Abstract
Black pepper, a commonly utilized culinary condiment, holds significant importance in Ayurvedic and Chinese medicinal practices due to its various biological benefits, including antioxidant, antimicrobial, and anti-inflammatory properties. To amplify these attributes and enhance their efficacy in formulations, the utilization of nanocarriers presents a promising approach. Thus, the objective of this study was to obtain a pepper extract, evaluate its constituents, and encapsulate it in polymeric nanoparticles. The ethanol extract of the grains powder had a higher concentration of piperine and better antioxidant activity when compared to whole grains. Pepper extract encapsulation efficiency in terms of piperine concentration was 84.8 ± 3.5%, and a sustained and prolonged release profile was observed, as well as other studies in the literature using polycaprolactone (PCL). The presence of the extract did not change the instability index and the sedimentation velocity of the nanoparticles, as well as the polydispersity index and the zeta potential of nanoparticles. However, there was a difference in the mean size and concentration of particles. This study highlights the potential of PCL nanoparticles as a promising delivery system for black pepper extract, which could have various applications in the cosmetic and pharmaceutical industries, maximizing the benefits of black pepper extract.
1. Introduction
Black pepper, also known as pepper or Indian pepper, belongs to the Piperaceae family [1], originates from the forest of Kerala in southern Africa [2], and was introduced in Brazil during colonization in the 17th century. Its extracts have been used in folk medicine in different cultures. In India, it was one of the most used herbs in Ayurvedic medicine to treat gastrointestinal disorders and, more recently, chronic malaria [2,3,4]. Black pepper has also been used to treat epilepsy in traditional Chinese medicine [4]. Typically, the extracts obtained from black pepper contain alkaloids, flavonoids, tannins, and terpenes, which are substances with recognized biological activities, such as anti-inflammatory and antioxidant [5].
The cosmetic industries already use alkaloids in different types of formulations due to their antimicrobial and antioxidant activities, for lightening discoloration, reducing cellulite and wrinkles, soothing and anti-inflammatory properties, and protecting against UV radiation [6]. Piperine, one of the main components of black pepper, is an alkaloid responsible for its characteristic pungency. Throughout history, studies have demonstrated the pharmacological effects of piperine, including antidepressant, antipyretic, and anti-inflammatory activities [7,8], antimicrobial [9], antioxidant [10], analgesic, and wound-healing aid [11]. Cosmetic uses of piperine have also been reported, as permeation enhancers of other active ingredients through the skin [6], photoprotection [12], and potential use in treating vitiligo [13] and alopecia areata [14]. Nonetheless, the inherent pungency associated with piperine and its low aqueous solubility may be the main barriers to its topical use.
Carrier systems such as nanoparticles are highlighted in the literature as a promising alternative to protect compounds from degradation, improve their solubility, absorption, and subsequent bioavailability, and modify their tissue distribution profile, in addition to improving organoleptic characteristics and increasing the stability of natural compounds [15], and also as a platform to combine multiple actives [16]. Thus, encapsulation in nanocarriers has already been used to overcome piperine drawbacks. For example, polymeric nanoparticles composed of Eudragit and Poloxamer [17] and Poly(lactic-co-glycolic acid) (PLGA) [18] were used for the treatment of inflammatory skin disease and a nanoemulsion used for hypopigmentation disorders such as vitiligo [19]. Piperine anticancer activity has also been evaluated after its encapsulation in nanoemulsion [20], PLGA nanoparticles [21], and lipid polymer hybrid nanoparticles [22].
Among nanocarrier systems, polymeric nanoparticles are suitable for topical drug delivery, once they accumulate more in the stratum corneum, when compared to other systems (i.e., lipidic or emulsions) [16,23]. This accumulation not only reduces permeation into deeper layers, but also mitigates the risk of systemic off-target delivery. This promotes a targeted local drug delivery and allows the drug to be gradually released providing a period of retention in the skin [16,23]. Polymeric nanoparticles can be composed of various types of polymers, both synthetic and natural. Poly-ε-caprolactone (PCL) is a semi-crystalline aliphatic polyester of great interest, since it is a hydrophobic polymer with a lower cost than other aliphatic polyesters [24,25]. Due to its slow degradation and absence of toxicity, PCL has been investigated for use in drug and vaccine delivery systems [26,27] and has already been studied for piperine delivery as an electrospun nanofiber for cancer therapy [28,29]. PCL nanoparticles have been used for topical applications, with studies showing that they can prolong the residence time of actives in upper layers of skin, and even increase the rate and extent of transport across the skin [23,27,30,31,32]. PCL nanoparticles also present sustained release at a specific site, biocompatibility and biodegradability, and the possibility of modifications in the nanoparticles. Furthermore, PCL nanoparticles are easy and flexible to formulate, showing reproducibility and achieving various sizes and surface properties [33,34,35].
Given the biological functionalities attributed to pepper extract and its principal alkaloid, piperine, it becomes interesting to study the extraction process of this compound and its subsequent integration into pharmaceutical formulations. Therefore, the development of PCL nanoparticles as a carrier system for pepper extract can be considered an alternative to increasing the possibility of piperine application, aiming at its therapeutic use.
2. Materials and Methods
2.1. Materials
Black pepper grains were purchased at a local market in Campinas/SP. Piperine standard (98% purity) was purchased from Fluorochem (Hadfield, UK). Polyvinyl alcohol (PVA) was purchased from Dinâmica Química Contemporânea Ltd. (Indaiatuba, Brazil). 2,2-Diphenyl-1-picrylhydrazyl (DPPH), quercetin (95% purity), and polycaprolactone (PCL, average molecular weight 80,000 g/mol) were purchased from Sigma-Aldrich (Atlanta, GA, USA), and gallic acid and Folin–Ciocalteu reagent were purchased from Dinâmica Química Contemporânea Ltd. (São Paulo, Brazil), and tannic acid and sodium carbonate were purchased from Êxodo Científica (Sumaré, Brazil). All other reagents were of analytical grade.
2.2. Piperine Extraction
To obtain the pepper powder, the grains were crushed using a blender and the resulting powder was sieved to standardize the sizes. Ethanol p.a. was used for extraction, following the ratio of 50 g of plant material (grain or powder) to 200 mL of solvent. Both extractions were in constant agitation at 200 rpm for 72 h. Then, the extracts were filtered and then rotary evaporated to concentrate to a final volume of 100 mL.
2.3. Extract Characterization
2.3.1. Piperine Concentration
Piperine concentration was determined by spectrophotometer (Multiscan GO, Thermo Fisher Scientific, Waltham, MA, USA) at 342 nm [36]. The calibration curve was constructed with piperine (2–10 µg/mL). All assays were performed in triplicate.
2.3.2. Antioxidant Activity
In vitro antioxidant activity was evaluated by 2,2-diphenyl-1-picrylhydrazyl (DPPH) and ferric-reducing antioxidant power (FRAP) assays. The extract (20 µL) was added to a 96-well plate with 280 µL of DPPH solution (32 μg/mL) and the nanoparticle with extract (150 µL) was added to 150 µL of DPPH solution (32 μg/mL). Following a 30 min incubation period at room temperature in the absence of light, the absorbance of the samples was measured at 517 nm using a spectrophotometer. The assay was executed in triplicate [37].
To perform FRAP assay, the extract (20 µL) was added to a 96-well plate with 265 µL of FRAP reagent and 15 µL of ultrapure water. The microplate was incubated in the dark for 30 min, followed by measuring the absorbance at 595 nm in a spectrophotometer. The assay was executed in triplicate [38].
2.3.3. Tannin, Flavonoid and Phenol Concentration
To determine the concentration of tannins, 1 mL of the extracts was mixed with 0.5 mL of the Folin–Ciocalteu reagent, 1 mL of Na2CO3 solution, and 8 mL of distilled water. The samples were left at room temperature for 30 min, and their absorbance was then measured at 725 nm using a spectrophotometer. The tannin concentration was determined by utilizing the calibration curve of tannic acid (10–50 mg/mL). The tests were carried out in triplicate [39].
To determine the flavonoid content, 150 µL of the extracts were mixed with an equal volume of a 2% solution of aluminum chloride (AlCl3) in methanol. To prepare a blank sample, 150 µL of AlCl3 and 150 µL of methanol were mixed. The spectrophotometer was used to measure the absorbance at 425 nm after 10 min. The flavonoid content was calculated using a quercetin standard curve ranging from 20–100 µg/mL [40].
The Folin–Ciocalteu method was utilized to determine the total phenolic compound quantity. To the 96-well plate, 20 µL of the extracts were added along with 180 µL of ultrapure water, 20 µL of 1 N Folin–Ciocalteu, 20 µL of methanol, and 60 µL of sodium carbonate. The experiments were conducted in triplicate, and a standard curve was prepared with gallic acid (0–10 µg/mL). The samples were incubated at room temperature in the dark for 20 min, and the absorbance was measured at 760 nm using a spectrophotometer [41].
2.4. Extract-Loaded PCL Nanoparticles Formulation
PCL nanoparticles were produced by nanoprecipitation in an aqueous phase containing PVA as a steric stabilizer, according to Tundisi et al. [27], with modifications. PVA (0.25% m/v) was solubilized at 96 °C in ultrapure water and PCL (2.5 mg/mL) was solubilized in acetone at 37 °C in a sealed recipient to avoid solvent evaporation. To produce extract-loaded nanoparticles, black pepper extract (2 mg/mL) was added to the PCL solution. Ethanol (10 times the volume of extract added) was used as a cosolvent in the organic phase to avoid precipitation of extract components due to the acetone. For producing the nanoparticle suspension, 50 mL of the aqueous phase (PVA solution) was placed under magnetic stirring (300 rpm) and 12.5 mL of PCL solution (plus ethanol, with or without the extract) was poured in one quick motion into the aqueous phase, at room temperature. Nanoparticle suspensions were placed under magnetic stirring at 37 °C overnight for solvent removal. The final volume was adjusted to 50 mL with ultrapure water.
2.5. Nanoparticles Characterization
2.5.1. Nanoparticle’s Size Distribution and Zeta Potential
The mean diameter and size distribution of PCL nanoparticles was evaluated by dynamic light scattering (DLS) and zeta potential was determined by the electrophoretic mobility of particles in an electric field. All measurements were performed in the Zetasizer Nano ZS (Malvern, UK) at 25 °C in triplicates.
The mean diameter, dispersity, and concentration of PCL nanoparticles were evaluated at a 1:1000 (v/v) dilution in ultrapure water by nanoparticle tracking analysis (NTA) in the NanoSight NS300 (NanoSight Ltd., Salisbury, UK) and analyzed with NanoSight NTA software v3.44 (NanoSight Ltd., Salisbury, UK).
2.5.2. Nanoparticle’s Morphology
The morphology of empty and extract-loaded PCL nanoparticles was analyzed by cryo-transmission electron microscopy (cryo-TEM) in the equipment Talos F200C (Thermo Fischer Scientific, Waltham, MA, USA), operating at 200 kV. Samples (~3 µL) without dilution were added to thin carbon grades (300 mesh) and vitrified using Vitrobot (Thermo Fischer Scientific) with the following parameters: 2 blots and 3 s of blotting time. Images were acquired in a camera CETA 4k × 4k (Thermo Fischer Scientific), with −1 to 5 µm defocus, exposition time between 1 and 1.5 s, and pixel size of 0.413 nm.
2.5.3. Piperine Encapsulation Efficiency
A sample of extract-loaded PCL nanoparticles (1 mL) was centrifuged at 15,000× g for 1 h at 25 °C in a refrigerated centrifuge (Centrifuge 5810 R, Eppendorf, Hamburg, Germany). The concentration of non-encapsulated piperine was quantified in the supernatant as previously described. Empty PCL nanoparticles were also centrifuged, and the supernatant was used as a blank for the quantification. The encapsulation efficiency (EE) was determined by comparing the initial concentration of piperine in the extract at 2 mg/mL and the piperine in the supernatant (Equation (1)).
2.5.4. Piperine In Vitro Release
The in vitro release test of piperine from pepper extract, PCL nanoparticles, and piperine standard was conducted on vertical diffusion Franz cell system (PermeGear, Hellertown, PA, USA), with a diffusion area of 0.64 cm2 [27,42]. All samples were prepared to contain the same initial concentration of piperine by previous dilution in the same buffer (PBS) used as the receiving medium, to compare and to respect the sink conditions. The acceptor compartment (5 mL) was filled with phosphate buffer solution kept at a temperature of 33 °C by a thermostatic bath. The membranes (cellulose dialysis membrane, 14 kDa molecular weight cut-off) were previously soaked in ultra-purified water and then stored in the receiving medium to keep the pores open. A 1 mL sample was applied to the donor compartment. A sample (0.2 mL) of the acceptor fluid was withdrawn at fixed time intervals (30 min, 1, 2, 4, 6, 8, 24, 32, 48, 56, 72, 80, and 96 h) and replaced with an equal volume of the fresh PBS solution into the cell. The presence of piperine was determined by spectrophotometry at 342 nm. Each sample was evaluated in triplicate. Permeation of piperine across the membrane (%) was calculated considering the initial amount of piperine placed in the donor compartment, following Equation (2):
Steady-state permeation flux (Jss, µg/cm2/h) was determined following Equation (3):
where (dQ/dt)ss is the amount of TBH (µg) permeated through time (h), and A is the diffusion area (cm2).
2.5.5. Nanoparticles Stability
The accelerated stability and separation behavior was assessed using LUMiSizer® (LUM GmbH, Berlin, Germany). Freshly prepared PCL dispersions (0.4 mL) were slowly added to the bottom of the cell (2 mm polycarbonate sample cell with a rectangular cross-section). The cell was placed horizontally into the instrument after capping and exposed to centrifugal force under 2100 g (4000 rpm rotor speed) at 25 °C. A total of 1000 profiles were recorded in intervals of 20 s. Instability indexes were calculated using the SEPView™ version 6.4 software, and tests were carried out in triplicate.
Empty and extract-loaded PCL nanoparticles were stored in sealed recipients at 4 °C and evaluated periodically, at 1, 7, 15, 30, 60, 90, 120, and 150 days after production, regarding mean diameter, size distribution, and zeta potential at the Zetasizer Nano ZS (Malvern, UK). The encapsulation efficiency of piperine in the extract-loaded PCL nanoparticles was also evaluated.
2.6. Statistical Analysis
Data are expressed as means ± standard deviations. All statistical comparisons were made with Student’s t-test unpaired for independent samples or ANOVA followed by a suitable posttest using Prism 5 version 5.01 software (GraphPad Software Inc., San Diego, CA, USA). A p value < 0.05 was considered to indicate statistical significance.
3. Results
3.1. Piperine Concentration
The concentration of piperine in the extract using the grains was 41.2 ± 5.8 mg/g, and the grain powder showed a concentration of 743.8 ± 24.5 mg/g. The particle size influenced the yield of the extractive process, since smaller particles have a greater contact surface with the extracting solvent, allowing a greater amount of bioactive compounds to be extracted [43]. Other studies found values of 12.37 mg/g using methanol in an ultrasound bath for 30 min [44], and 53.71 mg/g using ethanol 70% in the maceration process for 24 h [45]. The difference found in this study and the literature may be due to the analytical method employed. Although UV-Vis spectroscopy is a fast and economical method, there may be interference from other compounds absorbing at the same wavelength, while high-performance liquid chromatography, used in other studies, presents the most specific measurement for piperine. Thus, not only the extraction method and the solvent influences the yield of the piperine extracts [46,47], but also the growing region and the harvest season [48], which can be the reason for the differences between the studies.
3.2. Antioxidant Activity
The antioxidant activity using the DPPH free radical method was determined for black pepper grain and powder. The results indicated that the ability to scavenge DPPH radicals was influenced by concentration. Based on the information presented in Figure 1, it can be inferred that higher concentrations led to greater scavenging effects. The grain extract showed higher antioxidant activity, reaching a maximum of 89% inhibition of the DPPH radical at the highest concentration used (0.25 µL/µL). On the other hand, the extract obtained from black pepper powder showed 77% inhibition of DPPH with a concentration 10 times lower (0.025 µL/µL) than the seed extract. This difference may be associated with the performance of the extractive process. The effective concentration (EC50) values for the grain were 0.15 mg/mL and for the powder, it was 0.02 mg/mL. The lower the EC50 value, the better the sample, as it means that a lower concentration is needed to inhibit 50% of the initial free radicals.
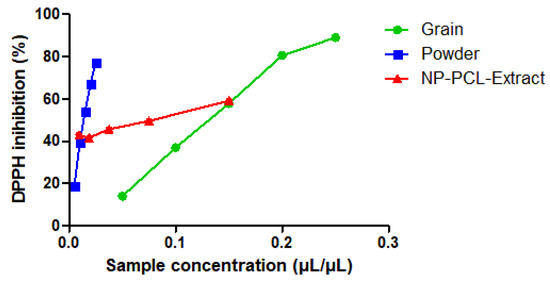
Figure 1.
Antioxidant activity in DPPH assay for grain, powder pepper extracts, and extract-containing nanoparticles (NP-PCL-Extract).
In another study, black pepper powder showed 48% antioxidant activity at 75 µg/mL [49]. This variation in results could be attributed to various factors, such as the use of different solvents, extraction techniques, and other related aspects. Thus, the findings suggest that the powder has a potential antioxidant activity.
For the extract-loaded PCL nanoparticle, the highest concentration also showed the best antioxidant activity with 58% at 0.15 µL/µL. It exhibited an EC50 of 0.15 mg/mL. The extract-loaded PCL nanoparticle exhibited lower antioxidant activity compared to the free extract, as it was encapsulated, which causes the release of the extract to be slower due to a different release profile, thereby resulting in lower antioxidant activity over the same period of time.
In the FRAP test, the powder also showed a better antioxidant activity than the grain, with a value of 2.08 ± 0.94 mg GAE/g sample (gallic acid equivalent) compared to 0.26 ± 0.04 mg GAE/g sample, respectively. Onyesife et al. [50] reported values of 0.08 ± 9.06 mg GAE/g sample for black pepper powder extracted with methanol and 0.01 ± 0.58 mg GAE/g sample for n-hexane extraction.
3.3. Tannin, Flavonoid and Phenol Concentration
The results obtained for the extracts from grain and powder of pepper regarding the quantification of tannins, flavonoids, and phenolic compounds are presented in Table 1. The extract obtained with the pepper powder showed the best results in all experiments performed, with a higher concentration of tannins, flavonoids, and phenolic compounds.

Table 1.
Tannins, flavonoids, and total phenolics for black pepper samples.
Other studies have reported the presence of tannins, flavonoids, and phenolic compounds, Tedasen et al. [51] found 201.82 ± 17.78 mg catechin equivalents/mg extract and 402.46 ± 7.49 mg of GAE/g extract for tannins and total phenolics, respectively. Saha and Verma [52] reported 4.30 ± 0.59 mg of rutin equivalent/g dry weight and 7.36 ± 0.20 mg of quercetin equivalent/g dry weight for tannins and flavonoids, respectively. In the present study, the grain and powder of black pepper found higher values for flavonoids than those reported by Saha and Verma [52]. However, the total phenolic content was found to be lower than that reported in the literature. Both results can be related to differences in solvent, extraction time, sample harvesting time, or other factors.
Tannins, flavonoids, and phenolic compounds are important secondary metabolites of plants with antioxidant and anti-inflammatory actions, among others. Therefore, since the extract obtained with black pepper powder showed the best results, this sample was chosen to be incorporated into nanoparticles.
3.4. Extract-Loaded PCL Nanoparticles Formulation
Piperine is an alkaloid from black pepper extract that can enhance the absorption of actives through the skin, an interesting feature for topical, cosmetical applications, and skincare [6]. Here, we aimed to use nanotechnology as a strategy to enhance the stability and reduce the pungency of the extract. Empty and extract-loaded PCL nanoparticles were successfully produced using PVA as a stabilizer. Both PCL and PVA are biocompatible polymers used in dosage forms for topical application [26,53]. Nanoparticles were characterized by DLS (Table 2) and NTA (Table 3) after production.

Table 2.
Characterization of PCL nanoparticles without and with black pepper extract by dynamic light scattering (ZetaSizer NanoZS) after production.

Table 3.
Characterization of PCL nanoparticles without and with black pepper extract by nanoparticles tracking analysis (NanoSight NS300) after production.
The addition of black pepper extract led to a decrease in size measured by DLS by around 20 nm (p < 0.05) and no change in polydispersity index (p > 0.05). This suggests the interaction between the extract and polymeric chains of PCL. The polydispersity index, which measures the heterogeneity of a sample’s size, was below 0.1 for both unloaded and loaded formulations, which translates to a monodispersed size distribution, favorable for the distribution, cellular uptake, and release of the active from the particles [54].
Size measurement in NTA was different than in DLS, which might be due to the high dilution needed for analysis (1:1000) and the difference in techniques. Both DLS and NTA measure the hydrodynamic diameter of nanoparticles. However, DLS detects the hydrodynamic diameter by correlating the fluctuation in scattered light intensity over time, while NTA registers the particle motion of scattered light using a camera, leading to the difference in particle size parameters detected in each technique [55,56]. Overall, the size of PCL nanoparticles was around 200–300 nm, which means it is on the nanometer scale, but favorable for topical residency and cosmetic application [57].
The zeta potential of empty and extract-loaded nanoparticles was small and negative (−1 mV), with no significant statistical difference between formulations (p > 0.05). The observed zeta potential is due to PVA acetate groups that yield a negative charge [58]. A highly negative or positive zeta potential (+20 mV or −20 mV) is often sought after for the stability of formulations, but other factors such as steric stabilization play an important role in stability. PVA acts as a stabilizer because it reduces surface tension and can link with PCL from the nanoparticles [27,53].
Encapsulation efficiency of piperine in extract-loaded PCL nanoparticles was 84.8 ± 3.5%. Previous studies demonstrated high encapsulation efficiency of piperine in polymeric nanoparticles, such as 87% with chitosan [59], and 92% [60] and 99% [18] with poly(lactic-co-glycolic) acid. The high entrapment capacity of piperine may be associated with a high affinity with hydrophobic polymeric chains.
Morphology analysis by cryo-EM demonstrated that nanoparticles were mostly oval and spherical (Figure 2). It was possible to observe fiber-like structures around some particles, which are attributed to PCL’s elasticity and ability to create nanofibers. The mean size of empty PCL nanoparticles was 166.7 nm while extract loaded was 102.9 nm. The smaller results compared to DLS and NTA measurements are expected since both measure the hydrodynamic size of particles. However, the reduction in size after pepper extract incorporation was consistent between techniques.
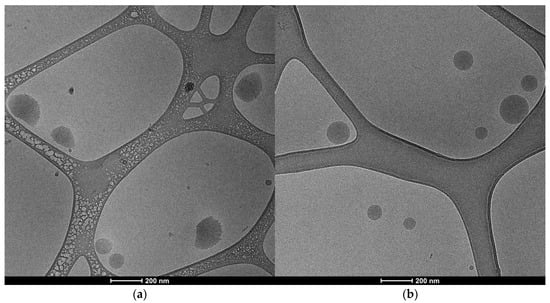
Figure 2.
Representative cryo-TEM images of empty PCL nanoparticles (a) and extract-loaded nanoparticles (b) from an aqueous suspension.
3.5. Piperine In Vitro Release
Figure 3 shows the piperine release profile from the pepper extract, the nanoparticle suspension containing pepper extract (NP-PCL-Extract), and the piperine standard. It is possible to see that there is a difference (statistically significant, p < 0.01) in almost all samples’ times, except for 30 min, 1 h, and 2 h times. From the release data, the piperine release flow (µg/cm2/h) was calculated from the extract and the nanoparticles, and there was a statistical difference (p < 0.01) between the samples (Figure 4), indicating that nanoparticles slowed piperine release under used experiment conditions.
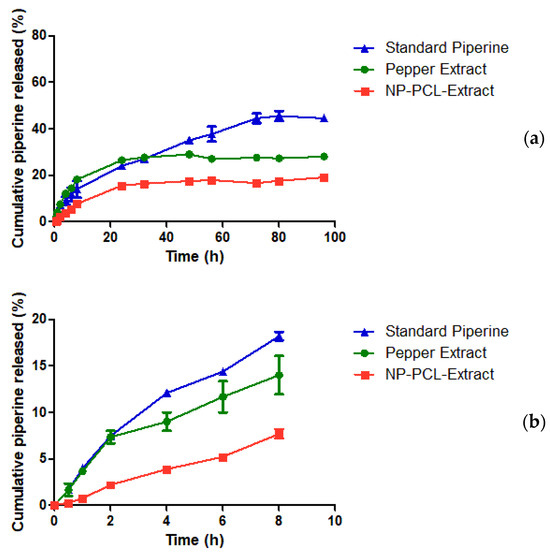
Figure 3.
Cumulative piperine in vitro released (%) from piperine standard, pepper extract, and extract-containing nanoparticles (NP-PCL-Extract) throughout pre-determined time intervals (a) and in detail of the first 8 h (b).

Figure 4.
Piperine flux during piperine release assay from piperine standard, pepper extract, and extract-containing nanoparticles (NP-PCL-Extract). The * symbol indicates significant statistical difference between samples (one-way ANOVA with Tukey posttest, p < 0.05).
According to Figure 3, both the standard piperine and the pepper extract had a higher % release at all sampling times compared to NP-PCL-Extract, with values of 28% for the piperine standard, 26% for pepper extract and 16% for NP-PCL-Extract, all in 24 h. The use of the PCL polymer for the nanoencapsulation of the extract can justify the lower % of release, as well as the lower flux of the NP-PCL-Extract (Figure 4); this polymer has been used in other studies with piperine [28,29] and also with other drugs, such as melatonin [42], methotrexate [61], and terbinafine [27], which proved to be promising for a sustained and prolonged release profile of the drug. Babadi et al. carried out studies producing nanofibers containing mixtures of PCL and collagen for the release of piperine for postsurgical breast cancer treatment. In this work, the release of the drug obtained a similar profile in the first 8 h, which showed a release of approximately 12% in 8 h sampling time compared to NP-PCL-Extract which was 8% in the same sampling time (Figure 3). This test was observed for 16 days (with less than 70% piperine being released) [28], which confirms the trend of a prolonged release profile of piperine using PCL as a carrier polymer. On the other hand, another study reached more than 90% release of piperine from PCL nanoparticles in 48 h when using purified water as a release medium [62].
3.6. Nanoparticles Stability
The transmission profiles showed that both formulations tend to precipitate the nanoparticles (Figure 5) since there was an increase in transmittance in the upper portion of the cell over time.
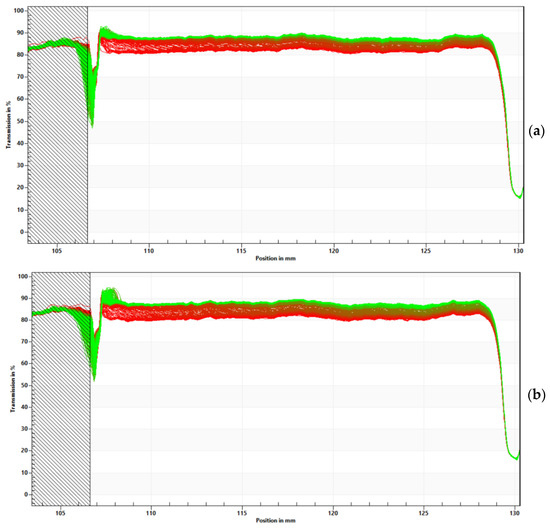
Figure 5.
Recorded evolution of transmission profiles of empty (a) and extract-loaded nanoparticles (b). The abscissa shows the cell position, and the ordinate represents the percentage of light transmission. The initial profiles are indicated by red lines, while the most recent profiles are represented by green lines over time.
The comparison of instability index and sedimentation velocity for empty and loaded nanoparticles was performed. Empty nanoparticles showed an instability index of 0.496 ± 0.045 and sedimentation velocity of 1.625 ± 0.069 µm/s, while extract-loaded nanoparticles showed values of 0.529 ± 0.016 and 1.762 ± 0.115 µm/s, respectively. These values were not statistically different (p > 0.05), meaning that the incorporation of pepper extract did not alter the physical stability of the nanoparticles.
The storage stability of PCL nanoparticles was evaluated by measuring the size distribution and polydispersity index by DLS, and EE for extract-loaded nanoparticles, over the course of 180 days (Figure 6). Empty PCL nanoparticles presented statistical differences in size at days 30, 90, 120, 150, and 180 when compared to the first day of production (one-way ANOVA, p < 0.05). Meanwhile, extract-loaded nanoparticle size presented statistical differences at days 60 and 150 (one-way ANOVA, p < 0.05). This observed size variation was below 5% for NP-PCL and NP-PCL-Extract, and the polydispersity index of both formulations was stable throughout the storage period evaluated. In addition, there was no variation regarding the encapsulation efficiency of piperine during storage, ranging from 84.8 ± 3.5% on the first day, to 86.7 ± 0.3% after 6 months (one-way ANOVA p > 0.05). Therefore, black pepper extract-loaded nanoparticles stored at 4 °C were considered stable for 180 days.
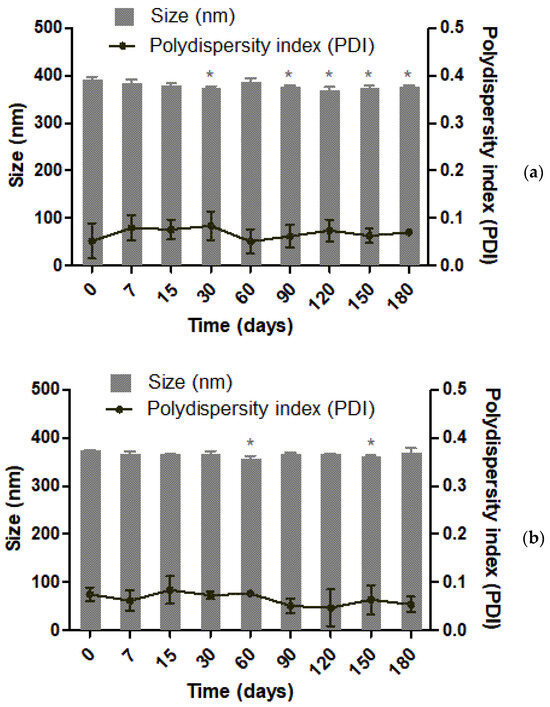
Figure 6.
Size and polydispersity index measured by dynamic light scattering of (a) unloaded PCL nanoparticles (NP-PCL) and (b) loaded with black pepper extract (NP-PCL-Extract) over 180 days. The * symbol indicates significant statistical difference when compared to first day of production (one-way ANOVA followed by Dunnett’s multiple comparison test, p < 0.05).
4. Conclusions
To summarize, this study indicates that using black pepper grain powder results in a higher concentration of piperine in the extract than using whole grains, as well as higher antioxidant acidity, and higher concentration of tannins, flavonoids, and phenolic compounds. Therefore, it was chosen for incorporation into nanoparticles, which were successfully produced using PVA as a stabilizer. The addition of black pepper extract decreased the nanoparticle size but did not interfere with nanoparticle stability under accelerated or storage conditions. Encapsulation promoted a sustained and prolonged release profile of piperine when compared with the extract. Thus, PCL nanoparticles can be considered an effective delivery system for black pepper extract, being a promising ingredient for skincare and cosmetic applications.
Author Contributions
Conceptualization, J.A.A. and P.G.M.; methodology, J.C.C., L.A.L.S., É.M.d.S., A.C.S. and J.A.A.; validation, J.C.C., L.A.L.S., É.M.d.S., A.C.S. and J.A.A.; formal analysis, J.C.C., L.A.L.S., É.M.d.S., A.C.S. and J.A.A.; investigation, J.C.C., L.A.L.S., É.M.d.S. and A.C.S.; resources, P.G.M.; data curation, J.A.A. and P.G.M.; writing—original draft preparation, J.C.C., L.A.L.S., É.M.d.S., A.C.S. and J.A.A.; writing—review and editing, A.C.P.-S. and P.G.M.; visualization, J.C.C., L.A.L.S., É.M.d.S., A.C.S., J.A.A. and A.C.P.-S.; supervision, P.G.M.; project administration, J.A.A. and P.G.M.; funding acquisition, P.G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), grant numbers 2018/07707-6, 2018/06475-4, 2020/05292-3, 2020/11333-4, and 2021/00319-3.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Authors acknowledge the contribution of former student Bruna Cezario Evangelista dos Santos, who started the project. This research used facilities of the Brazilian Nanotechnology National Laboratory (LNNano), part of the Brazilian Centre for Research in Energy and Materials (CNPEM), a private non-profit organization under the supervision of the Brazilian Ministry for Science, Technology, and Innovations (MCTI). The TEM-FT and CRYO-EM-FT staff are acknowledged for the assistance during the experiments (proposal numbers 20230954 and 20231889).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Garcia, J.; Kamada, T.; Jacobson, T.; Curado, M.; Oliveira, S. Super ação de dormência em sementes de pimenta-do-reino (Piper nigrum L.). Pesqui. Agropecuária Trop. 2007, 30, 51–54. [Google Scholar] [CrossRef]
- Pissinate, K. Atividade Citotóxica de Piper Nigrum e Struthanthusmarginatus. Estudo Preliminar da Correlação Entre a Citotoxicidade e Hidrofobicidade da Piperina e Derivados Sintéticos; Universidade Federal Rural do Rio de Janeiro: Rio de Janeiro, Brazil, 2006. [Google Scholar]
- Mailoo, V. The Ayurvedic Model of Human Occupation. Asian J. Occup. Ther. 2007, 6, 1–13. [Google Scholar] [CrossRef]
- Singletary, K. Black Pepper: Overview of Health Benefits. Nutr. Today 2010, 45, 43–47. [Google Scholar] [CrossRef]
- Houcine, B.; Romdhane, M.; Souchard, J.-P.; Cazaux, S.; Bouajila, J. Chemical Composition and Anticancer and Antioxidant Activities of Schinus Molle, L. and Schinus Terebinthifolius Raddi Berries Essential Oils. J. Food Sci. 2010, 75, C466–C472. [Google Scholar] [CrossRef]
- Stępniowska, A.; Cieplińska, P.; Fac, W.; Górska, J. Selected Alkaloids Used in the Cosmetics Industry. J. Cosmet. Sci. 2021, 72, 229–245. [Google Scholar]
- Abbasi, B.; Ahmad, N.; Fazal, H.; Mahmood, T. Conventional and modern propagation techniques in Piper nigrum. J. Med. Plants Res. 2010, 4, 7–12. [Google Scholar]
- Gupta, S.K.; Bansal, P.; Bhardwaj, R.K.; Velpandian, T. Comparative anti-nociceptive, anti-inflammatory and toxicity profile of nimesulide vs nimesulide and piperine combination. Pharmacol. Res. 2000, 41, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Masood Ahmed Chaudhry, N.; Tariq, P. Bactericidal activity of black pepper, bay leaf, aniseed and coriander against oral isolates. Pak. J. Pharm. Sci. 2006, 19, 214–218. [Google Scholar]
- Mittal, R.; Gupta, R. In vitro antioxidant activity of piperine. Methods Find. Exp. Clin. Pharmacol. 2000, 22, 271–274. [Google Scholar] [CrossRef]
- Parmar, V.; Jain, S.; Bisht, K.; Jain, R.; Taneja, P.; Jha, A.; Tyagi, O.; Prasad, A.; Wengel, J.; Olsen, C.; et al. Phytochemistry of genus Piper. Phytochemistry 1997, 46, 597–673. [Google Scholar] [CrossRef]
- Verma, A.; Kushwaha, H.N.; Srivastava, A.K.; Srivastava, S.; Jamal, N.; Srivastava, K.; Ray, R.S. Piperine attenuates UV-R induced cell damage in human keratinocytes via NF-kB, Bax/Bcl-2 pathway: An application for photoprotection. J. Photochem. Photobiol. B Biol. 2017, 172, 139–148. [Google Scholar] [CrossRef]
- Faas, L.; Venkatasamy, R.; Hider, R.C.; Young, A.R.; Soumyanath, A. In vivo evaluation of piperine and synthetic analogues as potential treatments for vitiligo using a sparsely pigmented mouse model. Br. J. Dermatol. 2008, 158, 941–950. [Google Scholar] [CrossRef]
- Mao, Y.; Xu, Z.; Song, J.; Xie, Y.; Mei, X.; Shi, W. Efficacy of a mixed preparation containing piperine, capsaicin and curcumin in the treatment of alopecia areata. J. Cosmet. Dermatol. 2022, 21, 4510–4514. [Google Scholar] [CrossRef]
- Ye, Y.-J.; Wang, Y.; Lou, K.; Chen, Y.-Z.; Chen, R. The preparation, characterization, and pharmacokinetic studies of chitosan nanoparticles loaded with paclitaxel/dimethyl-β-cyclodextrin inclusion complexes. Int. J. Nanomed. 2015, 10, 4309–4319. [Google Scholar] [CrossRef]
- Ataide, J.A.; Coco, J.C.; dos Santos, É.M.; Beraldo-Araujo, V.; Silva, J.R.A.; de Castro, K.C.; Lopes, A.M.; Filipczak, N.; Yalamarty, S.S.K.; Torchilin, V.P.; et al. Co-Encapsulation of Drugs for Topical Application-A Review. Molecules 2023, 28, 1449. [Google Scholar] [CrossRef] [PubMed]
- Politi, F.A.S.; Carvalho, S.G.; Rodero, C.F.; dos Santos, K.P.; Meneguin, A.B.; Sorrechia, R.; Chiavacci, L.A.; Chorilli, M. Piperine-loaded nanoparticles incorporated into hyaluronic acid/sodium alginate-based membranes for the treatment of inflammatory skin diseases. Int. J. Biol. Macromol. 2023, 227, 736–748. [Google Scholar] [CrossRef] [PubMed]
- Budama-Kilinc, Y. Piperine Nanoparticles for Topical Application: Preparation, Characterization, In vitro and In silico Evaluation. ChemistrySelect 2019, 4, 11693–11700. [Google Scholar] [CrossRef]
- Ozkan, B.; Altuntas, E.; Cakir Koc, R.; Budama-Kilinc, Y. Development of piperine nanoemulsions: An alternative topical application for hypopigmentation. Drug Dev. Ind. Pharm. 2022, 48, 117–127. [Google Scholar] [CrossRef]
- Alshehri, S.; Bukhari, S.I.; Imam, S.S.; Hussain, A.; Alghaith, A.F.; Altamimi, M.A.; AlAbdulkarim, A.S.; Almurshedi, A. Formulation of Piperine-Loaded Nanoemulsion: In Vitro Characterization, Ex Vivo Evaluation, and Cell Viability Assessment. ACS Omega 2023, 8, 22406–22413. [Google Scholar] [CrossRef]
- Kaur, J.; Singh, R.R.; Khan, E.; Kumar, A.; Joshi, A. Piperine-Loaded PLGA Nanoparticles as Cancer Drug Carriers. ACS Appl. Nano Mater. 2021, 4, 14197–14207. [Google Scholar] [CrossRef]
- Kazmi, I.; Al-Abbasi, F.A.; Imam, S.S.; Afzal, M.; Nadeem, M.S.; Altayb, H.N.; Alshehri, S. Formulation of Piperine Nanoparticles: In Vitro Breast Cancer Cell Line and In Vivo Evaluation. Polymers 2022, 14, 1349. [Google Scholar] [CrossRef]
- Alvarez-Román, R.; Naik, A.; Kalia, Y.N.; Guy, R.H.; Fessi, H. Skin penetration and distribution of polymeric nanoparticles. J. Control. Release 2004, 99, 53–62. [Google Scholar] [CrossRef]
- Leroueil-Le Verger, M.; Fluckiger, L.; Kim, Y.-I.; Hoffman, M.; Maincent, P. Preparation and characterization of nanoparticles containing an antihypertensive agent. Eur. J. Pharm. Biopharm. 1998, 46, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Sinha, V.R.; Bansal, K.; Kaushik, R.; Kumria, R.; Trehan, A. Poly-ϵ-caprolactone microspheres and nanospheres: An overview. Int. J. Pharm. 2004, 278, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.; Laurencin, C. Biodegradable Polymers as Biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Tundisi, L.L.; Ataide, J.A.; da Fonseca, J.H.L.; Silvério, L.A.L.; Lancellotti, M.; Paiva-Santos, A.C.; d’Ávila, M.A.; Kohane, D.S.; Mazzola, P.G. Terbinafine Nanohybrid: Proposing a Hydrogel Carrying Nanoparticles for Topical Release. Pharmaceutics 2023, 15, 841. [Google Scholar] [CrossRef]
- Babadi, D.; Dadashzadeh, S.; Shahsavari, Z.; Shahhosseini, S.; ten Hagen, T.L.M.; Haeri, A. Piperine-loaded electrospun nanofibers, an implantable anticancer controlled delivery system for postsurgical breast cancer treatment. Int. J. Pharm. 2022, 624, 121990. [Google Scholar] [CrossRef]
- Jain, S.; Meka, S.R.K.; Chatterjee, K. Engineering a Piperine Eluting Nanofibrous Patch for Cancer Treatment. ACS Biomater. Sci. Eng. 2016, 2, 1376–1385. [Google Scholar] [CrossRef]
- Alvarez-Román, R.; Barré, G.; Guy, R.H.; Fessi, H. Biodegradable polymer nanocapsules containing a sunscreen agent: Preparation and photoprotection. Eur. J. Pharm. Biopharm. 2001, 52, 191–195. [Google Scholar] [CrossRef]
- Javaid, S.; Ahmad, N.M.; Mahmood, A.; Nasir, H.; Iqbal, M.; Ahmad, N.; Irshad, S. Cefotaxime Loaded Polycaprolactone Based Polymeric Nanoparticles with Antifouling Properties for In-Vitro Drug Release Applications. Polymers 2021, 13, 2180. [Google Scholar] [CrossRef] [PubMed]
- Kondiah, P.P.D.; Rants’o, T.A.; Mdanda, S.; Mohlomi, L.M.; Choonara, Y.E. A Poly(Caprolactone)-Cellulose Nanocomposite Hydrogel for Transdermal Delivery of Hydrocortisone in Treating Psoriasis Vulgaris. Polymers 2022, 14, 2633. [Google Scholar] [CrossRef]
- Łukasiewicz, S.; Mikołajczyk, A.; Błasiak, E.; Fic, E.; Dziedzicka-Wasylewska, M. Polycaprolactone Nanoparticles as Promising Candidates for Nanocarriers in Novel Nanomedicines. Pharmaceutics 2021, 13, 191. [Google Scholar] [CrossRef]
- El Yousfi, R.; Brahmi, M.; Dalli, M.; Achalhi, N.; Azougagh, O.; Tahani, A.; Touzani, R.; El Idrissi, A. Recent Advances in Nanoparticle Development for Drug Delivery: A Comprehensive Review of Polycaprolactone-Based Multi-Arm Architectures. Polymers 2023, 15, 1835. [Google Scholar] [CrossRef] [PubMed]
- Pawar, R.; Pathan, A.; Nagaraj, S.; Kapare, H.; Giram, P.; Wavhale, R. Polycaprolactone and its derivatives for drug delivery. Polym. Adv. Technol. 2023, 34, 3296–3316. [Google Scholar] [CrossRef]
- Bhairy, S.; Shaikh, A.; Nalawade, V.; Hirlekar, R. Development and validation of bivariate UV-visible spectroscopic method for simultaneous estimation of curcumin and piperine in their combined nanoparticulate system. J. Appl. Pharm. Sci. 2021, 11, 064–070. [Google Scholar]
- Pires, J.; Torres, P.B.; Santos, D.Y.A.C.d.; Chow, F. Ensaio em microplaca do potencial antioxidante através do método de sequestro do radical livre DPPH para extratos de algas. Inst. Biociências Univ. São Paulo 2017, 12, 1–6. [Google Scholar]
- Urrea-Victoria, V.; Pires, J.; Torres, P.B.; Alves, D.Y.; Santos, C.d.; Chow, F. Ensaio Antioxidante em Microplaca do Poder de Redução do Ferro (FRAP) Para Extratos de Algas; Instituto de Biociências, Universidade de São Paulo: São Paulo, Brazil, 2016; pp. 1–6. ISBN 978-85-85658-62-5. [Google Scholar]
- Shad, M.; Nawaz, H.; Rehman, T.; Badar, H.; Hussain, M. Optimization of extraction efficiency of tannins from Cichorium intybus L.: Application of response surface methodology. J. Med. Plant Res. 2012, 6, 4467–4474. [Google Scholar]
- Alves, E.; Kubota, E.H. Conteúdo de fenólicos, flavonoides totais e atividade antioxidante de amostras de própolis comerciais. Rev. Ciências Farm. Básica E Apl. 2013, 34, 37–41. [Google Scholar]
- Caetano, K.; Ceotto, J.; Ribeiro, A.; de Morais, F.; Ferrari, R.; Pacheco, M.; Capitani, C.; Caetano, K.A.; Ceotto, J.M.; Badan Ribeiro, A.P.; et al. Effect of baru (Dipteryx alata Vog.) addition on the composition and nutritional quality of cookies. Food Sci. Technol. 2017, 37, 239–245. [Google Scholar] [CrossRef]
- Massella, D.; Leone, F.; Peila, R.; Barresi, A.A.; Ferri, A. Functionalization of Cotton Fabrics with Polycaprolactone Nanoparticles for Transdermal Release of Melatonin. J. Funct. Biomater. 2018, 9, 1. [Google Scholar] [CrossRef]
- Gorgani, L.; Mohammadi, M.; Najafpour, G.D.; Nikzad, M. Sequential Microwave-Ultrasound-Assisted Extraction for Isolation of Piperine from Black Pepper (Piper nigrum L.). Food Bioprocess Technol. 2017, 10, 2199–2207. [Google Scholar] [CrossRef]
- Yu, L.; Hu, X.; Xu, R.; Ba, Y.; Chen, X.; Wang, X.; Cao, B.; Wu, X. Amide alkaloids characterization and neuroprotective properties of Piper nigrum L.: A comparative study with fruits, pericarp, stalks and leaves. Food Chem. 2022, 368, 130832. [Google Scholar] [CrossRef] [PubMed]
- Narendra Babu, K.; Hemalatha, R.; Satyanarayana, U.; Shujauddin, M.; Himaja, N.; Bhaskarachary, K.; Dinesh Kumar, B. Phytochemicals, polyphenols, prebiotic effect of Ocimum sanctum, Zingiber officinale, Piper nigrum extracts. J. Herb. Med. 2018, 13, 42–51. [Google Scholar] [CrossRef]
- Grinevicius, V.M.A.S.; Andrade, K.S.; Ourique, F.; Micke, G.A.; Ferreira, S.R.S.; Pedrosa, R.C. Antitumor activity of conventional and supercritical extracts from Piper nigrum L. cultivar Bragantina through cell cycle arrest and apoptosis induction. J. Supercrit. Fluids 2017, 128, 94–101. [Google Scholar] [CrossRef]
- Subramanian, R.; Subbramaniyan, P.; Noorul Ameen, J.; Raj, V. Double bypasses soxhlet apparatus for extraction of piperine from Piper nigrum. Arab. J. Chem. 2016, 9, S537–S540. [Google Scholar] [CrossRef]
- Vieira, L.V.; Juvenato, M.M.E.; Krause, M.; Heringer, O.A.; Ribeiro, J.S.; Brandão, G.P.; Kuster, R.M.; Carneiro, M.T.W.D. The effects of drying methods and harvest season on piperine, essential oil composition, and multi-elemental composition of black pepper. Food Chem. 2022, 390, 133148. [Google Scholar] [CrossRef]
- Gülçin, İ. The antioxidant and radical scavenging activities of black pepper (Piper nigrum) seeds. Int. J. Food Sci. Nutr. 2005, 56, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Onyesife, C.O.; Chukwuma, I.F.; Okagu, I.U.; Ndefo, J.C.; Amujiri, N.A.; Ogugua, V.N. Nephroprotective effects of Piper nigrum extracts against monosodium glutamate-induced renal toxicity in rats. Sci. Afr. 2023, 19, e01453. [Google Scholar] [CrossRef]
- Tedasen, A.; Khoka, A.; Madla, S.; Sriwiriyajan, S.; Graidist, P. Anticancer effects of piperine-free Piper nigrum extract on cholangiocarcinoma cell lines. Pharmacogn. Mag. 2020, 16, S28–S38. [Google Scholar]
- Saha, S.; Verma, R.J. In vitro and in silico study of Piper nigrum on cyclooxygenase-2, inducible nitric oxide synthase and antioxidant enzymes. J. Herb. Med. 2015, 5, 86–98. [Google Scholar] [CrossRef]
- Cortés, H.; Hernández-Parra, H.; Bernal-Chávez, S.A.; Prado-Audelo, M.L.D.; Caballero-Florán, I.H.; Borbolla-Jiménez, F.V.; González-Torres, M.; Magaña, J.J.; Leyva-Gómez, G. Non-Ionic Surfactants for Stabilization of Polymeric Nanoparticles for Biomedical Uses. Materials 2021, 14, 3197. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.M.; Jäger, E.; Jäger, A.; Stepánek, P.; Giacomelli, F.C. Physicochemical aspects behind the size of biodegradable polymeric nanoparticles: A step forward. Colloids Surf. A Physicochem. Eng. Asp. 2013, 436, 1092–1102. [Google Scholar] [CrossRef]
- Bell, N.C.; Minelli, C.; Tompkins, J.; Stevens, M.M.; Shard, A.G. Emerging Techniques for Submicrometer Particle Sizing Applied to Stöber Silica. Langmuir 2012, 28, 10860–10872. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Bolzinger, M.-A.; Briançon, S.; Chevalier, Y. Nanoparticles through the skin: Managing conflicting results of inorganic and organic particles in cosmetics and pharmaceutics. WIREs Nanomed. Nanobiotechnol. 2011, 3, 463–478. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Bogatyrov, V.; Ostolska, I.; Szewczuk-Karpisz, K.; Terpiłowski, K.; Nosal-Wiercińska, A. Impact of poly(vinyl alcohol) adsorption on the surface characteristics of mixed oxide MnxOy–SiO2. Adsorption 2016, 22, 417–423. [Google Scholar] [CrossRef]
- Baspinar, Y.; Üstündas, M.; Bayraktar, O.; Sezgin, C. Curcumin and piperine loaded zein-chitosan nanoparticles: Development and in-vitro characterisation. Saudi Pharm. J. 2018, 26, 323–334. [Google Scholar] [CrossRef]
- Li, S.; Guo, J.; Tian, Z.; Chen, J.; Gou, G.; Niu, Y.; Li, L.; Yang, J. Piperine-Loaded Glycyrrhizic Acid- and PLGA-Based Nanoparticles Modified with Transferrin for Antitumor. AAPS PharmSciTech 2021, 22, 239. [Google Scholar] [CrossRef]
- Hashemi, S.; Mortazavi, S.A.; Moghimi, H.R.; Darbasizadeh, B. Development and evaluation of a novel methotrexate-loaded electrospun patch to alleviate psoriasis plaques. Drug Dev. Ind. Pharm. 2022, 48, 355–366. [Google Scholar] [CrossRef]
- de Oliveira, J.G.; Pilz-Júnior, H.L.; de Lemos, A.B.; da Silva da Costa, F.A.; Fernandes, M.; Gonçalves, D.Z.; Variza, P.F.; de Moraes, F.M.; Morisso, F.D.P.; Magnago, R.F.; et al. Polymer-based nanostructures loaded with piperine as a platform to improve the larvicidal activity against Aedes aegypti. Acta Trop. 2022, 230, 106395. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).