Abstract
Oenanthe javanica (OJ) is a perennial herb that grows wildly or is cultivated in Asia, and it is used as food or in traditional medicine. The antioxidant and anti-inflammatory effects of OJ-derived materials have been extensively explored previously, but their effects on the cytotoxicity of air pollution are currently unknown. Therefore, the present study aimed to evaluate the effect of the hot water extract of OJ on atmospheric particulate matter 10 (PM10)-induced cytotoxicity and oxidative damage in human HaCaT keratinocytes, and to identify its active ingredient and mechanism of action. When the hot water extract of OJ was divided into methylene chloride, ethyl acetate (EA), n-butanol (BA), and water fractions, caffeic acid was enriched in the EA fraction and chlorogenic acid was enriched in the BA fraction. PM10 increased reactive oxygen species (ROS) production, lipid peroxidation, protein carbonylation, and inflammatory prostaglandin (PG) E2 production in cells. The BA fraction reduced the PM10-induced ROS production in cells more effectively than the total extract and other solvent fractions. Chlorogenic acid was more effective in reducing ROS levels than caffeic acid and N-acetyl cysteine (NAC). Chlorogenic acid attenuated the increase in lipid peroxidation and the PG E2 production of cells due to PM10 exposure. Of the genes involved in PG E2 production, phospholipase A2 group IVA (PLA2G4A), Prostaglandin-endoperoxide synthase 1 (PTGS1), and 2 (PTGS2) were transcriptionally up-regulated by PM10, whereas phospholipase A2 group IIA (PLA2G2A) was down-regulated and prostaglandin E synthetase 1 (PTGES1) and 2 (PTGES2) were a little altered. The PM10-induced increase in PLA2G4A mRNA was alleviated by chlorogenic acid and NAC. Accordingly, PM10 increased the expression levels of cytosolic phospholipase A2 (cPLA2) protein and its phosphorylated form, which were attenuated by chlorogenic acid and NAC. Thus, chlorogenic acid may attenuate the PM10-induced PG E2 production through the suppression of PLA2G4A mRNA and cPLA2 protein expressions. This study suggests that chlorogenic acid contained in OJ extract may help alleviate the oxidative damage to and inflammatory responses of the skin cells due to exposure to air pollutants.
1. Introduction
Air pollution has a detrimental effect on skin health. The particulate matter (PM) that is suspended in the atmosphere with an approximate diameter of less than 10 and 2.5 μm is called PM10 and PM2.5, respectively [1]. PM is a mixture of various organic compounds, heavy metals, and biological constituents [1,2]. PM is capable of penetrating the skin through pores and weak skin barriers [3,4]. PM can exacerbate various skin diseases, such as atopic dermatitis, acne, and psoriasis [5,6]. PM can also cause premature skin aging [7] and hyperpigmentation [8]. Combined exposure to PM and ultraviolet (UV) rays exerts a synergistic harmful effect, accelerating skin photo-aging and cancer development [9,10].
PM exposure stimulates Ca2+ signaling and increases the production of reactive oxygen species (ROS), such as superoxide radical (O2•−), hydrogen peroxide (H2O2), and hydroxyl radical (•OH), which mediate the oxidative damage to and inflammatory responses of cells [11,12]. ROS can be generated in the aryl hydrocarbon receptor-mediated metabolism of organic components of PM and the chemical reactions catalyzed by its transition metal components [13,14]. PM induces the activation of the NADPH oxidase family, including dual oxidase 2, which plays a critical role in the production of ROS and the maintenance of redox balance in cells [12,15].
PM-derived ROS can cause the activation of the mitogen-activated protein kinase (MAPK) family, including extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 kinase, and the stimulation of the nuclear factor-kappa B (NF-κB) signaling pathway, leading to the activation of redox-sensitive transcription factors [16]. This increases the gene expression and secretion of the inflammatory eicosanoid mediators, such as prostaglandin (PG) E2 [17,18], and the inflammatory cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, and IL-8 [19]. PM stimulates the expression of matrix metalloproteinases (MMPs), causing the loss of the extracellular matrix, including collagen [20], and decreases the expression of filaggrin, causing the loss of the skin barrier function [21,22]. Thus, there is a need for antioxidants that alleviate the adverse effects of PM.
Plant-derived phenolic compounds may function as antioxidants in cells that alleviate oxidative stress due to PM10 [23]. Phenolic antioxidants can prevent ROS production, scavenge exiting ROS, or enhance the antioxidant capacity of cells by regulating gene expression [24]. We have shown that extracts derived from various plants, such as green tea, pomegranate, Siegesbeckiae herba, propolis, and Ecklonia cava, and their phenolic compounds, such as (−)-epigallocatechin-3-gallate, punicalagin, chlorogenic acid, ferulic acid, and dieckol, reduce ROS production, lipid peroxidation, and inflammatory responses in HaCaT cells exposed to PM10 [18,19,25,26].
Oenanthe javanica (Blume) DC, abbreviated to OJ herein, is generally called water dropwort, water parsley, water celery, or ‘minari’ in Korean. It is a dicotyledonous perennial plant that grows in wetlands or watersides and is cultivated for food or medicinal purposes [27,28]. OJ contains a substantial amount of phenolic compounds, with chlorogenic acid being in the highest concentration, followed by caffeic acid [29,30,31]. The extract of OJ alleviates the hepatic steatosis and oxidative damage induced by ethanol poisoning [32] via acting as an antioxidant with free radical scavenging capabilities and reducing power [29], enhancing cellular antioxidant enzymes, such as superoxide dismutase (SOD)-1, SOD-2, catalase, and glutathione peroxidase [33], or accelerating the metabolic removal of ethanol [34]. Its anti-inflammatory effects have also been demonstrated in RAW 264.7 macrophages, which were stimulated by lipopolysaccharide [35,36] in the skin of mice irradiated with UV rays [37], and in mice with sodium dextran sulfate-induced colitis [31].
Although the antioxidant and anti-inflammatory effects of OJ-derived materials have been explored in various experimental models, no previous studies have reported their protective effects against the cytotoxicity of air pollution. Therefore, the present study aimed to evaluate the effect of the hot water extract of OJ on PM10-induced cytotoxicity and oxidative damage in human HaCaT keratinocytes, and to identify its active ingredient and mechanism of action. The results of this study demonstrated that chlorogenic acid, a main component of OJ, attenuated ROS production, lipid peroxidation, and protein carbonylation in PM10-stimulated cells. It also attenuated PM10-induced PG E2 production in cells, which was associated with the suppressed expression of cytosolic phospholipase A2 (cPLA2). Thus, the chlorogenic acid contained in OJ extract is suggested to help alleviate the oxidative damage to and inflammatory responses of the skin cells caused by exposure to air pollutants.
2. Materials and Methods
2.1. Reagents
Chlorogenic acid, caffeic acid, standardized fine dust (PM10-like, European standard ERM-CZ120), 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT), 2’,7’-dichlorodihydrofluorescein diacetate (DCFH-DA), 2-thiobarbituric acid, and 1,1,3,3-tetramethoxypropane (TMP) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Preparation of the Hot Water Extract and Fractions of OJ
Dried Leaves and stems of OJ (Oenanthe javanica (Blume) DC.) purchased from Cheongmyeongyakcho (Chungbuk, Republic of Korea) (http://www.good1075.com, accessed on 31 March 2023) were used for extraction. The plant material (100 g) was extracted with water (600 mL) at 90 °C for 2 h. After filtering, the filtrate was concentrated to dryness using a rotary evaporator (Eyela, Bohemia, NY, USA) under reduced pressure, and the total extract of OJ (3.702 g) was obtained. The total extract was suspended in water (100 mL) and transferred to a separating funnel where the aqueous suspension was partitioned with an equal volume of methylene chloride (MC), ethyl acetate (EA), and n-butyl alcohol (BA), sequentially [34]. The evaporation of each solvent fraction under reduced pressure yielded an MC fraction (0.168 g), EA fraction (0.181 g), BA fraction (0.555 g), water (WT) fraction (13.781 g), and insoluble materials (8.096 g). Each fraction was re-dissolved in 30% (v/v) aqueous ethanol at a 10% concentration and kept at −20 °C until use.
2.3. High-Performance Liquid Chromatography with Photodiode Array Detection (HPLC-DAD)
The HPLC-DAD profiles of the total extract of OJ and its solvent fractions were compared using a Waters Alliance HPLC system (Waters, Milford, MA, USA) equipped with an e2695 separation module and a 2996 photodiode array detector [30]. Chromatographic separation was performed using a Hector-M C18 column (4.6 mm × 250 mm, 5 μm) (RS Tech Co. Daejeon, Republic of Korea) as the stationary phase, and a mixture of 0.1% phosphoric acid (solvent A) and acetonitrile (solvent B) as the mobile phase. The mobile phase composition was changed as follows: 0–30 min, a linear gradient from 0 to 100% B; 30–40 min, 100% B; 40–45 min, a linear gradient from 100 to 0% B. The flow rate of the mobile phase was set at 0.6 mL min−1. Samples were diluted with water, filtered, and injected into HPLC in 10 μL aliquots.
2.4. Cell Culture and Treatments
An immortalized human keratinocyte HaCaT cell line (CLS Cell Lines Service GmbH, Eppelheim, Germany) was cultured as previously described [26]. The growth medium consisted of DMEM/F-12 medium (GIBCO-BRL, Grand Island, NY, USA), 10% fetal bovine serum, antibiotics (100 U mL−1 penicillin, 100 µg mL−1 streptomycin, 0.25 µg mL−1 amphotericin B), and 10 μg mL−1 of hydrocortisone. In each experiment, cells were cultured in 96-well, 12-well, or 6-well culture plates (SPL Life Sciences, Pocheon, Republic of Korea) for 24 h and then subjected to various treatments. PM10 was suspended in phosphate-buffered saline (PBS) at 100 times the final treatment concentrations. Other test materials were diluted or dissolved in 30% (v/v) aqueous ethanol at 100 times the final treatment concentrations. Cells were treated with the total extract of OJ, its fractions, compounds, and PM10 individually, in combination with each other at the specified concentrations, or with vehicles for the indicated time.
2.5. Cell Viability Assay
Cell viability was assessed by measuring the reduction of MTT to water-insoluble formazan in the viable cells [38,39]. Cells were seeded on 96-well culture plates (4 × 103 cells/well) and cultured in a growth medium (200 μL) for 24 h. After various treatments with the total extract of OJ, its fractions, compounds, and PM10 for 48 h, the cells were washed with PBS and incubated in 100 μL of growth medium containing 1 mg mL−1 of MTT at 37 °C for 2 h. After discarding the medium, cells were washed with PBS, and the formazan dye that had accumulated inside the cells was extracted with 100 μL of dimethyl sulfoxide per well. Then, the absorbance was measured at 570 nm using a Spectrostar Nano microplate reader (BMG LABTECH GmbH, Ortenberg, Germany).
2.6. Cellular Reactive Oxygen Species (ROS) Production Assay
Cellular ROS production was assessed by measuring the oxidation of DCFH-DA to a fluorescent compound inside the cells [40]. The cells were seeded on 12-well culture plates (1.4 × 105 cells/well) and cultured in a growth medium (1 mL) for 24 h. Cells were treated with 10 μM DCFH-DA for 30 min. The spent medium was replaced with the growth medium containing various test materials, which was followed by the incubation of cells for 60 min. Cells were then washed with PBS and the fluorescing cells were observed under a LEICA DMI3000 B microscope (Leica Microsystems GmbH, Wetzlar, Germany). For a quantitative analysis of the fluorescing compound formed inside the cells, the washed cells were extracted with 150 µL of the cell lysis buffer (1% sodium dodecyl sulfate, 20 mM of Tris-Cl, 2.5 mM of EDTA, pH of 7.5), followed by centrifugation at 14,500× g for 15 min. After 200 µL aliquots of the supernatants were transferred to black 96-well plates (SPL Life Sciences, Pocheon, Republic of Korea), the fluorescence intensity was measured at an excitation wavelength of 485 nm and an emission wavelength of 538 nm with a Gemini EM fluorescence microplate reader (Molecular Devices, Sunnyvale, CA, USA).
2.7. Cellular Lipid Peroxidation Assay
Cellular lipid peroxidation was assessed by measuring the production of malondialdehyde (MDA) [41]. Cells were seeded on 6-well culture plates (2 × 105 cells per well) and cultured in the growth medium (2 mL) for 24 h. Cells were then treated with various test materials, such as the total extract of OJ, its fractions, compounds, and PM10, for 48 h. Then, cells were washed with PBS and harvested using 150 µL of the cell lysis buffer (1% sodium dodecyl sulfate, 20 mM of Tris-Cl, 2.5 mM of EDTA, pH of 7.5). The reaction mixture (500 μL) consisting of 100 μL of cell lysate (ca. 200 μg protein), 50 μL of 1.0% m-phosphoric acid, and 350 μL of 0.9% 2-thiobarbituric acid was heated at 95 °C in a water bath for 45 min. The reaction was also run with 100 to 400 nM of TMP as a donor of MDA to construct a standard curve. After the reaction mixture was cooled to room temperature, 500 μL of BA was added to the reaction mixture, mixed vigorously, and centrifuged at 14,500× g for 15 min to separate the mixture into a BA layer and an aqueous layer. After 200 µL of the BA layer was transferred to black 96-well plates, the fluorescence intensity was measured at an excitation wavelength of 544 nm and an emission wavelength of 590 nm using a Gemini EM fluorescence microplate reader. Data are presented as MDA levels normalized to protein content.
2.8. Protein Carbonylation Assay
Protein carbonylation was measured using a fluorometric assay kit (ab235631) from Abcam (Cambridge, MA, USA). The whole cell lysate (75 μg of protein in 50 μL) was mixed with 50 µL of 0.2 mM fluorescein-5-thiosemicarbazide (FTC) fluorophore in an assay buffer in microcentrifuge tubes, followed by incubation overnight at 25 °C in the dark. Then, proteins were precipitated by adding 200 µL of ice-cold 20% trichloroacetic acid solution, keeping the tubes on ice for 10 min. The tubes were centrifuged at 14,500× g for 15 min and the supernatant was removed by suction aspiration. The pellet was washed with 200 µL of ice-cold isopropanol 3 times and then dissolved in 50 µL of 6 M guanidine solution by heating it at 50 °C for 1 h. The tubes were cooled to 50 °C and the samples were diluted with 70 µL of sample dilution buffer. Aliquots of the diluted samples (100 µL) were transferred to a 96-well plate and the fluorescence was measured at a 485 nm excitation and at a 535 nm emission using a Gemini EM fluorescence microplate reader. Protein carbonyl contents were estimated by comparing a standard curve that was prepared using FTC fluorophore. The protein carbonyl contents were normalized to the protein content.
2.9. Enzyme-Linked Immunosorbent Assay (ELISA) for PG E2
The secreted level of PG E2 protein in the culture medium was measured using a PG E2 ELISA kit (Enzo Life Sciences, Inc., Farmingdale, NY, USA). In this colorimetric competitive enzyme immunoassay, a fixed amount of PG E2-phosphatase conjugate is used as a PG E2 tracer, whose binding to the PG E2 monoclonal antibody is inversely proportional to the amount of PG E2 derived from the sample. Cells were seeded on 6-well culture plates (8 × 104 cells per well) and cultured in a growth medium (1 mL) for 24 h. Cells were then treated with various test materials for another 48 h. The conditioned culture medium was centrifuged at 14,500× g for 15 min, and the supernatants were used as samples in the assay. Briefly, 100 µL samples of the culture medium or a standard PG E2 solution were transferred to microtiter plates coated with a goat antibody specific to mouse IgG. To each well, 50 µL of assay buffer, 50 µL of PG E2 tracer solution, and 50 µL of PG E2 monoclonal antibody solution were added, and the mixtures were incubated at 25 °C for 2 h. The well was rinsed 3 times with wash buffer and 200 µL of p-nitrophenyl phosphate substrate solution was added to initiate the phosphatase reaction. After 45 min of incubation at 25 °C, absorbances were measured at 405 nm with a SPECTROstar Nano microplate reader (BMG LABTECH GmbH). The amount of PG E2 in the samples was estimated from a standard curve.
2.10. Quantitative Reverse Transcriptase-Polymerase Chain Reaction (qRT-PCR) Analysis
The mRNA level was analyzed by qRT-PCR, as described in a previous study [18]. Cells were treated with various test materials for 24 h and the total cellular RNA from the treated cells was used in the preparation of complementary DNA. The qRT-PCR was run using the gene-specific primers, whose nucleotide sequences are shown in Table 1. The mRNA level of each target gene was compared to that of the internal reference, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), using the comparative Ct method [42]. Data are presented as a percentage of the control group.

Table 1.
Forward (F) and reverse (R) primers for the quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR).
2.11. Western Blotting
The primary antibodies for cPLA2 (#2832) and phospho-cPLA2 (Ser505) (#2831) were purchased from Cell Signaling Technology (Danvers, MA, USA), and β-actin (#47778) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The anti-rabbit IgG (#7074) secondary antibody was purchased from Cell Signaling Technology. Protein samples were denatured by mixing them with a Laemmli sample buffer and heating them at 95 °C for 5 min. Equal amounts of proteins (20 μg) were resolved using 10% SDS-polyacrylamide gel electrophoresis at 80 V and were then electrically transferred to a polyvinylidene difluoride membrane (Amersham Pharmacia, Little Chalfont, UK) at 4 °C overnight. After blocking the incubation of the membrane with TBST (137 mM of sodium chloride, 20 mM of Tris, 0.1% Tween 20, pH of 7.6.) containing 5% skim milk, it was incubated with the primary antibody in TBST containing 5% skim milk at 4 °C overnight; this was followed by incubation with the secondary antibody in TBST containing 5% skim milk at room temperature (25 °C) for 1 h. The target protein bands were visualized with a chemiluminescence method using the picoEPD Western Reagent kit (ELPIS-Biotech, Daejeon, Republic of Korea). The captured blot images were analyzed using the Image J program provided by the U.S. National Institute of Health (Bethesda, MD, USA).
2.12. Statistical Analysis
Experimental data were analyzed using SigmaStat v.3.11 software (Systat Software Inc., San Jose, CA, USA) and presented as mean ± standard deviation (SD). The presence of group means that were significantly different from other groups was determined using a one-way analysis of variance (ANOVA) at the p < 0.05 level. Then, Duncan’s multiple range test was subsequently run to compare all groups to each other.
3. Results
The dried leaves and stems of OJ were used in the preparation of the total hot water extract of OJ and its MC, EA, BA, and WT fractions. HPLC-DAD analysis was performed to compare the overall composition of the total OJ extract and its solvent fractions. Chlorogenic acid and caffeic acid were used as the standards, as these compounds are known to be the main components of OJ [29,30,31]. As shown in Figure 1, two of the main peaks observed in the total OJ extract and its solvent fractions were identified as chlorogenic acid and caffeic acid by comparing their retention times and absorption spectra with those of the standards. The total OJ extract contained a substantial level of chlorogenic acid, while its caffeic acid content was relatively lower. Chlorogenic acid was highly enriched in the BA fraction and caffeic acid was enriched in the EA fraction, and their contents were relatively lower in the MC fraction and the WT fraction.
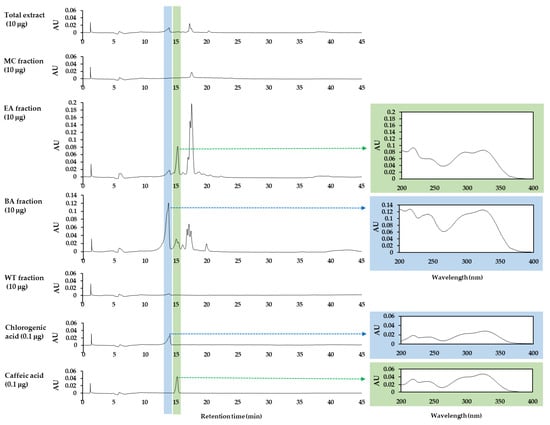
Figure 1.
High-performance liquid chromatography with photodiode array detection (HPLC-DAD) analysis of the total extract Oenanthe javanica (Blume) DC, abbreviated OJ, and its MC, EA, BA, and WT fractions. Authentic chlorogenic acid and caffeic acid were used as the standards for comparing the retention times and absorption spectra. Chromatograms at 330 nm and UV absorption spectra of the indicated peaks are shown.
As shown in Figure 2, in quantities up to 300 μg mL−1, PM10 decreased the viability of HaCaT cells in a concentration-dependent manner. The effects of the total OJ extract and each fraction on cell viability were evaluated at 10–1000 μg mL−1 in the absence and presence of 200 μg mL−1 PM10. The total extract of OJ significantly reduced cell viability at concentrations above 300 μg mL−1 in the absence of PM10, but had no additional effect on cell viability in the presence of PM10. None of the solvent fractions showed significant cytotoxicity up to 100 μg mL−1, but they did show reduced cell viability at 300 μg mL−1 in the order of the EA fraction, MC fraction, BA fraction, and WT fraction. In the presence of 200 μg mL−1 of PM10, the water fraction had no additional effect on cell viability, but the other fractions enhanced the cytotoxic effects of PM10 at their high concentrations (300 or 1000 μg mL−1). In the following experiments, cells were treated with the total OJ extract or each fraction at 30 and/or 100 μg mL−1 within non-cytotoxic ranges.
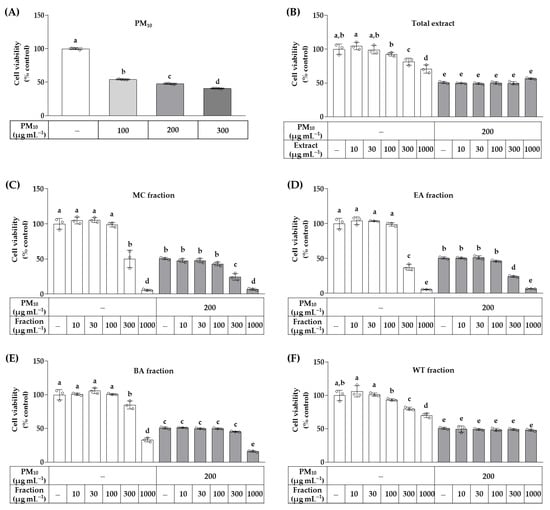
Figure 2.
Effects of the total OJ extract and its solvent fractions on the viability of HaCaT keratinocytes exposed to particulate matter 10 (PM10). In (A), cells were treated with PM10 at different concentrations for 48 h. In (B–F), cells were treated with the total OJ extract (B), MC fraction (C), EA fraction (D), BA fraction €, or WT fraction (F) at the specified concentrations, alone or in combination with PM10 (200 μg mL−1), for 48 h. Cell viability was determined using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT). Data are presented as mean ± SD (n = 4 for A, n = 3 for B–F). Duncan’s multiple range test was performed to compare all group means to each other. Groups that do not share the same lowercase alphabet letters (a–e) are considered to have significantly different means at the p < 0.05 level.
The effects of chlorogenic acid and caffeic acid, identified as major components of OJ, on cell viability were examined at 10–1000 μM in the absence and presence of 200 μg mL−1 of PM10, and were compared with that of NAC, a positive control. As shown in Figure 3, caffeic acid decreased cell viability at concentrations above 100 μM, and chlorogenic acid and NAC decreased cell viability at concentrations above 300 μM. Chlorogenic acid and caffeic acid at 1000 μM enhanced the cytotoxic effects of PM10, but NAC did not. In the following experiments, cells were treated with each of these compounds at 30 and/or 100 μM.
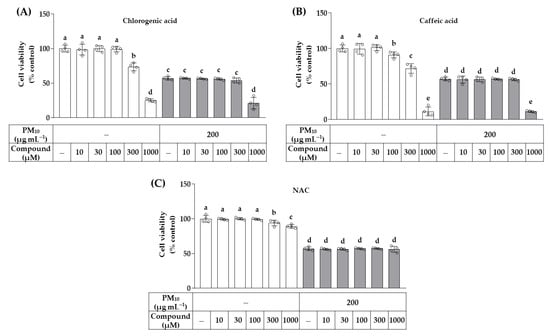
Figure 3.
Effects of chlorogenic acid, caffeic acid, and N-acetyl cysteine (NAC) on the viability of HaCaT keratinocytes exposed to PM10. Cells were treated with chlorogenic acid (A), caffeic acid (B), or NAC (C) at the specified concentrations, alone or in combination with PM10 (200 μg mL−1), for 48 h. Data are presented as mean ± SD (n = 3). Groups that do not share the same lowercase alphabet letters (a–e) are considered to have significantly different means at the p < 0.05 level.
The effects of the total OJ extract, solvent fractions, and compounds on PM10-induced ROS production were examined using a fluorescent probe. Cells were treated with the total extract and each fraction at 30 and 100 μg mL−1, or with chlorogenic acid, caffeic acid, and NAC at 30 and 100 μM in the absence and presence of 200 μg mL−1 PM10. As shown in Figure 4, PM10 at 100–300 μg mL−1 increased ROS production in a concentration-dependent manner. The total extract, MC fraction, EA fraction, BA fraction, and WT fraction at 30–100 μg mL−1 did not affect ROS production in the absence of PM10. However, the BA fraction (30–100 μg mL−1), EA fraction (30–100 μg mL−1), MC fraction (30–100 μg mL−1), and WT fraction (100 μg mL−1) attenuated the PM10-induced ROS production, in that order. Chlorogenic acid, caffeic acid, and NAC at 30–100 μM did not affect the basal level of ROS production, but reduced the PM10-induced ROS production, in that order. The images of cells fluorescing due to ROS confirmed that the basal ROS production was not affected by the total OJ extract (100 μg mL−1), BA fraction (100 μg mL−1), chlorogenic acid (100 μM), and NAC (100 μM), but that the PM10-induced ROS production was reduced by chlorogenic acid (100 μM), BA fraction (100 μg mL−1), and NAC (100 μM), in that order.
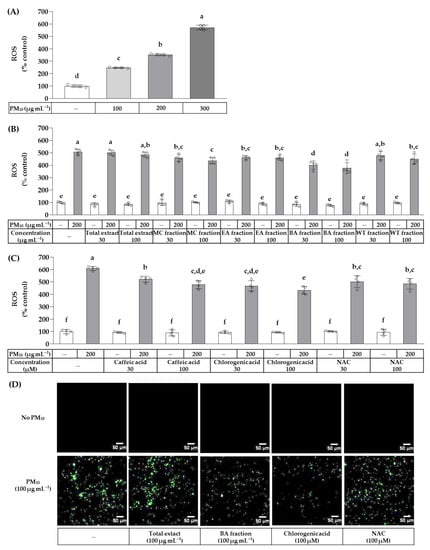
Figure 4.
Effects of the total OJ extract, its solvent fractions, chlorogenic acid, caffeic acid, and NAC on the production of reactive oxygen species (ROS) in HaCaT keratinocytes exposed to PM10. In (A), after pre-labeling with 2’,7’-dichlorodihydrofluorescein diacetate (DCFH-DA) for 30 min, cells were treated with PM10 at different concentrations for 60 min. In (B,C), the pre-labeled cells were treated with the total OJ extract, its solvent fractions, or compounds at the specified concentrations, alone or in combination with PM10 (200 μg mL−1), for 60 min. Typical images of cells fluorescing due to ROS production are shown in (D). The change in the fluorescence intensity due to cellular ROS production was determined. Data are presented as mean ± SD (n = 4). Groups that do not have the same lowercase alphabet letters (a–f) are considered to have significantly different means at the p < 0.05 level.
The effects of the total OJ extract, BA fraction, chlorogenic acid, and NAC on PM10-induced lipid peroxidation were examined by measuring MDA production. As shown in Figure 5, PM10 at 100–300 μg mL−1 increased lipid peroxidation in a concentration-dependent manner. The total OJ extract (100 μg mL−1), BA fraction (100 μg mL−1), chlorogenic acid (100 μM), and NAC (100 μM) did not affect the basal level of lipid peroxidation in the absence of PM10. The BA fraction (100 μg mL−1) and chlorogenic acid (100 μM) suppressed the lipid peroxidation induced by PM10 (200 μg mL−1), whereas the total OJ extract (100 μg mL−1) and NAC (100 μM) had no significant effects. The increases in the MDA levels due to PM10 exposure were smaller in groups treated with the BA fraction (100 μg mL−1) or chlorogenic acid (100 μM) compared to the control group.
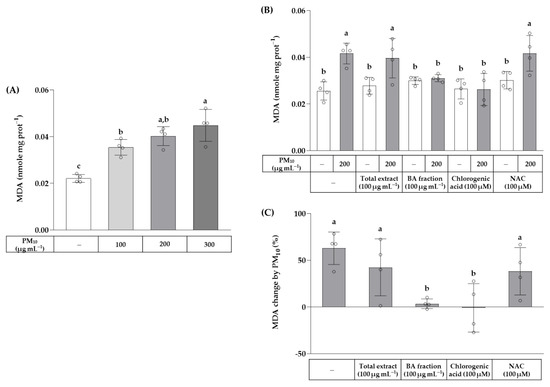
Figure 5.
Effects of the total OJ extract, its BA fraction, chlorogenic acid, and NAC on lipid peroxidation in HaCaT keratinocytes exposed to PM10. In (A), cells were treated with PM10 at different concentrations for 48 h. In (B,C), cells were treated with the total OJ extract (100 μg mL−1), BA fraction (100 μg mL−1), chlorogenic acid (100 μM), or NAC (100 μM), alone or in combination with PM10 (200 μg mL−1), for 48 h. Lipid peroxidation was determined by measuring malondialdehyde (MDA) levels in whole-cell lysates and the values were normalized to the protein contents (nmole mg protein−1) (B). The % change in the MDA values due to PM10 exposure in each group was calculated using the following equation: change (%) = (α − M)/M × 100, where α is a value in the presence of PM10 and M is the mean value of each group in the absence of PM10 (C). Data are presented as mean ± SD (n = 4). Groups that do not share the same lowercase alphabet letters (a–c) are considered to have significantly different means at the p < 0.05 level.
The effects of the total OJ extract, BA fraction, chlorogenic acid, and NAC on protein carbonylation were examined in HaCaT cells exposed to PM10. As shown in Figure 6, PM10 at 100–300 μg mL−1 increased the protein carbonyl content in a concentration-dependent manner. The total OJ extract (100 μg mL−1), BA fraction (100 μg mL−1), chlorogenic acid (100 μM), and NAC (100 μM) slightly increased the protein carbonyl content of cells in the absence of PM10, but attenuated an increase in the protein carbonyl contents due to the exposure of cells to PM10 (200 μg mL−1).
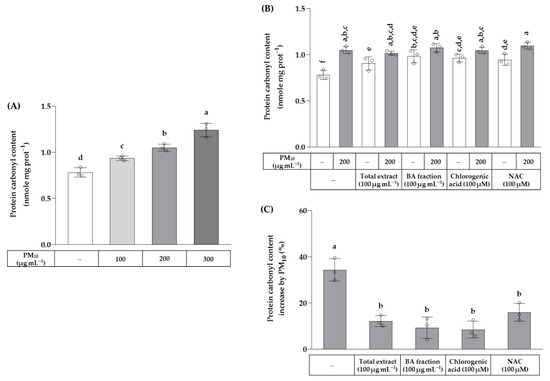
Figure 6.
Effects of the total OJ extract, its BA fraction, chlorogenic acid, and NAC on the protein carbonyl content in HaCaT keratinocytes exposed to PM10. In (A), cells were treated with different concentrations of PM10. In (B,C), cells were treated with the total OJ extract (100 μg mL−1), BA fraction (100 μg mL−1), chlorogenic acid (100 μM), and NAC (100 μM), alone or in combination with PM10 (200 μg mL−1), for 48 h. Protein carbonyl contents were normalized to the protein contents and presented in nmole mg protein−1 (B). The % change in the protein carbonyl contents due to PM10 exposure in each group was calculated using the following equation: change (%) = (α − M)/M × 100, where α is the value in the presence of PM10 and M is the mean value of each group in the absence of PM10 (C). Data are presented as mean ± SD (n = 3). Groups that do not share the same lowercase alphabet letters (a–f) are considered to have significantly different means at the p < 0.05 level.
The effects of the total OJ extract, BA fraction, chlorogenic acid, and NAC on the secreted level of PG E2 in HaCaT cells exposed to PM10 were examined using ELISA. As shown in Figure 7, PM10 at 100–300 μg mL−1 increased the secreted level of PG E2 up to 14-fold in a concentration-dependent manner. The total OJ extract (100 μg mL−1), BA fraction (100 μg mL−1), chlorogenic acid (100 μM), and NAC (100 μM) did not affect the secreted level of PG E2 in the absence of PM10. The BA fraction (100 μg mL−1) and chlorogenic acid (100 μM) inhibited the increase in the secreted level of PG E2 in cells stimulated by PM10 (200 μg mL−1), whereas the total OJ extract (100 μg mL−1) and NAC (100 μM) had no significant effects.
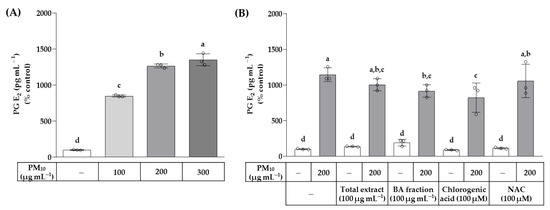
Figure 7.
Effects of the total OJ extract, its BA fraction, chlorogenic acid, and NAC on the secreted level of prostaglandin (PG) E2 in HaCaT keratinocytes exposed to PM10. In (A), cells were treated with different concentrations of PM10. In (B), cells were treated with the total OJ extract (100 μg mL−1), BA fraction (100 μg mL−1), chlorogenic acid (100 μM), and NAC (100 μM), alone or in combination with PM10 (200 μg mL−1), for 48 h. The secreted level of PG E2 was determined using a PG E2 enzyme-linked immunosorbent assay (ELISA). Data are presented as mean ± SD (n = 3). Groups that do not share the same lowercase alphabet letters (a–d) are considered to have significantly different means at the p < 0.05 level.
The effects of the total OJ extract, BA fraction, chlorogenic acid, and NAC on the mRNA expression levels of the genes involved in PG E2 production were examined. The qRT-PCR was run to analyze the mRNA expression levels of phospholipase A2 group IIA (PLA2G2A), phospholipase A2 group IVA (PLA2G4A), prostaglandin-endoperoxide synthase 1 (PTGS1), prostaglandin-endoperoxide synthase 2 (PTGS2), prostaglandin E synthetase 1 (PTGES1), and prostaglandin E synthetase 2 (PTGES2). As shown in Figure 8, PM10 at 200 μg mL−1 decreased the mRNA expression level of PLA2G2A, but increased that of PLA2G4A. The PM10-induced increase in PLA2G4A mRNA was reduced by chlorogenic acid (100 μM) and NAC (100 μM), whereas the total OJ extract (100 μg mL−1) and BA fraction (100 μg mL−1) had no effects. PM10 increased the mRNA expression levels of PTGS1 and PTGS2; these changes were not affected by being treated with the total OJ extract, BA fraction, chlorogenic acid, or NAC. PM10 and other test materials had little effect on the mRNA expression levels of PTGES1 and PTGES2.
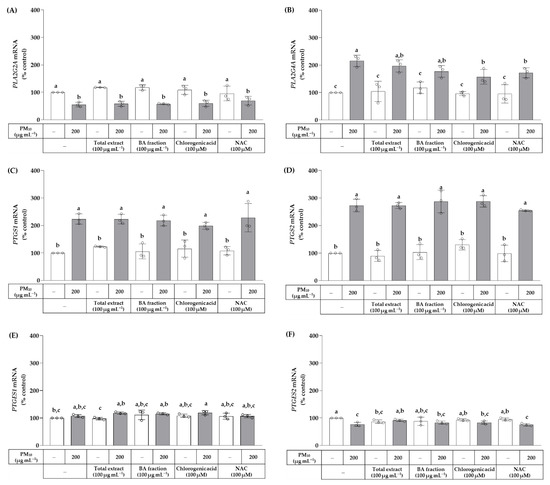
Figure 8.
Effects of the total OJ extract, its BA fraction, chlorogenic acid, and NAC on the mRNA expression levels of phospholipase A2 group IIA (PLA2G2A) (A), phospholipase A2 group IVA (PLA2G4A) (B), prostaglandin-endoperoxide synthase 1 (PTGS1) (C), prostaglandin-endoperoxide synthase 2 (PTGS2) (D), prostaglandin E synthetase 1 (PTGES1) (E), and prostaglandin E synthetase 2 (PTGES2) (F) in HaCaT keratinocytes exposed to PM10. Cells were treated with the total extract (100 μg mL−1), BA fraction (100 μg mL−1), chlorogenic acid (100 μM), and NAC (100 μM), alone or in combination with PM10 (200 μg mL−1), for 24 h. The mRNA expression level of each gene was analyzed by the quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) and normalized to that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Data are presented as % of control (means ± SD, n = 3). Groups that do not share the same lowercase alphabet letters (a–c) have statistically different means at the p < 0.05 level.
The effects of the total OJ extract, BA fraction, chlorogenic acid, and NAC on the protein expression levels of cPLA2 and its phosphorylated active form (phospho-cPLA2) were examined by Western blotting. As shown in Figure 9, PM10 increased the protein levels of cPLA2 and phospho-cPLA2. The total OJ extract (100 μg mL−1), BA fraction (100 μg mL−1), chlorogenic acid (100 μM), and NAC (100 μM) did not affect the basal levels of the cPLA2 protein. However, the BA fraction, chlorogenic acid, and NAC lowered the levels of cPLA2 protein under PM10-exposed conditions. In addition, chlorogenic acid and NAC lowered the levels of phospho-cPLA2 protein under basal and PM10-exposed conditions.
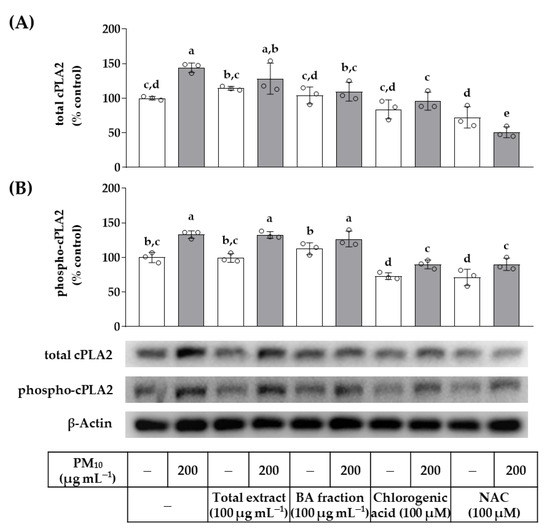
Figure 9.
Effects of the total OJ extract, its BA fraction, chlorogenic acid, and NAC on the protein levels of cytosolic phospholipase A2 (cPLA2) and phospho-cPLA2 in HaCaT keratinocytes under basal and PM10-exposed conditions. Cells were treated with the total OJ extract (100 μg mL−1), BA fraction (100 μg mL−1), chlorogenic acid (100 μM), and NAC (100 μM), alone or in combination with PM10 (200 μg mL−1), for 48 h. The protein levels of total cPLA2 (A) and phospho-cPLA2 (B) were determined by Western blotting and compared to that of β-actin. Representative blots are shown. Data are presented as percentages of the control (mean ± SD, n = 3). Duncan’s multiple range test was performed to compare all group means to each other. Groups that do not share the same lowercase alphabet letters (a–e) have significantly different means at the p < 0.05 level.
4. Discussion
This study, for the first time, has examined the antioxidant and anti-inflammatory effects of the total OJ extract and its solvent fractions on skin cells exposed to PM10. Neither the total extract nor the solvent fraction of OJ significantly reversed HaCaT cell death due to a high level of PM10 (200 μg mL−1), but some solvent fractions within a non-cytotoxic range significantly reduced the PM10-induced cellular ROS production. When the effect of reducing ROS production was compared between the solvent fractions, the BA fraction was more effective than the EA fraction and other fractions.
The BA fraction contained chlorogenic acid (49.0 mg g−1), whereas the EA fraction contained caffeic acid (18.7 mg g−1) as a major phenolic component. These two phenolic compounds did not significantly alleviate the cell death caused by PM10, but effectively reduced the cellular ROS production, and their effects were comparable to that of NAC, a positive control antioxidant. Thus, we speculated that chlorogenic acid and caffeic acid might have been the active components of OJ that reduced ROS levels in cells exposed to PM10. Chlorogenic acid showed a stronger effect than the same molar concentration of caffeic acid in the inhibition of cellular ROS production. These results explain why the BA fraction that was rich in chlorogenic acid was more effective than the EA fraction, which was rich in caffeic acid, in reducing cellular ROS levels under PM10 exposure.
Because plant-derived substances may have various effects on cell physiology, it is important to optimize preparations for targeting biological activity. In a previous study comparing the phytochemical composition and antioxidant activity of a 70% ethanol extract of OJ and its solvent fractions, the EA fraction showed a higher content of total phenolic compounds and flavonoids, and a higher free radical scavenging activity against 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical, 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid (ABTS) radical, and hydroxyl radical in vitro, compared to the BA fraction [29]. Another study showed that the BA fraction of the hot water extract of OJ was more effective than other fractions in reducing the blood ethanol concentration after the oral administration of ethanol in ICR mice [34]. In the current study, the BA fraction would be more effective and potentially useful in attenuating oxidative stress in human keratinocytes under PM10-exposed conditions.
Therefore, additional experiments were performed focusing on the antioxidant and anti-inflammatory effects of the BA fraction and its major component, chlorogenic acid. The results showed that the BA fraction (100 μg mL−1) and chlorogenic acid (100 μM) were effective in inhibiting lipid peroxidation and PG E2 secretion in cells exposed to PM10. The results suggest the potential utility of the BA fraction of OJ in attenuating the PM10-induced oxidative damage to and inflammatory responses of cells. It is also suggested that chlorogenic acid may be an active component that is responsible for the antioxidant and anti-inflammatory effects of the BA fraction of the OJ extract.
The arachidonic acid that is released from membrane phospholipids via the enzymatic action of phospholipases A2 (PLA2) is converted to PG G2 and then to PG H2 in a reaction that is catalyzed by prostaglandin-endoperoxide synthases (PTGS), also known as cyclooxygenase (COX); further, the conversion of PG H2 to PG E2 is catalyzed by prostaglandin E synthetases (PEGS) [48,49]. The PLA2G4A gene encodes a cytosolic phospholipase A2 (cPLA2), while the PLA2G2A gene encodes a secretory phospholipase A2 (sPLA2) [43,44]. Interestingly, PM10 decreased the mRNA expression of PLA2G2A and increased that of PLA2G4A. It also increased the mRNA expression of PTGS1 and PTGS2, which encode COX1 and COX2, respectively, but had no significant effects on the mRNA expression of PTGES1 and PTGES2, which encode microsomal prostaglandin E synthetase 1 (mPEGS1) and mPEGS2. This suggests that the increase in PG E2 production by PM10 could be mediated by cPLA2, COX1, and COX2 proteins. Since chlorogenic acid attenuated the expressions of PLA2G4A mRNA and cPLA2 protein induced by PM10, this is considered to be one of chlorogenic acid’s main mechanisms of action regarding its ability to reduce PM10-induced PG E2 production, as depicted in Figure 10.
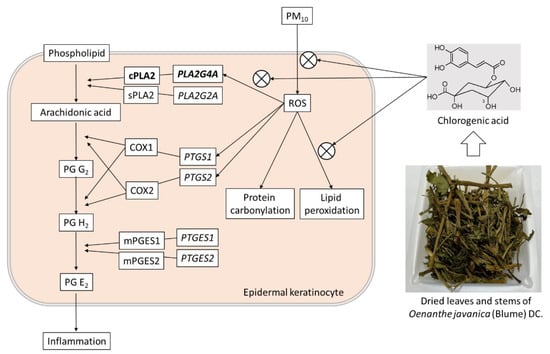
Figure 10.
Antioxidant and anti-inflammatory effects of chlorogenic acid in HaCaT keratinocytes exposed to PM10. PM10 increased ROS production and lipid peroxidation. It also increased PG E2 production through the enhanced expressions of PLA2G4A mRNA and cPLA2 protein. These changes were attenuated (⊗) by chlorogenic acid, a main component of OJ.
Although the ability of chlorogenic acid to reduce the PM10-induced mRNA expression of PTGS1 and PTGS2, which encode COX1 and COX2, respectively, was not observed in the present study, it is worth recalling that chlorogenic acid is a potent inhibitor of COX2 enzyme activity (IC50, 8.1 μM) [50]. Chlorogenic acid could reduce PG E2 production through the direct inhibition of COX-2 enzymatic activity as an additional mechanism of action.
The cPLA2 is activated by airborne agricultural particulate matter and mediates inflammatory responses in human lung epithelial A549 cells [51]. The differential regulation of the gene expression of PLA2G4A versus PLA2G2A by PM10 observed in the present study suggests that each isozyme may play a distinct role under different cellular contexts. The cPLA2 is a calcium-dependent enzyme that is additionally activated by phosphorylation at Ser505 and other sites [52]. In the present study, PM10 increased the total cPLA2 protein and phospho-cPLA2 (Ser505), and these changes were reduced by chlorogenic acid and NAC. A more expanded study is needed to elucidate the differential role of phospholipase A2 isoenzymes in association with the PM10-induced inflammatory reaction.
Previous studies demonstrated the anti-inflammatory effects of OJ extracts on the attenuation of the NF-kB-mediated expression of COX2, and on the secretion of TNF-α and PG E2 in lipopolysaccharide-stimulated RAW 264.7 macrophages [35,36]. The topical application of OJ extracts alleviated the decrease in skin collagen caused by UV irradiation in male ICR mice, and this effect was associated with the restoration of the expression of collagen types I and III, MMP1, MMP3, TNF-α, and COX2 [37]. The present study additionally showed that the BA fraction purified from the OJ extract has anti-inflammatory effects, by reducing the secretion of PG E2 in keratinocytes stimulated by PM10. Taken together, data from these previous studies and the current study suggest that OJ extract is a useful natural product to alleviate skin inflammation caused by UV rays, atmospheric fine dust, or a combination thereof. In addition, it is suggested that the efficacy can be further improved by purifying the OJ extract and increasing the content of chlorogenic acid, one of its active ingredients.
Chlorogenic acid has been previously reported to attenuate oxidative stress and inflammatory responses in various in vitro and in vivo models [53,54]. In our recent study, chlorogenic acid was identified as a major component of the Siegesbeckiae herba extract, which has now been shown to mitigate PM10-induced cytotoxicity by activating the NRF2 pathway [25]. It has also been shown to suppress the production of nitric oxide (NO) and PG E2 by inhibiting the expression of inducible nitric oxide synthase (iNOS) and COX2 in lipopolysaccharide-stimulated RAW 264.7 macrophages [55], and in human chondrocytes stimulated by IL-1β [56]. In the present study, chlorogenic acid was shown to reduce the production of PG E2 in human keratinocytes induced by PM10. Thus, there is consolidating evidence that chlorogenic acid exhibits antioxidant and anti-inflammatory activities in cells.
OJ contains a variety of compounds that exhibit anti-inflammatory activity [27]. In previous studies, ten biphenyl derivatives were isolated from the cyclohexane fraction of an 85% ethanol extract of the aerial part of OJ, and some significantly inhibited COX2 activity in vitro [57]. Thirteen kinds of phenylpropanoids were isolated from the EA fraction of the extract, and some of these compounds exhibited anti-inflammatory activities that inhibited NO production in RAW 264.7 macrophages [58]. Therefore, it should not be overlooked that OJ has various components that exhibit anti-inflammatory capabilities via various mechanisms, in addition to chlorogenic acid and caffeic acid.
In this study, OJ extract and its fractions showed different degrees of cytotoxicity at high concentrations in the absence of fine dust. Currently, the identity and mechanism of action of these toxic components are unknown. Nonetheless, it is desirable to remove toxic components as much as possible, while leaving active components, when using OJ extract to protect cells. Since even active components can be toxic at high concentrations, it is important to find the optimal concentration range, considering both toxicity and efficacy.
5. Conclusions
This study demonstrated that the BA fraction of OJ extract reduces PM10-induced ROS production in HaCaT cells most effectively among the solvent fractions. Of its major phenolic components, chlorogenic acid was more effective than caffeic acid in reducing ROS levels. The BA fraction and chlorogenic acid attenuated lipid peroxidation, protein carbonylation, and PG E2 production due to the exposure of cells to PM10. The PM10-induced expressions of PLA2G4A mRNA and cPLA2 protein were alleviated by chlorogenic acid, and this action of chlorogenic acid is suggested to be the main mechanism that enables a reduction in PM10-induced PG E2 production. This study suggests that the chlorogenic acid contained in OJ extract may help to alleviate the oxidative damage to and inflammatory responses produced by the skin cells when exposed to air pollutants.
Author Contributions
Investigation, I.A.B., J.W.H.; writing, I.A.B., J.W.H. and Y.C.B.; conceptualization, supervision, and funding acquisition, Y.C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HP20C0004).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| BA | n-butanol |
| COX | cyclooxygenase |
| cPLA2 | cytosolic phospholipase A2 |
| DCFH-DA | 2′,7′-dichlorodihydrofluorescein diacetate |
| EA | ethyl acetate |
| ELISA | enzyme-linked immunosorbent assay |
| FTC | fluorescein-5-thiosemicarbazide |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| HPLC-DAD | high-performance liquid chromatography with photodiode array detection |
| IL | interleukin |
| iNOS | inducible nitric oxide synthase |
| MC | methylene chloride |
| MDA | malondialdehyde |
| MMP | matrix metalloproteinase |
| MTT | 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide |
| NAC | N-acetyl cysteine |
| NF-κB | nuclear factor-κB |
| NO | nitric oxide |
| OJ | Oenanthe javanica (Blume) DC. |
| PBS | phosphate-buffered saline |
| PG | prostaglandin |
| PEGS | prostaglandin E synthetase |
| PLA2 | phospholipases A2 |
| PM | particulate matter |
| qRT-PCR | quantitative reverse transcriptase-polymerase chain reaction |
| ROS | reactive oxygen species |
| sPLA2 | secretory phospholipase A2 |
| SOD | superoxide dismutase |
| TNF-α | tumor necrosis factor-α |
| TMP | 1,1,3,3-tetramethoxypropane |
| UV | ultraviolet |
| WT | water |
References
- Zhu, X.; Qiu, H.; Wang, L.; Duan, Z.; Yu, H.; Deng, R.; Zhang, Y.; Zhou, L. Risks of hospital admissions from a spectrum of causes associated with particulate matter pollution. Sci. Total Environ. 2019, 656, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Fuzzi, S.; Baltensperger, U.; Carslaw, K.; Decesari, S.; van Der Gon, H.D.; Facchini, M.C.; Fowler, D.; Koren, I.; Langford, B.; Lohmann, U.; et al. Particulate matter, air quality and climate: Lessons learned and future needs. Atmos. Chem. Phys. 2015, 15, 8217–8299. [Google Scholar] [CrossRef]
- Song, S.; Lee, K.; Lee, Y.M.; Lee, J.H.; Lee, S.I.; Yu, S.D.; Paek, D. Acute health effects of urban fine and ultrafine particles on children with atopic dermatitis. Environ. Res. 2011, 111, 394–399. [Google Scholar] [CrossRef]
- Jin, S.P.; Li, Z.; Choi, E.K.; Lee, S.; Kim, Y.K.; Seo, E.Y.; Chung, J.H.; Cho, S. Urban particulate matter in air pollution penetrates into the barrier-disrupted skin and produces ROS-dependent cutaneous inflammatory response in vivo. J. Dermatol. Sci. 2018, 91, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Ngoc, L.T.N.; Park, D.; Lee, Y.; Lee, Y.C. Systematic Review and Meta-Analysis of Human Skin Diseases Due to Particulate Matter. Int. J. Environ. Res. Public Health 2017, 14, 1458. [Google Scholar] [CrossRef]
- Kim, K.E.; Cho, D.; Park, H.J. Air pollution and skin diseases: Adverse effects of airborne particulate matter on various skin diseases. Life Sci. 2016, 152, 126–134. [Google Scholar] [CrossRef]
- Vierkotter, A.; Schikowski, T.; Ranft, U.; Sugiri, D.; Matsui, M.; Kramer, U.; Krutmann, J. Airborne particle exposure and extrinsic skin aging. J. Investig. Dermatol. 2010, 130, 2719–2726. [Google Scholar] [CrossRef]
- Roberts, W.E. Pollution as a risk factor for the development of melasma and other skin disorders of facial hyperpigmentation is there a case to be made? J. Drugs Dermatol. 2015, 14, 337–341. [Google Scholar]
- Soeur, J.; Belaidi, J.P.; Chollet, C.; Denat, L.; Dimitrov, A.; Jones, C.; Perez, P.; Zanini, M.; Zobiri, O.; Mezzache, S.; et al. Photo-pollution stress in skin: Traces of pollutants (PAH and particulate matter) impair redox homeostasis in keratinocytes exposed to UVA1. J. Dermatol. Sci. 2017, 86, 162–169. [Google Scholar] [CrossRef]
- Datzmann, T.; Markevych, I.; Trautmann, F.; Heinrich, J.; Schmitt, J.; Tesch, F. Outdoor air pollution, green space, and cancer incidence in Saxony: A semi-individual cohort study. BMC Public Health 2018, 18, 715. [Google Scholar] [CrossRef]
- Lee, D.U.; Ji, M.J.; Kang, J.Y.; Kyung, S.Y.; Hong, J.H. Dust particles-induced intracellular Ca2+ signaling and reactive Oxygen species in lung fibroblast cell line MRC5. Korean J. Physiol. Pharmacol. 2017, 21, 327–334. [Google Scholar] [CrossRef]
- Seok, J.K.; Cho, M.A.; Ha, J.W.; Boo, Y.C. Role of Dual Oxidase 2 in Reactive Oxygen Species Production Induced by Airborne Particulate Matter PM10 in Human Epidermal Keratinocytes. J. Soc. Cosmet. Sci. Korea 2018, 45, 57–67. [Google Scholar]
- Tsuji, G.; Takahara, M.; Uchi, H.; Takeuchi, S.; Mitoma, C.; Moroi, Y.; Furue, M. An environmental contaminant, benzo(a)pyrene, induces oxidative stress-mediated interleukin-8 production in human keratinocytes via the aryl hydrocarbon receptor signaling pathway. J. Dermatol. Sci. 2011, 62, 42–49. [Google Scholar] [CrossRef]
- Verma, V.; Shafer, M.M.; Schauer, J.J.; Sioutas, C. Contribution of transition metals in the reactive oxygen species activity of PM emissions from retrofitted heavy-duty vehicles. Atmos. Environ. 2010, 44, 5165–5173. [Google Scholar] [CrossRef]
- Ryu, Y.S.; Kang, K.A.; Piao, M.J.; Ahn, M.J.; Yi, J.M.; Hyun, Y.M.; Kim, S.H.; Ko, M.K.; Park, C.O.; Hyun, J.W. Particulate matter induces inflammatory cytokine production via activation of NFkappaB by TLR5-NOX4-ROS signaling in human skin keratinocyte and mouse skin. Redox Biol. 2019, 21, 101080. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, R.; Cao, L.; Shen, Z.X.; Cao, Y.X. The Role of MAPK Pathways in Airborne Fine Particulate Matter-Induced Upregulation of Endothelin Receptors in Rat Basilar Arteries. Toxicol. Sci. 2016, 149, 213–226. [Google Scholar] [CrossRef]
- Lee, C.W.; Lin, Z.C.; Hsu, L.F.; Fang, J.Y.; Chiang, Y.C.; Tsai, M.H.; Lee, M.H.; Li, S.Y.; Hu, S.C.; Lee, I.T.; et al. Eupafolin ameliorates COX-2 expression and PGE2 production in particulate pollutants-exposed human keratinocytes through ROS/MAPKs pathways. J. Ethnopharmacol. 2016, 189, 300–309. [Google Scholar] [CrossRef]
- Ha, J.W.; Song, H.; Hong, S.S.; Boo, Y.C. Marine Alga Ecklonia cava Extract and Dieckol Attenuate Prostaglandin E2 Production in HaCaT Keratinocytes Exposed to Airborne Particulate Matter. Antioxidants 2019, 8, 190. [Google Scholar] [CrossRef]
- Seok, J.K.; Lee, J.W.; Kim, Y.M.; Boo, Y.C. Punicalagin and (−)-Epigallocatechin-3-Gallate Rescue Cell Viability and Attenuate Inflammatory Responses of Human Epidermal Keratinocytes Exposed to Airborne Particulate Matter PM10. Skin Pharmacol. Physiol. 2018, 31, 134–143. [Google Scholar] [CrossRef]
- Kim, M.; Kim, J.H.; Jeong, G.J.; Park, K.Y.; Lee, M.K.; Seo, S.J. Particulate matter induces pro-inflammatory cytokines via phosphorylation of p38 MAPK possibly leading to dermal inflammaging. Exp. Dermatol. 2019, 28, 809–815. [Google Scholar] [CrossRef]
- Lee, C.W.; Lin, Z.C.; Hu, S.C.; Chiang, Y.C.; Hsu, L.F.; Lin, Y.C.; Lee, I.T.; Tsai, M.H.; Fang, J.Y. Urban particulate matter down-regulates filaggrin via COX2 expression/PGE2 production leading to skin barrier dysfunction. Sci. Rep. 2016, 6, 27995. [Google Scholar] [CrossRef] [PubMed]
- Alfaro-Moreno, E.; Martinez, L.; Garcia-Cuellar, C.; Bonner, J.C.; Murray, J.C.; Rosas, I.; Rosales, S.P.; Osornio-Vargas, A.R. Biologic effects induced in vitro by PM10 from three different zones of Mexico City. Environ. Health Perspect. 2002, 110, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. Can Plant Phenolic Compounds Protect the Skin from Airborne Particulate Matter? Antioxidants 2019, 8, 379. [Google Scholar] [CrossRef]
- Boo, Y.C. Natural Nrf2 Modulators for Skin Protection. Antioxidants 2020, 9, 812. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.W.; Boo, Y.C. Siegesbeckiae Herba Extract and Chlorogenic Acid Ameliorate the Death of HaCaT Keratinocytes Exposed to Airborne Particulate Matter by Mitigating Oxidative Stress. Antioxidants 2021, 10, 1762. [Google Scholar] [CrossRef]
- Bae, I.A.; Ha, J.W.; Choi, J.Y.; Boo, Y.C. Antioxidant Effects of Korean Propolis in HaCaT Keratinocytes Exposed to Particulate Matter 10. Antioxidants 2022, 11, 781. [Google Scholar] [CrossRef]
- Lu, C.L.; Li, X.F. A Review of Oenanthe javanica (Blume) DC. as Traditional Medicinal Plant and Its Therapeutic Potential. Evid.-Based Complement. Altern. Med. 2019, 2019, 6495819. [Google Scholar] [CrossRef]
- Liu, J.X.; Jiang, Q.; Tao, J.P.; Feng, K.; Li, T.; Duan, A.Q.; Wang, H.; Xu, Z.S.; Liu, H.; Xiong, A.S. Integrative genome, transcriptome, microRNA, and degradome analysis of water dropwort (Oenanthe javanica) in response to water stress. Hortic. Res. 2021, 8, 262. [Google Scholar] [CrossRef]
- Hwang, C.-R.; Hwang, I.-G.; Kim, H.-Y.; Kang, T.-S.; Kim, Y.-B.; Joo, S.-S.; Lee, J.-S.; Jeong, H.-S. Antioxidant Component and Activity of Dropwort (Oenanthe javanica) Ethanol Extracts. J. Korean Soc. Food Sci. Nutr. 2011, 40, 316–320. [Google Scholar] [CrossRef]
- Hwang, S.-J.; Park, S.-J.; Kim, J.-D. Component Analysis and Antioxidant Activity of Oenanthe javanica Extracts. Korean J. Food Sci. Technol. 2013, 45, 227–234. [Google Scholar] [CrossRef]
- Bae, U.-J.; Jang, H.-N.; Lee, S.-H.; Kim, J.-Y.; Kim, G.-C. Oenanthe javanica Ethanolic Extract Alleviates Inflammation and Modifies Gut Microbiota in Mice with DSS-Induced Colitis. Antioxidants 2022, 11, 2429. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, J.S.; Lee, I.H.; Hong, J.T. Therapeutic potency of fermented field water-dropwort (Oenanthe javanica (Blume) DC.) in ethanol-induced liver injury. RSC Adv. 2020, 10, 1544–1551. [Google Scholar] [CrossRef]
- Lee, C.-H.; Park, J.-H.; Cho, J.-H.; Kim, I.-H.; Ahn, J.-H.; Lee, J.-C.; Chen, B.H.; Shin, B.-N.; Tae, H.-J.; Bae, E.J.; et al. Effect of Oenanthe Javanica Extract on Antioxidant Enzyme in the Rat Liver. Chin. Med. J. 2015, 128, 1649–1654. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, K.H.; Lee, Y.J.; Lee, S.H.; Park, J.C.; Nam, D.H. Oenanthe javanica extract accelerates ethanol metabolism in ethanol-treated animals. BMB Rep. 2009, 42, 482–485. [Google Scholar] [CrossRef]
- Lee, J.-M.; Kim, N.-J.; Cho, D.-H.; Chung, M.-Y.; Hwang, K.-T.; Kim, H.-J.; Jun, W.-J.; Park, C.-S. Ethanol Extract of Oenanthe javanica Modulates Inflammatory Response by Inhibiting NF-kappaB Mediated Cyclooxygenase-2 Expression in RAW 264.7 Macrophage. Food Sci. Biotechnol. 2006, 15, 303–307. [Google Scholar]
- Lee, J.; Kim, H.J.; Choi, H.; You, Y.; Hwang, K.T.; Lee, M.Y.; Park, C.S.; Jun, W. Effects of Oenanthe javanica on transcriptional regulation of COX-2 by inhibiting translocation of p65 subunit in LPS-stimulated murine peritoneal macrophages. Food Sci. Biotechnol. 2006, 15, 975–979. [Google Scholar]
- Her, Y.; Shin, B.N.; Lee, Y.L.; Park, J.H.; Kim, D.W.; Kim, K.S.; Kim, H.; Song, M.; Kim, J.D.; Won, M.H.; et al. Oenanthe Javanica Extract Protects Mouse Skin from UVB Radiation via Attenuating Collagen Disruption and Inflammation. Int. J. Mol. Sci. 2019, 20, 1435. [Google Scholar] [CrossRef]
- Hsouna, A.B.; Boye, A.; Ackacha, B.B.; Dhifi, W.; Saad, R.B.; Brini, F.; Mnif, W.; Kačániová, M. Thiamine Demonstrates Bio-Preservative and Anti-Microbial Effects in Minced Beef Meat Storage and Lipopolysaccharide (LPS)-Stimulated RAW 264.7 Macrophages. Animals 2022, 12, 1646. [Google Scholar] [CrossRef]
- Ben Hsouna, A.; Michalak, M.; Ben Akacha, B.; Dhifi, W.; Ben Saad, R.; Brini, F.; Mnif, W. Assessment of the phytochemical composition, antimicrobial activity and anti-inflammatory effects of Lobularia maritima extracts on lipopolysaccharide-stimulated RAW 264.7 cells and their capacity to extend the shelf life of raw minced beef. J. Funct. Foods 2022, 99, 105327. [Google Scholar] [CrossRef]
- Eruslanov, E.; Kusmartsev, S. Identification of ROS using oxidized DCFDA and flow-cytometry. Methods Mol. Biol. 2010, 594, 57–72. [Google Scholar]
- Lee, J.W.; Seok, J.K.; Boo, Y.C. Ecklonia cava Extract and Dieckol Attenuate Cellular Lipid Peroxidation in Keratinocytes Exposed to PM10. Evid.-Based Complement. Altern. Med. 2018, 2018, 8248323. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Q.; Xiang, R.; Glorieux, C.; Huang, P. PLA2G2A Phospholipase Promotes Fatty Acid Synthesis and Energy Metabolism in Pancreatic Cancer Cells with K-ras Mutation. Int. J. Mol. Sci. 2022, 23, 11721. [Google Scholar] [CrossRef] [PubMed]
- Baihau, D.; Khaniani, M.S. Supplementation with omega fatty acids increases the mRNA expression level of PLA2G4A in patients with gastric cancer. J. Gastrointest. Oncol. 2018, 9, 1176–1183. [Google Scholar]
- Lin, W.; Li, Z. Blueberries inhibit cyclooxygenase-1 and cyclooxygenase-2 activity in human epithelial ovarian cancer. Oncol. Lett. 2017, 13, 4897–4904. [Google Scholar] [CrossRef]
- Xu, J.; Wu, W.; Zhang, H.; Yang, L. Berberine alleviates amyloid beta25-35-induced inflammatory response in human neuroblastoma cells by inhibiting proinflammatory factors. Exp. Ther. Med. 2018, 16, 4865–4872. [Google Scholar]
- Molloy, E.S.; Morgan, M.P.; Doherty, G.A.; McDonnell, B.; O’Byrne, J.; Fitzgerald, D.J.; McCarthy, G.M. Microsomal prostaglandin E2 synthase 1 expression in basic calcium phosphate crystal-stimulated fibroblasts: Role of prostaglandin E2 and the EP4 receptor. Osteoarthr. Cartil. 2009, 17, 686–692. [Google Scholar] [CrossRef]
- Ferrer, M.D.; Busquests-Cortes, C.; Capo, X.; Tejada, S.; Tur, J.A.; Pons, A.; Sureda, A. Cyclooxygenase-2 inhibitors as a therapeutic target in inflammatory diseases. Curr. Med. Chem. 2018, 26, 3225–3241. [Google Scholar] [CrossRef]
- Hara, S. Prostaglandin terminal synthases as novel therapeutic targets. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2017, 93, 703–723. [Google Scholar] [CrossRef]
- Yang, L.; Liu, S.Z.; Liu, R.H.; He, J.W. Bioassay-guided isolation of cyclooxygenase-2 inhibitory and antioxidant phenylpropanoid derivatives from the roots of Dendropanax dentiger. Bioorganic Chem. 2020, 104, 104211. [Google Scholar] [CrossRef]
- Malireddy, S.; Lawson, C.; Steinhour, E.; Hart, J.; Kotha, S.R.; Patel, R.B.; Zhao, L.; Wilkins, J.R.; Marsh, C.B.; Magalang, U.J.; et al. Airborne agricultural particulate matter induces inflammatory cytokine secretion by respiratory epithelial cells: Mechanisms of regulation by eicosanoid lipid signal mediators. Indian J. Biochem. Biophys. 2013, 50, 387–401. [Google Scholar]
- Pavicevic, Z.; Leslie, C.C.; Malik, K.U. cPLA2 phosphorylation at serine-515 and serine-505 is required for arachidonic acid release in vascular smooth muscle cells. J. Lipid Res. 2008, 49, 724–737. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Role of Chlorogenic Acids in Controlling Oxidative and Inflammatory Stress Conditions. Nutrients 2016, 8, 16. [Google Scholar] [CrossRef]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The Biological Activity Mechanism of Chlorogenic Acid and Its Applications in Food Industry: A Review. Front. Nutr. 2022, 9, 943911. [Google Scholar] [CrossRef]
- Shan, J.; Fu, J.; Zhao, Z.; Kong, X.; Huang, H.; Luo, L.; Yin, Z. Chlorogenic acid inhibits lipopolysaccharide-induced cyclooxygenase-2 expression in RAW264.7 cells through suppressing NF-kappaB and JNK/AP-1 activation. Int. Immunopharmacol. 2009, 9, 1042–1048. [Google Scholar] [CrossRef]
- Chen, W.-P.; Wu, L.-D. Chlorogenic acid suppresses interleukin-1β-induced inflammatory mediators in human chondrocytes. Int. J. Clin. Exp. Pathol. 2014, 7, 8797–8801. [Google Scholar]
- Ma, Q.G.; Wei, R.R.; Sang, Z.P. Biphenyl Derivatives from the Aerial Parts of Oenanthe javanica and Their COX-2 Inhibitory Activities. Chem. Biodivers. 2019, 16, e1800480. [Google Scholar] [CrossRef]
- Ma, Q.G.; Guan, Y.; Sang, Z.P.; Wei, R.R. Anti-inflammatory phenylpropanoid derivatives from the aerial parts of Oenanthe javanica. Chem. Nat. Compd. 2021, 57, 752–756. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).