Phenolic Composition and Wound Healing Potential Assessment of Moroccan Henna (Lawsonia inermis) Aqueous Extracts
Abstract
1. Introduction
2. Material and Methods
2.1. Leaves of Lawsonia inermis
2.2. Aqueous Extract (AQ) Obtention
2.3. Ointment Formulation
2.4. Total Phenolic Compounds Content
2.5. Total Flavonoid Content
2.6. Total Tannin Content
2.7. Total Saponin Content
2.8. Gel Filtration Chromatography
2.9. Wound Healing Activity Assessment
- -
- Ethics statement
- -
- Animal preparationWe used groups of mice consisting of 10 males and 15 females as an experimental model to induce thermal burns, with a weight range between 40 and 41.4 g. Each animal was housed in an individual polystyrene cage with ad libitum access to food and water. The animals were divided into 5 groups, each containing 5 mice:
- ▪
- Negative control group (NC): mice were left untreated and treated with white soft paraffin;
- ▪
- Positive control group (PC): mice were treated with 1% silver sulfadiazine, also known as Flamazine;
- ▪
- LI1 group: mice were treated with the Alnif ointment formulation;
- ▪
- LI2 group: mice were treated with the Tafraoute Sidi Ali ointment formulation;
- ▪
- LI3 group: mice were treated with the Tazarine ointment formulation.
- -
- Performance of experimental burns
- -
- Treatment application
- -
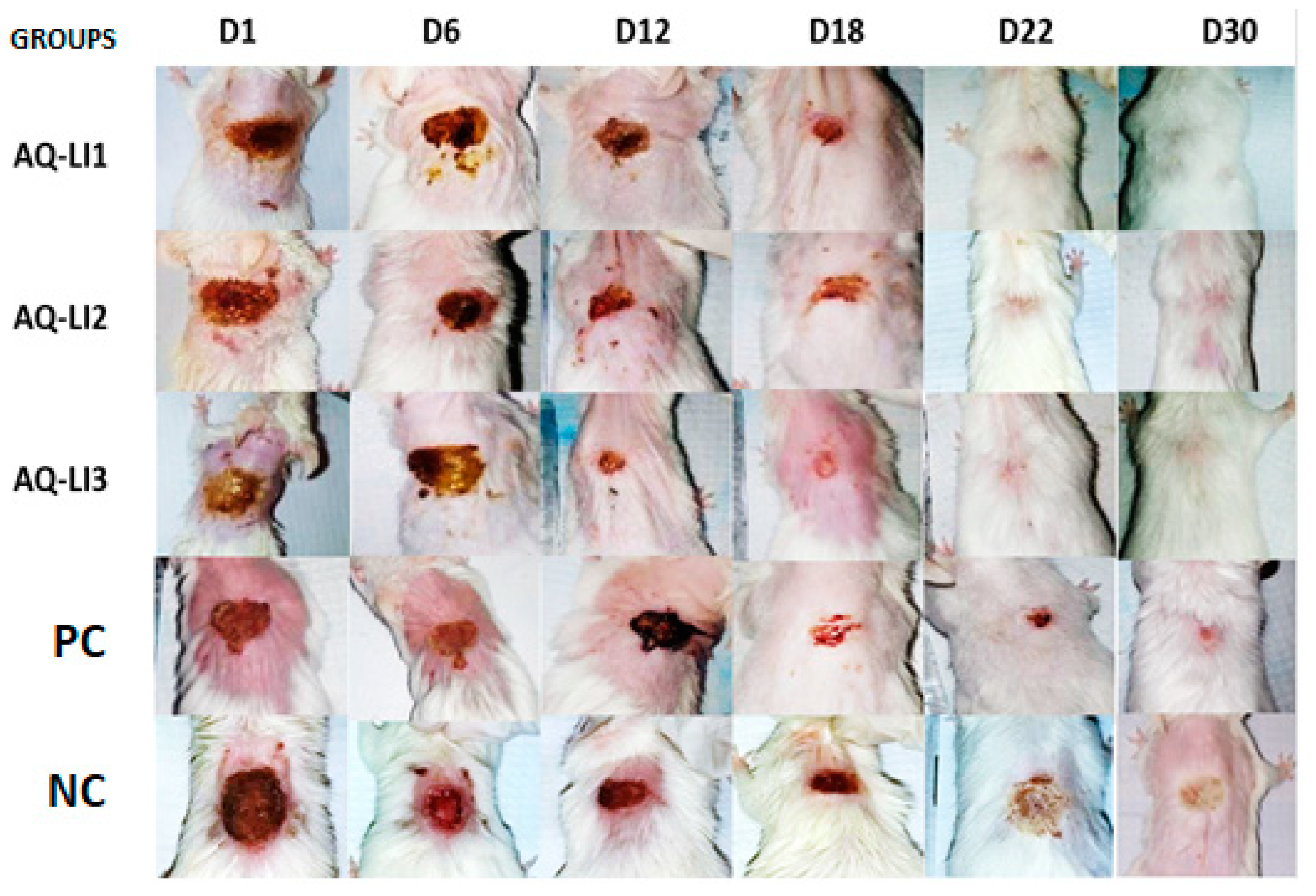
- Study of scar evolution using digital planimetry
- -
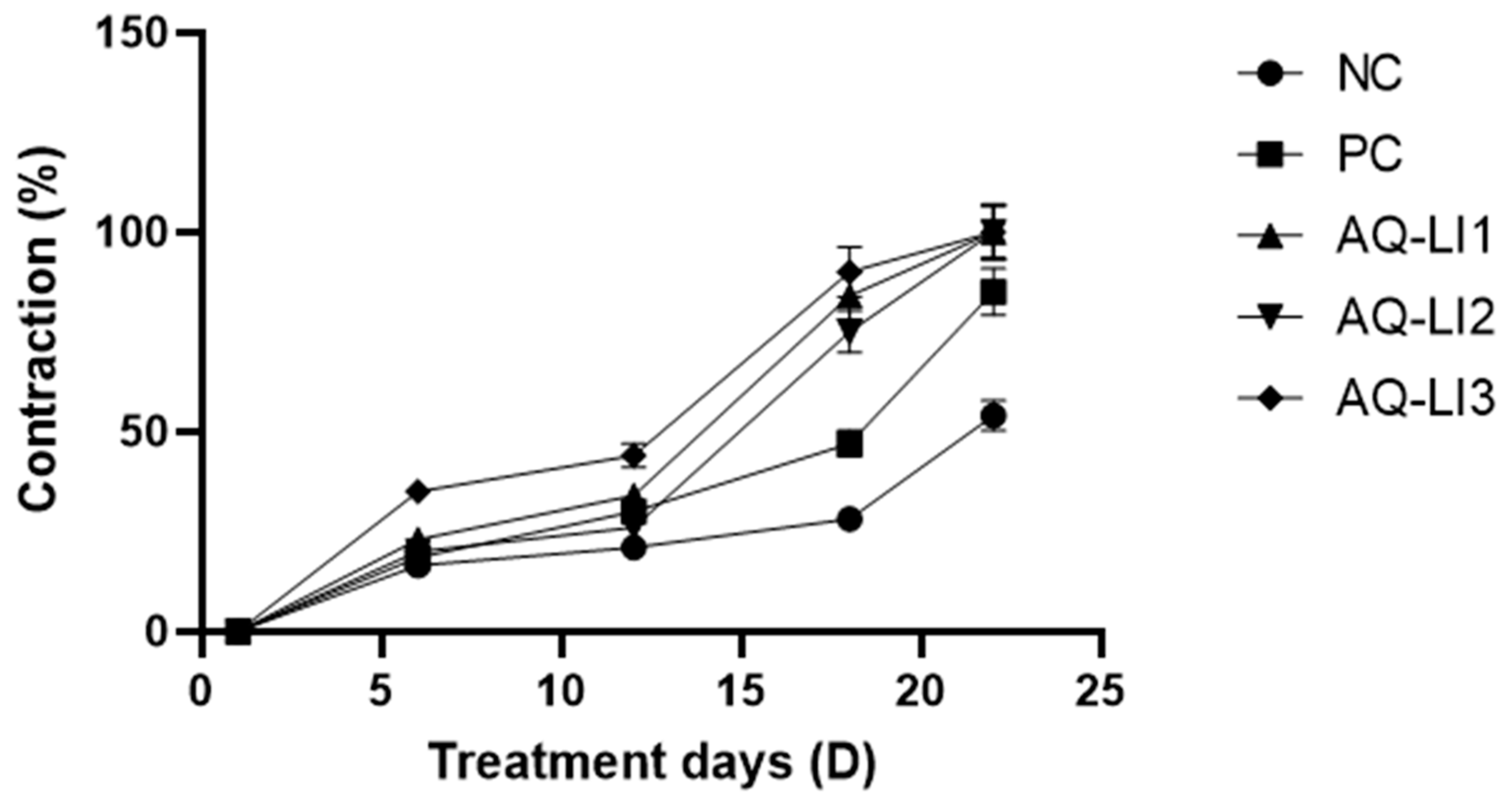
- Percentage of contraction
- -
- WCP: Wound contraction percentage;
- -
- D0: Day 0;
- -
- Dn: Day n.
- -
- Epithelialization period
2.10. Statistical Analyses
3. Results and Discussion
3.1. Phenolic Compounds Analysis
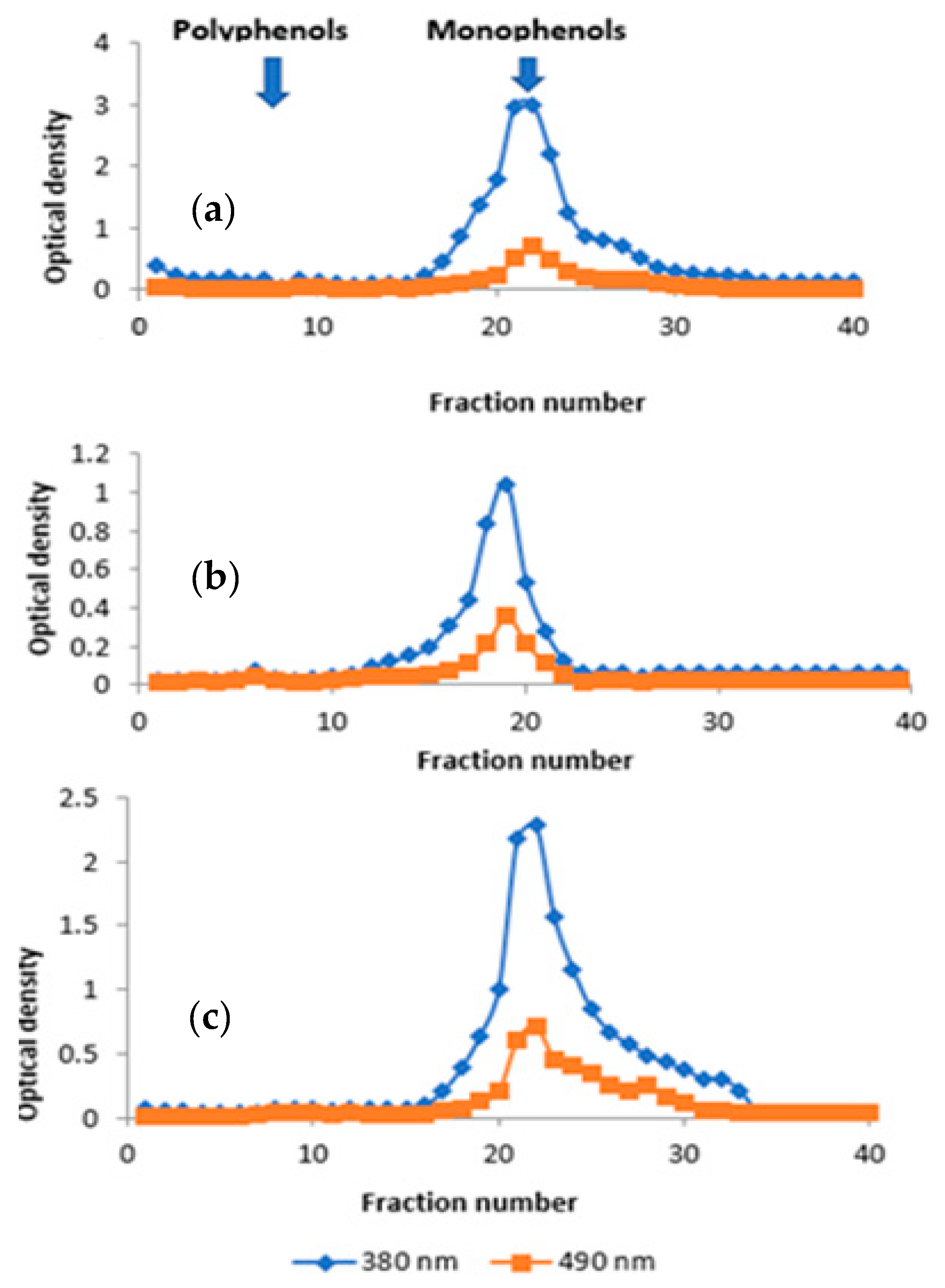
3.2. Gel Filtration Chromatography
3.3. Evaluation of In Vivo Wound Healing Activity
- -
- Epithelialization period
- -
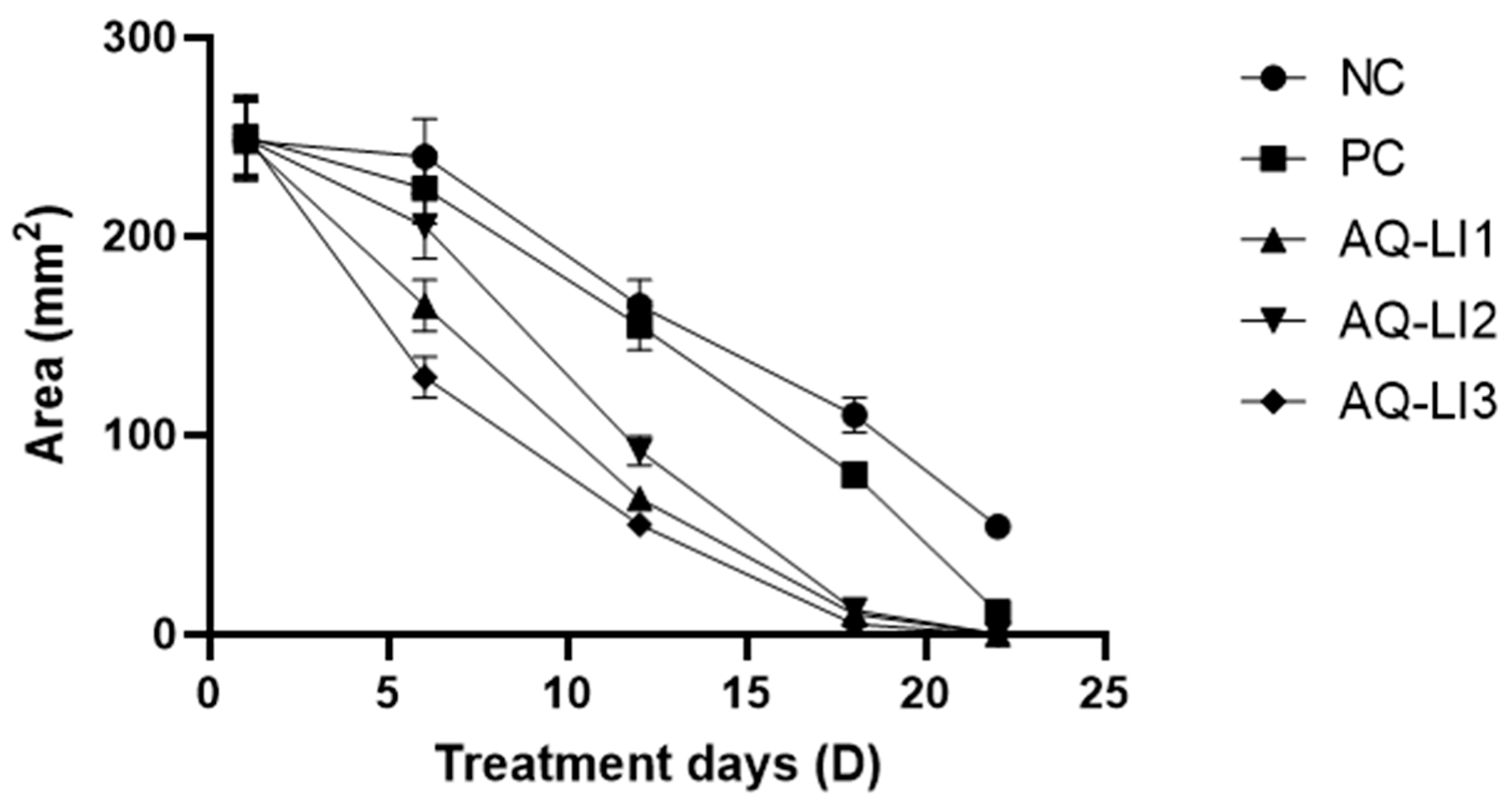
- Average surface and mean percentage of burn contraction
- -
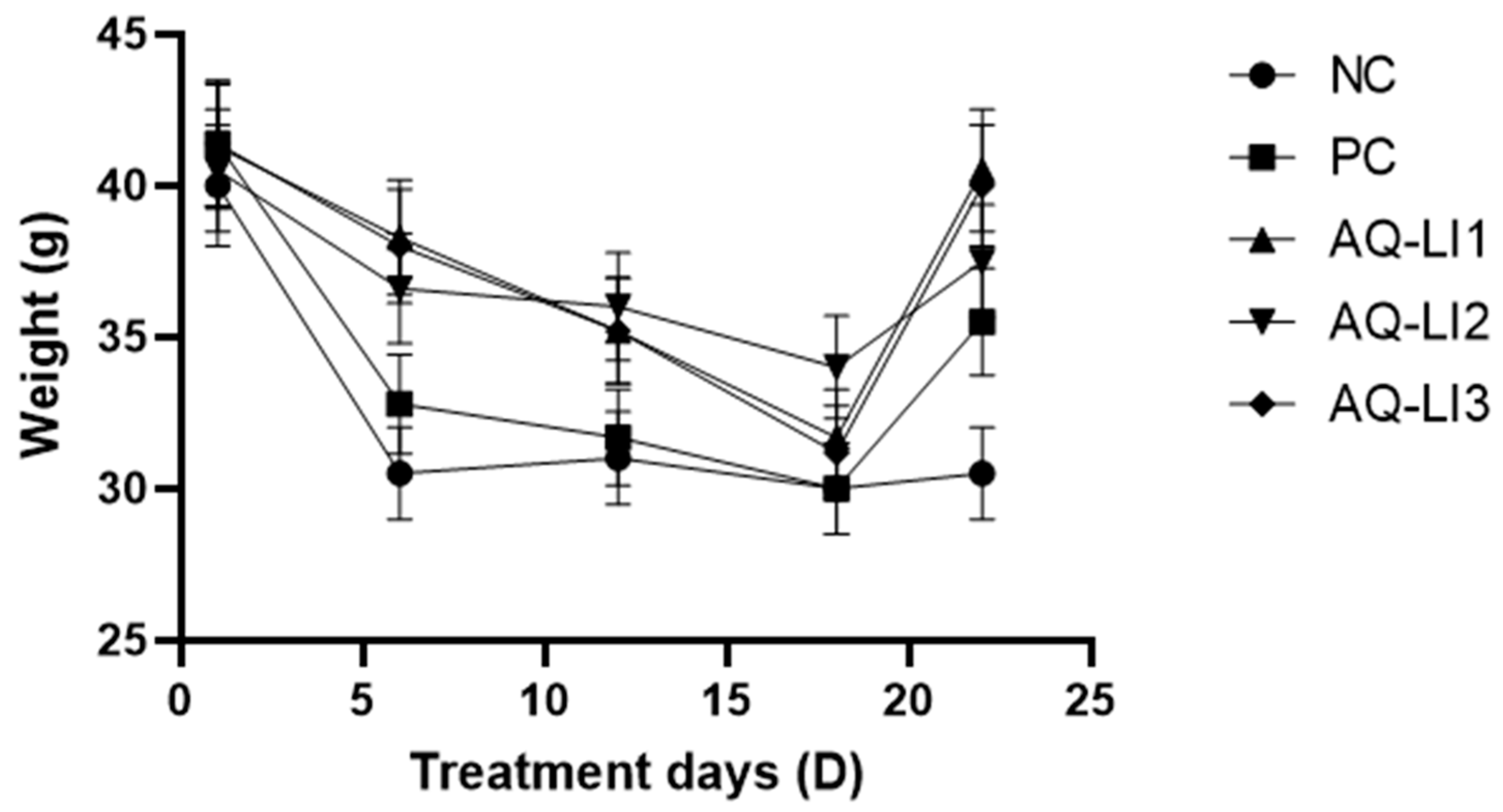
- Variation in weight of mice during treatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lydia, B.Y. Petite Histoire Du Maquillage; Stock: Paris, France, 1999. [Google Scholar]
- Hughes, K.; Ho, R.; Butaud, J.-F.; Filaire, E.; Ranouille, E.; Berthon, J.-Y.; Raharivelomanana, P. A selection of eleven plants used as traditional Polynesian cosmetics and their development potential as anti-aging ingredients, hair growth promoters and whitening products. J. Ethnopharmacol. 2019, 245, 112159. [Google Scholar] [CrossRef] [PubMed]
- Walter, P.; Martinetto, P.; Tsoucaris, G.; Brniaux, R.; Lefebvre, M.A.; Richard, G.; Talabot, J.; Dooryhee, E. Making make-up in Ancient Egypt. Nature 1999, 397, 483–484. [Google Scholar] [CrossRef]
- González-Minero, F.J.; Bravo-Díaz, L. The Use of Plants in Skin-Care Products, Cosmetics and Fragrances: Past and Present. Cosmetics 2018, 5, 50. [Google Scholar] [CrossRef]
- Bouhlal, T.; Benkhnigue, O.; Zidane, L.; Sobh, M.; Fadli, M. Vasculat Plants Used in Tradional Cosmetic by the Human Population in the Plain of the Gharb (Morocco) Full Paper. Nat. Prod. Indian J. 2013, 9, 326–330. [Google Scholar]
- Zrira, S. Some Important Aromatic and Medicinal Plants of Morocco. Med. Aromat. Plants World 2017, 3, 91–125. [Google Scholar] [CrossRef]
- Saive, M.; Frederich, M.; Fauconnier, M.-L. Plants Used in Traditional Medicine and Cosmetics in Mayotte Island (France): An Ethnobotanical Study. Indian J. Tradit. Knowl. 2018, 17, 645–653. [Google Scholar]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Cartwright-Jones, C.; Viljoen, A. Lawsonia inermis L. (henna): Ethnobotanical, phytochemical and pharmacological aspects. J. Ethnopharmacol. 2014, 155, 80–103. [Google Scholar] [CrossRef]
- El Ghanjaoui Mohammed Cervera, M.L.; El Rhazi, M.; De La Guardia, M. Assessment of Trace Elements in Traditional Moroccan Cosmetics by Inductively Coupled Plasma Atomic Emission Spectroscopy. Int. J. Sci. Technol. Res. 2014, 3, 104–112. [Google Scholar]
- Bafghi, M.F.; Salary, S.; Mirzaei, F.; Mahmoodian, H.; Meftahizade, H.; Zareshahi, R. Antibacterial and anti-trichomunas characteristics of local landraces of Lawsonia inermis L. BMC Complement. Med. Ther. 2022, 22, 203. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. A review on Lawsonia inermis: A potential medicinal PLANT. Int. J. Curr. Pharm. Res. 2019, 11, 1–13. [Google Scholar] [CrossRef]
- Tang, Y.; He, W.; Yang, S.; Liu, L. Stabilisation and detoxification of henna (Lawsonia inermis L.) extract for hair dye cosmetics by spray-drying encapsulation. Color. Technol. 2019, 135, 439–450. [Google Scholar] [CrossRef]
- Obat, R.; Bosire, C. Formulation and evaluation of herbal lipstick using beta vulgaris and Lawsonia inermis as natural colorants. J. Phys. Appl. Sci. (JPAS) 2022, 1, 28–37. [Google Scholar] [CrossRef]
- Benzeid, H.; Gouaz, F.; Touré, A.H.; Bouatia, M.; Idrissi, M.O.B.; Draoui, M. Inventory of Toxic Plants in Morocco: An Overview of the Botanical, Biogeography, and Phytochemistry Studies. J. Toxicol. 2018, 2018, 4563735. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Ba, K.; Tine, E.; Destain, J.; Cissé, N.; Thonart, P. Étude Comparative Des Composés Phénoliques, Du Pouvoir Antioxydant De Différentes Variétés De Sorgho Sénégalais Et Des Enzymes Amylolytiques De Leur Malt. Biotechnol. Agron. Soc. Environ. 2010, 14, 131–139. [Google Scholar]
- Hiai, S.; Oura, H.; Nakajima, T. Color reaction of some sapogenins and saponins with vanillin and sulfur1c acid. Planta Medica 1976, 29, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Mottaghipisheh, J.; Iriti, M. Sephadex® LH-20, Isolation, and Purification of Flavonoids from Plant Species: A Comprehensive Review. Molecules 2020, 25, 4146. [Google Scholar] [CrossRef]
- Uddin, N.; Siddiqui, B.S.; Begum, S.; Ali, M.I.; Marasini, B.P.; Khan, A.; Choudhary, M.I. Bioassay-guided isolation of urease and α-chymotrypsin inhibitory constituents from the stems of Lawsonia alba Lam. (Henna). Fitoterapia 2013, 84, 202–207. [Google Scholar] [CrossRef]
- Ettayebi, K.; Errachidi, F.; Jamai, L.; Tahri-Jouti, M.A.; Sendide, K.; Ettayebi, M. Biodegradation of polyphenols with immobilized Candida tropicalis under metabolic induction. FEMS Microbiol. Lett. 2003, 223, 215–219. [Google Scholar] [CrossRef]
- Davidson, J.M. Animal Models for Wound Repair. Arch. Dermatol. Res. 1998, 290, S1–S11. [Google Scholar] [CrossRef] [PubMed]
- Cai, E.Z.; Ang, C.H.; Raju, A.; Tan, K.B.; Hing, E.C.H.; Loo, Y.; Wong, Y.C.; Lee, H.; Lim, J.; Moochhala, S.M.; et al. Creation of Consistent Burn Wounds: A Rat Model. Arch. Plast. Surg. 2014, 41, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Nunes, J.P.S.; Dias, A.A.M. ImageJ macros for the user-friendly analysis of soft-agar and wound-healing assays. Biotechniques 2017, 62, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Abeje, B.A.; Bekele, T.; Getahun, K.A.; Asrie, A.B. Evaluation of Wound Healing Activity of 80% Hydromethanolic Crude Extract and Solvent Fractions of the Leaves of Urtica simensis in Mice. J. Exp. Pharmacol. 2022, 14, 221–241. [Google Scholar] [CrossRef]
- Shivananda Nayak, B.; Isitor, G.; Davis, E.M.; Pillai, G.K. Wound healing activity of Lawsonia inermis linn. 827 The Evidence Based Wound Healing Activity of Lawsonia Inermis Linn. Phytother. Res 2007, 21, 827–831. [Google Scholar] [CrossRef]
- Dev, S.K.; Choudhury, P.; Srivastava, R.; Sharma, M. Antimicrobial, anti-inflammatory and wound healing activity of polyherbal formulation. Biomed. Pharmacother. 2019, 111, 555–567. [Google Scholar] [CrossRef]
- Rekik, D.M.; Ben Khedir, S.; Daoud, A.; Moalla, K.K.; Rebai, T.; Sahnoun, Z. Wound Healing Effect of Lawsonia inermis. Ski. Pharmacol. Physiol. 2019, 32, 295–306. [Google Scholar] [CrossRef]
- Mssillou, I.; Bakour, M.; Slighoua, M.; Laaroussi, H.; Saghrouchni, H.; Amrati, F.E.-Z.; Lyoussi, B.; Derwich, E. Investigation on wound healing effect of Mediterranean medicinal plants and some related phenolic compounds: A review. J. Ethnopharmacol. 2022, 298, 115663. [Google Scholar] [CrossRef]
- Hadisi, Z.; Nourmohammadi, J.; Nassiri, S.M. The antibacterial and anti-inflammatory investigation of Lawsonia inermis-gelatin-starch nano-fibrous dressing in burn wound. Int. J. Biol. Macromol. 2018, 107, 2008–2019. [Google Scholar] [CrossRef]
- Al-Snafi, A.E.; Talab, T.A.; Alfuraiji, N. The analgesic and anti-inflammatory effect of lawsone isolated from Lawsonia inermis. Sci. Pharm. Sci. 2022, 1, 77–84. [Google Scholar] [CrossRef]
- Goldenheim, P.D. An Appraisal of Povidone-Iodine and Wound Healing. Postgrad Med. J. 1993, 69, 97–105. [Google Scholar]
- De Francesco, F.; Riccio, M.; Jimi, S. Contribution of Topical Agents such as Hyaluronic Acid and Silver Sulfadiazine to Wound Healing and Management of Bacterial Biofilm. Medicina 2022, 58, 835. [Google Scholar] [CrossRef] [PubMed]
- Atiyeh, B.S.; Costagliola, M.; Hayek, S.N.; Dibo, S.A. Effect of silver on burn wound infection control and healing: Review of the literature. Burns 2007, 33, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Klasen, H.J. A historical review of the use of silver in the treatment of burns. II. Renewed interest for silver. Burns 2000, 26, 131–138. [Google Scholar] [CrossRef]
- Seyed, J.H.; Ghasemali, K.; Azadbakht, M.; Peyman, Z.; Ghasemi, M.; Amirhossein, A. Effect of Aloe Cream versus Silver Sulfadiazine for Healing Burn Wounds in Rats. Acta Dermatovenerol. Croat. 2010, 18, 2–7. [Google Scholar]
- Khorasani, G.; Hosseinimehr, S.J.; Zamani, P.; Ghasemi, M.; Ahmadi, A. The Effect of Saffron (Crocus sativus) Extract for Healing of Second-degree Burn Wounds in Rats. Keio J. Med. 2008, 57, 190–195. [Google Scholar] [CrossRef]
- Bahramsoltani, R.; Farzaei, M.H.; Rahimi, R. Medicinal plants and their natural components as future drugs for the treatment of burn wounds: An integrative review. Arch. Dermatol. Res. 2014, 306, 601–617. [Google Scholar] [CrossRef]
- El Massoudi, S.; Benidir, M.; Chabir, R.; Benjelloun, M.; El Ghadraoui, L.; Errachidi, F. Morphological, Biochemical, and Climatological Analysis of Three Moroccan Henna Verities. Sci. World J. 2019, 2019, 1418456. [Google Scholar] [CrossRef]
- Manuja, A.; Rathore, N.; Choudhary, S.; Kumar, B. Phytochemical Screening, Cytotoxicity and Anti-inflammatory Activities of the Leaf Extracts from Lawsonia inermis of Indian Origin to Explore their Potential for Medicinal Uses. Med. Chem. 2020, 17, 576–586. [Google Scholar] [CrossRef]
- Raisagar, A.; Kaur, C.D.; Sawarkar, H.A.; Kumar, L.; Raisagar, A.; Karmakar, A.; Sahu, M. Comparative Study of Wound-Healing Effect of Bark Extracts of Ficus religiosa & Ficus benghalensis by Mice Model. J. Pharmacogn. Phytochem. 2019, 8, 1815–1821. [Google Scholar]
- Sahib, A.; Al-Jawad, F.; Alkaisy, A. Effect of Antioxidants on the Incidence of Wound Infection in Burn Patients. Ann. Burn. Fire Disasters 2010, 23, 199–205. [Google Scholar]
- Platon, J.F. Lipids in Cosmetology. Ol. Corps Gras Lipides 1997, 4, 275–281. [Google Scholar]
| Extracts | AQ-LI 1 | AQ-LI 2 | AQ-LI 3 |
|---|---|---|---|
| Aqueous extract (AQ) Yields (%) | 50.89 ± 3.05 | 24.80 ± 1.74 | 37.41 ± 1.90 |
| Total phenolic compounds (g GAE)/100 g DM) | 13.48 ± 0.81 | 6.46 ± 0.50 | 8.34 ± 0.42 |
| Total flavonoids (g QE/100 g DM) | 9.25 ± 0.55 | 4.42 ± 0.40 | 5.54 ± 0.28 |
| Total tannin (g TAE/100 g DM) | 2.57 ± 0.15 | 1.43 ± 0.15 | 0.39 ± 0.05 |
| Saponins (mg/100 g DM) | 291.70 ± 17.50 | 83.38 ± 5.84 | 321.50 ± 16.10 |
| Ointment Formulation | AQ-LI1 | AQ-LI2 | AQ-LI3 | PC | NC |
|---|---|---|---|---|---|
| Epithelialization period (day) | 20.33 ± 4.65 | 21.67 ± 5.1 | 19.33 ± 0.05 | 30.67 ± 3.63 | 42.33 ± 2.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Massoudi, S.; Zinedine, A.; Rocha, J.M.; Benidir, M.; Najjari, I.; El Ghadraoui, L.; Benjelloun, M.; Errachidi, F. Phenolic Composition and Wound Healing Potential Assessment of Moroccan Henna (Lawsonia inermis) Aqueous Extracts. Cosmetics 2023, 10, 92. https://doi.org/10.3390/cosmetics10030092
El Massoudi S, Zinedine A, Rocha JM, Benidir M, Najjari I, El Ghadraoui L, Benjelloun M, Errachidi F. Phenolic Composition and Wound Healing Potential Assessment of Moroccan Henna (Lawsonia inermis) Aqueous Extracts. Cosmetics. 2023; 10(3):92. https://doi.org/10.3390/cosmetics10030092
Chicago/Turabian StyleEl Massoudi, Soukaina, Abdellah Zinedine, João Miguel Rocha, Meryem Benidir, Ilham Najjari, Lahsen El Ghadraoui, Meryem Benjelloun, and Faouzi Errachidi. 2023. "Phenolic Composition and Wound Healing Potential Assessment of Moroccan Henna (Lawsonia inermis) Aqueous Extracts" Cosmetics 10, no. 3: 92. https://doi.org/10.3390/cosmetics10030092
APA StyleEl Massoudi, S., Zinedine, A., Rocha, J. M., Benidir, M., Najjari, I., El Ghadraoui, L., Benjelloun, M., & Errachidi, F. (2023). Phenolic Composition and Wound Healing Potential Assessment of Moroccan Henna (Lawsonia inermis) Aqueous Extracts. Cosmetics, 10(3), 92. https://doi.org/10.3390/cosmetics10030092









