The Assessment of the Skin-Whitening and Anti-Wrinkling Effects of Anemarrhena asphodeloides Bunge Root Extracts and the Identification of Nyasol in a Developed Cream Product
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Sample Preparation
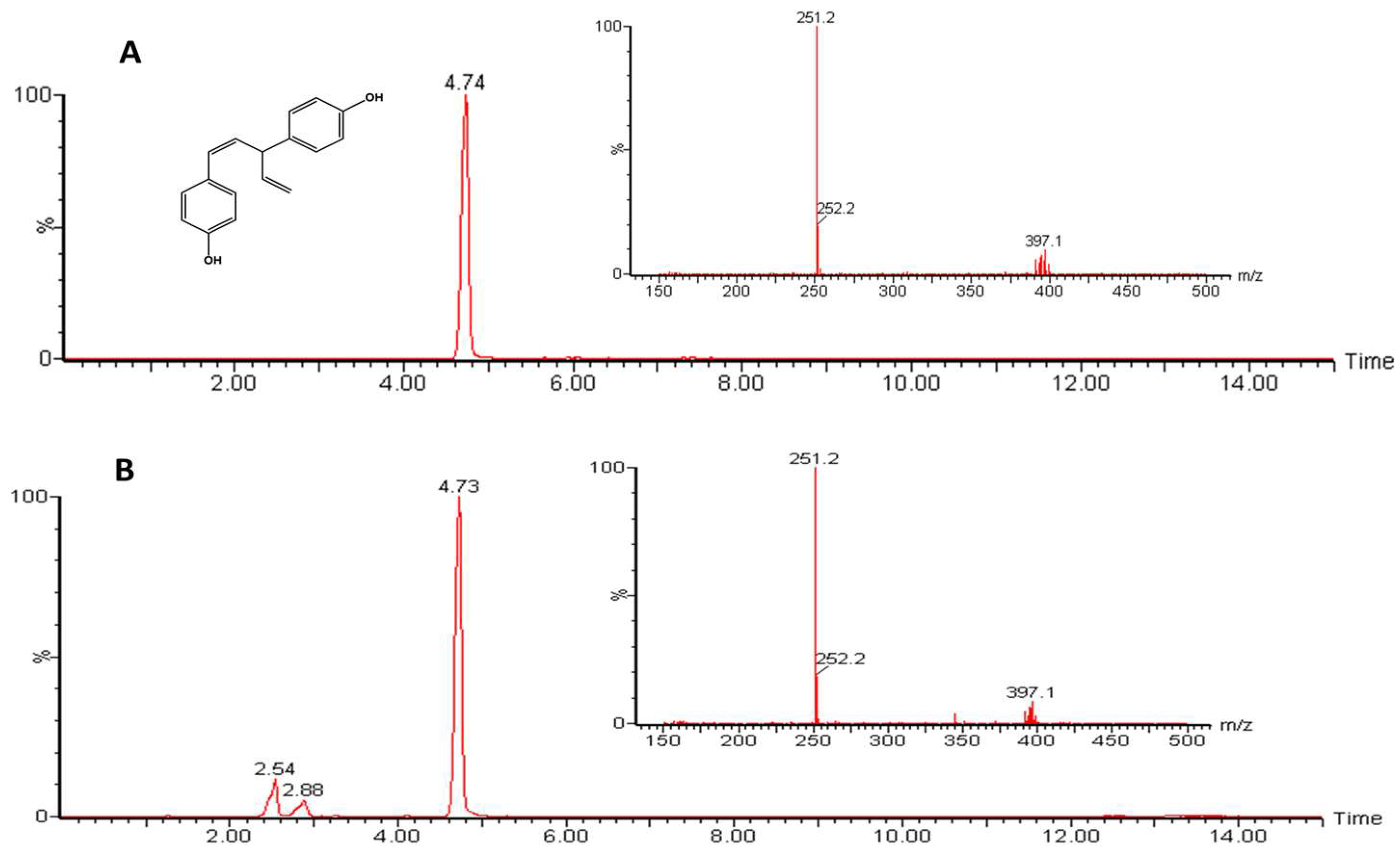
2.3. Identification of Nyasol in AAREs Using UPLC-ESI-MS
2.4. Calibration Curve of Nyasol
2.5. Quantitative Analysis of Nyasol in the Cream with AARE by HPLC-PDA
2.6. Statistical Analysis
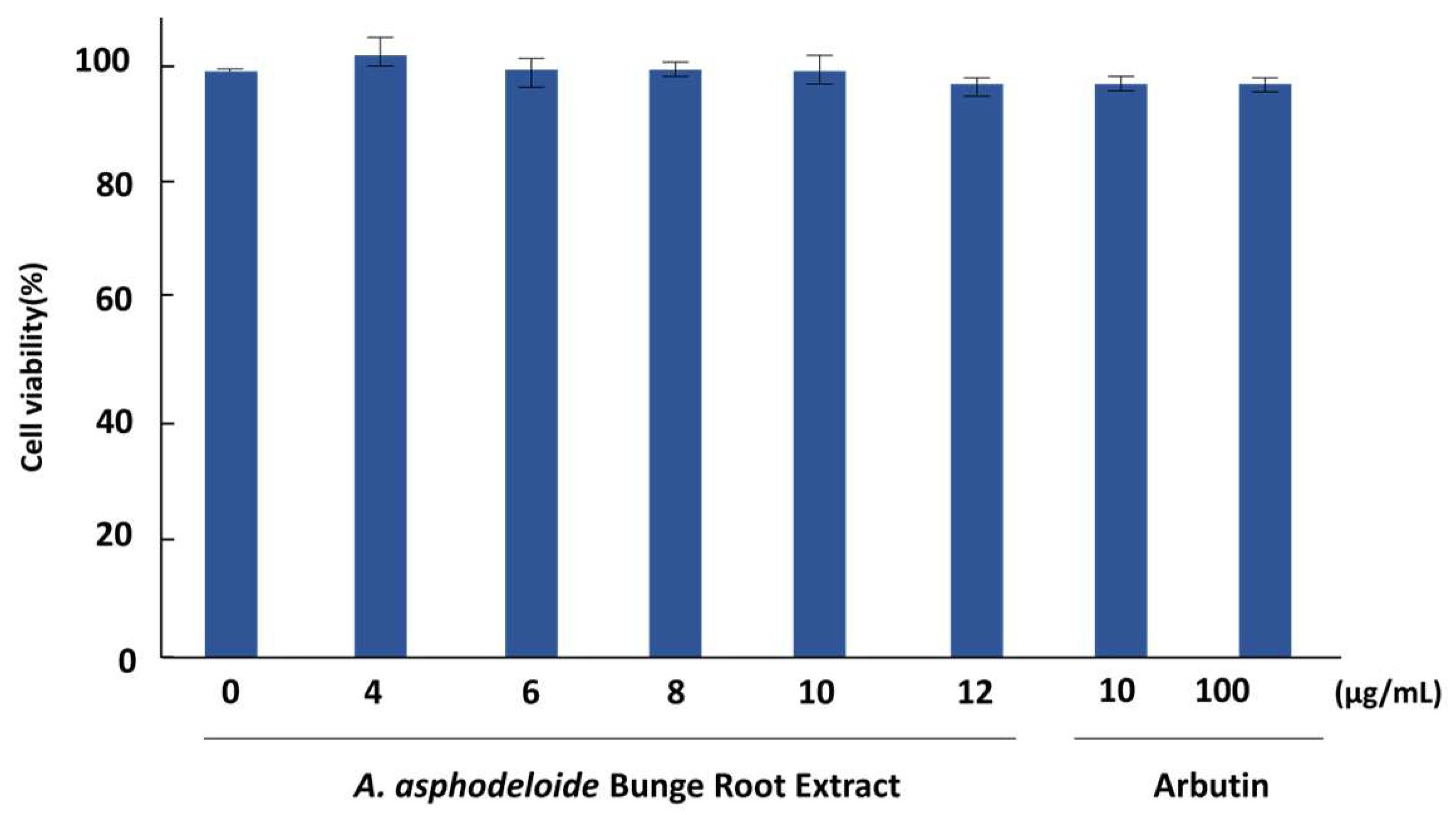
2.7. Cell Viability Assessment
2.8. Measurement of Inhibitory Activity of Melanin Biosynthesis
2.9. Assessment of MMP-1 Expression-Inhibition Activity
2.10. DPPH Radical Scavenging Activity
3. Results and Discussion
3.1. Identification of Nyasol in AARE Using UPLC-ESI-MS
3.2. Quantification of Nyasol by HPLC-PDA
3.3. Cell Viability and Melanin Inhibition
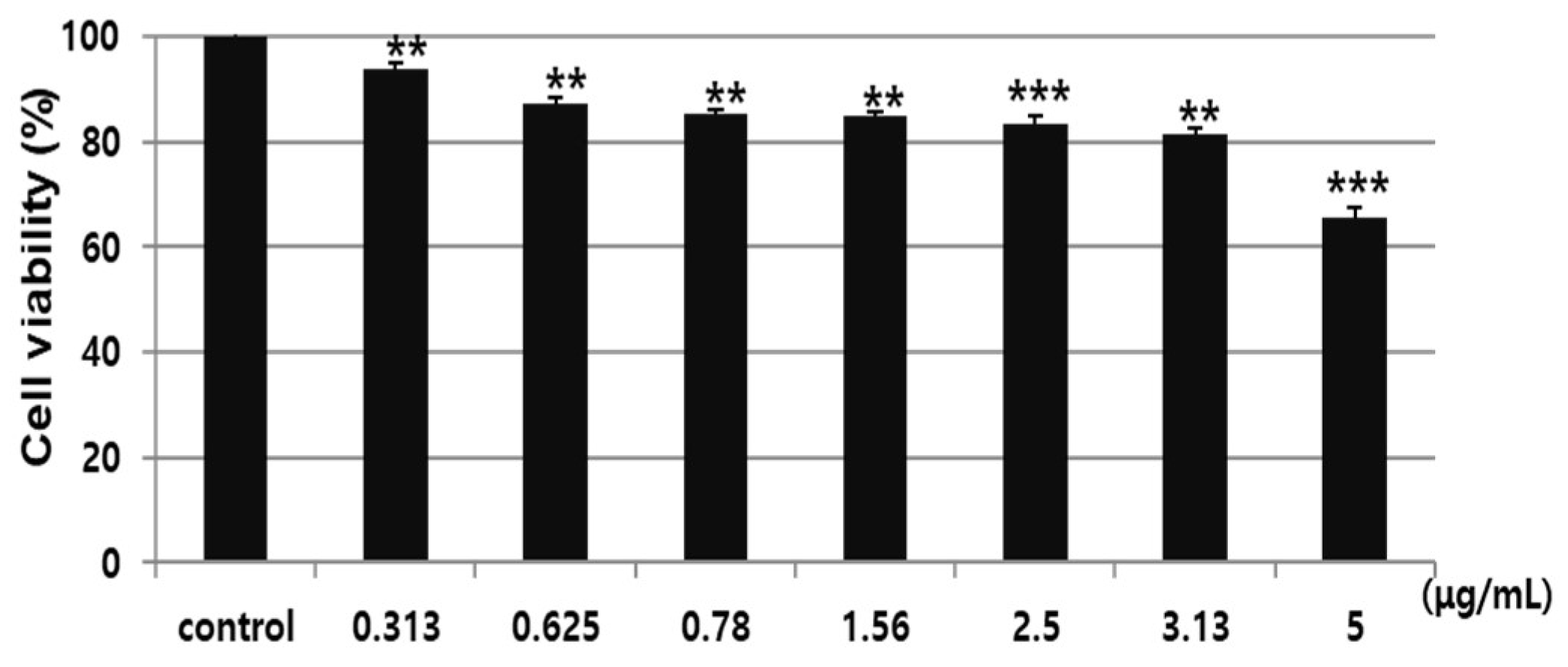
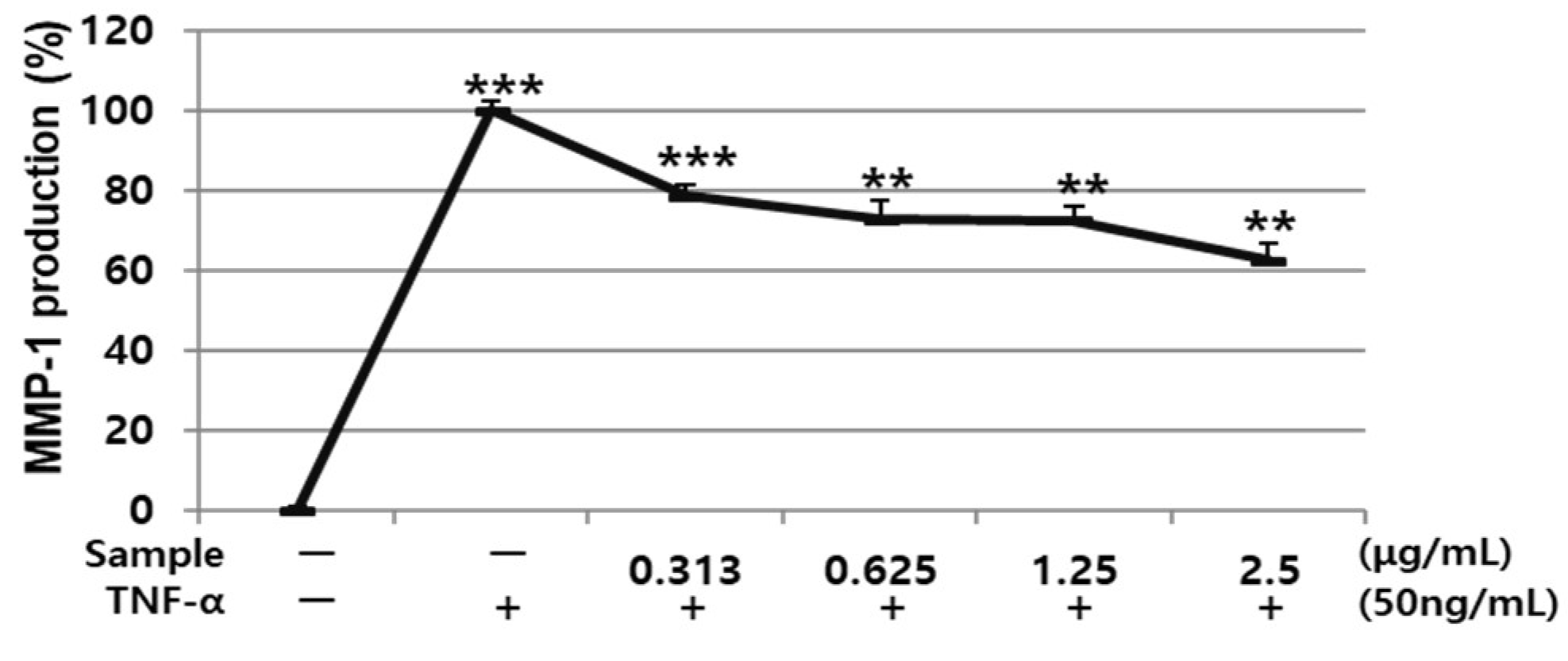
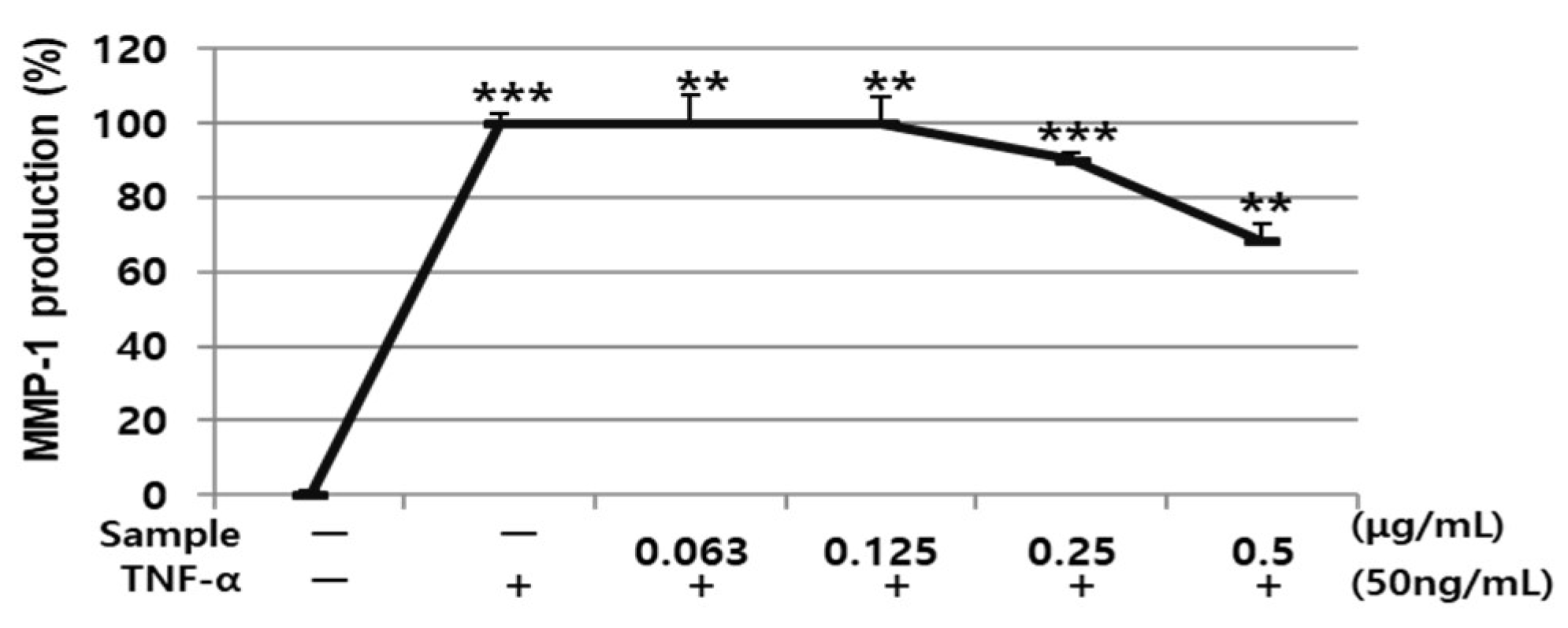
3.4. Effects of AARE and Nyasol on HDF Cell Viability and TNF-α-induced MMP-1 Protein Expression
3.5. Antioxidant Effects Using DPPH Free Radical Scavenging Activity Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, G.S.; Park, C.K.; Seong, J.D.; Kim, H.T.; Han, S.I.; Kwack, Y.H. Isolation and HPLC analysis of timosaponin A from rhizomes of Anemarrhena asphodeloides Bunge. Korean J. Med. Crop Sci. 1999, 7, 45–50. [Google Scholar]
- Lee, J.M.; Lee, S.H.; Park, J.H.; Kang, S.J.; Chang, S.Y.; Lee, K.S.; Son, K.H. Isolation and quantitative determination of anemarsaponin B from the rhizomes of Anemarrhena asphodeloides Bunge. Korean J. Pharmacogn. 1999, 30, 163–167. [Google Scholar]
- Kawasaki, T.; Yamauchi, T. Saponins of timo (Anemarrhena rizoma). II. Structure of timosaponin A-III. Chem. Pharm. Bull. 1963, 11, 1221–1224. [Google Scholar] [CrossRef]
- Kawasaki, T.; Yamauchi, T.; Itakura, N. Saponins of timo (Anemarrhena rizoma). Yakugaku Zasshi 1963, 83, 892–896. [Google Scholar] [CrossRef]
- Niwa, A.; Takeda, O.; Ishimaru, M.; Nakamoto, Y.; Yamasaki, K.; Kohda, H.; Nishio, H.; Segawa, T.; Fujumura, K.; Kuramoto, A. Screening test for platelet aggregation inhibitor in natural products. The active principle of Anemarrhena rizoma. Yakugaku Zasshi 1988, 108, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Nahumo, S.; Kishi, S.I.; Inoue, T.; Nagai, M. Saponins of Anemarrhena rizoma. Yakugaku Zasshi 1991, 111, 306–310. [Google Scholar]
- Lin, Y.; Zhao, W.R.; Shi, W.T.; Zhang, J.; Zhang, K.Y.; Ding, Q.; Chen, X.L.; Tang, J.Y.; Zhou, Z.Y. Pharmacological activity, pharmacokinetics, and toxicity of timosapoin A III, a natural product isolated from Anemarrhena asphodeloides bunge, A review. Front. Pharmacol. 2020, 11, 1–16. [Google Scholar] [CrossRef]
- Nakashima, N.; Kimura, I.; Kimura, M. Isolation of pseudoprototimosaponin A III from rhizomes of Anemarrhena asphodeloides and its hypoglycemic activity in streptozotocin-induced diabetic mice. J. Nat. Prod. 1993, 56, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Kimura, F.; Chen, J. Combined potentiating effects of Byakko-ka-ninjin-to, its constituents, rhizomes of Anemarrhena asphodeloides, timosaponin A III, and calcium on pilocarpine-induced saliva secretion in streptozotocin-induced diabetic mice. Biol. Pharm. Bull. 1996, 19, 926–931. [Google Scholar] [CrossRef] [PubMed]
- Morita, N.; Shimizu, M.; Fukuta, M. Studies on the medical resources. XXIV. Chimonin in Anemarrhena rizoma. Yakugaku Zasshi 1965, 85, 374–375. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.H.; Kizu, H.; Tommory, T. Quantitative analysis of timosaponin B-II, timosaponin A-III and mangferin in Anemarrhena rizoma and Kampo prescriptions containing this crude drug. Shoyakugaku Zasshi 1992, 46, 19–24. [Google Scholar]
- Park, Y.J.; Ku, C.S.; Kim, M.J.; Lee, M.K.; Kim, K.O.; Ryu, H.W.; Song, H.H.; Kim, D.Y.; Oh, S.R. Cosmetic activities of nyasol from the rhizomes of Anemarrhena asphodeloide. J. Appl. Biol. Chem. 2015, 58, 31–38. [Google Scholar] [CrossRef]
- Lee, Y.J.; Song, B.R.; Lee, S.L.; Shin, H.S.; Park, S.N. Antimicrobial, antioxidant and cellular protective effects against oxidative stress of Anemarrhena asphodeloides Bunge extract and fraction. Microbiol. Biotechnol. Lett. 2018, 46, 360–371. [Google Scholar] [CrossRef]
- Seo, S.K.; Han, S.J.; Ku, C.S.; Kim, D.H.; Ryu, J.H.; Baek, J.H.; Koh, J.S.; Jang, D.I. New development of natural depigmentation agent from Anemarrhena asphodeloides root extracts by inhibition of melanin biosynthesis. Asian J. Beauty Cosmetol. 2018, 16, 1–9. [Google Scholar] [CrossRef]
- Jang, T.W.; Park, J.H. Antioxidative activities and whitening effects of ethyl acetate fractions from the immature seeds of Abeliophyllum distichum. J. Life Sci. 2017, 27, 536–544. [Google Scholar] [CrossRef]
- Lee, J.Y.; Cho, Y.R.; Park, J.H.; Ahn, E.K.; Jeong, W.; Shin, H.S.; Kim, M.S.; Yang, S.H.; Oh, J.S. Anti-melanogenic and anti-oxidant activities of ethanol extract of Kummerowia striata, Kummerowia striata regulate anti-melanogenic activity through down-regulation of TRP-1, TRP-2 and MITF expression. Toxicol. Rep. 2018, 3, 10–17. [Google Scholar] [CrossRef]
- Choi, C.; Jeong, H.W. Study on skin whitening and antioxidant effect of Anemarrhenae rhizoma extract. J. Physiol. Pathol. Korean Med. 2020, 34, 67–74. [Google Scholar] [CrossRef]
- Yoo, J.M.; Kang, Y.J.; Pyo, H.B.; Choung, E.S.; Park, S.Y.; Choi, J.H.; Han, G.J.; Lee, C.H.; Kim, T.J. Anti-wrinkle effects of Korean rice wine cake on human fibroblast. J. Life Sci. 2010, 20, 1838–1843. [Google Scholar] [CrossRef]
- Kim, E.J.; Kim, M.K.; Jin, X.J.; Oh, J.H.; Kim, J.E.; Chung, J.H. Skin aging and photoaging alter fatty acids composition, including 11,14,17-eicosatrienoic acid, in the epidermis of human skin. J. Korean Med. Sci. 2010, 25, 980–983. [Google Scholar] [CrossRef]
- Park, K.J.; Park, S.H.; Kim, J.K. Anti-wrinkle activity of acanthopanax senticosus extract in ultraviolet B (UVB)-induced photoaging. J. Korean Soc. Food Sci. Nutr. 2010, 39, 42–46. [Google Scholar] [CrossRef]
- Fraizer, K.; Williams, S.; Kothapal, D.; Klapper, H.; Grotendorst, G.R. Stimulation of fibroblast cell-growth, matrix production, and granulation-tissue formation by connective-tissue growth-factor. J. Invest. Dermatol. 1996, 107, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.H. Inhibitory effect of gallic acid on TNF-α-induced matrix metalloproteinase-1 (MMP-1) in HaCaT Cells. J. Korea Acad. Ind. Coop. Soc. 2013, 14, 5778–5784. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Meth 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Jeon, M.J.; Kim, M.Y.; Jang, H.J.; Lee, S.W.; Kim, J.H.; Kim, H.S.; Lee, S.H. Whitening effect of Hizikia fusiformis ethanol extract and its fractions. J. Life Sci. 2012, 22, 889–896. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determination by the use of a stable free radical. Nature 1958, 25, 1199–1200. [Google Scholar] [CrossRef]
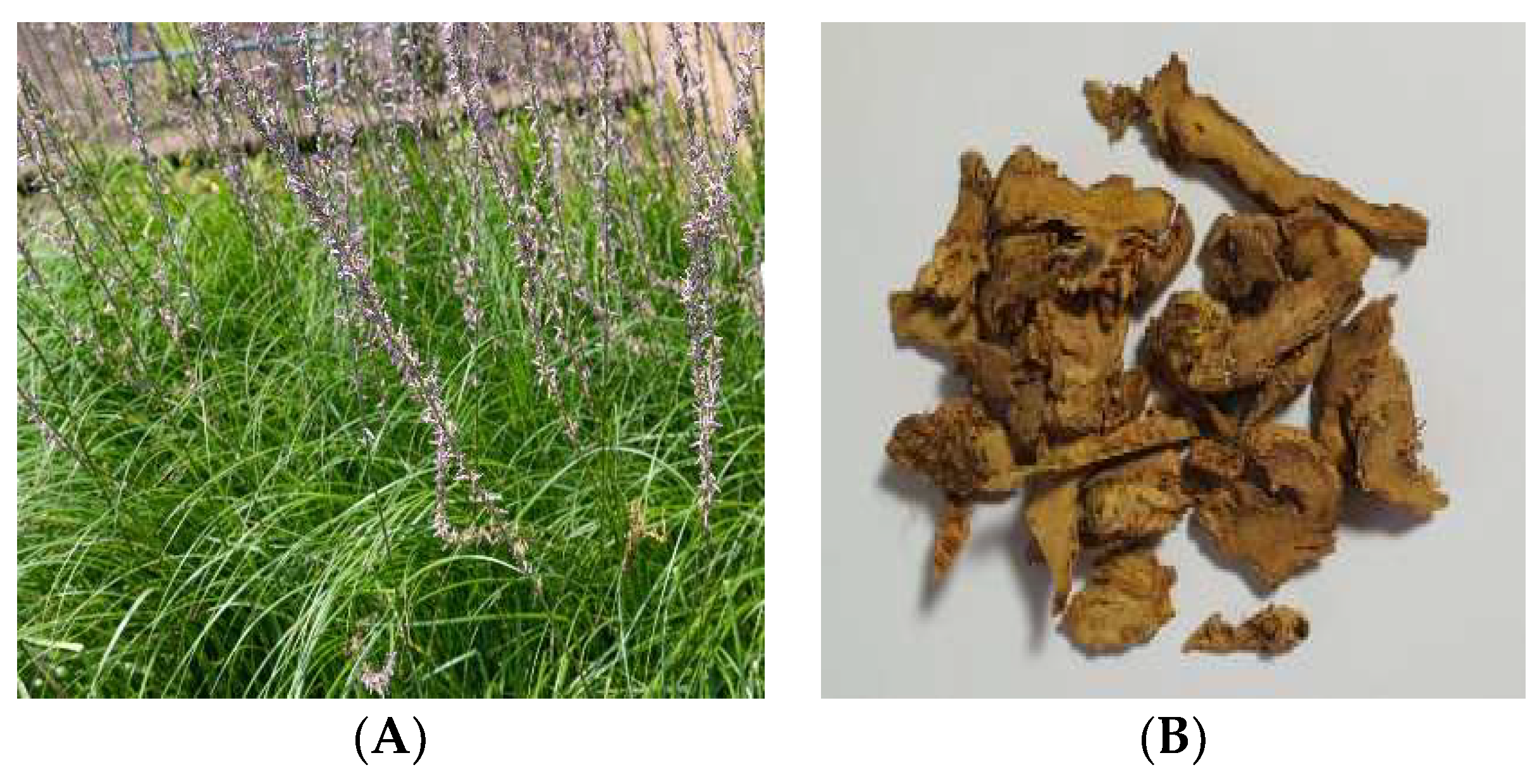




| Product Name | Manufacturer (Country) | Ingredient |
|---|---|---|
| Premier Anemarrhena 100% Extract | Premier Specialties Inc. (USA) | AARE |
| Volufiline | Sederma SAS (France) | AARE and hydrogenated polyisobutene |
| Gimo Extract | The Garden of Naturalsolution Co. (Korea) | AARE, water, and butylene glycol |
| Chimo Liquid E | Ichimaru Pharcos Co. (Japan) | AARE, water, and alcohol |
| Boumdan | SK Bioland (Korea) | AARE, 6 types of plant extracts, water, and butylene glycol |
| Time (min) | Flow Rate (min) | A (%) | B (%) | |
|---|---|---|---|---|
| 1 | 0 | 0.4 | 70 | 30 |
| 2 | 12.0 | 0.4 | 0 | 100 |
| 3 | 13.0 | 0.4 | 0 | 100 |
| 4 | 13.5 | 0.4 | 70 | 30 |
| 5 | 18.0 | 0.4 | 70 | 30 |
| Time (min) | Flow Rate (min) | A (%) | B (%) | |
|---|---|---|---|---|
| 1 | 0 | 1 | 50 | 50 |
| 2 | 10 | 1 | 0 | 100 |
| 3 | 13 | 1 | 0 | 100 |
| 4 | 14 | 1 | 50 | 50 |
| 5 | 20 | 1 | 50 | 50 |
| Sample | Nyasol Content (%) | Recovery Rate (%) | RSD of Recovery Rate (%) |
|---|---|---|---|
| 1 | 0.0974 ± 0.0001 | 96.44 | 0.33 |
| 2 | 0.0973 ± 0.0006 | 96.34 | |
| 3 | 0.0979 ± 0.0006 | 96.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.H.; Jang, D.I.; Choi, J. The Assessment of the Skin-Whitening and Anti-Wrinkling Effects of Anemarrhena asphodeloides Bunge Root Extracts and the Identification of Nyasol in a Developed Cream Product. Cosmetics 2023, 10, 73. https://doi.org/10.3390/cosmetics10030073
Lee MH, Jang DI, Choi J. The Assessment of the Skin-Whitening and Anti-Wrinkling Effects of Anemarrhena asphodeloides Bunge Root Extracts and the Identification of Nyasol in a Developed Cream Product. Cosmetics. 2023; 10(3):73. https://doi.org/10.3390/cosmetics10030073
Chicago/Turabian StyleLee, Myoung Hee, Dong IL Jang, and Jongkeun Choi. 2023. "The Assessment of the Skin-Whitening and Anti-Wrinkling Effects of Anemarrhena asphodeloides Bunge Root Extracts and the Identification of Nyasol in a Developed Cream Product" Cosmetics 10, no. 3: 73. https://doi.org/10.3390/cosmetics10030073
APA StyleLee, M. H., Jang, D. I., & Choi, J. (2023). The Assessment of the Skin-Whitening and Anti-Wrinkling Effects of Anemarrhena asphodeloides Bunge Root Extracts and the Identification of Nyasol in a Developed Cream Product. Cosmetics, 10(3), 73. https://doi.org/10.3390/cosmetics10030073






