The Effect of Cysteine Peptide Ingestion on Skin Brightness, a Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Human Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population (Participants)
2.1.1. Efficacy Trial
- Japanese males and females aged 20–65 years old.
- Those who have received a full explanation of the purpose and content of the study, and have agreed to participate in the study in writing.
- Subjects currently receiving medication or outpatient treatment for any serious disease.
- Subjects currently undergoing exercise or diet therapy under the supervision of a physician.
- Subjects currently undergoing an esthetic salon and beauty treatment (laser treatment, Zeo Skin, and so on) under the supervision of a physician.
- Subjects that may develop allergies to the test food.
- Subjects with a current or history of drug dependence or alcohol dependence.
- Subjects hospitalized for mental disorders (depression and so on) or sleep disorders or having a history of mental disorders.
- Subjects with an irregular rhythm of life due to night work or shift work.
- Subjects with extremely irregular eating, sleeping, or other habits.
- Subjects with an extremely unbalanced diet.
- Subjects who have or had serious diseases such as brain diseases, malignant tumors, immune diseases, diabetes, liver diseases (hepatitis), kidney diseases, heart diseases, thyroid diseases, adrenal diseases, or other metabolic diseases.
- Subjects that use health foods, supplements, or medicines (ingredients: glutathione, cysteine, yeast extract, vitamins, placenta, hyaluronic acid, collagen, and so on) that may improve skin quality.
- Subjects that live in an environment that is prone to sunburn daily (those who often work outdoors or have a habit of exercising outdoors).
- Subjects that participated in other clinical trials (research) within 3 months from the date of obtaining consent, or those who have plans to participate in other clinical trials (research) during the study period.
- Subjects that received over 200 mL of blood or donated over 400 mL of blood within 1 month or 3 months prior to the date of obtaining consent.
- Subjects that are currently pregnant or breastfeeding or are likely to become pregnant during the study period.
- Subjects who have difficulty complying with the recording of various questionnaires.
- Subjects who were judged to be unsuitable based on clinical laboratory values and measurements at the time of screening (SCR).
- Subjects that are judged unsuitable by the principal investigator.
2.1.2. Safety Trial
- 1.
- Japanese males and females aged 30–64 years old.
- 2.
- Subjects who have received a full explanation of the purpose and content of the study and have agreed to participate in the study in writing.
- 3.
- Subjects who are not currently receiving treatment or medication.
- 4.
- Subjects whose liver function marker values correspond to either of the following A or B (U/L):
- A:
- 20 ≤ ALT ≤ 30;
- B:
- 31 ≤ ALT ≤ 50.
- 5.
- Subjects who are not expected to meet the exclusion criteria based on the results of background questionnaires in the 1st SCR.
- Subjects who have been diagnosed with serious liver disease (viral hepatitis, drug-induced liver injury, liver cirrhosis, etc.) by a doctor, or those who have a medical history or are suspected (positive hepatitis virus test, etc.).
- Subjects who are currently undergoing medication or outpatient treatment due to some serious illness.
- Subjects who are currently undergoing exercise or diet therapy under the supervision of a physician.
- Subjects that may develop allergies to the test food.
- Subjects with a current or history of drug dependence or alcohol dependence.
- Subjects with excessive alcohol consumption.
- Subjects hospitalized for mental disorders (depression and so on) or sleep disorders or having a history of mental disorders.
- Subjects with an irregular rhythm of life due to night work or shift work.
- Subjects with extremely irregular eating, sleeping, or other habits.
- Subjects with an extremely unbalanced diet.
- Subjects that have or had serious diseases such as brain diseases, malignant tumors, immune diseases, diabetes, liver diseases (hepatitis), kidney diseases, heart diseases, thyroid diseases, renal diseases, or other metabolic diseases.
- Subjects that use health foods, supplements, or medicines (ingredients: Ornithine, sulforaphane, turmeric, etc.).
- Subjects that participated in other clinical trials within 2 months from the date of obtaining consent, or those who have plans to participate in other clinical trials during the study period.
- Subjects who cannot take the test food as instructed during the intervention period.
- Subjects that received over 200 mL of blood or donated over 400 mL of blood within 1 month or 3 months prior to the date of obtaining consent.
- Subjects that are currently pregnant or breastfeeding or are likely to become pregnant during the study period.
- Subjects who have difficulty complying with the recording of various questionnaires.
- Subjects were judged to be unsuitable based on clinical laboratory values and measurements at the time of screening.
- Subjects that are judged unsuitable by the principal investigator.
2.2. Design of the Human Clinical Study
2.2.1. Efficacy Trial
2.2.2. Safety Trial
2.3. Clinical Measurements
2.3.1. Efficacy Trial
Main Outcomes
Secondary Outcomes
Physical Measurement
Adverse Events
2.3.2. Safety Evaluation
Physical Measurements
2.3.3. Urinalysis
2.3.4. Hematology
2.3.5. Blood Biochemistry Test
2.3.6. Adverse Events
2.4. Test Sample
2.4.1. Efficacy Trial
2.4.2. Safety Trial
2.5. Statistical Analysis
2.5.1. Efficacy Trial
2.5.2. Safety Trial
3. Results
3.1. Flow Diagram
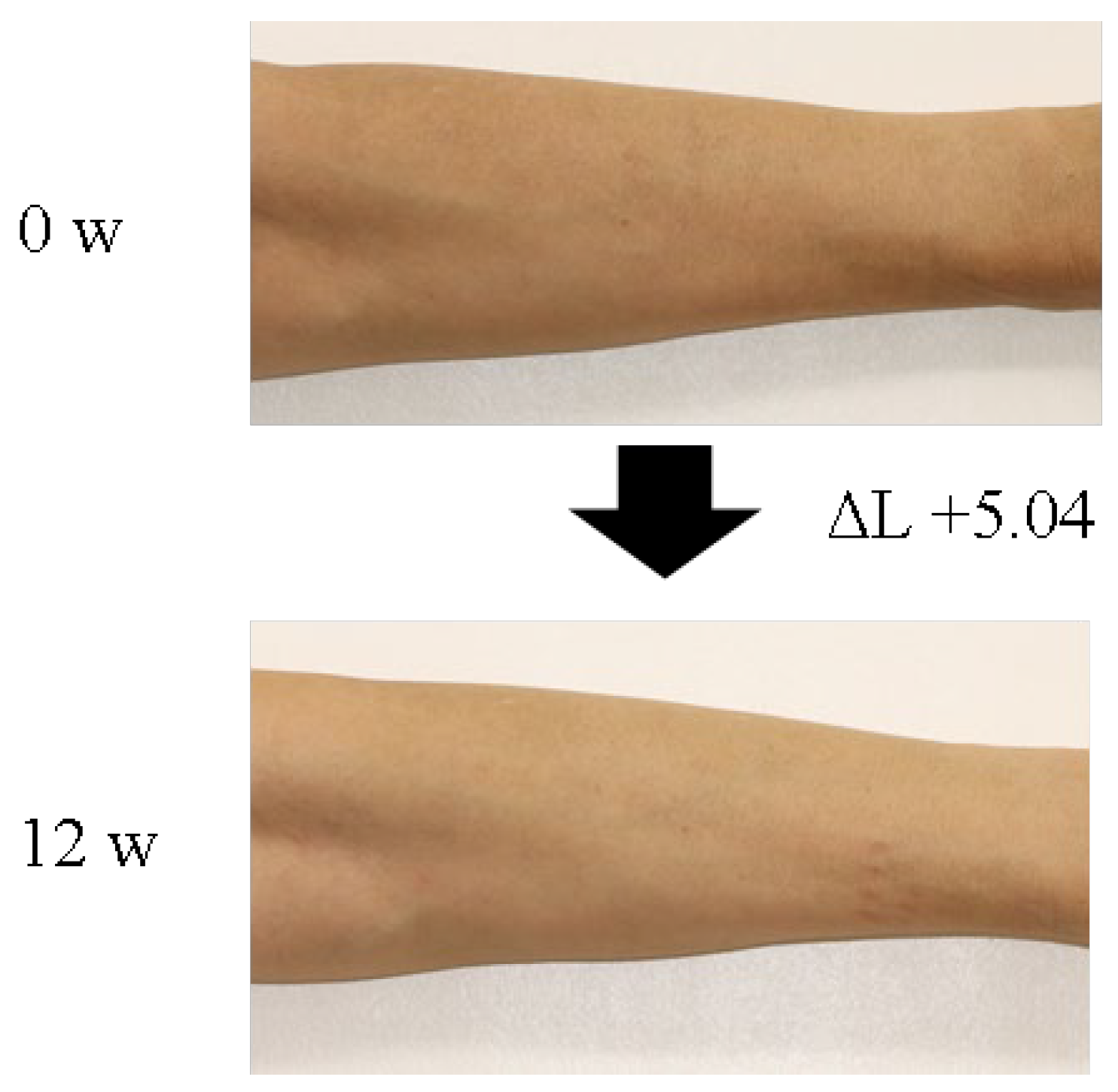
3.2. Effect of Cysteine Peptide on Human Skin Brightness
3.3. Effect of Cysteine Peptide on Human Skin Spots and Wrinkles
3.4. Safety Evaluation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 11551228. [Google Scholar] [CrossRef]
- Maddaleno, A.S.; Camargo, J.; Mitjans, M.; Vinardell, M.P. Melanogenesis and melasma treatment. Cosmetics 2021, 8, 82. [Google Scholar] [CrossRef]
- D’Mello, S.A.N.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling pathways in melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef] [PubMed]
- Arslanbaeva, L.R.; Santoro, M.M. Adaptive redox homeostasis in cutaneous melanoma. Redox Biol. 2020, 37, 101753. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403425. [Google Scholar] [CrossRef] [PubMed]
- Praetorius, C.; Grill, C.; Stacey, S.N.; Metcalf, A.M.; Gorkin, D.U.; Robinson, K.C.; Van Otterloo, E.; Kim, R.S.Q.; Bergsteinsdottir, K.; Ogmundsdottir, M.H.; et al. A polymorphism in IRF4 affects human pigmentation through a tyrosinase-dependent MITF/TFAP2A pathway. Cell 2013, 155, 10221033. [Google Scholar] [CrossRef]
- Qian, W.; Liu, W.; Zhu, D.; Cao, Y.; Tang, A.; Gong, G.; Su, H. Natural skinwhitening compounds for the treatment of melanogenesis (Review). Exp. Ther. Med. 2020, 20, 173185. [Google Scholar] [CrossRef]
- Kamakshi, R. Fairness via formulations: A review of cosmetic skin-lightening ingredients. J. Cosmet. Sci. 2012, 63, 4354. [Google Scholar]
- Anderson, M.E. Glutathione: An overview of biosynthesis and modulation. Chem. Biol. Interact. 1998, 111–112, 114. [Google Scholar] [CrossRef]
- Sonthalia, S.; Sarkar, R. Glutathione for skin lightning: An update. Pigment. Int. 2017, 4, 3–6. [Google Scholar] [CrossRef]
- Ballatori, N.; Krance, S.M.; Notenboom, S.; Shi, S.; Tieu, K.; Hammond, C.L. Glutathione dysregulation and the etiology and progression of human diseases. Biol. Chem. 2009, 390, 191–214. [Google Scholar] [CrossRef]
- Honda, Y.; Kessoku, T.; Sumida, Y.; Kobayashi, T.; Kato, T.; Ogawa, Y.; Tomeno, W.; Imajo, K.; Fujita, K.; Yoneda, M.; et al. Efficacy of glutathione for the treatment of nonalcoholic fatty liver disease: An open-label, single-arm, multicenter, pilot study. BMC Gastroenterol. 2017, 17, 96. [Google Scholar] [CrossRef]
- Pocernich, C.B.; Butterfield, D.A. Elevation of glutathione as a therapeutic strategy in Alzheimer disease. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2012, 1822, 625630. [Google Scholar] [CrossRef]
- Villarama, C.D.; Maibach, H.I. Glutathione as a depigmenting agent: An overview. Int. J. Cosmet. Sci. 2005, 27, 147153. [Google Scholar] [CrossRef] [PubMed]
- Weschawalit, S.; Thongthip, S.; Phutrakool, P.; Asawanonda, P. Glutathione and its antiaging and antimelanogenic effects. Clin. Cosmet. Investig. Dermatol. 2017, 10, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Arjinpathana, N.; Asawanonda, P. Glutathione as an oral whitening agent: A randomized, double-blind, placebo-controlled study. J. Dermatol. Treat. 2012, 23, 97102. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shertzer, H.G.; Schneider, S.N.; Nebert, D.W.; Dalton, T.P. Glutamate cysteine ligase catalysis: Dependence on ATP and modifier subunit for regulation of tissue glutathione levels. J. Biol. Chem. 2005, 280, 3376633774. [Google Scholar] [CrossRef]
- Lino, T.; Harada, S.; Kim, M.; Sakashita, M.; Kim, J.-W.; Yamashita, Y.; Kosaka, H. Inhibition of melanin synthesis and skin whitening effect by heat—Induced yeast extract (Cerepron (R)). Pharmacol. Ther. 2016, 44, 221226. [Google Scholar]
- Griffith, O.W. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic. Biol. Med. 1999, 27, 922935. [Google Scholar] [CrossRef] [PubMed]
- Meister, A. Glutathione metabolism and its selective modification. J. Biol. Chem. 1988, 263, 1720517208. [Google Scholar] [CrossRef]
- Wu, G.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489492. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 31433153. [Google Scholar] [CrossRef] [PubMed]
- Teskey, G.; Abrahem, R.; Cao, R.; Gyurjian, K.; Islamoglu, H.; Lucero, M.; Martinez, A.; Paredes, E.; Salaiz, O.; Robinson, B.; et al. Glutathione as a marker for human disease. Adv. Clin. Chem. 2018, 87, 141–159. [Google Scholar] [CrossRef] [PubMed]
- Kovacs-Nolan, J.; Rupa, P.; Matsui, T.; Tanaka, M.; Konishi, T.; Sauchi, Y.; Sato, K.; Ono, S.; Mine, Y. In vitro and ex vivo uptake of glutathione (GSH) across the intestinal epithelium and fate of oral GSH after in vivo supplementation. J. Agric. Food Chem. 2014, 62, 9499–9506. [Google Scholar] [CrossRef]
- Park, E.Y.; Shimura, N.; Konishi, T.; Sauchi, Y.; Wada, S.; Aoi, W.; Nakamura, Y.; Sato, K. Increase in the protein-bound form of glutathione in human blood after the oral administration of glutathione. J. Agric. Food Chem. 2014, 62, 61836189. [Google Scholar] [CrossRef]
- Richie, J.P., Jr.; Nichenametla, S.; Neidig, W.; Calcagnotto, A.; Haley, J.S.; Schell, T.D.; Muscat, J.E. Randomized controlled trial of oral glutathione supplementation on body stores of glutathione. Eur. J. Nutr. 2015, 54, 251263. [Google Scholar] [CrossRef]
- Jablonski, N.G.; Chaplin, G. Human skin pigmentation as an adaptation to UV radiation. Proc. Natl. Acad. Sci. USA 2010, 107 (Suppl. 2), 89628968. [Google Scholar] [CrossRef]
- Miyauchi, M.; Hirai, C.; Nakajima, H. The solar exposure time required for vitamin D3 synthesis in the human body estimated by numerical simulation and observation in Japan. J. Nutr. Sci. Vitaminol. 2013, 59, 257263. [Google Scholar] [CrossRef]
- Pullar, J.M.; Carr, A.C.; Vissers, M.C. The roles of vitamin C in skin health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef]
- Linster, C.L.; Van Schaftingen, E. Vitamin C: Biosynthesis, recycling and degradation in mammals. FEBS J. 2007, 274, 1–22. [Google Scholar] [CrossRef]

| Measurement | SCR | During Intervention | ||||
|---|---|---|---|---|---|---|
| (Visit 1) SCR | (Visit 2) Before Start of Intake | (Visit 3) 4 Weeks after Intake | (Visit 4) 8 Weeks after Intake | (Visit 5) 12 Weeks after intake | ||
| Main outcome | L value | ○ | ○ | ○ | ○ | ○ |
| Secondary outcomes | Spot (number) | ○ | ○ | ○ | ○ | |
| Wrinkle (number) | ○ | ○ | ○ | ○ | ||
| Physical measurement | Height | ○ | ||||
| Weight | ○ | ○ | ○ | ○ | ○ | |
| Body fat percentage | ○ | ○ | ○ | ○ | ○ | |
| BMI | ○ | ○ | ○ | ○ | ○ | |
| Vital signs | ○ | ○ | ○ | ○ | ○ | |
| Cysteine Peptide | Placebo | ||
|---|---|---|---|
| Group | 90 mg group | 45 mg group | Placebo group |
| n | 16 | 15 | 16 |
| Gender (Male = 1, Female = 2) | 1.80 ± 0.10 | 1.80 ± 0.11 | 1.81 ± 0.10 |
| Age | 42.94 ± 3.69 | 46.6 ± 2.87 | 44.94 ± 2.83 |
| L value (Face) | 62.51 ± 1.03 | 61.57 ± 0.84 | 61.83 ± 0.89 |
| L value (Arm) | 60.05 ± 1.38 | 59.06 ± 0.82 | 59.47 ± 1.56 |
| Task | Group | n | Intervention Period (Week) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 4 | 8 | 12 | |||||||||
| Mean ± SE | p-value | Mean ± SE | p-value | Mean ± SE | p-value | |||||||
| vs. 0 w | vs. pla. | vs. 0 w | vs. pla. | vs. 0 w | vs. pla. | |||||||
| ΔL value (arm) | Cysteine peptide (90 mg) | 15 | 0 | 0.98 ± 0.30 | 0.077 | 0.922 | 1.54 ± 0.33 | 0.002 | 0.938 | 2.63 ± 0.44 | <0.001 | 0.111 |
| Cysteine peptide (45 mg) | 14 | 0 | 1.08 ± 0.18 | 0.003 | 0.996 | 1.74 ± 0.21 | <0.001 | 0.673 | 3.02 ± 0.35 | <0.001 | 0.028 | |
| Placebo | 16 | 0 | 1.11 ± 0.30 | 0.038 | 1.42 ± 0.31 | 0.005 | 1.52 ± 0.45 | 0.002 | ||||
| Task | Group | N | Intervention Period (Week) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 4 | 8 | 12 | |||||||||
| Mean ± SE | p-value | Mean ± SE | p-value | Mean ± SE | p-value | |||||||
| vs.0 w | vs. pla. | vs.0 w | vs. pla. | vs.0 w | vs. pla. | |||||||
| ΔL value (face) | Cysteine peptide (90 mg) | 15 | 0 | 1.99 ± 0.37 | <0.001 | 0.614 | 2.75 ± 0.45 | <0.001 | 0.452 | 3.86 ± 0.46 | <0.001 | 0.439 |
| Cysteine peptide (45 mg) | 14 | 0 | 2.02 ± 0.50 | 0.006 | 0.660 | 2.99 ± 0.49 | <0.001 | 0.709 | 3.53 ± 0.55 | <0.001 | 0.761 | |
| Placebo | 16 | 0 | 2.48 ± 0.40 | <0.001 | 3.44 ± 0.42 | <0.001 | 3.09 ± 0.48 | <0.001 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uchida, Y.; Kaneda, T.; Ono, M.; Matsuoka, M.; Nakamura, U.; Ishida, A.; Yamasaki, Y.; Takeo, H.; Sakurai, T. The Effect of Cysteine Peptide Ingestion on Skin Brightness, a Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Human Clinical Trial. Cosmetics 2023, 10, 72. https://doi.org/10.3390/cosmetics10030072
Uchida Y, Kaneda T, Ono M, Matsuoka M, Nakamura U, Ishida A, Yamasaki Y, Takeo H, Sakurai T. The Effect of Cysteine Peptide Ingestion on Skin Brightness, a Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Human Clinical Trial. Cosmetics. 2023; 10(3):72. https://doi.org/10.3390/cosmetics10030072
Chicago/Turabian StyleUchida, Yoshiaki, Tomomi Kaneda, Mio Ono, Masao Matsuoka, Utano Nakamura, Akiko Ishida, Yoshimitsu Yamasaki, Hiroki Takeo, and Takanobu Sakurai. 2023. "The Effect of Cysteine Peptide Ingestion on Skin Brightness, a Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Human Clinical Trial" Cosmetics 10, no. 3: 72. https://doi.org/10.3390/cosmetics10030072
APA StyleUchida, Y., Kaneda, T., Ono, M., Matsuoka, M., Nakamura, U., Ishida, A., Yamasaki, Y., Takeo, H., & Sakurai, T. (2023). The Effect of Cysteine Peptide Ingestion on Skin Brightness, a Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Human Clinical Trial. Cosmetics, 10(3), 72. https://doi.org/10.3390/cosmetics10030072





