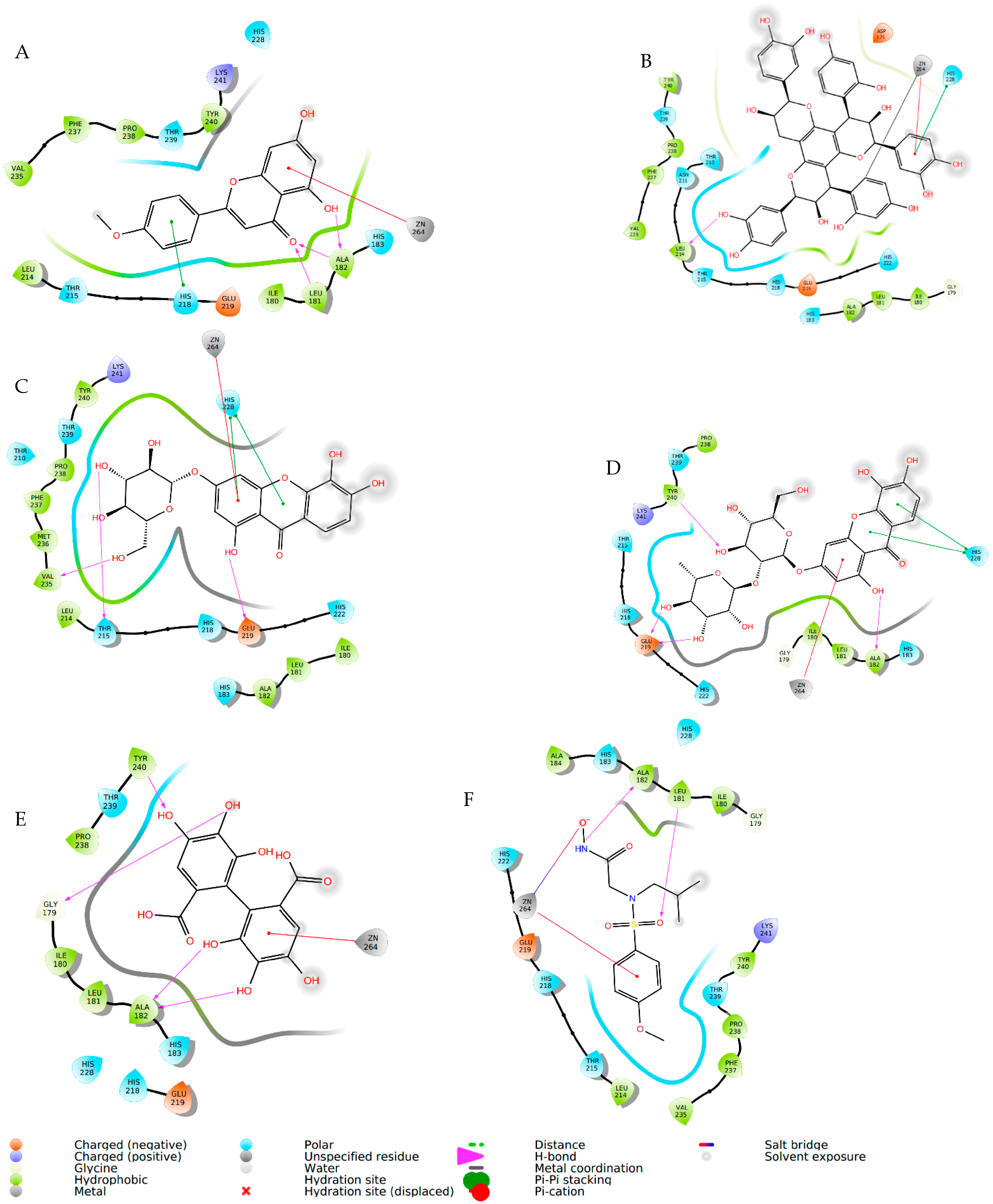
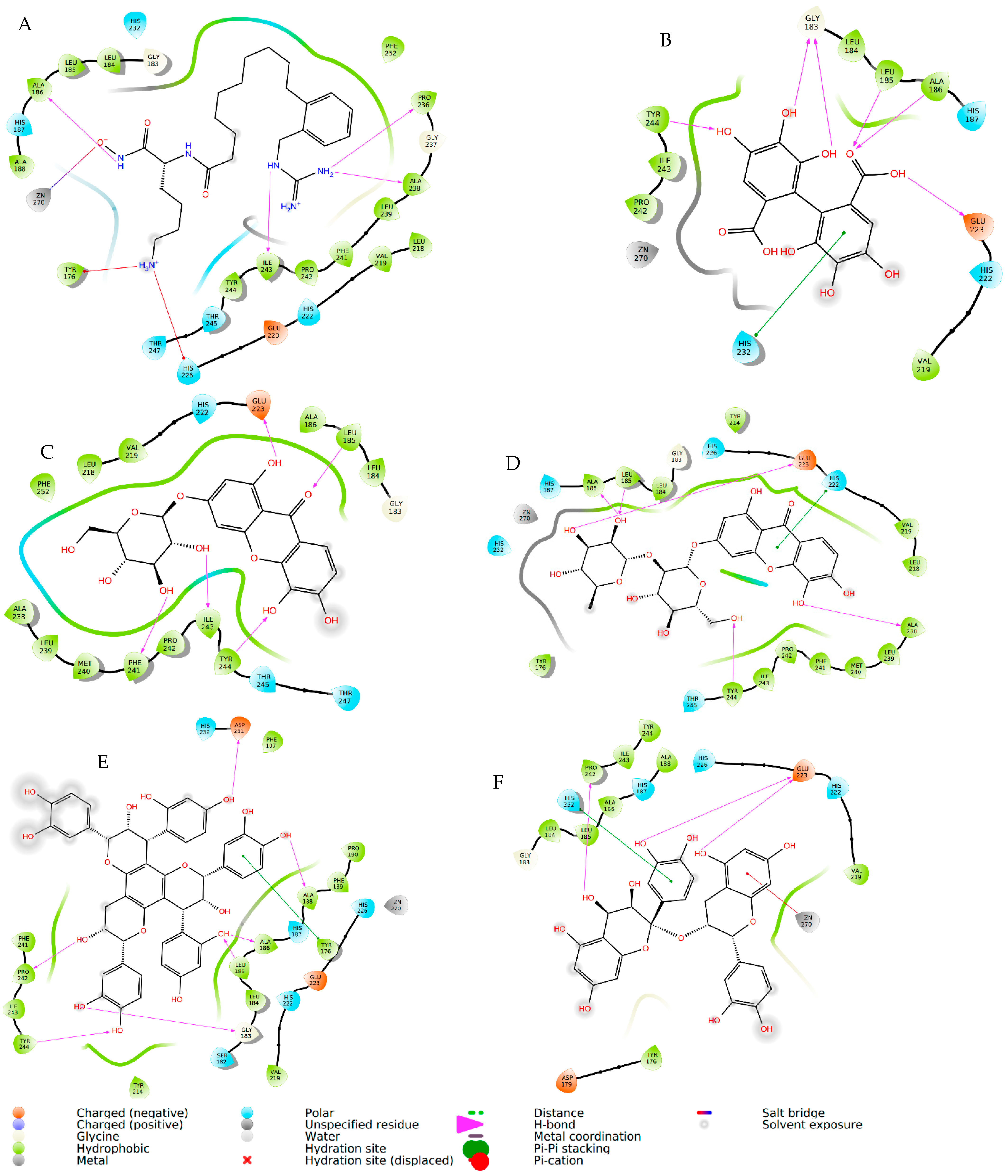
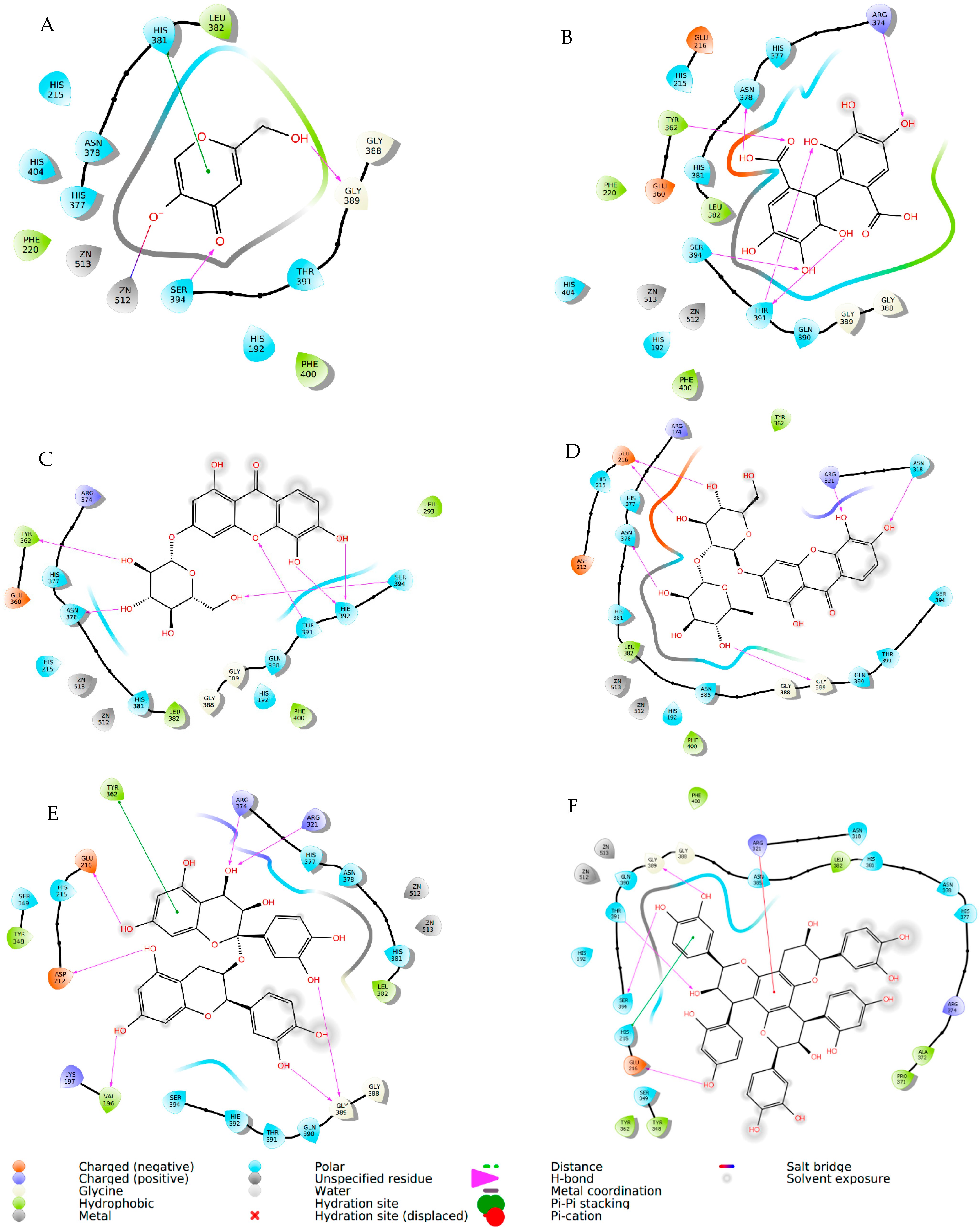
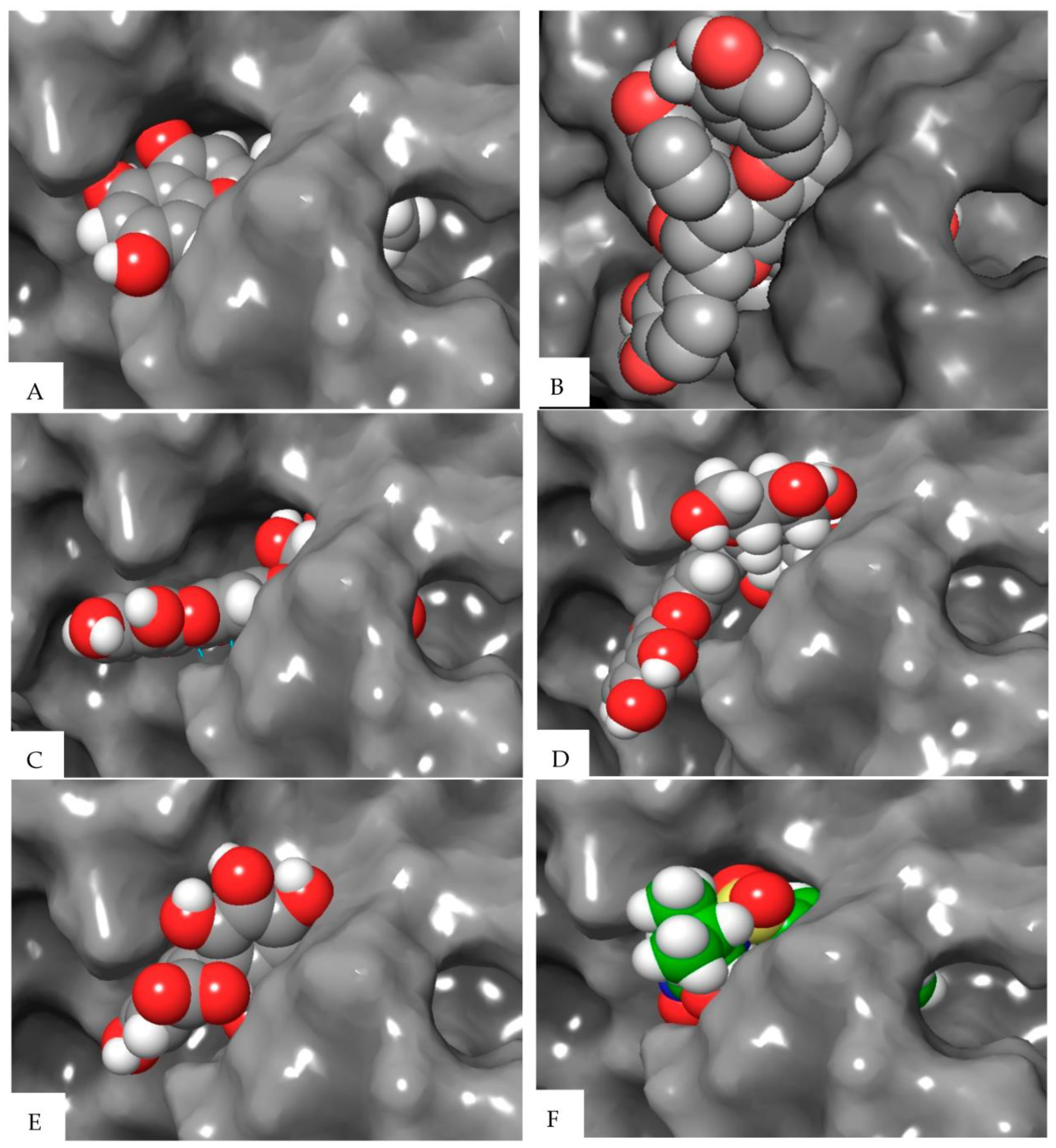
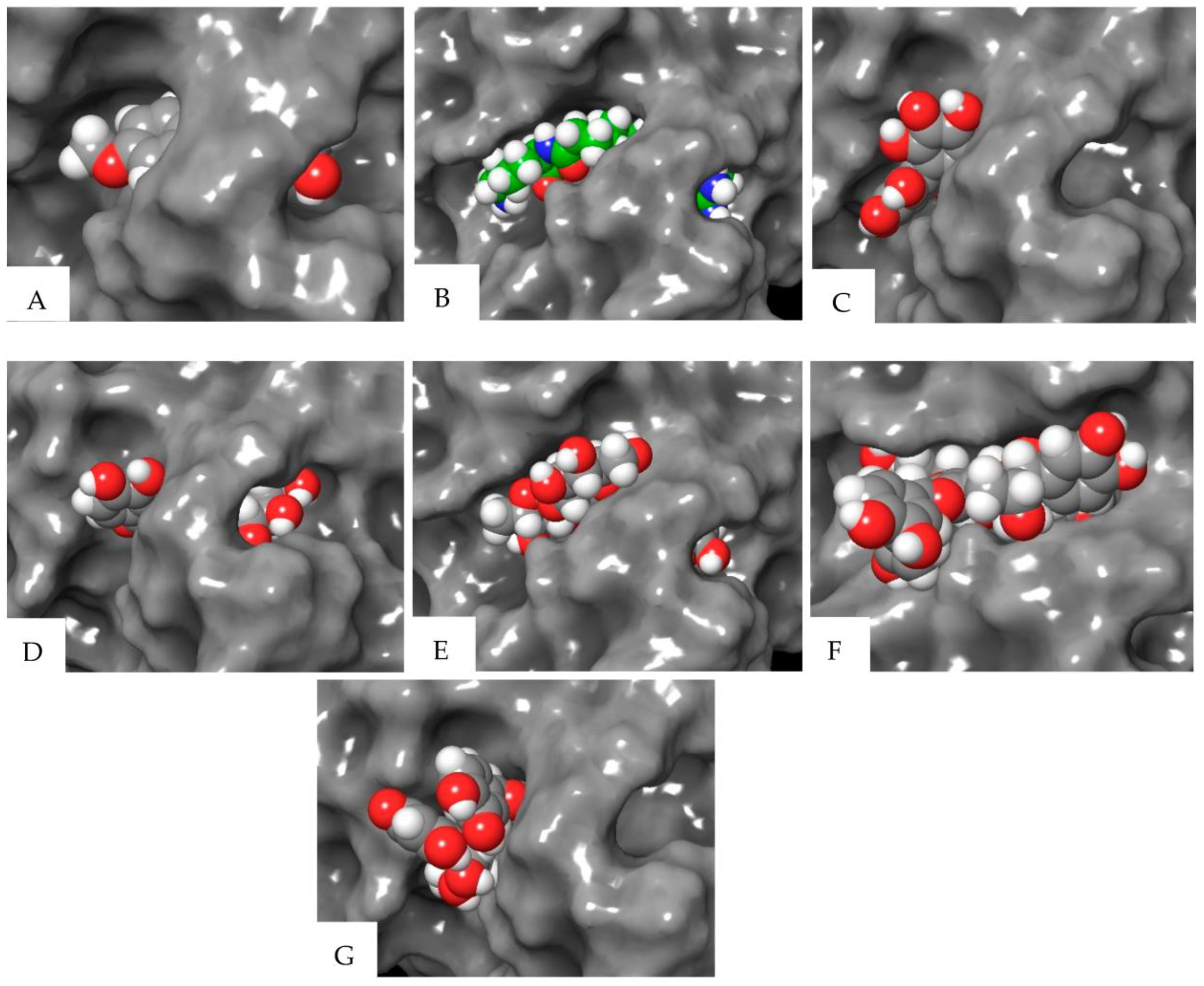
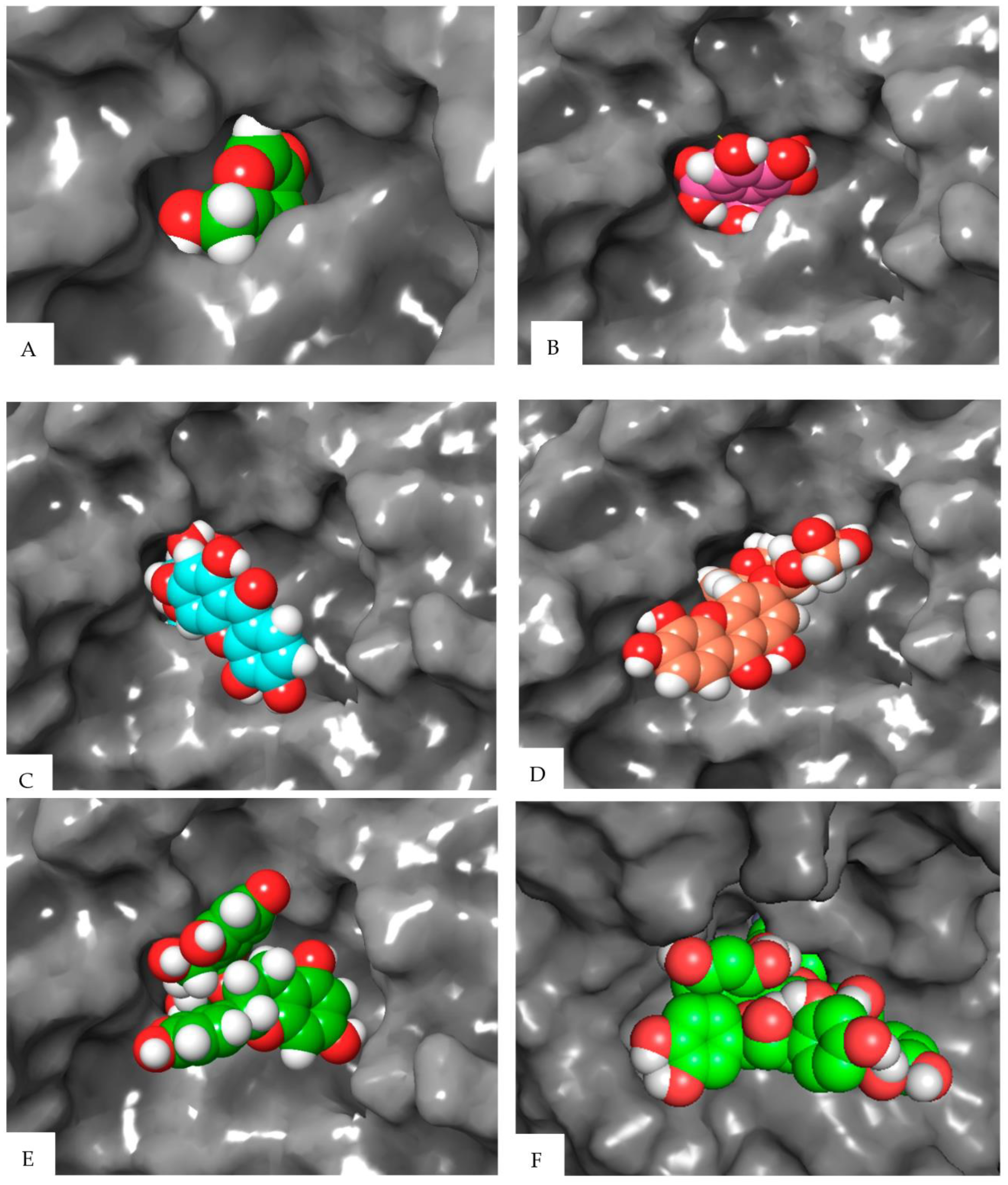
| Compound | Elastase (1RMZ) | Collagenase (2D1N) | Tyrosinase (5M8M) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Score | Ki (nM) | Key Interactions | Score | Ki (nM) | Key Interactions | Score | Ki (nM) | Key Interactions | |
| Co-crystallized ligands (CCL)/synthetic inhibitors | −10.32 | 24.48 | Zn chelation–Zn264 H-bond–LEU181, ALA 182 | −12.00 | 1.67 | Zn chelation–Zn270 H-bond–ALA186, PRO236, ALA238, ILE243. π-cat–TYR176, HIS226 | −8.19 | 898.03 | Zn chelation–Zn512 H-bond–SER394 π–π–HIS381 |
| Hexahydroxydiphenic acid | −9.33 | 137.99 | Zn chelation–Zn264 H-bond–GLY179, ALA 182, TYR240 | −8.07 | 1210 | H-bond–GLY183, LEU185, ALA 186, TYR244. π-π–HIS232 | −8.03 | 1258.0 | H-bond–TYR362, ARG374, ASN378, THR391, SER394 |
| Patuloside A | −12.91 | 0.35 | Zn chelation–Zn264 H-bond–THR215, GLU219, VAL235. π–π–HIS228 | −11.03 | 8.29 | H-bond–LEU185, GLU223, PHE241, ILE243, TYR244. | −8.47 | 614.69 | H-bond–TYR362, ASN378, THR391, SER394 |
| Patuloside B | −12.01 | 1.56 | Zn chelation–Zn264 H-bond–ALA 182, GLU219, TYR240. π–π–HIS228 | −12.06 | 1.45 | H-bond–LEU185, ALA 186, GLU223, ALA238, TYR244. π–π–HIS222 | −9.43 | 121.40 | H-bond–GLU216, ASN318, ARG321, ASN378, GLY389. |
| Procyanidin | −9.57 | 96.11 | Zn chelation–Zn264 H-bond–LEU181, ALA 182, GLU219, TYR240. π–π–HIS228 | −10.96 | 9.33 | Zn chelation–Zn270 H-bond–GLU223, PRO242. π–π–HIS232 | −9.32 | 193.48 | H-bond–VAL196, ASP212, GLU216, ARG321, ARG374, GLY389. π–π–TYR362 |
| Phlobaphene | −12.83 | 0.39 | Zn chelation–Zn264 H-bond–LEU214. π–π–HIS228 | −12.36 | 0.87 | H-bond–GLY183, LEU185, ALA 186, ALA188, ASP231, PRO242, TYR244. π–π–TYR176 | −8.51 | 582.93 | H-bond–GLU216, GLY389, THR391, SER394 π–π–HIS215 |
| Acacetin | −9.60 | 91.86 | Zn chelation–Zn264 H-bond–LEU181, ALA 182. π–π–HIS218 | −9.40 | 129.05 | H-bond–ALA238. π–π–HIS222 | −7.34 | 4180 | H-bond–ARG321, 374 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oyinloye, B.E.; Agbebi, E.A.; Agboola, O.E.; Ubah, C.S.; Owolabi, O.V.; Aruleba, R.T.; Onikanni, S.A.; Ejeje, J.N.; Ajiboye, B.O.; Omotuyi, O.I. Skin Anti-Aging Potentials of Phytochemicals from Peperomia pellucida against Selected Metalloproteinase Targets: An In Silico Approach. Cosmetics 2023, 10, 151. https://doi.org/10.3390/cosmetics10060151
Oyinloye BE, Agbebi EA, Agboola OE, Ubah CS, Owolabi OV, Aruleba RT, Onikanni SA, Ejeje JN, Ajiboye BO, Omotuyi OI. Skin Anti-Aging Potentials of Phytochemicals from Peperomia pellucida against Selected Metalloproteinase Targets: An In Silico Approach. Cosmetics. 2023; 10(6):151. https://doi.org/10.3390/cosmetics10060151
Chicago/Turabian StyleOyinloye, Babatunji Emmanuel, Emmanuel Ayodeji Agbebi, Oluwaseun Emmanuel Agboola, Chukwudi Sunday Ubah, Olutunmise Victoria Owolabi, Raphael Taiwo Aruleba, Sunday Amos Onikanni, Jerius Nkwuda Ejeje, Basiru Olaitan Ajiboye, and Olaposi Idowu Omotuyi. 2023. "Skin Anti-Aging Potentials of Phytochemicals from Peperomia pellucida against Selected Metalloproteinase Targets: An In Silico Approach" Cosmetics 10, no. 6: 151. https://doi.org/10.3390/cosmetics10060151
APA StyleOyinloye, B. E., Agbebi, E. A., Agboola, O. E., Ubah, C. S., Owolabi, O. V., Aruleba, R. T., Onikanni, S. A., Ejeje, J. N., Ajiboye, B. O., & Omotuyi, O. I. (2023). Skin Anti-Aging Potentials of Phytochemicals from Peperomia pellucida against Selected Metalloproteinase Targets: An In Silico Approach. Cosmetics, 10(6), 151. https://doi.org/10.3390/cosmetics10060151







