Protective Effects of Naringenin against UVB Irradiation and Air Pollution-Induced Skin Aging and Pigmentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Viability
2.2. Cell Culture
2.3. UVB Keratinocyte/Fibroblast Assay
2.4. Pollution-Induced Keratinocyte/Fibroblast Assay
2.5. Pollution-Induced CYP1A1 Assay
2.6. RNA Isolation and cDNA Synthesis
2.7. qPCR Analysis of CYP1A1 Gene Expression
2.8. Gene Expression Analysis of Pigmentation Genes in Melanocytes
2.9. Pigmentation Analysis, Reconstructed Human Epidermis
2.9.1. Cell Culture and Reconstruction of Epidermis
2.9.2. Pigmentation Assay, with or without UV Challenge
2.10. Statistical Analysis
3. Results
3.1. Viability
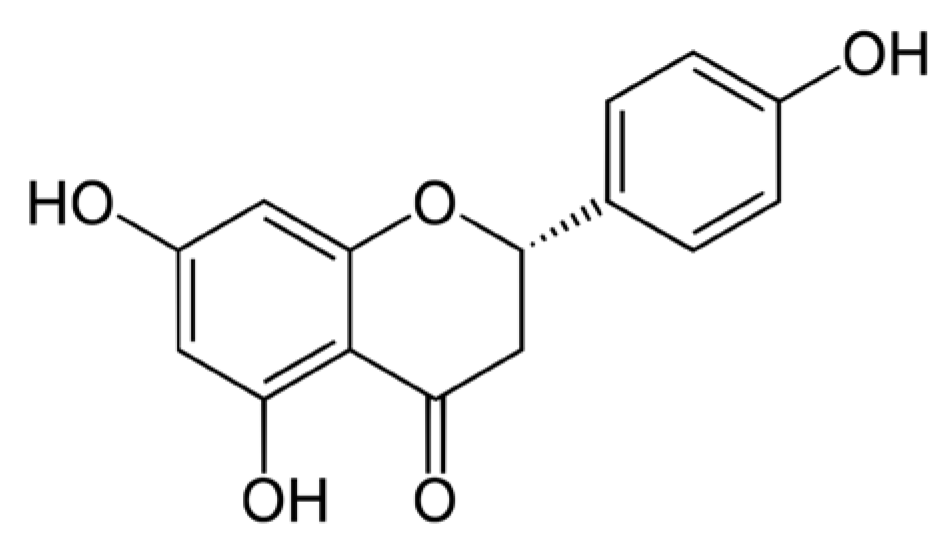
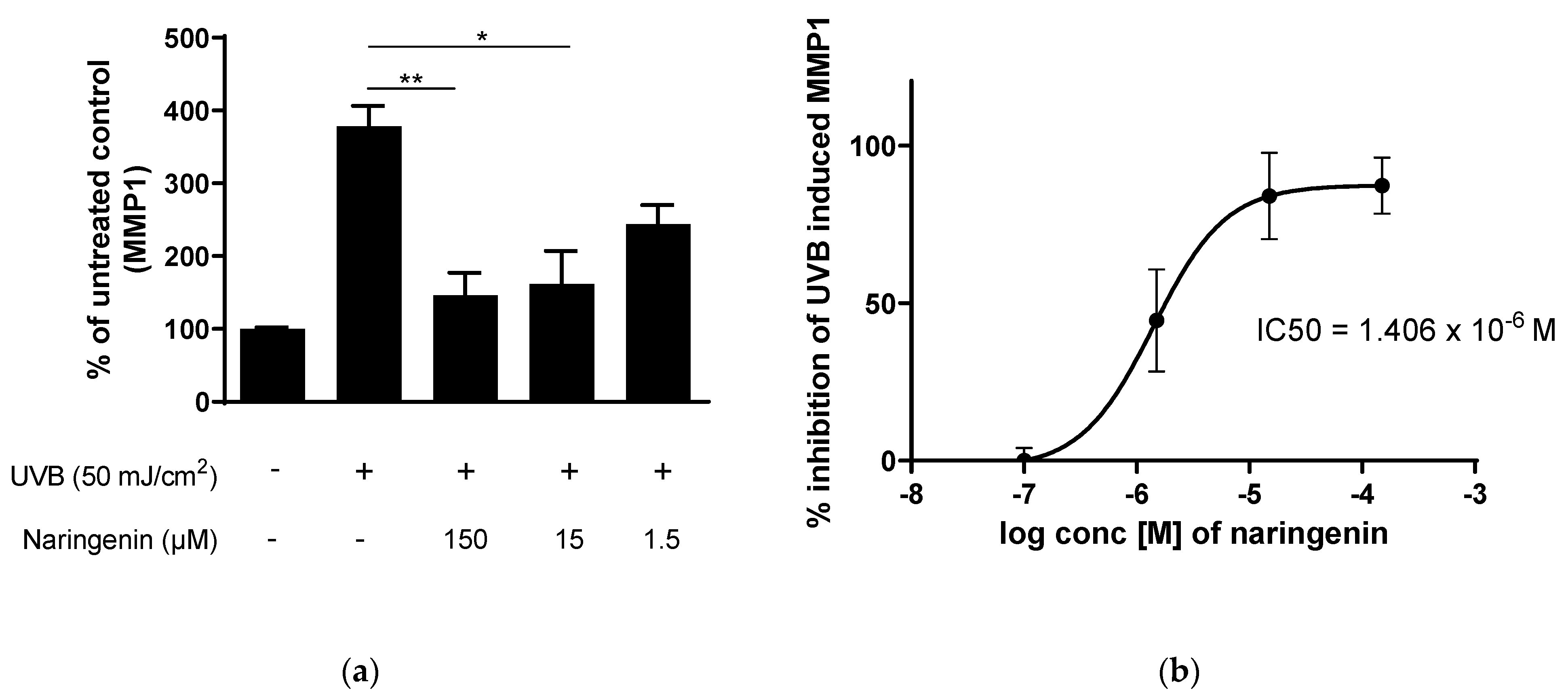
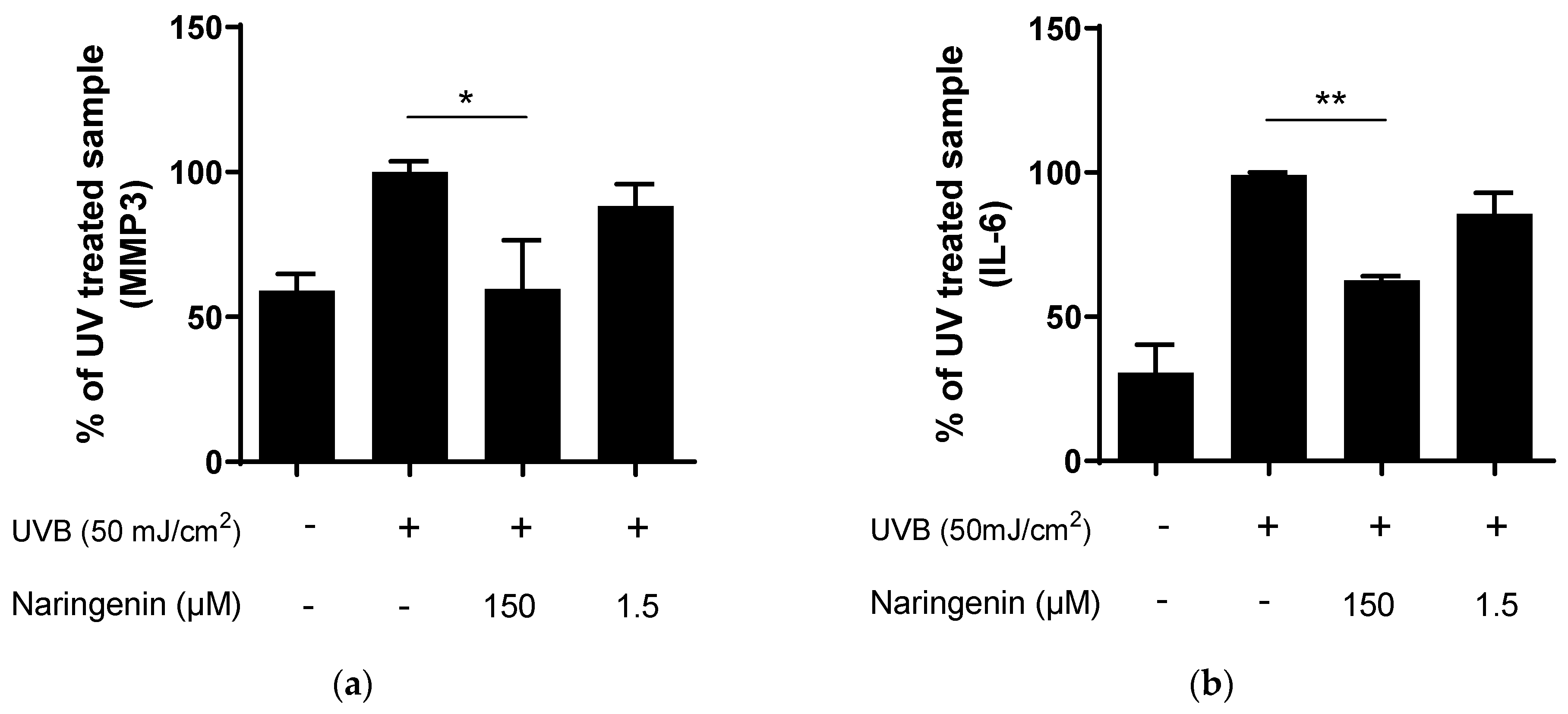
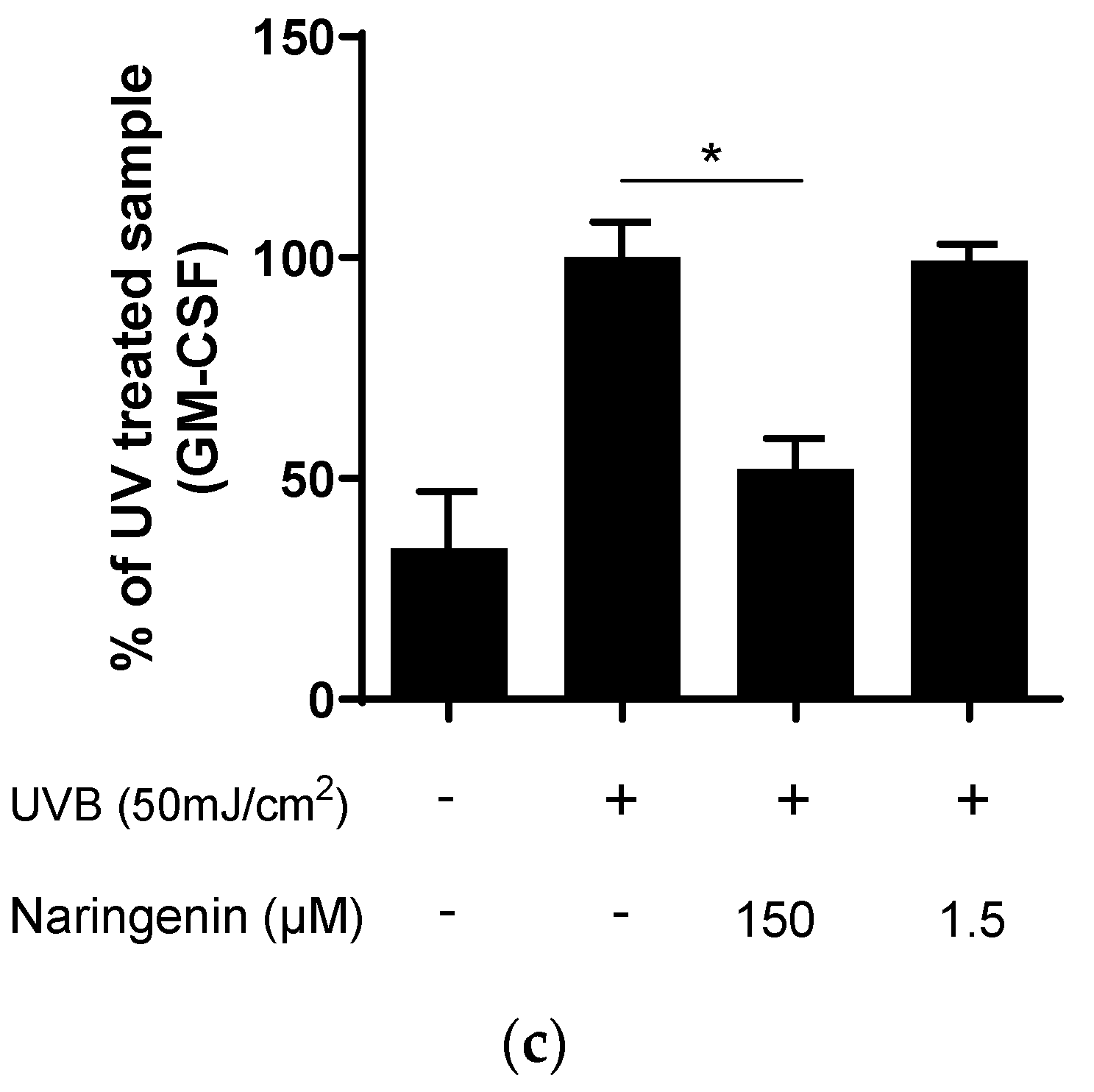
3.2. Naringenin Inhibits UVB Induced Inflammatory Response in Human Primary Skin Cells
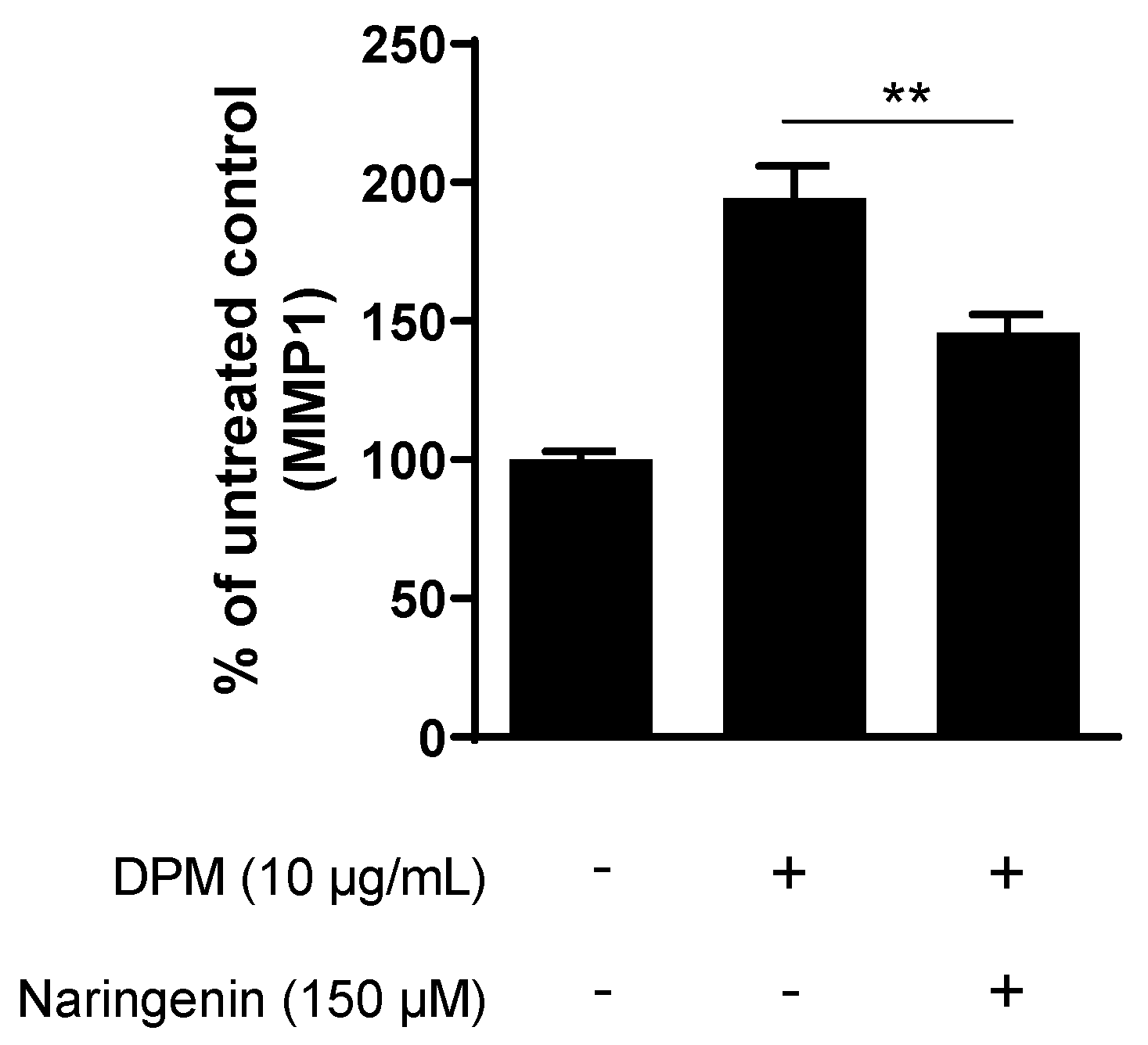
3.3. Naringenin Inhibits Pollution-Induced MMP1 in Human Dermal Fibroblasts
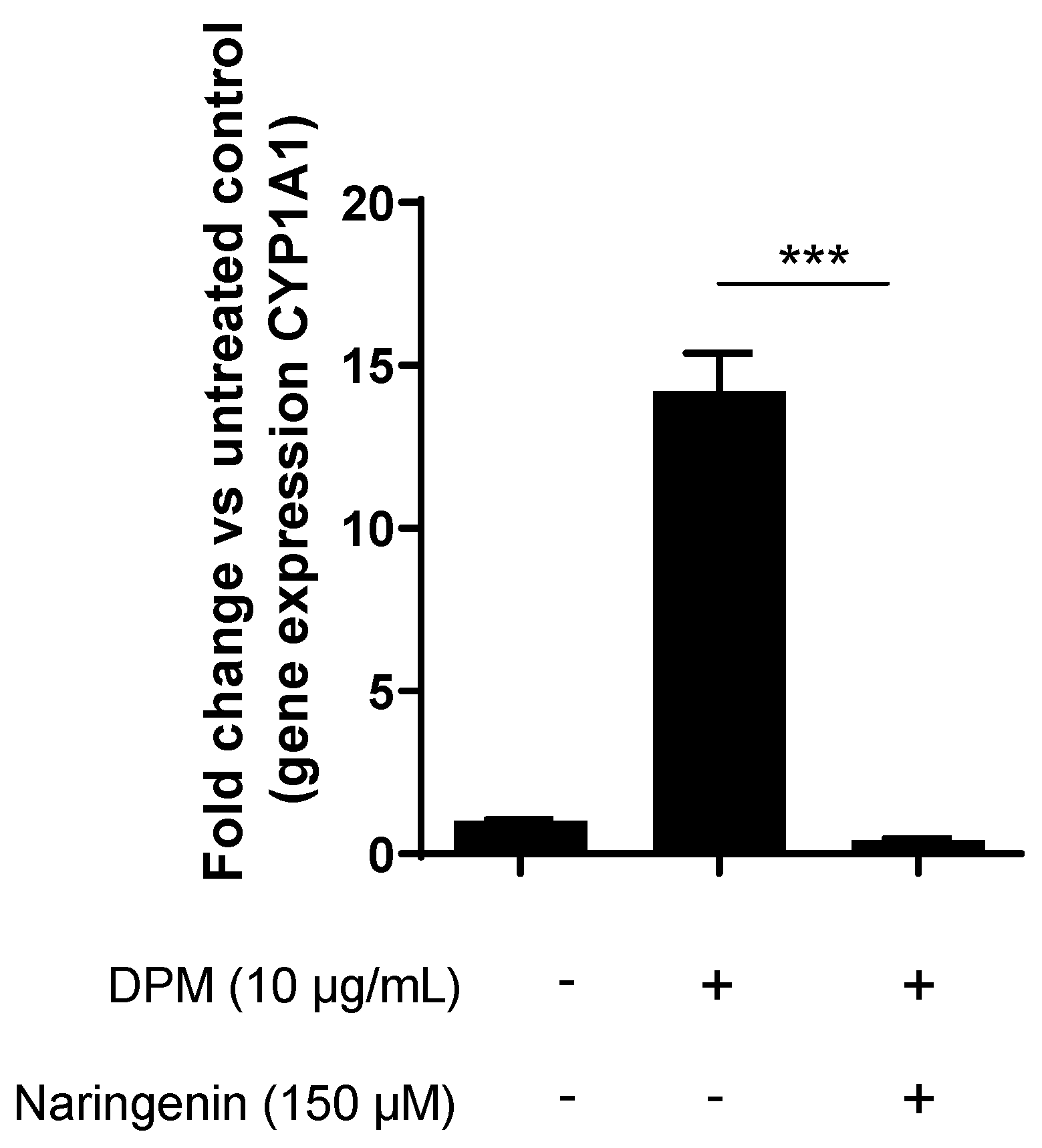
3.4. Naringenin Inhibits Pollution-Induced CYP1A1 in Human Keratinocytes
3.5. Naringenin Downregulates Key Melanogenesis Genes
3.6. Naringenin Inhibits Basal and UV-Induced Melanogenesis in Human Pigmented Reconstructed Epidermis
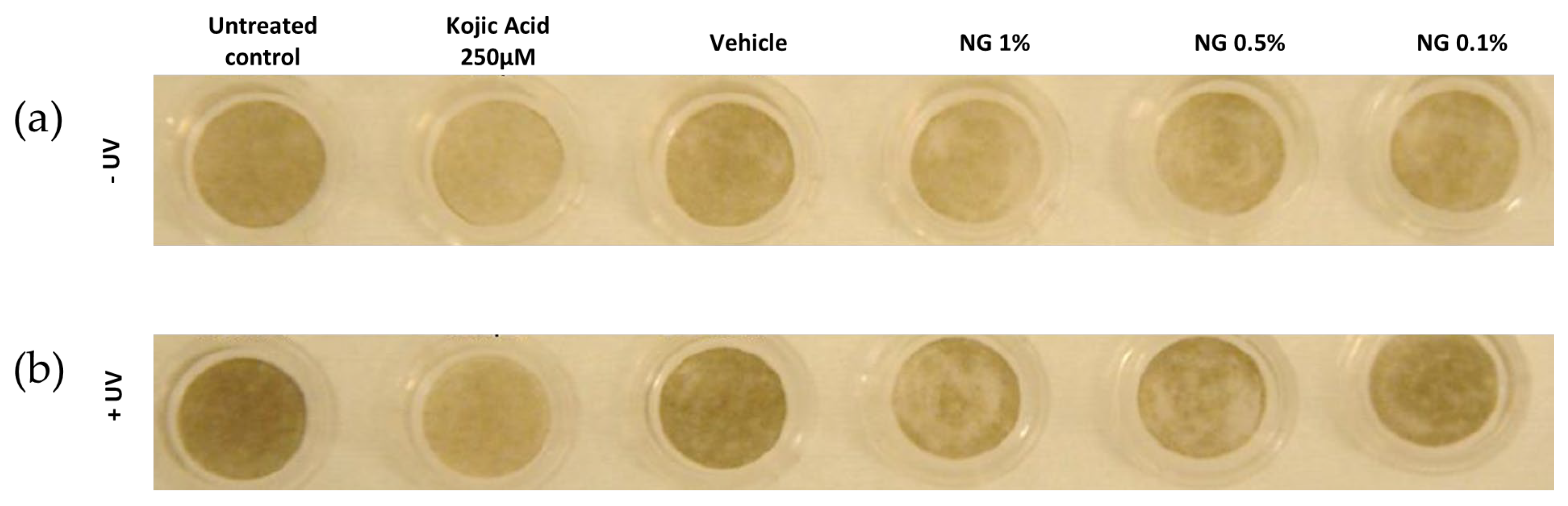
3.6.1. Visual Analysis
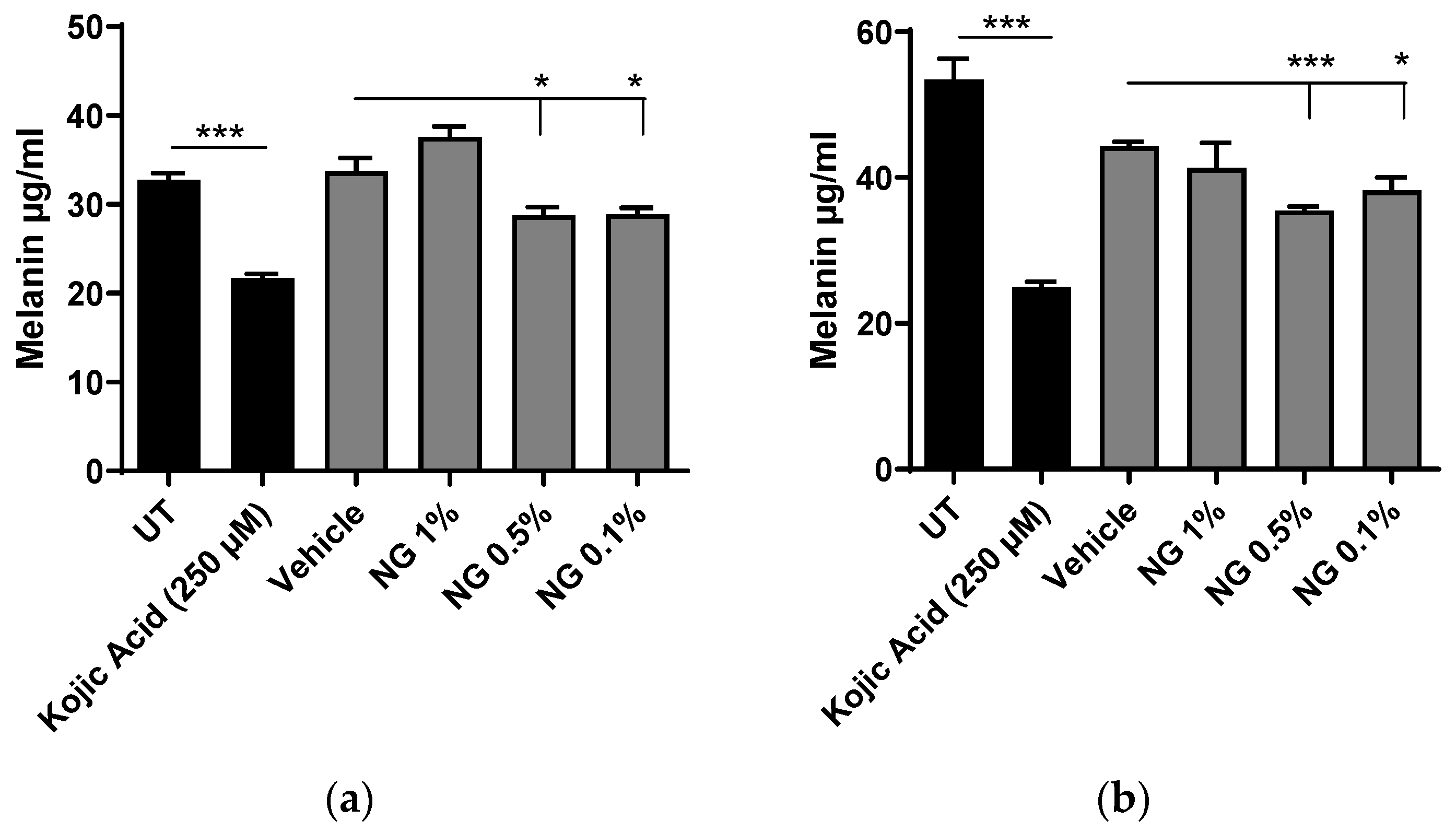
3.6.2. Melanin Content Analysis after Chemical Extraction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krutmann, J.; Liu, W.; Li, L.; Pan, X.; Crawford, M.; Sore, G.; Seite, S. Pollution and Skin: From Epidemiological and Mechanistic Studies to Clinical Implications. J. Dermatol. Sci. 2014, 76, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Velasco, M.V.R.; Sauce, R.; de Oliveira, C.A.; de Oliveira Pinto, C.A.S.; Martinez, R.M.; Baah, S.; Almeida, T.S.; Rosado, C.; Baby, A.R. Active Ingredients, Mechanisms of Action and Efficacy Tests of Antipollution Cosmetic and Personal Care Products. Brazilian J. Pharm. Sci. 2018, 54, e01003. [Google Scholar] [CrossRef]
- Khmaladze, I.; Leonardi, M.; Fabre, S.; Messaraa, C.; Mavon, A. The Skin Interactome: A Holistic “Genome-Microbiome-Exposome” Approach to Understand and Modulate Skin Health and Aging. Clin. Cosmet. Investig. Dermatol. 2020, 13, 1021–1040. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of Photoaging and Chronological Skin Aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Vierkötter, A.; Schikowski, T.; Ranft, U.; Sugiri, D.; Matsui, M.; Krämer, U.; Krutmann, J. Airborne Particle Exposure and Extrinsic Skin Aging. J. Investig. Dermatol. 2010, 130, 2719–2726. [Google Scholar] [CrossRef]
- Ahn, Y.; Lee, E.J.; Luo, E.; Choi, J.; Kim, J.Y.; Kim, S.; Kim, S.H.; Bae, Y.J.; Park, S.; Lee, J.; et al. Particulate Matter Promotes Melanin Production through Endoplasmic Reticulum Stress–Mediated IRE1α Signaling. J. Investig. Dermatol. 2022, 142, 1425–1434.e6. [Google Scholar] [CrossRef]
- Nguyen, L.P.; Bradfield, C.A. The Search for Endogenous Activators of the Aryl Hydrocarbon Receptor. Chem. Res. Toxicol. 2008, 21, 102–116. [Google Scholar] [CrossRef]
- Costa, C.; Catania, S.; De Pasquale, R.; Stancanelli, R.; Scribano, G.M.; Melchini, A. Exposure of Human Skin to Benzo[a]Pyrene: Role of CYP1A1 and Aryl Hydrocarbon Receptor in Oxidative Stress Generation. Toxicology 2010, 271, 83–86. [Google Scholar] [CrossRef]
- Ono, Y.; Torii, K.; Fritsche, E.; Shintani, Y.; Nishida, E.; Nakamura, M.; Shirakata, Y.; Haarmann-Stemmann, T.; Abel, J.; Krutmann, J.; et al. Role of the Aryl Hydrocarbon Receptor in Tobacco Smoke Extract–Induced Matrix Metalloproteinase-1 Expression. Exp. Dermatol. 2013, 22, 349–353. [Google Scholar] [CrossRef]
- Tigges, J.; Haarmann-Stemmann, T.; Vogel, C.F.A.; Grindel, A.; Hübenthal, U.; Brenden, H.; Grether-Beck, S.; Vielhaber, G.; Johncock, W.; Krutmann, J.; et al. The New Aryl Hydrocarbon Receptor Antagonist E/Z-2-Benzylindene-5,6- Dimethoxy-3,3-Dimethylindan-1-One Protects against UVB-Induced Signal Transduction. J. Investig. Dermatol. 2014, 134, 556–559. [Google Scholar] [CrossRef]
- Barreca, D.; Mandalari, G.; Calderaro, A.; Smeriglio, A.; Trombetta, D.; Felice, M.R.; Gattuso, G. Citrus Flavones: An Update on Sources, Biological Functions, and Health Promoting Properties. Plants 2020, 9, 288. [Google Scholar] [CrossRef]
- de Lima Cherubim, D.J.; Buzanello Martins, C.V.; Oliveira Fariña, L.; da Silva de Lucca, R.A. Polyphenols as Natural Antioxidants in Cosmetics Applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Nunes, A.R.; Vieira, Í.G.P.; Queiroz, D.B.; Leal, A.L.A.B.; Maia Morais, S.; Muniz, D.F.; Calixto-Junior, J.T.; Coutinho, H.D.M. Use of Flavonoids and Cinnamates, the Main Photoprotectors with Natural Origin. Adv. Pharmacol. Sci. 2018, 2018, 5341487. [Google Scholar] [CrossRef]
- Takayama, K.S.; Monteiro, M.C.; Saito, P.; Pinto, I.C.; Nakano, C.T.; Martinez, R.M.; Thomaz, D.V.; Verri, W.A.; Baracat, M.M.; Arakawa, N.S.; et al. Rosmarinus Officinalis Extract-Loaded Emulgel Prevents UVB Irradiation Damage to the Skin. An. Acad. Bras. Cienc. 2022, 94, e20201058. [Google Scholar] [CrossRef]
- Velasco, M.V.R.; Sarruf, F.D.; Salgado-Santos, I.M.N.; Haroutiounian-Filho, C.A.; Kaneko, T.M.; Baby, A.R. Broad Spectrum Bioactive Sunscreens. Int. J. Pharm. 2008, 363, 50–57. [Google Scholar] [CrossRef]
- Lee, C.-H.; Jeong, T.-S.; Choi, Y.-K.; Hyun, B.-H.; Oh, G.-T.; Kim, E.-H.; Kim, J.-R.; Han, J.-I.; Bok, S.-H. Anti-Atherogenic Effect of Citrus Flavonoids, Naringin and Naringenin, Associated with Hepatic ACAT and Aortic VCAM-1 and MCP-1 in High Cholesterol-Fed Rabbits. Biochem. Biophys. Res. Commun. 2001, 284, 681–688. [Google Scholar] [CrossRef]
- Al-Roujayee, A.S. Naringenin Improves the Healing Process of Thermally-Induced Skin Damage in Rats. J. Int. Med. Res. 2017, 45, 570–582. [Google Scholar] [CrossRef]
- Martinez, R.M.; Pinho-Ribeiro, F.A.; Steffen, V.S.; Silva, T.C.C.; Caviglione, C.V.; Bottura, C.; Fonseca, M.J.V.; Vicentini, F.T.M.C.; Vignoli, J.A.; Baracat, M.M.; et al. Topical Formulation Containing Naringenin: Efficacy against Ultraviolet B Irradiation-Induced Skin Inflammation and Oxidative Stress in Mice. PLoS ONE 2016, 11, e0146296. [Google Scholar] [CrossRef]
- Pinho-Ribeiro, F.A.; Zarpelon, A.C.; Fattori, V.; Manchope, M.F.; Mizokami, S.S.; Casagrande, R.; Verri, W.A. Naringenin Reduces Inflammatory Pain in Mice. Neuropharmacology 2016, 105, 508–519. [Google Scholar] [CrossRef]
- Ali, R.; Shahid, A.; Ali, N.; Hasan, S.K.; Majed, F.; Sultana, S. Amelioration of Benzo[a]Pyrene-Induced Oxidative Stress and Pulmonary Toxicity by Naringenin in Wistar Rats: A Plausible Role of COX-2 and NF-ΚB. Hum. Exp. Toxicol. 2016, 36, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bi, Z.; Chu, W.; Wan, Y. IL-1 Receptor Antagonist Attenuates MAP Kinase/AP-1 Activation and MMP1 Expression in UVA-Irradiated Human Fibroblasts Induced by Culture Medium from UVB-Irradiated Human Skin Keratinocytes. Int. J. Mol. Med. 2005, 16, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.; Bhatti, H.; Nerusu, K.C.; Bhagavathula, N.; Kang, S.; Fisher, G.J.; Varani, J.; Voorhees, J.J. Matrix Metalloproteinase-1 Is the Major Collagenolytic Enzyme Responsible for Collagen Damage in UV-Irradiated Human Skin. Photochem. Photobiol. 2007, 78, 43–48. [Google Scholar] [CrossRef]
- Lu, J.; Guo, J.H.; Tu, X.L.; Zhang, C.; Zhao, M.; Zhang, Q.W.; Gao, F.H. Tiron Inhibits UVB-Induced AP-1 Binding Sites Transcriptional Activation on MMP-1 and MMP-3 Promoters by MAPK Signaling Pathway in Human Dermal Fibroblasts. PLoS ONE 2016, 11, e0159998. [Google Scholar] [CrossRef]
- Choi, Y.J.; Uehara, Y.; Park, J.Y.; Kim, S.J.; Kim, S.R.; Lee, H.W.; Moon, H.R.; Chung, H.Y. MHY884, a Newly Synthesized Tyrosinase Inhibitor, Suppresses UVB-Induced Activation of NF-ΚB Signaling Pathway through the Downregulation of Oxidative Stress. Bioorg. Med. Chem. Lett. 2014, 24, 1344–1348. [Google Scholar] [CrossRef]
- Sharma, S.D.; Katiyar, S.K. Dietary Grape Seed Proanthocyanidins Inhibit UVB-Induced Cyclooxygenase-2 Expression and Other Inflammatory Mediators in UVB-Exposed Skin and Skin Tumors of SKH-1 Hairless Mice. Pharm. Res. 2010, 27, 1092–1102. [Google Scholar] [CrossRef]
- Nebert, D.W.; Dalton, T.P.; Okey, A.B.; Gonzalez, F.J. Role of Aryl Hydrocarbon Receptor-Mediated Induction of the CYP1 Enzymes in Environmental Toxicity and Cancer. J. Biol. Chem. 2004, 279, 23847–23850. [Google Scholar] [CrossRef]
- Furue, M.; Uchi, H.; Mitoma, C.; Hashimoto-Hachiya, A.; Tanaka, Y.; Ito, T.; Tsuji, G. Implications of Tryptophan Photoproduct FICZ in Oxidative Stress and Terminal Differentiation of Keratinocytes. G. Ital. Dermatol. Venereol. 2019, 154, 37–41. [Google Scholar] [CrossRef]
- Manchope, M.F.; Casagrande, R.; Verri, W.A. Naringenin: An Analgesic and Anti-Inflammatory Citrus Flavanone. Oncotarget 2017, 8, 3766–3767. [Google Scholar] [CrossRef]
- Kumar, R.; Bhan Tiku, A. Naringenin Suppresses Chemically Induced Skin Cancer in Two-Stage Skin Carcinogenesis Mouse Model. Nutr. Cancer 2020, 72, 976–983. [Google Scholar] [CrossRef]
- Sun, R.; Liu, C.; Liu, J.; Yin, S.; Song, R.; Ma, J.; Cao, G.; Lu, Y.; Zhang, G.; Wu, Z.; et al. Integrated Network Pharmacology and Experimental Validation to Explore the Mechanisms Underlying Naringenin Treatment of Chronic Wounds. Sci. Rep. 2023, 13, 132. [Google Scholar] [CrossRef]
- Jung, S.K.; Ha, S.J.; Jung, C.H.; Kim, Y.T.; Lee, H.K.; Kim, M.O.; Lee, M.H.; Mottamal, M.; Bode, A.M.; Lee, K.W.; et al. Naringenin Targets ERK2 and Suppresses UVB-Induced Photoaging. J. Cell. Mol. Med. 2016, 20, 909–919. [Google Scholar] [CrossRef]
- Martinez, R.M.; Pinho-Ribeiro, F.A.; Steffen, V.S.; Caviglione, C.V.; Vignoli, J.A.; Barbosa, D.S.; Baracat, M.M.; Georgetti, S.R.; Verri, W.A.; Casagrande, R. Naringenin Inhibits UVB Irradiation-Induced Inflammation and Oxidative Stress in the Skin of Hairless Mice. J. Nat. Prod. 2015, 78, 1647–1655. [Google Scholar] [CrossRef]
- Chang, H.L.; Chang, Y.M.; Lai, S.C.; Chen, K.M.; Wang, K.C.; Chiu, T.T.; Chang, F.H.; Hsu, L.S. Naringenin Inhibits Migration of Lung Cancer Cells via the Inhibition of Matrix Metalloproteinases-2 and-9. Exp. Ther. Med. 2017, 13, 739–744. [Google Scholar] [CrossRef]
- Wang, C.C.; Guo, L.; Tian, F.D.; An, N.; Luo, L.; Hao, R.H.; Wang, B.; Zhou, Z.H. Naringenin Regulates Production of Matrix Metalloproteinases in the Knee-Joint and Primary Cultured Articular Chondrocytes and Alleviates Pain in Rat Osteoarthritis Model. Brazilian. J. Med. Biol. Res. 2017, 50, e5714. [Google Scholar] [CrossRef]
- Quan, T.; Little, E.; Quan, H.; Qin, Z.; Voorhees, J.J.; Fisher, G.J. Elevated Matrix Metalloproteinases and Collagen Fragmentation in Photodamaged Human Skin: Impact of Altered Extracellular Matrix Microenvironment on Dermal Fibroblast Function. J. Investig. Dermatol. 2013, 133, 1362. [Google Scholar] [CrossRef]
- Fingleton, B. Matrix Metalloproteinases as Regulators of Inflammatory Processes. Biochim. Biophys. Acta Mol. Cell. Res. 2017, 1864, 2036–2042. [Google Scholar] [CrossRef]
- Onodera, S.; Kaneda, K.; Mizue, Y.; Koyama, Y.; Fujinaga, M.; Nishihira, J. Macrophage Migration Inhibitory Factor Up-Regulates Expression of Matrix Metalloproteinases in Synovial Fibroblasts of Rheumatoid Arthritis. J. Biol. Chem. 2000, 275, 444–450. [Google Scholar] [CrossRef]
- Imokawa, G.; Nakajima, H.; Ishida, K. Biological Mechanisms Underlying the Ultraviolet Radiation-Induced Formation of Skin Wrinkling and Sagging II: Over-Expression of Neprilysin Plays an Essential Role. Int. J. Mol. Sci. 2015, 16, 7776–7795. [Google Scholar] [CrossRef]
- El-Mahdy, M.A.; Zhu, Q.; Wang, Q.E.; Wani, G.; Patnaik, S.; Zhao, Q.; Arafa, E.S.; Barakat, B.; Mir, S.N.; Wani, A.A. Naringenin Protects HaCaT Human Keratinocytes against UVB-Induced Apoptosis and Enhances the Removal of Cyclobutane Pyrimidine Dimers from the Genome. Photochem. Photobiol. 2008, 84, 307–316. [Google Scholar] [CrossRef]
- Kim, K.E.; Cho, D.; Park, H.J. Air Pollution and Skin Diseases: Adverse Effects of Airborne Particulate Matter on Various Skin Diseases. Life Sci. 2016, 152, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Nakatsuru, Y.; Ichinose, M.; Takahashi, Y.; Kume, H.; Mimura, J.; Fujii-Kuriyama, Y.; Ishikawa, T. Benzo[a]Pyrene Carcinogenicity Is Lost in Mice Lacking the Aryl Hydrocarbon Receptor. Proc. Natl. Acad. Sci. USA 2000, 97, 779. [Google Scholar] [CrossRef] [PubMed]
- Grether-Beck, S.; Felsner, I.; Brenden, H.; Marini, A.; Jaenicke, T.; Aue, N.; Welss, T.; Uthe, I.; Krutmann, J. Air Pollution-Induced Tanning of Human Skin. Br. J. Dermatol. 2021, 185, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zeng, Z.; Liu, J.; Pi, Z.; Zou, P.; Deng, Q.; Ma, X.; Qiao, F.; Xiong, W.; Zhou, C.; et al. Particulate Matter Promotes Hyperpigmentation via AhR/MAPK Signaling Activation and by Increasing α-MSH Paracrine Levels in Keratinocytes. Environ. Pollut. 2021, 278, 116850. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Kim, M.J.; Choi, Y.H.; Kim, B.K.; Kim, K.S.; Park, K.J.; Park, S.M.; Lee, N.H.; Hyun, C.G. Down-Regulation of Tyrosinase, TRP-1, TRP-2 and MITF Expressions by Citrus Press-Cakes in Murine B16 F10 Melanoma. Asian Pac. J. Trop. Biomed. 2013, 3, 617–622. [Google Scholar] [CrossRef]
- Murata, K.; Takahashi, K.; Nakamura, H.; Itoh, K.; Matsuda, H. Search for Skin-Whitening Agent from Prunus Plants and the Molecular Targets in Melanogenesis Pathway of Active Compounds. Nat. Prod. Commun. 2014, 9, 185–188. [Google Scholar] [CrossRef]
- Huang, Y.C.; Yang, C.H.; Chiou, Y.L. Citrus Flavanone Naringenin Enhances Melanogenesis through the Activation of Wnt/β-Catenin Signalling in Mouse Melanoma Cells. Phytomedicine 2011, 18, 1244–1249. [Google Scholar] [CrossRef]
- Nasr Bouzaiene, N.; Chaabane, F.; Sassi, A.; Chekir-Ghedira, L.; Ghedira, K. Effect of Apigenin-7-Glucoside, Genkwanin and Naringenin on Tyrosinase Activity and Melanin Synthesis in B16F10 Melanoma Cells. Life Sci. 2016, 144, 80–85. [Google Scholar] [CrossRef]
- Vance, K.W.; Goding, C.R. The Transcription Network Regulating Melanocyte Development and Melanoma. Pigment. Cell. Res. 2004, 17, 318–325. [Google Scholar] [CrossRef]
- Wu, X.; Bowers, B.; Rao, K.; Wei, Q.; Hammer, J.A. Visualization of Melanosome Dynamics within Wild-Type and Dilute Melanocytes Suggests a Paradigm for Myosin V Function In Vivo. J. Cell Biol. 1998, 143, 1899–1918. [Google Scholar] [CrossRef]
- Hume, A.N.; Ushakov, D.S.; Tarafder, A.K.; Ferenczi, M.A.; Seabra, M.C. Rab27a and MyoVa Are the Primary Mlph Interactors Regulating Melanosome Transport in Melanocytes. J. Cell Sci. 2007, 120, 3111–3122. [Google Scholar] [CrossRef]
| Gene ID | Naringenin | Vehicle | Ratio | p Value | ||
|---|---|---|---|---|---|---|
| MLPH | XM_006712737.1 | 192.88 | 226.91 | 0.85 | 0.049 | |
| 159.27 | 226.91 | 0.7 | 0.028 | |||
| MYO5A | NM_000259.3 | 544.97 | 808.06 | 0.67 | 0.03 | |
| 549.92 | 808.06 | 0.68 | 0.024 | |||
| MITF | NM_000248.3 | 298.35 | 401.2 | 0.74 | 0.026 | |
| 302.99 | 401.2 | 0.76 | 0.049 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Österlund, C.; Hrapovic, N.; Lafon-Kolb, V.; Amini, N.; Smiljanic, S.; Visdal-Johnsen, L. Protective Effects of Naringenin against UVB Irradiation and Air Pollution-Induced Skin Aging and Pigmentation. Cosmetics 2023, 10, 88. https://doi.org/10.3390/cosmetics10030088
Österlund C, Hrapovic N, Lafon-Kolb V, Amini N, Smiljanic S, Visdal-Johnsen L. Protective Effects of Naringenin against UVB Irradiation and Air Pollution-Induced Skin Aging and Pigmentation. Cosmetics. 2023; 10(3):88. https://doi.org/10.3390/cosmetics10030088
Chicago/Turabian StyleÖsterlund, Christina, Nina Hrapovic, Virginie Lafon-Kolb, Nahid Amini, Sandra Smiljanic, and Lene Visdal-Johnsen. 2023. "Protective Effects of Naringenin against UVB Irradiation and Air Pollution-Induced Skin Aging and Pigmentation" Cosmetics 10, no. 3: 88. https://doi.org/10.3390/cosmetics10030088
APA StyleÖsterlund, C., Hrapovic, N., Lafon-Kolb, V., Amini, N., Smiljanic, S., & Visdal-Johnsen, L. (2023). Protective Effects of Naringenin against UVB Irradiation and Air Pollution-Induced Skin Aging and Pigmentation. Cosmetics, 10(3), 88. https://doi.org/10.3390/cosmetics10030088






