Abstract
The dietary bacillus natto productive protein (BNPP) is a functional food ingredient that contains Bacillopeptidase F. BNPP is reported to improve blood flow. Based on previous research, we investigated the effect of BNPP on the skin. In vitro tests were performed to evaluate BNPP for its inhibitory effects on tyrosinase, elastase, and active oxygen (2,2-diphenyl-1-picrylhydrazyl (DPPH)) radical scavenging activities. In addition, a small-scale, single-armed trial of 15 female participants aged 40–65 years were conducted to assess the effects on human skin of BNPP, administered 250 mg/day orally, for 6 weeks. The beneficial effects of BNPP on the skin were shown by the evaluation of the tyrosinase inhibitory (0.01% and 0.1%), elastase inhibitory (0.00001% to 0.001%), and DPPH radical scavenging (1% and 10%) activities. In addition, the results suggested that the oral administration of BNPP may significantly enhance skin rosiness and also achieve significant improvement in skin conditions, defined as complexion, skin elasticity and resilience, moist feeling, skin texture, cosmetic adhesion, fine lines, under-eye darkness, eye bags, sagging cheeks, and sagging mouth. Furthermore, to investigate the use of BNPP as cosmetics, a skin irritation study was conducted using a cultured human skin model. The results showed that BNPP is non-irritant. In addition, to confirm the stability of BNPP, the quality of BNPP at the time of manufacture and three years and six months after manufacture was examined. The results showed no quality problems. These results suggest that the BNPP could be used as cosmetic purposes.
1. Introduction
Japanese traditional foods are known to be healthy overall and have the effects of reducing all-cause mortality, cardiovascular mortality, and combined cancer mortality [1]. Natto is a traditional Japanese fermented food rich in nutrients, including vitamins, proteins, amino acids, and dietary fiber. It has a long history in the traditional diet of Japanese people. However, some people are unwilling to eat natto because of its distinctive odor. To address this issue, Daiwa Pharmaceutical Co., Ltd. (Tokyo, Japan) developed BNPP as a functional food ingredient containing Bacillopeptidase F (EC 3.4.21.), one of the proteases produced by natto bacteria, and produces it under the trade name NKCP®. BNPP is a white or light-yellow powder with a slight fermented odor. The natto bacteria are removed from BNPP. The distinctive odor and vitamin K produced by natto are also removed from BNPP to negligible levels, creating a product that is easier to ingest than natto. BNPP has anti-coagulation, thrombolytic, and blood viscosity-lowering effects [2], and the oral administration of BNPP to rats showed an effect of promoting thrombolysis [3]. In addition, in a four-week study of oral BNPP administration, participants reported symptom improvements associated with blood circulation, such as blood flow; elevation in the skin surface temperature of the neck and shoulders; an improvement of neck muscle stiffness, shoulder stiffness, and low back pain; the amelioration of coldness of the extremities and headache; and decreased blood pressure [4,5,6,7].
The causal association between skin aging and blood flow has been demonstrated [8]; therefore, maintaining good blood flow is essential to skin care. Since BNPP is known to be effective in improving blood flow [7], it is expected to exhibit a skincare effect as well. To date, however, there has been no study demonstrating the effect of BNPP on skin. With this background, the present study evaluated the inhibitory effects on tyrosinase (EC 1.14.18.1), elastase (EC 3.4.21.36), and active oxygen (2,2-diphenyl-1-picrylhydrazyl (DPPH)) radical scavenging activities of BNPP. Furthermore, a small-scale trial with adult female participants was also performed to evaluate the effect of BNPP on the skin. The potential for the future use of BNPP as cosmetics was also investigated.
2. Materials and Methods
2.1. Measurement of Tyrosinase Inhibitory Activity
The effect of tyrosinase inhibitory activity on the BNPP test samples was evaluated using dihydroxyphenylalanine (DOPA) as a substrate [9,10]. The test samples were prepared as follows. First, 80 μL of BNPP solution or 80 μL of the control were put into individual 1.5 mL test tubes, and 160 μL of tyrosinase (40 units/mL) (CAS No. 9002-10-2, Sigma-Aldrich, St. Louis, MO, USA) solution and 400 μL of 100 mM phosphate buffer (pH 6.8) were added. The solution was vortexed and incubated at 23 °C for 3 min. For the blank, 100 mM phosphate buffer was used. Next, 200 μL of 2.5 mM 3,4-L-DOPA (CAS No. 59-92-7, Wako, Japan) solution was added, mixed with the vortex mixer, and incubated at 23 °C for 10 min. The solution was then centrifuged at 12,000× g for 3 min. The supernatant was recovered into a 1.5 mL test tube, and 210 μL of the recovered supernatant was dispensed into each well of a 96-well plate in triplicate. The absorbance (optical density (OD) 490) was measured at 490 nm with a microplate reader. The activity rate of tyrosinase was calculated from the OD 490 values of each sample well and control using the following formula:
where C is the OD 490 of the control; CB is the OD 490 of the blank for the control; S is the OD 490 of the test sample; SB is the OD 490 of the blank for the test sample.
Tyrosinase activity rate (%) = (S − SB)/(C − CB) × 100
2.2. Measurement of Elastase Inhibitory Activity
The effect of BNPP on the elastase activity was evaluated using N-succinyl-ala-ala-ala-p-nitroanilide as a substrate [11]. First, 200 μL of BNPP solution or control, 200 μL of elastase (1.25 μg/mL) (CAS No. 39445-21-1, Sigma-Aldrich, St. Louis, MO, USA) solution, and 400 μL of 0.555 mM N-succinyl-Ala-Ala-Ala-p-nitroanilide (CAS No. 52299-14-6, Sigma-Aldrich, St. Louis, MO, USA) solution were put into a 1.5 mL test tube. The solution was vortexed and incubated at 37 °C for 15 min. For the blank, 0.05 M Tris-HCl buffer (pH 8.0) was used. The solution was then centrifuged at 12,000× g for 3 min. The supernatant was recovered into a 1.5 mL test tube, and 200 μL of the recovered supernatant was dispensed into each well of a 96-well plate in triplicate. The absorbance (OD 415) was measured at 415 nm with a microplate reader.
The activity rate of elastase was calculated from OD 490 values of each sample well and control using the following formula:
where C is the OD 415 of the control; CB is the OD 415 of the blank for the control; S is the OD 415 of the test sample; SB is the OD 415 of the blank for the test sample.
Elastase activity rate (%) = (S − SB)/(C − CB) × 100
2.3. Evaluation of DPPH Radical Scavenging Activity
The DPPH radical scavenging activity of BNPP was evaluated as follows [12]. First, 240 μL of BNPP solution or control, 240 μL of ethanol (CAS No. 64-17-5, Japan Alcohol, Japan), and 160 μL of 0.25 M acetate buffer (pH 5.5) were put into a 1.5 mL test tube. The solution was vortexed and incubated at 37 °C for 5 min. Ethanol was used for the blank. Next, 160 μL of 78.4 μM DPPH (CAS No. 1898-66-4, Sigma-Aldrich, St. Louis, MO, USA) solution was added into the test tube, and the solution was mixed with the vortex mixer and incubated at 37 °C for 30 min. The solution was then centrifuged at 12,000× g for 3 min. The supernatant was recovered into a 1.5 mL test tube, and 200 μL of the recovered supernatant was dispensed into each well of a 96-well plate in triplicate. The absorbance (OD 517) was measured at 517 nm with a microplate reader.
Active oxygen residual rate was calculated from the OD 517 values of each sample well and control using the following formula:
where C is the OD 517 of the control; CB is the OD 517 of the blank for the control; S is the OD 517 of the test sample; SB is the OD 517 of the blank for the test sample.
Active oxygen residual rate (%) = (S − SB)/(C − CB) × 100
2.4. Skin Irritation Test
A skin irritation test was performed using a LabCyte EPI-MODEL24 (Japan Tissue Engineering Co., Ltd., Aichi, Japan) [13]. LabCyte EPI-MODEL24 tissues were transferred into 24-well plates with the assay medium (500 µL) and incubated overnight (37 °C, 5% CO2, humidified atmosphere). Three tissues were used to assess of BNPP. To ensure good contact with the epidermal surface, the epidermal surface was moistened with 25 µL distilled water and then 25 mg of BNPP was added. Three tissues serving as negative controls were treated with 25 µL distilled water, and three tissues serving as positive controls were exposed to 5% sodium lauryl sulfate (SLS, CAS No. 151-21-3, FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan). After 15 min, each tissue was washed with phosphate buffered saline (PBS, FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan). The tissues were then transferred to new wells containing 1 mL of fresh assay medium. The treated and control tissues were post-incubated for 42 h (37 °C, 5% CO2, humidified atmosphere). After 42 h post-incubation, tissues were transferred to new wells on 24-well plates containing 500 µL of fresh prepared MTT medium (0.5 mg/mL) for the MTT assay. Tissues were incubated for three hours (37 °C, 5% CO2, humidified atmosphere) and were then transferred to microtubes containing 300 µL isopropanol, completely immersing the tissue. Each tissue was refrigerated overnight and the pigment was extracted. Subsequently, 200 µL of extracts were transferred to a 96-well plate. The optical density was measured at 570 nm and at 650 nm as a reference absorbance, with isopropanol as a blank.
Cell viability was calculated from the absorbance of the test substance relative to the absorbance of the negative control using the following formula:
where Sa is the absorbance of BNPP or positive control; NC is the mean absorbance of the negative control (n = 3).
Cell viability (%) = Sa/NC × 100
Cell viability was assessed as irritating if the mean value of cell viability (n = 3) was less than 50%, and non-irritating if the mean value of cell viability (n = 3) was more than 50%.
2.5. Clinical Study
2.5.1. Inclusion/Exclusion Criteria for Participants
Participants included were healthy women aged 40–65 who were concerned about the “darkness” of their skin, such as under-eye pigmentation or bags (hereafter referred to as darkness).
The exclusion criteria were as follows:
- a.
- Dermatological diseases, such as atopic dermatitis, contact dermatitis, and/or cutaneous hypersensitivity;
- b.
- Use of an oral drug or topical drug applied to the test skin site within two weeks before starting the study with a potential impact on the study intent;
- c.
- Injury or tanned area that may interfere with measurement;
- d.
- Past medical history of malignant tumors, heart failure, and/or myocardial infarction;
- e.
- Chronic diseases under medical treatment, as follows: atrial fibrillation, arrhythmia, liver failure, kidney disease, cerebrovascular disease, rheumatoid arthritis, diabetes mellitus, dyslipidemia, hypertension, and other chronic diseases;
- f.
- Regular intake of medicines (herbal medicines), food for specified health uses, foods with functional claims, health foods, and supplements;
- g.
- Allergies to medicines or test food-related foods; and
- h.
- Pregnancy, breastfeeding, or intention to become pregnant during the study period.
Based on these criteria, 15 participants were evaluated for this study.
2.5.2. Ethics Approval and Informed Consent
The present study was conducted in consideration of medical ethics in accordance with the Declaration of Helsinki and the Ethical Guidelines for Life Sciences and Medical Research Involving Human Subjects, and was approved by the Ethics Committees of NISHI-UMEDA Clinic for Asian Medical Collaboration (Japan, IRB No. 20000026). The study protocol was registered with the University Hospital Medical Information Network (UMIN-CTR) (UMIN000049095). The study was conducted from October 2022 to January 2023 in Japan.
Prior to the study, the participants received explanation on the study orally and in writing, and signed the informed consent to participate in the study. The participants could withdraw their consent at any time.
2.5.3. Test Food Samples
The test food samples were supplied by Daiwa Pharmaceutical Co., Ltd. (Tokyo, Japan). The samples were formulated as tablets containing 125 mg of BNPP per tablet and compounded ingredients. These ingredients included BNPP, maltodextrin, crystalline cellulose, sucrose fatty acid ester, silicon dioxide granules, and shellac.
2.5.4. Study Design
In this single-arm study, the participants were instructed to take 2 BNPP-containing tablets per day after dinner (250 mg/day) for the 6-week intervention period. All outcome variables were assessed at baseline and at the end of 6 weeks. The primary endpoints were defined as skin brightness, hue, and chroma. The secondary endpoints were defined as dark circle status, stratum corneum moisture content, skin texture, and skin viscoelasticity and responses to the questionnaire about skin conditions. Before skin site testing, participants were asked to wash their test skin sites and faces, followed by 15 min habituation in an environmental chamber controlled at 20 ± 2 °C, 50 ± 5% humidity. The measurements were taken after habituation.
Skin lightness, hue, and chroma were calculated by measuring Lab values under the left eye using a CM-26d® spectrophotometer (Konica Minolta Japan, Inc., Tokyo, Japan). A higher value of L* means brighter; a value of +a* indicates a red tint and −a* indicates a green tint; a value of +b* indicates a yellow tint and −b* indicates a blue tint; a higher value of c* means brighter shades; and a lower value of c* means duller shades.
The dark circles status score was based on the naked eye observation of the degree of dark circles under the left eye and rated by the physician responsible, on a seven-point scale: very prominent, 1; prominent, 2; somewhat prominent, 3; neither prominent nor not prominent, 4; not very prominent, 5; not prominent, 6; and not prominent at all, 7.
The stratum corneum moisture content was measured five times at the same location on the inner left forearm using a Corneometer CM825® (Courage + Khazaka Electronic, Cologne, Germany) and the average of the measurements was used.
The skin texture was measured using the VISIA Evolution (Canfield Scientific, Parsippany-Troy Hills, NJ, USA) to determine the number of texture marks on the left side of the face.
The skin viscoelasticity was measured five times at the same location on the inner left forearm using a Cutometer MPA580® (Courage + Khazaka Electronic, Cologne, Germany) and the average of the measured values was used. R2 (Ua/Uf, width of return after release of negative pressure/maximum elongation value at negative pressure = skin restoration rate), R5 (Ur/Ue, immediate width of return after release of negative pressure/elastic part of relaxation phase = elastic modulus at suction phase), and R7 (Ur/Uf, immediate width of return after release of negative pressure/maximum elongation value at negative pressure = elastic modulus at contraction).
After a macroscopic self-observation of their skin conditions, participants answered the survey questions about skin conditions, defined as complexion, skin elasticity and resilience, moist feeling, skin texture, cosmetic adhesion, fine lines, under-eye darkness, eye bags, sagging cheeks, and sagging mouth on the following 7-point scale: 1 = very bad; 2 = bad; 3 = slightly bad; 4 = acceptable; 5 = good; 6 = fairly good; and 7 = very good.
2.6. Statistical Analysis
The statistical analysis of tyrosinase inhibitory activity, elastase inhibitory activity, and DPPH radical scavenging activity was performed by Student t-test of parametric tests. The statistical analysis of skin lightness, hue, and chroma, stratum corneum moisture content, skin texture, and skin viscoelasticity was performed by Student-paired samples t-test of parametric tests. For dark circles status score and skin condition, the statistical analysis was performed using the nonparametric Wilcoxon signed-rank test. For all items, p < 0.05 was considered significant.
3. Results
3.1. Tyrosinase Inhibitory Activity, Elastase Inhibitory Activity, and DPPH Radical Scavenging Activity
Tyrosinase is an enzyme that induces skin melanogenesis. Elastase is an enzyme that degrades elastin, which plays a key role in keeping skin resilient (termed elasticity). In addition, active oxygen is involved in melanogenesis and photoaging by ultraviolet rays. BNPP exhibited significant inhibitory activity on tyrosinase at 0.01% and 0.1% (Figure 1a), while on elastase at 0.00001% to 0.001% (Figure 1b), and DPPH radical scavenging at 1% and 10% (Figure 1c).
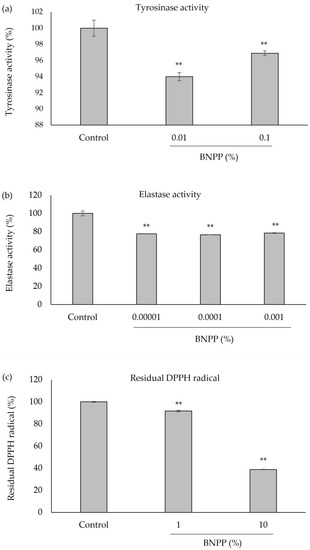
Figure 1.
Inhibitory effect of BNPP on tyrosinase, elastase, and reactive oxygen species (DPPH) radical scavenging activity. (a) Tyrosinase activity; (b) elastase activity; (c) residual DPPH radical. Vertical bars indicate standard deviations. **: Compared with control, p < 0.01.
3.2. Participant Tests
All fifteen participants (all female; average age 52.5 ± 7.29 years) completed the study without any issues, such as failing to remember to take the tablets or developing a poor physical condition.
3.2.1. Primary Endpoints
Brightness (L*) enhanced from 67.24 to 68.56 with no significant difference, and the complexion became slightly brighter, showing a trend toward improvement in skin conditions. Whereas skin hue a* significantly increased from 16.27 to 17.04, skin hue b* significantly decreased from 22.07 to 21.11. An increase in skin hue a* value indicates an increase in the skin’s red tone, whereas a decrease in skin hue b* indicates a reduced yellow tone. Croma (c*) slightly decreased from 27.46 to 27.20, with no significant difference (Table 1).

Table 1.
Effects of BNPP on skin brightness, hue, and chroma.
3.2.2. Secondary Endpoints
The dark circles status score slightly improved from 2.93 to 3.07, with no significant difference. Also, the stratum corneum moisture content increased from 25.9 to 27.3, with no significant difference. The skin texture was slightly enhanced from 1327.5 to 1335.0, with no significant difference. The skin viscoelasticity was slightly reduced in R2 (0.745 to 0.723) and in R7 (0.546 to 0.517), with no significant difference, whereas skin viscoelasticity was slightly but significantly reduced in R5 (0.753 to 0.707) (p = 0.025). A significant enhancement was observed in all responses to the questions of the questionnaire survey, suggesting that the skin conditions were improved (Table 2).

Table 2.
Effects of BNPP on skin conditions reported by participants on the ten-question survey.
3.3. Skin Irritation Test
The cell viability was assessed as irritating if the mean value of cell viability was less than 50%, and non-irritating if the mean value of cell viability was more than 50%. The mean value of cell viability for BNPP was 93.6%, and therefore, the skin irritancy was assessed as non-irritant (Figure 2).
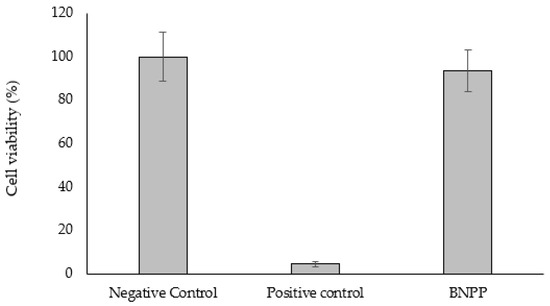
Figure 2.
Skin irritation test of BNPP. The skin irritancy of BNPP was assessed using the LabCyte EPI-MODEL24. Cell viability was assessed as irritating if the mean value of cell viability (n = 3) was less than 50%, and non-irritating if the mean value of cell viability (n = 3) was more than 50%.
3.4. Stability of BNPP
To investigate the stability of BNPP, properties (color, odor), aerobic plate count, and coliform bacteria of BNPP (Lot No. B2HJ0101) at production and after 3 years and 6 months of storage at room temperature were tested. The results showed little difference between the two test results, confirming their high stability (Table 3).

Table 3.
The stability of BNPP (Lot No. B2HJ0101).
4. Discussion
In previous studies, BNPP has demonstrated an effect of improving blood flow, an increase in skin surface temperature of the neck and shoulders, an amelioration of muscle stiffness in the neck, an amelioration of shoulder stiffness, low back pain improvement, improvement in the coldness of the extremities, an amelioration of headaches, and a decrease in blood pressure [4,5,6,7]. The present study demonstrated that BNPP has tyrosinase inhibitory, elastase inhibitory, and DPPH radical scavenging activities.
Melanin is the major pigment present in human skin and is the most important photoprotective factor in response to UV damage from the sun [14]. Melanin is synthesized in melanosomes of melanocytes [15]. Tyrosinase is a multifunctional copper-containing polyphenol oxidase and is key to melanin biosynthesis [16]. Tyrosinase is closely involved in melanin synthesis via the hydroxylation of tyrosine to 3,4-dihydroxyphenylalanine (l-DOPA) and the oxidation of l-DOPA to dopaquinone [17]. As tyrosinase overactivation is correlated with pigmentation, the inhibition of this enzyme is the most effective approach to control melanin overproduction and its dangerous effects. Therefore, tyrosinase inhibitors are being developed for use as whitening agents [18].
Elastin is a protein with elastic properties that exists in connective tissue, including the skin, and plays an important role in maintaining tissue structure after stretching or recoil. Excessive UV irradiation and the excessive production of active oxygen both trigger the expression of elastase, which reduces skin elasticity and causes wrinkles and sagging [19,20]. Elastase inhibitors have been reported to increase the linearity of dermal elastin fibers and skin elasticity, thereby preventing wrinkles and sagging [19].
A major factor in exogenous skin ageing is exposure to UV radiation [21,22,23]. Oxidative stress is considered to have a major influence on extrinsic skin ageing, with reactive oxygen species (ROS) being one of the main contributors [24]. Excessive sun exposure to the skin induces the production of large amounts of ROS, reduces the production of peroxidase and glutathione reductase and, in addition, triggers oxidative damage to elastin and collagen, resulting in reduced skin elasticity, rough skin, and wrinkle formation. Research is underway to reduce photo-ageing of the skin by reducing reactive oxygen species [25].
In this study, we found that BNPP has tyrosinase inhibitory, elastase inhibitory, and DPPH radical scavenging activities in vitro. It is reasonable to suggest that BNPP has the effects of preventing brown spot formation, lightening the skin, and improving fine lines/wrinkles. Based on these findings, we have further examined the efficacy of BNPP in humans. Participants who took oral BNPP 250 mg/day for 6 weeks achieved a significant increase in skin hue a* but a significant decrease in skin hue b* compared to baseline. These results indicate the increased red and decreased yellow tones in the skin. The brightness appeared to be enhanced, leading to a brighter skin tone, although no significant difference was observed. The red tone of the skin reflects the hemoglobin level in the microvascular system of the skin [26]. In a clinical study on healthy subjects, Sunagawa et al. showed that the ingestion of BNPP for four weeks increased skin surface temperature [6]. Fujita et al. also showed in a clinical study on healthy subjects that the ingestion of BNPP for four weeks improved blood flow after cold water immersion [7]. It is suggested that the blood flow rate of the skin may be increased by the supplementation of BNPP and thus enhance the skin’s red tone. Blood flow in the skin decreases after the age of 40, and there is a link between rough skin and blood flow, with rough skin reported to have less blood flow than normal skin [27]. BNPP intake was suggested to improve blood flow and improve skin condition.
The values obtained from evaluating the secondary endpoints—dark circles status, stratum corneum moisture content, and skin texture—all increased, but showed no significant differences. Although the skin viscoelasticity decreased in R2, R5, and R7, only a decrease in R5 level was statistically significant. Notably, viscoelasticity is reported to deteriorate in the medial forearm skin because of dry air from autumn to winter [28]. Because this study was conducted during the period from fall to winter (October 2022 to January 2023), this finding may have been subject to seasonal influences; therefore, a further controlled study is needed to address this issue.
In the questionnaire survey, significant improvement was observed in all endpoints, which reflected the participant’s responses to the ten survey questions about skin conditions, defined as complexion, skin elasticity and resilience, moist feeling, skin texture, cosmetic adhesion, fine lines, under-eye darkness, eye bags, sagging cheeks, and sagging mouth. The p-values for all items were very low, suggesting that participants clearly felt that the consumption of BNPP had a positive effect on their skin.
Skin irritation and stability tests were conducted to explore the potential use of BNPP as cosmetics. The results showed that BNPP was not irritating to the skin and could be applied to the skin. In addition, a high stability of more than three years was confirmed.
5. Conclusions
In this study, we revealed that BNPP has tyrosinase inhibitory, elastase inhibitory, and DPPH radical scavenging activities, suggesting that it has the effects of preventing brown spot formation, lightening the skin, and improving fine lines/wrinkles and sagging. In addition, from the participant tests for BNPP, we showed a statistically significant increase in the skin red tone and a decrease in yellow tone, as well as a significant improvement of skin conditions: complexion, skin elasticity and resilience, moist feeling, skin texture, cosmetic adhesion, fine lines, under-eye darkness, eye bags, sagging cheeks, and sagging mouth. As described above, this study suggests that BNPP has a positive effect on the skin. However, this study has several limitations. The small number of participants could have limited the findings of our study. It should be also noted that the current results of the skin tests are difficult to be extrapolated to other skin complexion types. Further large-scale trials with various skin complexion types are required. The results of the skin irritation test and the stability test suggest that BNPP could be used for cosmetic purposes. However, further tests are required for its use as a cosmetic.
Author Contributions
Conceptualization, N.I.; methodology, N.I.; validation, N.I., R.N. and S.K.; formal analysis, N.I.; investigation, N.I.; resources, N.I. and R.N.; data curation, N.I.; writing—original draft preparation, N.I.; writing—review and editing, N.I., R.N. and S.K.; supervision, S.K.; project administration, N.I. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Daiwa Pharmaceutical Co., Ltd. and received no external funding.
Institutional Review Board Statement
The clinical study reported above was conducted in consideration of medical ethics in accordance with the Declaration of Helsinki and the Ethical Guidelines for Life Sciences and Medical Research Involving Human Subjects, and was approved by the Ethics Committees of NISHI-UMEDA Clinic for Asian Medical Collaboration (Japan, IRB No. 20000026, approval date: 22 Sep. 2022). The study protocol was registered with the University Hospital Medical Information Network (UMIN-CTR) (UMIN000049095).
Informed Consent Statement
Prior to the study, the participants received explanation on the study orally and in writing, and signed the informed consent to participate in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We gratefully acknowledge the support of Kirei Testing Labo Co., Ltd. and M&I Science CORP.
Conflicts of Interest
N.I. and R.N. are employees of Daiwa Pharmaceutical Co., Ltd. The authors have no other conflict of interest in this work. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Abe, C.; Imai, T.; Sezaki, A.; Miyamoto, K.; Kawase, F.; Shirai, Y.; Sanada, M.; Inden, A.; Kato, T.; Sugihara, N.; et al. Global association between traditional Japanese diet score and All-Cause, cardiovascular disease, and total cancer mortality: A cross-sectional and longitudinal ecological study. J. Am. Nutr. Assoc. 2023, 42, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Omura, K.; Hitosugi, M.; Zhu, X.; Ikeda, M.; Maeda, H.; Tokudome, S. A newly derived protein from Bacillus subtilis natto with both antithrombotic and fibrinolytic effects. J. Pharmacol. Sci. 2005, 99, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Oda, E.; Giddings, J.C.; Yamamoto, J. The effect of dietary Bacillus natto productive protein on in vivo endogenous thrombolysis. Pathophysiol. Haemost. Thromb. 2003, 33, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Hitosugi, M. Effects of Bacillus Natto Products on Blood Pressure in Patients with Lifestyle Diseases. J. Hypertens. Open Access 2014, 3, 1000135. [Google Scholar] [CrossRef]
- Hitosugi, M.; Hamada, K.; Misaka, K. Effects of Bacillus subtilis var. natto products on symptoms caused by blood flow disturbance in female patients with lifestyle diseases. Int. J. Gen. Med. 2015, 8, 41–46. [Google Scholar] [CrossRef]
- Sunagawa, Y.; Okamura, N.; Miyazaki, Y.; Shimizu, K.; Genpei, M.; Funamoto, M.; Shimizu, S.; Katanasaka, Y.; Morimoto, E.; Yamakage, H.; et al. Effects of products containing Bacillus subtilis var. natto on healthy subjects with neck and shoulder stiffness, a double-blind, placebo-controlled, randomized crossover study. Biol. Pharm. Bull. 2018, 41, 504–509. [Google Scholar] [CrossRef]
- Fujita, C.; Usui, Y.; Inoue, M. Effects of Bacillus subtilis var. natto products on capillary blood flow in healthy subjects with peripheral coldness: A double-blind, placebo-controlled, randomized parallel study. Food Nutr. Sci. 2022, 13, 211–223. [Google Scholar] [CrossRef]
- Sawane, M.; Ota, M.; Yamanishi, H.; Motoyama, A.; Takakura, N.; Kajiya, K. The molecular basis of skin aging triggered by lymphatic and blood vascular dysfunction. J. Soc. Cosmet. Chem. Jpn. 2012, 46, 188–196. [Google Scholar] [CrossRef]
- Chang, T.S. An Updated Review of Tyrosinase Inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef]
- Mason, H.S. The Chemistry of melanin; Mechanism of the oxidation of dihydroxyphenylalanine by tyrosinase. J. Biol. Chem. 1948, 172, 83–99. [Google Scholar] [CrossRef]
- Castillo, M.J.; Nakajima, K.; Zimmerman, M.; Powers, J.C. Sensitive substrates for human leukocyte and porcine pancreatic elastase: A study of the merits of various chromophoric and fluorogenic leaving groups in assays for serine proteases. Anal. Biochem. 1979, 99, 53–64. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Katoh, M.; Hata, K. Refinement of LabCyte EPI-MODEL24 skin irritation test method for adaptation to the requirement of OECD Test Guideline 439. AATEX 2011, 16, 111–122. [Google Scholar]
- Mohania, D.; Chandel, S.; Kumar, P.; Verma, V.; Digvijay, K.; Tripathi, D.; Choudhury, K.; Mitten, S.K.; Shah, D. Ultraviolet Radiations: Skin Defense-Damage Mechanism. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2017; Volume 996, pp. 71–87. [Google Scholar]
- Nerya, O.; Musa, R.; Khatib, S.; Tamir, S.; Vaya, J. Chalcones as potent tyrosinase inhibitors: The effect of hydroxyl positions and numbers. Phytochemistry 2004, 65, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Shiino, M.; Watanabe, Y.; Umezawa, K. Synthesis and tyrosinase inhibitory activity of novel N-hydroxybenzyl-N-nitrosohydroxylamines. Bioorg. Chem. 2003, 31, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, K.U.; Ali, A.S.; Ali, S.A.; Naaz, I. Microbial tyrosinases: Promising enzymes for pharmaceutical, food bioprocessing, and environmental industry. Biochem. Res. Int. 2014, 2014, 854687. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef]
- Tsukahara, K.; Takema, Y.; Moriwaki, S.; Tsuji, N.; Suzuki, Y.; Fujimura, T.; Imokawa, G. Selective inhibition of skin fibroblast elastase elicits a concentration-dependent prevention of ultraviolet B-induced wrinkle formation. J. Investig. Dermatol. 2001, 117, 671–677. [Google Scholar] [CrossRef]
- Imokawa, G.; Ishida, K. Biological Mechanisms Underlying the Ultraviolet Radiation-Induced Formation of Skin Wrinkling and Sagging I: Reduced Skin Elasticity, Highly Associated with Enhanced Dermal Elastase Activity, Triggers Wrinkling and Sagging. Int. J. Mol. Sci. 2015, 16, 7753–7775. [Google Scholar] [CrossRef]
- Han, A.; Chien, A.L.; Kang, S. Photoaging. Dermatol. Clin. 2014, 32, 291–299. [Google Scholar] [CrossRef]
- Bosch, R.; Philips, N.; Suárez-Pérez, J.A.; Juarranz, A.; Devmurari, A.; Chalensouk-Khaosaat, J.; González, S. Mechanisms of photoaging and cutaneous photocarcinogenesis, and photoprotective strategies with phytochemicals. Antioxidants 2015, 4, 248–268. [Google Scholar] [CrossRef] [PubMed]
- Kammeyer, A.; Luiten, R.M. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Poljšak, B.; Dahmane, R.G.; Godić, A. Intrinsic skin aging: The role of oxidative stress. Acta Dermatovenerol. Alp. Pannonica Adriat. 2012, 21, 33–36. [Google Scholar] [PubMed]
- Chen, X.; Yang, C.; Jiang, G. Research progress on skin photoaging and oxidative stress. Adv. Dermatol. Alergol. 2021, 38, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Kitada, Y.; Kaneda, Y.; Muramatsu, Y.; Ogawa, H.; Iijima, S.; Takakura, N. Skin colors in the four seasons. J. Soc. Cosmet. Chem. Jpn. 1996, 30, 169–175. [Google Scholar] [CrossRef]
- Tsukada, H.; Takeda, A.; Uyama, M. Skin and Blood Flow of Microcirculation. J. Soc. Cosmet. Chem. Jpn. 1996, 30, 184–189. [Google Scholar] [CrossRef]
- Ohnami, H.; Morimoto, T.; Urushibata, O.; Ikeda, S.; Okouchi, S. Effects of Bathing in the Hot Spring Waters with Reductive Characteristic on the Elasticity of the Skins. Jpn. Soc. Hot Spring Sci. 2008, 57, 215–225. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).