In-Vial Micro-Matrix-Solid Phase Dispersion for the Analysis of Fragrance Allergens, Preservatives, Plasticizers, and Musks in Cosmetics
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals, Materials and Samples
| Fragrance Allergens | Chemical Names | Purity (%) | CAS | Maximum Concentration Permitted [1] |
|---|---|---|---|---|
| Pinene | Bicyclo[3.1.1]hept-2-ene, 2,6,6-trimethyl | ≥99 b | 80-56-8 | n.r |
| Limonene a | (4R)-1-Methyl-4-(1-methylethenyl)cyclohexene | 97 b | 5989-27-5 | n.r |
| Benzyl alcohol a | Benzene methanol | ≥99 b | 100-51-6 | 1% (as preservative) |
| Linalool a | 3,7-Dimethyl-1,6-octadien-3-ol | 97 b | 78-70-6 | n.r |
| Methyl-2-octynoate a | Methyl heptin carbonate | ≥99 b | 111-12-6 | n.r |
| Citronellol a | (±)-3,7-Dimethyoct-6-en-1-ol | 95 b | 106-22-9 | n.r |
| Citral a | 3,7-Dimethyl-2,6-octadienal | 95 b | 5392-40-5 | n.r |
| Geraniol a | 3,7-Dimethyl-(2E)-2,6-octadien-1-ol | ≥96 b | 106-24-1 | n.r |
| Cinnamal a | 3-Phenyl-2-propenal | ≥93 b | 104-55-2 | n.r |
| Hydroxycitronellal a | 7-Hydroxy-3,7-dimethyloctanal | ≥95 b | 107-75-5 | 1% |
| Anise alcohol a | 4-Methoxybenzyl alcohol | 98 b | 105-13-5 | n.r |
| Cinnamyl alcohol a | 3-Phenyl-2-propen-1-ol | 98 b | 104-54-1 | n.r |
| Eugenol a | 2-Methoxy-4-(2-propenyl)-phenol | 99 b | 97-53-0 | n.r |
| Methyleugenol a | 1,2-Dimethoxy-4-(2-propenyl)-benzene | 99 b | 93-15-2 | 0.01% (fine fragrance); 0.004% (eau de toilette); 0.002% (fragrance cream); 0.0002% (other leave-on products); 0.001% (rinse-off products) |
| Isoeugenol a | 2-Methoxy-4-(1-propenyl)phenol | 98 b | 97-54-1 | 0.02% |
| Coumarin a | 2H-1-benzopyran-2-one | ≥99 b | 91-64-5 | n.r |
| α-isomethyl ionone a | 3-Methyl-4-(2,6,6-trimethyl-2-cyclohexen-1yl)-3-buten-2-one | ≥85 b | 127-51-5 | n.r |
| Lilial® a | 2-(4-tert-Butylbenzyl)propionaldehyde | ≥90 b | 80-54-6 | n.r |
| Amyl cinnamala | 2-Benzylideneheptanal | 97 b | 122-40-7 | n.r |
| Lyral® a,g | Hydroxyhexyl-3-cyclohexene carboxaldehyde | ≥97 b | 31906-04-4 | n.r |
| Amylcinnamyl alcohol a | 2-Pentyl-3-phenylprop-2-en-1-ol | ≥85 b | 101-85-9 | n.r |
| Farnesol a | 3,7,11-trimethyldodeca-2,6,10-trien-1-ol | 95 b | 4602-84-0 | n.r |
| Hexylcinnamal a | 2-Benzylideneoctanal | ≥95 b | 101-86-0 | n.r |
| Chemical Names | Purity (%) | CAS | Maximum Concentration Permitted [1] | |
| Benzyl benzoate a | Phenylmethyl benzoate | ≥99 b | 120-51-4 | n.r |
| Benzyl salicylate a | Benzyl-2-hydroxybenzoate | ≥99 b | 118-58-1 | n.r |
| Benzyl cinnamate a | 3-Phenyl-2-propenoic acid phenylmethyl ester | 99 b | 103-41-3 | n.r |
| Preservatives | ||||
| Bronidox | 5-Bromo-5-nitro-1,3-dioxane | ≥99 c | 30007-47-7 | 0.1% (rinse-off products) |
| Phenoxyethanol (phEtOH) | 2-Phenoxyethanol | 99 c | 122-99-6 | 1% |
| Methyl paraben (MeP) | Methyl 4-hydroxibenzoate | 99 b | 99-76-3 | 0.4% as acid (for single ester) 0.8% as acid (for mixtures of esters) |
| BHA | Butylated hidroxyanisole | 98.5 c | 25013-16-5 | n.r |
| BHT | Butylated hydroxytoluene | 99 c | 128-37-0 | n.r |
| Ethyl paraben (EtP) | Ethyl 4-hydroxybenzoate | 99 b | 120-47-8 | 0.4% as acid (for single ester) 0.8% as acid (for mixtures of esters) |
| Isopropyl paraben (iPrP) * | Isopropyl 4-hydroxybenzoate | ≥99 b | 4191-73-5 | 0.4% as acid (for single ester) 0.8% as acid (for mixtures of esters) |
| Propyl paraben (PrP) | Propyl 4-hydroxybenzoate | 99 b | 94-13-3 | 0.4% as acid (for single ester) 0.8% as acid (for mixtures of esters) |
| IPBC | Carbamic acid, butyl-3-iodo-2-propynyl ester | 97 c | 55406-53-6 | Prohibited in products for children under 3 years, except in bath products. Prohibited in oral and lip products. 0.02% (rinse-off products); 0.01% (leave-on products); 0.0075% (deodorants). |
| Isobutyl paraben (iBuP) * | Isobutyl 4-hydroxybenzoate | ≥97 b | 4247-02-3 | 0.4% as acid (for single ester) 0.8% as acid (for mixtures of esters) |
| Butyl paraben (BuP) | Butyl 4-hydroxybenzoate | 99 b | 94-26-8 | 0.4% as acid (for single ester) 0.8% as acid (for mixtures of esters) |
| Chemical Names | Purity (%) | CAS | Maximum Concentration Permitted [1] | |
| Triclosan | 2,4,4′-Trichloro-2′-hydroxydiphenyl ether | ≥97 c | 3380-34-5 | 0.3% (toothpastes, hand soaps, shower gels, deodorants, face powders and blemish concealers, nail products); 0.2% (mouthwashes) |
| Benzyl paraben (BzP) * | Benzyl hydroxybenzoate | 99 b | 94-18-8 | 0.4% as acid (for single ester) 0.8% as acid (for mixtures of esters) |
| Plasticizers | ||||
| DMA | 1,6-Dimethylhexanedioate | 99 c | 627-93-0 | n.r |
| DEA | 1,6-Diethylhexanedioate | 99 c | 141-28-6 | n.r |
| DMP | Dimethyl phthalate | 98 c | 131-11-3 | n.r |
| DEP | Diethyl phthalate | 98 b | 84-66-2 | n.r |
| DIBP | Diisobutyl phthalate | 99 f | 84-69-5 | n.r |
| DBP | Dibutyl phthalate | 99 b | 84-74-2 | Prohibited |
| DMEP | Dimethoxyethyl phthalate | 94 f | 117-82-8 | Prohibited |
| DPP | Dipentyl phthalate | 99.2 b | 131-18-0 | Prohibited |
| BBP | Benzylbutyl phthalate | 98 b | 85-68-7 | Prohibited |
| DEHA | Di(2-ethylhexyl) adipate | 98.5 c | 103-23-1 | n.r |
| DIHP | Diisoheptylphthalate | 99 b | 41451-28-9 | n.r |
| DEHP | Di(2-ethylhexyl) phthalate | 99.5 c | 117-81-7 | Prohibited |
| DCHP | Diclohexyl phthalate | 99 b | 84-61-7 | n.r |
| DPhP | Diphenyl phthalate | 98 b | 84-62-8 | n.r |
| DNOP | Di-noctyl phthalate | ≥ 98 d | 117-84-0 | n.r |
| Musks | ||||
| Cashmeran | 1,1,2,3,3-Pentamethyl-2,5,6,7-tetrahydroinden-4-one | ≥ 95 f | 33704-61-9 | n.r |
| Celestolide | 4-Acetyl-6-tert-butyl-1,1-dimethylindane | ≥ 98 f | 13171-00-1 | n.r |
| Phantolide | 6-Acetyl-1,1,2,3,3,5-hexamethylindan | ≥ 98 f | 15323-35-0 | 2% (leave-on products) |
| Chemical Names | Purity (%) | CAS | Maximum Concentration Permitted [1] | |
| Musk Ambrette | 6-tert-Butyl-3-methyl-2,4-dinitroanisole | 99 f | 83-66-9 | Prohibited |
| Traseolide | 5-Acetyl-3-isopropyl-1,1,2,6-tetramethylindane | 99 f | 68140-48-7 | n.r |
| Galaxolide | 1,3,4,6,7,8-Hexahydro-4,6,6,7,8,8-hexamethylcyclopenta(g)-2-benzopyran | 55.5 b | 1222-05-5 | n.r |
| Musk Xylene | 1-tert-Butyl-3,5-dimethyl-2,4,6-trinitrobenzene | 100 ng·mL−1 c | 81-15-2 | Prohibited in oral products. 1.0% (fine fragrance); 0.4% (eau de toilette); 0.03% (other products) |
| Tonalide | 6-Acetyl-1,1,2,4,4,7-hexamethyltetralin | 98 f | 1506-02-1 | Prohibited in oral products. 0.2% (rinse-off products) 0.1% (leave-on products, except: 1% hydroalcoholic products; 2.5% fine fragrance; 0.5% fragrance cream) |
| Musk Moskene | 1,1,3,3,5-Pentamethyl-4,6-dinitro-2H-indene | ≥99 f | 116-66-5 | Prohibited |
| Musk Tibetene | 1-tert-Butyl-3,4,5-trimethyl-2,6-dinitrobenzen | ≥99 f | 145-39-1 | Prohibited |
| Ambrettolide | 17-Oxacycloheptadec-6-en-1-one | ≥ 97 b | 7779-50-2 | n.r |
| Musk Ketone | 4-tert-Butyl-3,5-dinitro-2,6-dimethyl acetophenone | ≥98 b | 81-14-1 | Prohibited in oral products. 1.4% (fine fragrance) 0.56% (eau de toilette) 0.042% (other products) |
2.2. Micro-Matrix Solid-Phase Dispersion (MSPD)
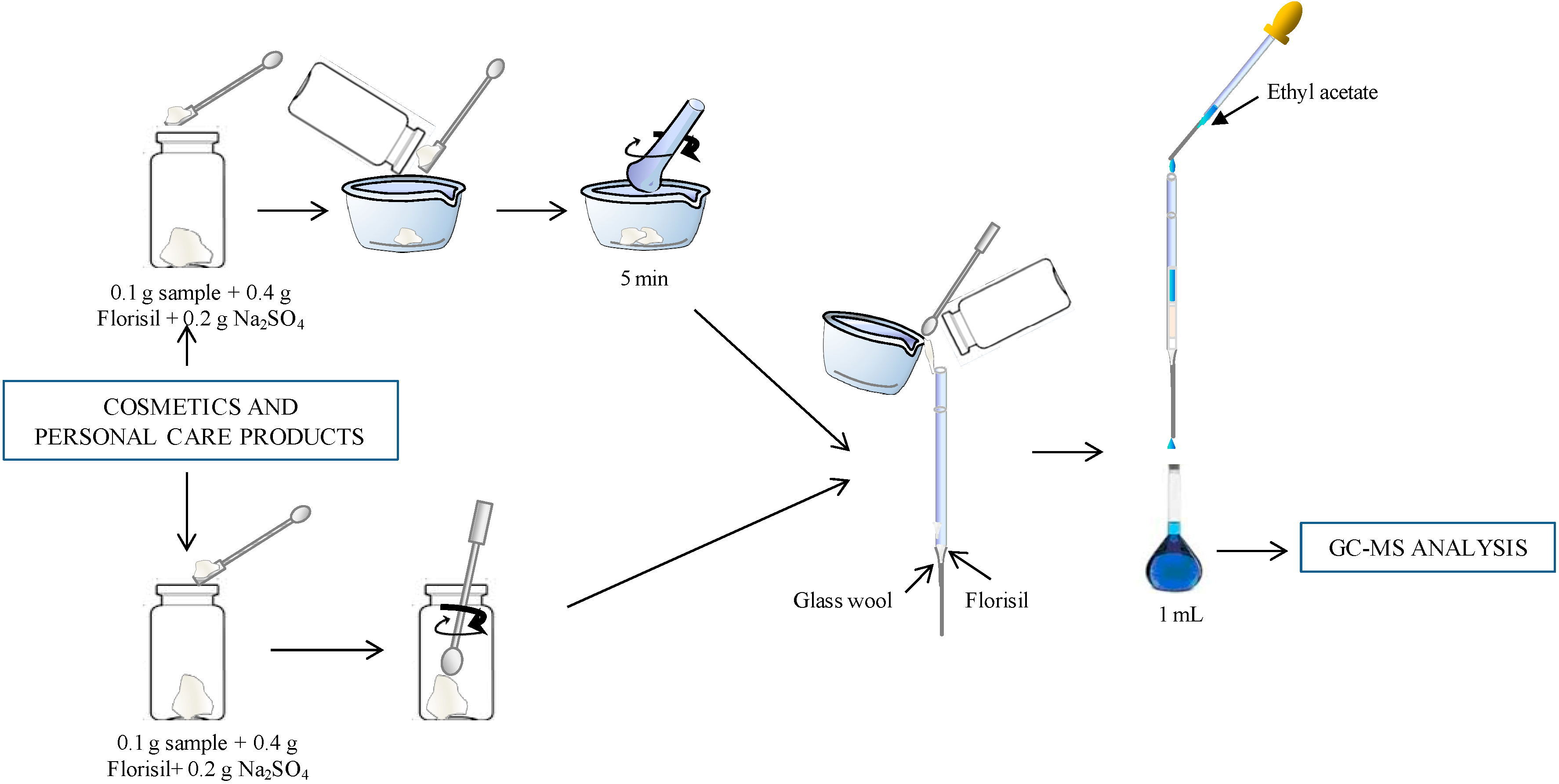
2.3. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
3. Results and Discussion
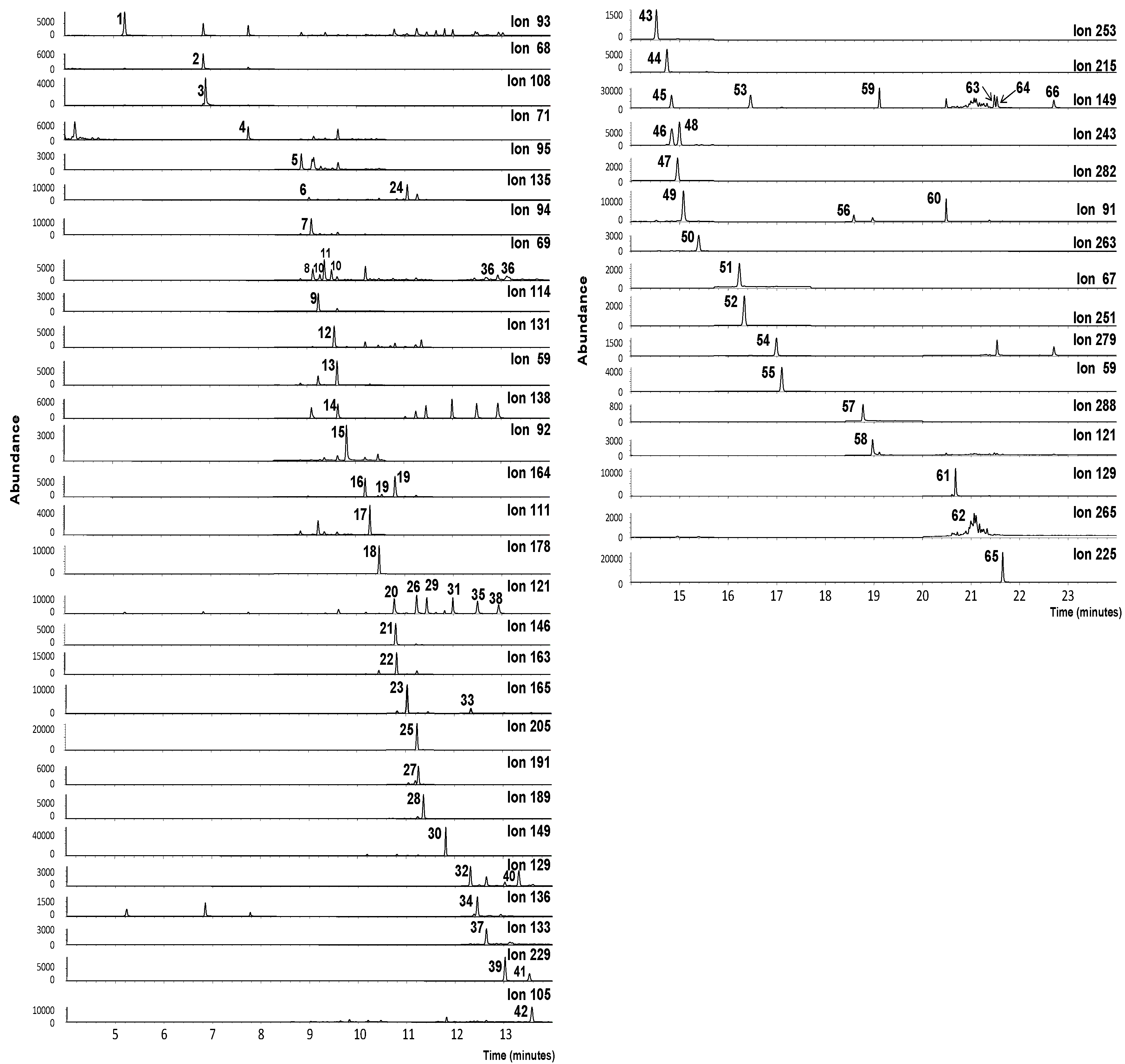
3.1. GC-MS Performance
| Key | Target Compounds | Retention Time (min) | Quantification and Identification Ions | Key | Target Compounds | Retention Time (min) | Quantification and Identification Ions |
|---|---|---|---|---|---|---|---|
| 1 | Pinene | 5.23 | 77 (27), 93 (100), 121 (13) | 34 | Lyral® | 12.45 | 79 (74), 93 (78), 136 (100) |
| 2 | Limonene | 6.85 | 68 (100), 93 (76),121 (25) | 35 | iBuP | 12.49 | 93 (12), 121 (100), 138 (58) |
| 3 | Benzyl alcohol | 6.90 | 77 (73), 79 (115), 108 (100) | 36 | Farnesol | 12.63/12.93 | 69 (100), 93 (27), 107 (15) |
| 4 | Linalool | 7.77 | 71 (100) ,93 (84) ,121 (24) | 37 | Amylcinnamyl alcohol | 12.64 | 91(88), 115 (60), 133 (100) |
| 5 | Methyl-2-octynoate | 8.87 | 79 (66), 95 (100) ,123 (73) | 38 | BuP | 12.93 | 121 (100), 138 (84), 194 (6) |
| 6 | Bronidox | 9.03 | 85 (27), 107 (49), 135 (100) | 39 | Celestolide | 13.03 | 173 (22), 229 (100), 244 (44) |
| 7 | PhEtOH | 9,09 | 77 (28), 94 (100), 138 (31) | 40 | Hexylcinnamal | 13.31 | 129 (100), 145 (51), 216 (40) |
| 8 | Citronellol | 9.12 | 69 (100) ,95 (49) ,109 (18) | 41 | Phantolide | 13.53 | 187 (11), 229 (100), 244 (24) |
| 9 | DMA | 9.23 | 101 (72), 111 (77), 114 (100) | 42 | Benzyl benzoate | 13.58 | 77 (28), 91 (47), 105 (100) |
| 10 | Citral | 9.27/9.51 | 69 (100), 94 (17), 109 (10) | 43 | Ambrette | 14.53 | 253 (100), 254 (13), 268 (35) |
| 11 | Geraniol | 9.36 | 69 (100), 93 (18) ,111 (6) | 44 | Traseolide | 14.74 | 43 (41), 215 (100), 258 (14) |
| 12 | Cinnamal | 9.56 | 77 (35), 103 (50), 131 (100) | 45 | DIBP | 14.84 | 57 (12), 149 (100), 223 (6.8) |
| 13 | Hydroxycitronellal | 9.62 | 59 (100), 71 (13) , 81 (43) | 46 | Galaxolide | 14.84 | 213 (23), 243 (100), 258 (20) |
| 14 | Anise alcohol | 9.64 | 109 (77), 121 (55), 138 (100) | 47 | Xylene | 14.96 | 43 (62), 57 (16), 282 (100) |
| 15 | Cinnamyl alcohol | 9.82 | 92 (100), 105 (53), 115 (54) | 48 | Tonalide | 14.99 | 43 (48), 243 (100), 258 (26) |
| 16 | Eugenol | 10.21 | 103 (28),131 (27), 164 (100) | 49 | Benzyl salicylate | 15.08 | 65 (11), 91 (100), 228 (12) |
| 17 | DEA | 10.30 | 111 (100), 128 (63), 157 (81) | 50 | Moskene | 15.40 | 263 (100), 264 (20), 278 (8.9) |
| 18 | Methyleugenol | 10.47 | 147 (31), 163 (29), 178 (100) | 51 | Ambrettolide | 16.23 | 67 (100), 81 (98), 96 (89) |
| 19 | Isoeugenol | 10.54/10.82 | 103(22), 131 (20), 164 (100) | 52 | Tibetene | 16.33 | 43 (33), 251 (100), 266 (28) |
| 20 | MeP | 10.78 | 93.0 (21), 121 (100), 153 (35) | 53 | DBP | 16.46 | 149 (100), 150 (9), 223 (4.9) |
| 21 | Coumarin | 10.82 | 90 (42), 118 (110), 146 (100) | 54 | Ketone | 16.99 | 191 (24), 294 (26), 279 (100) |
| 22 | DMP | 10.83 | 77 (13), 194 (66), 163 (100) | 55 | DMEP | 17.10 | 59 (100), 104 (18), 149 (29) |
| 23 | BHA | 11.03 | 137 (63), 165 (100), 180 (51) | 56 | Benzyl cinnamate | 18.59 | 91 (100), 131 (90), 192 (63) |
| 24 | α-isomethyl ionone | 11.05 | 107 (58), 135 (100), 150 (61) | 57 | Triclosan | 18.78 | 218 (93), 288 (100), 290 (93) |
| 25 | BHT | 11.24 | 177 (8), 205 (100), 220 (25) | 58 | BzP | 18.98 | 91 (46), 121 (100), 228 (21) |
| 26 | EtP | 11.24 | 121 (100), 138 (21), 166 (18) | 59 | DPP | 19.12 | 71 (16), 149 (100), 237 (5.6) |
| 27 | Cashmeran | 11.26 | 135 (43), 191 (100), 206 (57) | 60 | BBP | 20.49 | 91 (53), 149 (100), 206 (24) |
| 28 | Lilial® | 11.36 | 131 (39), 147 (40), 189 (100) | 61 | DEHA | 20.69 | 112 (26), 129 (100), 147 (21) |
| 29 | iPrP | 11.45 | 121 (100), 138 (39), 180 (14) | 62 | DIHP | 21.07 | 149 (100), 223 (7), 265 (52) |
| 30 | DEP | 11.82 | 149 (100), 150 (12), 177 (24) | 63 | DCHP | 21.53 | 55 (19), 149 (100), 167 (31) |
| 31 | PrP | 11.99 | 121 (100), 138 (58), 180 (7) | 64 | DEHP | 21.54 | 167 (30), 149 (100), 279 (10) |
| 32 | Amyl cinnamal | 12.31 | 115 (89), 129 (100), 145 (57) | 65 | DPhP | 21.65 | 77 (19), 153 (4), 225 (100) |
| 33 | IPBC | 12.34 | 100 (15), 165 (100), 182 (50) | 66 | DNOP | 22.71 | 149 (100), 223 (22), 279 (6.2) |
| Fragrance Allergens | Correlation coefficient (R) | IDL (ng·mL−1) | LOD (%, w/w × 104) | LOQ (%, w/w × 104) | Intra-Day Precision (RSD, %) a | Inter-Day Precision (RSD, %) b |
|---|---|---|---|---|---|---|
| Pinene | 0.9997 | 1.02 | 0.0169 | 0.0563 | 1.8 | 1.8 |
| Limonene | 0.9993 | 0.99 | 0.0213 | 0.0709 | 2.5 | 2.1 |
| Benzyl alcohol | 0.9980 | 2.03 | 0.0232 | 0.0773 | 3.0 | 5.5 |
| Linalool | 0.9984 | 2.10 | 0.0260 | 0.0866 | 4.0 | 3.8 |
| Methyl-2-octynoate | 0.9969 | 2.75 | 0.0275 | 0.0916 | 4.6 | 5.0 |
| Citronellol | 0.9965 | 2.78 | 0.0313 | 0.1042 | 5.6 | 4.6 |
| Citral | 0.9973 | 2.80 | 0.0400 | 0.1332 | 5.2 | 4.3 |
| Geraniol | 0.9969 | 3.05 | 0.0400 | 0.1332 | 5.1 | 5.2 |
| Cinnamal | 0.9984 | 2.97 | 0.0300 | 0.0990 | 3.4 | 3.2 |
| Hydroxycitronellal | 0.9972 | 1.93 | 0.0197 | 0.0656 | 4.7 | 4.2 |
| Anise alcohol | 0.9972 | 4.04 | 0.0404 | 0.1345 | 4.0 | 3.9 |
| Cinnamyl alcohol | 0.9965 | 5.28 | 0.0528 | 0.1758 | 3.3 | 3.8 |
| Eugenol | 0.9964 | 1.91 | 0.0210 | 0.0693 | 5.0 | 4.7 |
| Methyleugenol | 0.9982 | 1.97 | 0.0197 | 0.0656 | 2.8 | 2.4 |
| Isoeugenol | 0.9976 | 2.95 | 0.0309 | 0.1029 | 3.7 | 3.5 |
| Coumarin | 0.9997 | 2.00 | 0.0220 | 0.0733 | 1.7 | 2.1 |
| α-isomethyl ionone | 0.9985 | 0.96 | 0.0118 | 0.0393 | 3.4 | 3.0 |
| Lilial® | 0.9984 | 1.05 | 0.0196 | 0.0653 | 3.5 | 2.8 |
| Amyl cinnamal | 0.9963 | 2.20 | 0.0320 | 0.1066 | 4.4 | 4.1 |
| Lyral® | 0.9937 | 2.40 | 0.0240 | 0.0799 | 4.6 | 5.5 |
| Amylcinnamyl alcohol | 0.9930 | 6.04 | 0.0604 | 0.2011 | 4.3 | 4.1 |
| Farnesol | 0.9978 | 70.0 | 0.7000 | 2.331 | 4.3 | 2.3 |
| Hexylcinnamal | 0.9954 | 3.01 | 0.0301 | 0.1002 | 5.0 | 4.2 |
| Benzyl benzoate | 0.9986 | 2.10 | 0.0343 | 0.1142 | 3.1 | 2.7 |
| Benzyl salicylate | 0.9926 | 2.93 | 0.0293 | 0.0976 | 5.2 | 4.9 |
| Benzyl cinnamate | 0.9945 | 2.93 | 0.0293 | 0.0976 | 4.1 | 3.3 |
| Preservatives | Correlation coefficient (R) | IDL (ng·mL−1) | LOD (%, w/w × 104) | LOQ (%, w/w × 104) | Intra-Day Precision (RSD, %) a | Inter-Day Precision (RSD, %) b |
| Bronidox | 0.9977 | 2.61 | 0.0261 | 0.0869 | 1.8 | 3.2 |
| PhEtOH | 0.9967 | 2.34 | 0.0234 | 0.0779 | 4.6 | 4.4 |
| MeP | 0.9972 | 2.02 | 0.0300 | 0.0999 | 4.2 | 5.3 |
| BHA | 0.9984 | 1.7 | 0.0170 | 0.0566 | 4.5 | 4.8 |
| BHT | 0.9990 | 0.53 | 0.0053 | 0.0176 | 3.1 | 2.6 |
| EtP | 0.9974 | 2.87 | 0.0375 | 0.1249 | 3.3 | 3.7 |
| iPrP | 0.9972 | 2.80 | 0.0380 | 0.1265 | 4.7 | 4.4 |
| PrP | 0.9956 | 2.92 | 0.0292 | 0.0972 | 5.2 | 5.3 |
| IPBC | 0.9956 | 10.0 | 0.150 | 0.4995 | 1.7 | 4.4 |
| iBuP | 0.9941 | 2.94 | 0.0310 | 0.1032 | 5.1 | 5.2 |
| BuP | 0.9942 | 3.02 | 0.0302 | 0.1006 | 4.4 | 5.4 |
| Triclosan | 0.9915 | 5.95 | 0.0595 | 0.1981 | 4.8 | 5.7 |
| BzP | 0.9942 | 5.90 | 0.0590 | 0.1947 | 5.0 | 7.9 |
| Plasticizers | ||||||
| DMA | 0.9994 | 0.90 | 0.0090 | 0.0299 | 1.8 | 2.1 |
| DEA | 0.9992 | 1.20 | 0.0260 | 0.0866 | 3.6 | 3.0 |
| DMP | 0.9996 | 0.47 | 0.0096 | 0.0319 | 2.5 | 2.1 |
| DEP | 0.9996 | 0.70 | 0.0070 | 0.0233 | 3.0 | 3.0 |
| DIBP | 0.9992 | 1.30 | 0.0203 | 0.0676 | 4.2 | 3.6 |
| DBP | 0.9990 | 0.75 | 0.0075 | 0.0250 | 4.4 | 3.9 |
| DMEP | 0.9991 | 2.00 | 0.0375 | 0.1238 | 4.3 | 4.3 |
| DPP | 0.9982 | 0.17 | 0.0064 | 0.0213 | 4.6 | 4.8 |
| BBP | 0.9976 | 2.00 | 0.0342 | 0.1139 | 2.5 | 3.6 |
| DEHA | 0.9974 | 0.93 | 0.0261 | 0.0869 | 3.0 | 6.7 |
| DIHP | 0.9989 | 24 | 0.4000 | 1.332 | 9.5 | 10 |
| Plasticizers | Correlation coefficient (R) | IDL (ng·mL−1) | LOD (%, w/w × 104) | LOQ (%, w/w × 104) | Intra-Day Precision (RSD, %) a | Inter-Day Precision (RSD, %) b |
| DEHP | 0.9976 | 0.95 | 0.0300 | 0.0999 | 3.9 | 6.3 |
| DCHP | 0.9990 | 0.70 | 0.0200 | 0.0666 | 6.3 | 5.4 |
| DPhP | 0.9990 | 0.45 | 0.0307 | 0.1022 | 3.6 | 5.2 |
| DNOP | 0.9966 | 0.40 | 0.0092 | 0.0306 | 1.7 | 3.0 |
| Musks | ||||||
| Cashmeran | 0.9996 | 0.60 | 0.0300 | 0.0999 | 3.7 | 3.0 |
| Celestolide | 0.9983 | 0.25 | 0.0026 | 0.0866 | 5.0 | 4.2 |
| Phantolide | 0.9983 | 0.52 | 0.0087 | 0.0289 | 4.5 | 4.3 |
| Ambrette | 0.9965 | 2.00 | 0.0300 | 0.0999 | 3.6 | 4.4 |
| Traseolide | 0.9970 | 0.80 | 0.0126 | 0.0419 | 5.0 | 4.8 |
| Galaxolide | 0.9995 | 0.83 | 0.0216 | 0.0719 | 3.6 | 2.8 |
| Xylene | 0.9946 | 2.05 | 0.0293 | 0.0976 | 4.1 | 4.3 |
| Tonalide | 0.9992 | 0.83 | 0.0162 | 0.0539 | 4.7 | 3.9 |
| Moskene | 0.9933 | 1.72 | 0.0480 | 0.1598 | 2.6 | 4.3 |
| Tibetene | 0.9964 | 1.90 | 0.0196 | 0.0652 | 4.2 | 4.0 |
| Ambrettolide | 0.9990 | 2.13 | 0.1200 | 0.3996 | 3.8 | 3.5 |
| Ketone | 0.9954 | 3.20 | 0.0706 | 0.2351 | 5.4 | 4.5 |
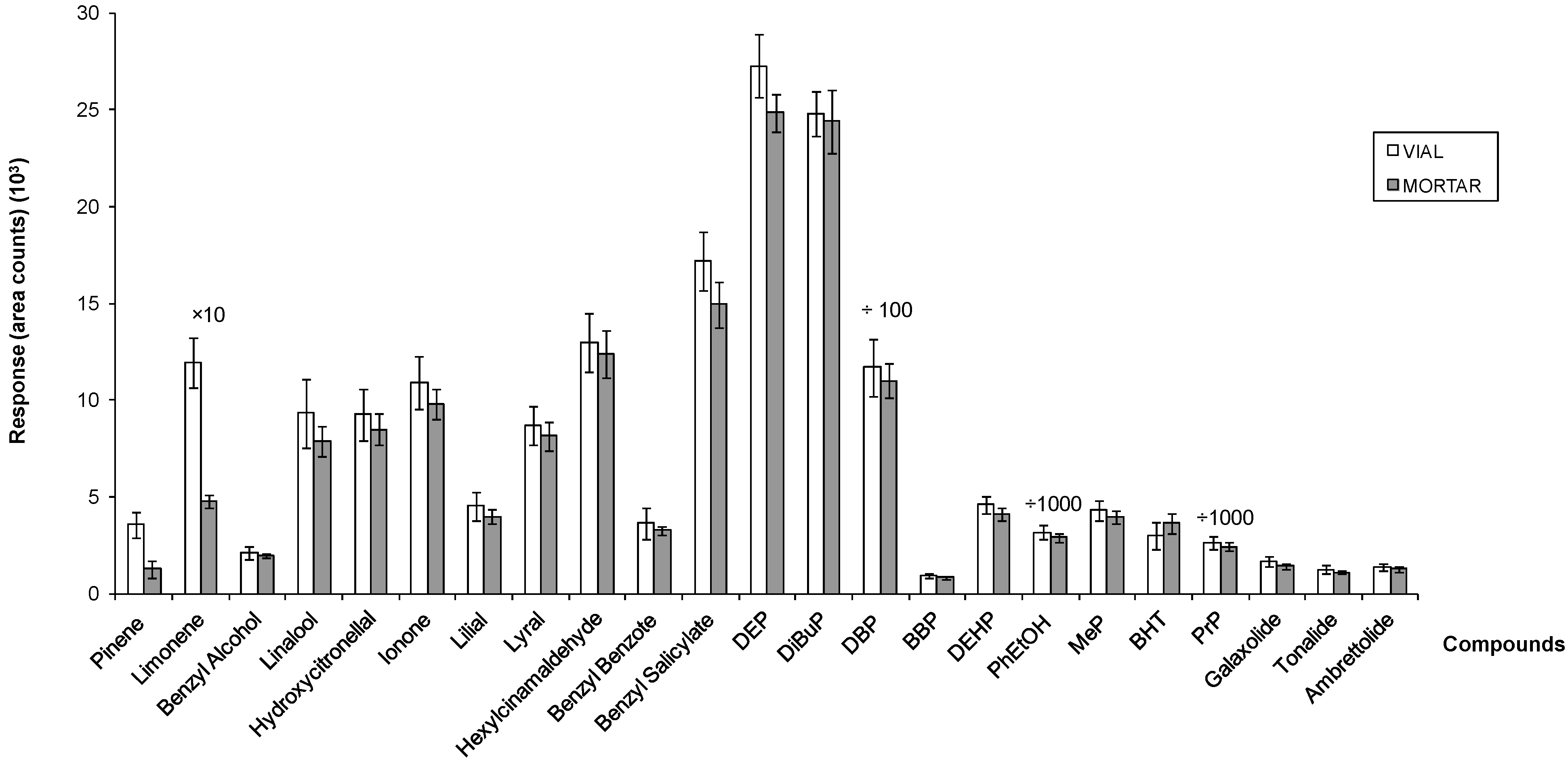
3.2. Analytical Method Performance
| Fragrance Allergens | Recoveries (%, RSD) | |||||
|---|---|---|---|---|---|---|
| 2 µg·g−1 | 10 µg·g−1 | 20 µg·g−1 | ||||
| Mortar | Vial | Mortar | Vial | Mortar | Vial | |
| Pinene | 23.2 (6.4) | 63.1 (3.9) | 23.5 (0.24) | 71.8 (13) | 34.0 (0.87) | 72.6 (4.3) |
| Limonene | 51.7 (2.0) | 73.8 (1.1) | 56.7 (7.1) | 79.5 (11) | 57.8 (0.016) | 78.6 (10) |
| Benzyl alcohol | 97.3 (0.76) | 97.3 (1.3) | 98.6 (0.80) | 90.4 (7.9) | 113 (0.15) | 110 (1.9) |
| Linalool | 105 (0.67) | 82.5 (0.11) | 96.7 (10) | 89.3 (14) | 107 (0.17) | 100 (13) |
| Methyl-2-octynoate | 87.8 (1.7) | 85.2 (2.9) | 97.5 (10) | 86.7 (14) | 112 (0.90) | 95.4 (11) |
| Citronellol | 101 (4.0) | 89.3 (11) | 97.6 (8.1) | 93.1 (13) | 110 (0.53) | 94.8 (10) |
| Citral | 99.0 (3.7) | 104 (1.8) | 97.5 (14) | 112 (14) | 112 (1.1) | 101 (10) |
| Geraniol | 114 (1.6) | 82.5 (7.7) | 82.0 (5.6) | 81.7 (7.6) | 102 (0.14) | 92.7 (10) |
| Cinnamal | 90.5 (4.5) | 87.8 (0.74) | 91.5 (12) | 84.5 (13) | 104 (0.88) | 96.2 (6.6) |
| Hydroxycitronellal | 81.9 (1.7) | 80.1 (0.60) | 101 (11) | 97.8 (2.9) | 114 (0.052) | 101 (11) |
| Anise alcohol | 93.8 (4.5) | 92.2 (2.6) | 96.2 (13) | 87.4 (13) | 111 (0.67) | 101 (6.3) |
| Cinnamyl alcohol | 96.3 (5.6) | 87.3 (13) | 94.0 (9.6) | 87.2 (15) | 110 (0.83) | 98.8 (13) |
| Eugenol | 87.2 (5.2) | 83.0 (4.0) | 93.8 (8.7) | 89.2 (15) | 105 (0.96) | 98.7 (12) |
| Methyleugenol | 85.8 (0.15) | 83.6 (3.1) | 95.5 (11) | 86.7 (14) | 109 (1.3) | 98.7 (8.4) |
| Isoeugenol | 80.9 (15) | 100 (12) | 114 (9.6) | 109 (14) | 87.2 (1.0) | 89.4 (9.1) |
| Coumarin | 91.1 (0.61) | 85.2 (2.7) | 95.5 (14) | 87.3 (11) | 109 (0.23) | 100 (2.7) |
| α-isomethyl ionone | 83.6 (6.2) | 86.3 (0.78) | 95.7 (11) | 89.6 (12) | 108 (0.82) | 101 (7.4) |
| Lilial® | 84.5 (3.3) | 83.2 (1.4) | 97.0 (11) | 88.8 (15) | 110 (1.7) | 98.2 (10) |
| Amyl cinnamal | 89.3 (4.4) | 89.5 (7.8) | 94.3 (5.2) | 85.8 (4.3) | 111 (3.5) | 95.9 (15) |
| Lyral® | 99.3 (11) | 83.0 (12) | 104 (6.9) | 94.3 (5.4) | 114 (2.8) | 95.4 (14) |
| Amylcinnamyl alcohol | 108 (10) | 95.2 (12) | 104 (7.4) | 95.9 (3.9) | 112 (3.1) | 102 (15) |
| Farnesol | <LOQ | <LOQ | 104 (2.7) | 97.1 (7.6) | 109 (7.5) | 86.8 (7.5) |
| Hexylcinnamal | 87.7 (1.8) | 107 (0.12) | 107 (4.5) | 103 (5.1) | 115 (3.8) | 97.3 (11) |
| Benzyl benzoate | 88.7 (7.6) | 90.8 (6.9) | 98.3 (14) | 90.8 (12) | 111 (0.16) | 104 (4.9) |
| Benzyl salicylate | 105 (3.1) | 109 (13) | 97.9 (14) | 90.7 (13) | 112 (1.9) | 106 (2.8) |
| Benzyl cinnamate | 94.5 (1.8) | 102 (0.27) | 102 (15) | 94.2 (12) | 112 (10) | 108 (1.6) |
| Preservatives | Recoveries (%, RSD) | |||||
| 2 µg·g−1 | 10 µg·g−1 | 20 µg·g−1 | ||||
| Mortar | Vial | Mortar | Vial | Mortar | Vial | |
| Bronidox | 92.1 (1.3) | 88.0 (4.4) | 95.6 (12) | 87.3 (13) | 108 (0.89) | 100 (10) |
| PhEtOH | 96.0 (3.8) | 91.6 (0.87) | 102 (12) | 92.1 (13) | 109 (0.91) | 97.1 (11) |
| MeP | 95.5 (4.2) | 90.2 (6.4) | 97.3 (11) | 93.6 (15) | 107 (1.7) | 96.6 (11) |
| BHA | 72.8 (6.6) | 80.2 (1.2) | 104 (9.4) | 104 (13) | 99.3 (0.53) | 97.7 (7.3) |
| BHT | 82.3 (0.16) | 81.4 (0.33) | 116 (0.83) | 115 (12) | 108 (0.67) | 105 (6.9) |
| EtP | 96.7 (10) | 83.9 (15) | 103 (13) | 95.2 (14) | 112 (0.36) | 102 (10) |
| iPrP | 100 (4.7) | 91.0 (6.5) | 100 (10) | 93.2 (14) | 109 (1.1) | 98.2 (11) |
| PrP | 111 (5.2) | 87.5 (14) | 99.3 (9.6) | 97.5 (7.8) | 109 (2.0) | 94.3 (13) |
| IPBC | 106 (15) | 83.0 (12) | 88.4 (1.6) | 101 (4.5) | 113 (4.0) | 89.0 (16) |
| iBuP | 113 (9.4) | 92.6 (14) | 103 (12) | 95.2 (2.2) | 111 (1.4) | 98.5 (11) |
| BuP | 96.8 (7.9) | 82.0 (6.7) | 99 (9.2) | 88.9 (2.7) | 110 (2.5) | 95.2 (15) |
| Triclosan | 109 (14) | 112 (3.4) | 100 (13) | 107 (9.1) | 111 (11) | 108 (12) |
| BzP | 105 (14) | 92.6 (1.5) | 111 (13) | 111 (6.8) | 117 ( 11) | 111 (8.4) |
| Plasticizers | ||||||
| DMA | 93.9 (7.0) | 105 (12) | 116 (9.8) | 108 (8.5) | 103 (0.60) | 97.5 (3.1) |
| DEA | 87.5 (0.038) | 81.4 (2.8) | 95.4 (15) | 87.5 (12) | 111 (0.22) | 100 (5.2) |
| DMP | 81.5 (3.6) | 83.3 (0.53) | 98.2 (15) | 90.5 (9.4) | 109 (0.77) | 102 (0.33) |
| DEP | 79.3 (5.2) | 83.1 (0.81) | 96.1 (13) | 87.2 (12) | 110 (0.36) | 101 (2.8) |
| DIBP | 83.1 (1.2) | 95.0 (2.1) | 98.4 (14) | 88.7 (14) | 111 (0.88) | 101 (3.2) |
| DBP | 91.0 (6.5) | 94.8 (3.3) | 100 (14) | 92.1 (8.8) | 114 (2.5) | 110 (3.6) |
| DMEP | 90.3 (6.8) | 103 (7.2) | 107 (12) | 99.2 (12) | 114 (3.7) | 108 (0.82) |
| DPP | 96.8 (11) | 98.3 (0.40) | 101 (14) | 96.3 (10) | 110 (6.6) | 107 (2.6) |
| BBP | 89.7 (3.5) | 83.7 (0.35) | 92.0 (16) | 86.7 (8.9) | 103 (0.34) | 96.9 (4.2) |
| DEHA | 83.9 (7.8) | 84.5 (2.7) | 87.8 (14) | 85.2 (8.5) | 86.4 (0.87) | 95.0 (0.76) |
| Plasticizers | Recoveries (%, RSD) | |||||
| 2 µg·g−1 | 10 µg·g−1 | 20 µg·g−1 | ||||
| Mortar | Vial | Mortar | Vial | Mortar | Vial | |
| DIHP | 104 (5.3) | 102 (1.1) | 106 (6.3) | 88.4 (6.0) | 96.9 (6.2) | 93.7 (5.1) |
| DEHP | 98.1 (3.6) | 81.1 (0.73) | 93.3 (13) | 92.4 (4.8) | 101 (8.8) | 96.4 (3.1) |
| DCHP | 88.5 (2.4) | 92.0 (5.1) | 93.0 (12) | 87.5 (6.4) | 105 (2.4) | 102 (3.4) |
| DPhP | 84.9 (4.6) | 84.4 (1.3) | 90.5 (0.61) | 84.3 (8.6) | 102 (0.29) | 96.9 (4.2) |
| DNOP | 87.1 (1.2) | 88.4 (2.8) | 89.0 (15) | 84.1 (9.2) | 103 (3.6) | 98.5 (2.2) |
| Musks | ||||||
| Cashmeran | 87.4 (2.0) | 82.5 (9.5) | 98.0 (13) | 89.1 (13) | 110 (1.5) | 103 (7.2) |
| Celestolide | 81.8 (5.7) | 82.9 (0.80) | 94.6 (9.9) | 87.4 (3.1) | 109 (1.9) | 96.9 (9.0) |
| Phantolide | 86.3 (4.9) | 90.4 (0.67) | 102 (10) | 94.3 (3.4) | 115 (3.3) | 102 (11) |
| Ambrette | 86.8 (9.3) | 83.7 (14) | 93.8 (0.23) | 104 (7.1) | 115 (6.1) | 91.5 (6.2) |
| Traseolide | 88.1 (7.6) | 90.7 (3.3) | 99.4 (9.4) | 91.0 (4.5) | 114 (2.6) | 97.9 (10) |
| Galaxolide | 88.9 (0.27) | 94.7 (0.52) | 103 (15) | 95.3 (13) | 114 (1.4) | 105 (3.3) |
| Xylene | 81.1 (12) | 82.1 (5.6) | 68.3 (2.1) | 79.1 (2.6) | 93.3 (3.1) | 80.0 (6.2) |
| Tonalide | 83.1 (1.4) | 88.4 (0.72) | 87.5 (14) | 83.1 (8.2) | 101 (0.92) | 96.4 (1.4) |
| Moskene | 89.8 (13) | 86.1 (15) | 93.5 (7.3) | 83.2 (4.4) | 114 (3.7) | 94.6 (15) |
| Tibetene | 103 (8.1) | 109 (3.8) | 100 (14) | 93.0 (13) | 115 (3.6) | 111 (2.6) |
| Ambrettolide | 96.0 (5.6) | 111 (7.9) | 106 (1.1) | 107 (12) | 113 (3.0) | 108 (8.8) |
| Ketone | 101 (14) | 109 (6.1) | 104 (10) | 97.9 (15) | 114 (5.9) | 109 (6.9) |
| Fragrance Allergens | Recoveries (%, RSD) | |||||
|---|---|---|---|---|---|---|
| 2 µg·g−1 | 10 µg·g−1 | 20 µg·g−1 | ||||
| Mortar | Vial | Mortar | Vial | Mortar | Vial | |
| Pinene | 4.08 (12) | 20.8 (0.59) | 5.13 (0.38) | 36.5 (1.2) | 9.5 (6.9) | 36.8 (9.4) |
| Limonene | 28.2 (11) | 64.7 (2.1) | 28.1 (7.5) | 75.8 (15) | 38.9 (5.7) | 65.9 (14) |
| Benzyl alcohol | 113 (9.3) | 90.8 (13) | 85.7 (1.7) | 92.2 (1.5) | 105 (8.7) | 102 (8.9) |
| Linalool | 81.3 (9.3) | 114 (6.9) | 85.4 (3.6) | 109 (2.5) | 95.5 (14) | 88.7 (0.53) |
| Methyl-2-octynoate | 92.4 (2.8) | 83.2 (5.2) | 94.0 (6.7) | 101 (5.6) | 106 (11) | 88.5 (15) |
| Citronellol | 108 (1.4) | 114 (5.0) | 90.9 (5.0) | 99.1 (3.8) | 100 (14) | 102 (6.8) |
| Citral | 100 (3.0) | 107 (10) | 83.3 (6.4) | 103 (7.0) | 103 (12) | 97.7 (4.3) |
| Geraniol | 109 (4.0) | 94.9 (13) | 90.4 (11) | 102 (3.4) | 93.9 (13) | 92.6 (0.44) |
| Cinnamal | 105 (3.6) | 89.1 (11) | 88.5 (5.3) | 94.2 (0.88) | 103 (7.2) | 96.0 (15) |
| Hydroxycitronellal | 100 (14) | 82.1 (5.0) | 80.1 (2.7) | 83.7 (4.7) | 89.9 (12) | 87.6 (3.7) |
| Anise alcohol | 112 (8.5) | 90.1 (15) | 86.6 (2.4) | 97.1 (0.76) | 101 (10) | 91.7 (9.3) |
| Cinnamyl alcohol | 113 (7.9) | 83.4 (15) | 87.6 (4.3) | 97.7 (0.40) | 97.9 (13) | 95.5 (13) |
| Eugenol | 106 (3.5) | 88.6 (13) | 87.5 (1.9) | 99.1 (1.2) | 98.6 (10) | 92.1 (15) |
| Methyleugenol | 110 (10) | 94.3 (1.5) | 96.4 (1.2) | 102 (0.33) | 104 (4.4) | 95.9 (10) |
| Isoeugenol | 87.9 (10) | 83.8 (6.9) | 107 (2.6) | 119 (0.42) | 96.2 (5.0) | 92.7 (10) |
| Coumarin | 111 (12) | 90.1 (3.8) | 89.7 (1.7) | 97.1 (3.5) | 104 (1.7) | 97.8 (7.9) |
| α-isomethyl ionone | 110 (10) | 97.4 (0.76) | 94.4 (0.84) | 98.4 (1.2) | 103 (4.3) | 93.4 (6.5) |
| Lilial® | 106 (12) | 91.7 (2.3) | 88.2 (0.10) | 93.3 (4.1) | 98.9 (5.3) | 88.3 (12) |
| Amyl cinnamal | 112 (5.8) | 106 (9.2) | 102 (3.6) | 109 (4.1) | 108 (11) | 102 (16) |
| Lyral® | 105 (1.7) | 112 (9.0) | 89.2 (1.2) | 99.4 (2.3) | 98.8 (11) | 82.6 (4.9) |
| Amylcinnamyl alcohol | 113 (8.3) | 118 (5.5) | 98.8 (1.8) | 114 (1.0) | 112 (7.7) | 113 (12) |
| Farnesol | <LOQ | <LOQ | 97 (3.2) | 111 (6.8) | 95.0 (15) | 108 (8.4) |
| Hexylcinnamal | 108 (1.2) | 105 (5.5) | 105 (3.1) | 111 (4.7) | 108 (9.5) | 104 (11) |
| Benzyl benzoate | 111 (11) | 98.4 (1.5) | 92.3 (1.6) | 103 (0.42) | 104 (3.1) | 97.6 (5.6) |
| Benzyl salicylate | 116 (1.2) | 113 (3.7) | 80.8 (0.51) | 102 (4.5) | 111 (8.4) | 104 (10) |
| Benzyl cinnamate | 102 (12) | 102 (2.1) | 102 (5.4) | 113 (3.9) | 119 (4.4) | 116 (3.3) |
| Preservatives | ||||||
| Bronidox | 104 (2.8) | 90.4 (11) | 94.4 (3.2) | 97 (5.3) | 101 (7.7) | 95.9 (16) |
| PhEtOH | 112 (10) | 90.5 (2.1) | 91.7 (3.1) | 94 (5.5) | 98.4 (12) | 92.6 (11) |
| MeP a | n.c | n.c | n.c | n.c | n.c | n.c |
| BHA | 106 (12) | 98.4 (1.3) | 105 (5.7) | 110 (1.1) | 100 (3.1) | 100 (8.3) |
| BHT a | n.c | n.c | n.c | n.c | n.c | n.c |
| EtP | 112 (1.0) | 103 (1.7) | 102 (2.5) | 102 (0.32) | 110 (3.9) | 108 (10) |
| iPrP | 118 (3.2) | 114 (15) | 102 (1.1) | 110 (3.4) | 111 (6.8) | 113 (7.4) |
| PrP a | n.c | n.c | n.c | n.c | n.c | n.c |
| IPBC | 113 (8.7) | 97.7 (14) | 103 (8.0) | 117 (0.50) | 111 (16) | 95.4 (7.9) |
| iBuP | 94.1 (15) | 103 (2.4) | 104 (2.9) | 109 (2.3) | 111 (0.13) | 113 (8.4) |
| BuP | 115 (1.0) | 110 (1.6) | 108 (2.7) | 114 (2.4) | 116 (1.6) | 113 (10) |
| Triclosan | 87.0 (6.9) | 84.4 (6.1) | 81.7 (6.7) | 91 (7.8) | 118 (6.4) | 113 (11) |
| BzP | 87.3 (11) | 104 (9.5) | 81.4 (2.0) | 112 (1.7) | 104 (12) | 103 (11) |
| Plasticizers | ||||||
| DMA | 98.6 (5.5) | 95.1 (2.7) | 104 (0.65) | 72.4 (0.65) | 104 (1.3) | 96.6 (9.4) |
| DEA | 101 (5.5) | 81.3 (6.9) | 94.8 (1.6) | 92.8 (1.1) | 103 (4.0) | 91.8 (11) |
| DMP | 109 (12) | 87.9 (1.5) | 95.4 (2.7) | 94.2 (0.72) | 103 (1.4) | 95.0 (7.6) |
| DEP | 105 (7.0) | 92.2 (0.34) | 102 (2.5) | 100 (0.20) | 101 (3.2) | 100 (8.6) |
| DIBP | 115 (4.9) | 93.3 (2.7) | 94.1 (2.4) | 102 (0.64) | 98.9 (4.6) | 102 (10) |
| DBP | 117 (12) | 109 (0.69) | 91.5 (2.0) | 108 (1.7) | 105 (5.9) | 110 (9.3) |
| DMEP | 112 (10) | 100 (1.6) | 112 (7.9) | 119 (4.9) | 113 (2.3) | 112 (8.7) |
| DPP | 113 (11) | 88.4 (4.1) | 94.7 (4.2) | 107 (0.56) | 114 (3.9) | 115 (5.9) |
| Plasticizers | Recoveries (%, RSD) | |||||
| 2 µg·g−1 | 10 µg·g−1 | 20 µg·g−1 | ||||
| Mortar | Vial | Mortar | Vial | Mortar | Vial | |
| BBP | 114 (5.6) | 115 (2.2) | 101 (5.4) | 108 (2.4) | 117 (3.0) | 119 (0.20) |
| DEHA | 96.9 (9.1) | 105 (7.0) | 101 (6.6) | 108 (8.5) | 112 (1.0) | 107 (2.8) |
| DIHP | 98.5 (1.4) | 102 (7.9) | 98.1 (1.7) | 102 (3.4) | 99.2 (6.8) | 113 (7.3) |
| DEHP | 116 (2.3) | 93.4 (3.5) | 103 (4.5) | 101 (4.6) | 109 (4.4) | 103 (5.2) |
| DCHP | 116 (8.7) | 107 (0.94) | 107 (6.9) | 107 (1.5) | 112 (10) | 114 (0.43) |
| DPhP | 116 (11) | 105 (1.5) | 95.9 (6.9) | 104 (3.2) | 113 (1.1) | 108 (2.2) |
| DNOP | 118 (4.4) | 87.8 (8.3) | 96.1 (6.5) | 106 (0.48) | 111 (2.3) | 120 (4.4) |
| Musks | ||||||
| Cashmeran | 112 (8.9) | 101 (1.8) | 113 (0.77) | 99 (1.2) | 103 (4.0) | 96.8 (11) |
| Celestolide | 113 (12) | 115 (1.1) | 114 (0.99) | 107 (0.47) | 104 (4.0) | 101 (6.8) |
| Phantolide | 109 (10) | 107 (3.4) | 113 (1.4) | 111 (4.2) | 102 (10) | 103 (14) |
| Ambrette | 118 (6.4) | 104 (10) | 118 (6.7) | 118 (11) | 100 (10) | 106 (10) |
| Traseolide | 113 (10) | 104 (7.3) | 95.2 (1.4) | 102 (3.8) | 103 (9.2) | 100 (12) |
| Galaxolide | 113 (13) | 100 (2.6) | 106 (2.0) | 98.9 (0.011) | 101 (4.0) | 97.2 (8.0) |
| Xylene | 118 (2.9) | 111 (8.2) | 101 (5.6) | 88.9 (14) | 104 (7.1) | 114 (4.3) |
| Tonalide | 117 (16) | 113 (2.6) | 90.7 (0.77) | 89.8 (1.3) | 101 (5.4) | 98.0 (7.6) |
| Moskene | 111 (15) | 96.7 (3.6) | 109 (4.0) | 105 (10) | 103 (16) | 96.0 (7.3) |
| Tibetene | 112 (12) | 108 (5.7) | 111 (1.7) | 99.0 (6.2) | 104 (10) | 100 (16) |
| Ambrettolide | 115 (3.8) | 111 (3.5) | 99.3 (1.3) | 101 82.7) | 104 (6.1) | 108 (14) |
| Ketone | 79.6 (4.5) | 114 (13) | 93.5 (0.75) | 109 (4.0) | 117 (8.0) | 113 (11) |
| Fragrance Allergens | Recoveries (%, RSD) | |||||
|---|---|---|---|---|---|---|
| S3 a | S6 a | S8 a | ||||
| Mortar | Vial | Mortar | Vial | Mortar | Vial | |
| Pinene | 4.2 (7.3) | 72.1 (1.5) | 11.2 (3.1) | 74.0 (0.013) | 21.2 (11) | 74.3 (4.1) |
| Limonene | 35.4 (5.6) | 90.3 (1.7) | 81.3 (3.1) | 77.0 (2.1) | 57.1 (2.5) | 93.0 (0.87) |
| Benzyl alcohol | 115 (6.9) | 109 (0.19) | 114 (4.2) | 91.2 (10) | 113 (0.51) | 116 (0.38) |
| Linalool | 102 (4.5) | 117 (0.021) | n.c | n.c | n.c | n.c |
| Methyl-2-octynoate | 106 (10.4) | 93.0 (3.4) | 94.9 (8.6) | 81.3 (9.7) | 103 (3.7) | 113 (6.1) |
| Citronellol | 89.8 (13) | 101 (2.8) | 110 (3.8) | 100 (2.2) | 90.0 (2.1) | 100 (2.8) |
| Citral | 113 (7.1) | 105 (6.7) | 115 (5.0) | 94.2 (4.0) | 107 (2.9) | 108 (8.1) |
| Geraniol | 80.4 (13) | 93.4 (1.9) | 107 (7.8) | 92.4 (4.1) | 91.2 (8.1) | 98.2 (9.7) |
| Cinnamal | 98.8 (9.4) | 103 (2.8) | 106 (7.4) | 98.2 (4.6) | 101 (2.4) | 108 (2.5) |
| Hydroxycitronellal | 108 (12) | 102 (6.8) | 93.6 (6.3) | 81.0 (7.4) | 112 (6.1) | 111 (6.8) |
| Anise alcohol | 98.3 (13) | 101 (4.2) | 95.4 (5.2) | 96.1 (6.2) | 83.3 (6.6) | 104 (0.041) |
| Cinnamyl alcohol | 81.7 (15) | 96.9 (6.7) | 112 (11) | 91.4 (8.8) | 106 (8.7) | 109 (3.5) |
| Eugenol | 100 (11) | 95.4 (4.2) | 115 (10) | 92.1 (3.6) | 101 (6.1) | 109 (5.9) |
| Methyleugenol | 100 (8.3) | 110 (0.45) | 96.4 (6.5) | 96.1 (4.9) | 100 (3.5) | 106 (3.7) |
| Isoeugenol | 102 (9.9) | 93.0 (2.9) | 114 (15) | 82.5 (5.3) | 86.2 (3.3) | 95.2 (0.45) |
| Coumarin | 107 (11) | 118 (0.62) | 95.8 (2.1) | 81.0 (1.5) | 91.3 (1.3) | 97.1 (0.52) |
| α-isomethyl ionone | 98.4 (7.5) | 104 (1.9) | 96.8 (3.7) | 94.6 (0.24) | 98.8 (3.1) | 103 (2.5) |
| Lilial® | 99.2 (8.6) | 98.1 (1.2) | 97.2 (4.5) | 85.0 (0.055) | 96.3 (3.7) | 102 (4.2) |
| Amyl cinnamal | 106 (10) | 107 (0.82) | 105 (8.1) | 96.4 (3.6) | 98.7 (4.5) | 106 (4.2) |
| Lyral® | 115 (7.3) | 98.0 (1.5) | 85.8 (12) | 82.0 (11) | 112 (6.7) | 114 (7.5) |
| Amylcinnamyl alcohol | 104 (10) | 98.3 (6.7) | 109 (9.7) | 96.5 (9.4) | 111 (4.3) | 111 (9.1) |
| Farnesol | 92.3 (13) | 95.0 (0.29) | 97.2 (1.8) | 95.9 (13) | 109 (8.5) | 106 (12) |
| Hexylcinnamal | 107 (14) | 119 (0.29) | n.c | n.c | 105 (5.9) | 112 (5.9) |
| Benzyl benzoate | 102 (9.0) | 105 (0.43) | 101 (2.4) | 95.4 (1.2) | 107 (3.8) | 113 (5.1) |
| Benzyl salicylate | 112 (10) | 115 (6.9) | n.c | n.c | 113 (3.5) | 114 (4.3) |
| Benzyl cinnamate | 115 (6.7) | 116 (0.14) | 113 (9.0) | 112 (0.40) | 113 (6.1) | 115 (4.8) |
| Preservatives | Recoveries (%, RSD) | |||||
| S3 a | S6 a | S8 a | ||||
| Mortar | Vial | Mortar | Vial | Mortar | Vial | |
| Bronidox | 93.9 (7.5) | 101 (2.7) | 106 (5.2) | 108 (0.94) | 105 (0.72) | 95.0 (5.2) |
| PhEtOH | n.c | n.c | n.c | n.c | n.c | n.c |
| MeP | 112 (11) | 100 (7.7) | n.c | n.c | n.c | n.c |
| BHA | 99.1 (9.6) | 94.2 (0.89) | 109 (6.2) | 83.2 (0.91) | 93.1 (3.1) | 102 (2.7) |
| BHT | 98.4 (3.1) | 92.0 (0.040) | n.c | n.c | 91.2 (3.4) | 100 (3.2) |
| EtP | 108 (7.7) | 92.0 (9.8) | n.c | n.c | n.c | n.c |
| iPrP | 105 (11) | 114 (8.5) | 114 (6.3) | 115 (1.2) | 94.9 (3.9) | 103 (3.8) |
| PrP | 115 (8.7) | 115 (1.14) | n.c | n.c | n.c | n.c |
| IPBC | 106 (14) | 84.2 (10) | 101 (7.4) | 113 (15) | 110 (18) | 114 (10) |
| iBuP | 105 (13) | 111 (5.3) | n.c | n.c | 112 (9.6) | 106 (1.4) |
| BuP | 103 (14) | 107 (0.79) | n.c | n.c | 107 (13) | 103 (6.8) |
| Triclosan | 119 (5.8) | 96.0 (3.0) | 114 (3.3) | 106 (13) | 104 (8.8) | 110 (3.5) |
| BzP | 96.0 (14) | 108 (7.9) | 95.1 (2.8) | 116 (9.0) | 110 (10) | 102 (3.5) |
| Plasticizers | ||||||
| DMA | 105 (8.1) | 105 (1.1) | 97.0 (3.7) | 84.0 (2.8) | 95.1 (0.59) | 98.2 (3.1) |
| DEA | 108 (6.5) | 100 (2.9) | 104 (5.2) | 96.1 (2.4) | 105 (4.6) | 113 (5.4) |
| DMP | 96.4 (7.6) | 104 (1.8) | 95.0 (5.8) | 88.3 (2.0) | 95.2 (0.75) | 101 (1.5) |
| DEP | 97.8 (7.4) | 107 (0.41) | n.c | n.c | n.c | n.c |
| DIBP | 97.6 (10) | 104 (0.24) | 100 (2.5) | 88.2 (1.5) | 96.4 (2.5) | 102 (3.1) |
| DBP | 109 (8.4) | 115 (1.1) | 108 (4.4) | 98.7 (2.1) | 106 (3.2) | 112 (4.1) |
| DMEP | 113 (8.7) | 119 (5.8) | 98.2 (7.0) | 103 (3.0) | 107 (7.9) | 114 (4.9) |
| DPP | 108 (7.3) | 108 (0.93) | 112 (3.7) | 101 (0.82) | 114 (2.7) | 115 (2.2) |
| BBP | 97.3 (8.1) | 97 (1.9) | 109 (3.6) | 86.0 (0.94) | 103 (4.8) | 110 (0.74) |
| DEHA | 112 (6.9) | 83 (3.3) | 83.1 (4.1) | 80.2 (1.7) | 96.4 (4.9) | 103 (0.70) |
| DIHP | 113 (6.6) | 115 (4.6) | 108 (7.3) | 107 (15) | 101 (10) | 89.0 (1.5) |
| Plasticizers | Recoveries (%, RSD) | |||||
| S3 a | S6 a | S8 a | ||||
| Mortar | Vial | Mortar | Vial | Mortar | Vial | |
| DEHP | 114 (13) | 93 (1.3) | 99.2 (4.4) | 90.3 (2.2) | 105 (2.1) | 112 (2.1) |
| DCHP | 113 (14) | 96 (0.80) | 94.0 (8.6) | 88.5 (1.5) | 97.2 (8.5) | 97.2 (7.5) |
| DPhP | 112 (7.6) | 102 (0.13) | 115 (3.0) | 92.0 (5.9) | 102 (3.1) | 108 (3.1) |
| DNOP | 102 (8.1) | 114 (0.88) | 113 (2.8) | 100 (0.31) | 114 (1.5) | 113 (2.4) |
| Musks | ||||||
| Cashmeran | 98.4 (8.6) | 96.0 (0.32) | 93.0 (7.0) | 88.3 (1.6) | 97.2 (2.4) | 103 (2.5) |
| Celestolide | 102 (8.5) | 103 (1.0) | 93.5 (6.9) | 93.5 (1.6) | 98.0 (4.8) | 105 (5.0) |
| Phantolide | 100 (8.1) | 102 (1.1) | 96.4 (4.4) | 93.9 (1.0) | 101 (4.8) | 107 (4.4) |
| Ambrette | 92.0 (13) | 84.2 (13) | 113 (6.3) | 112 (15) | 112 (10) | 113 (11) |
| Traseolide | 98.8 (10) | 100 (2.3) | 105 (7.7) | 101 (0.10) | 105 (5.3) | 113 (4.9) |
| Galaxolide | 98.0 (10) | 102 (0.72) | n.c | n.c | n.c | n.c |
| Xylene | 88.2 (9.2) | -- | 111 (8.9) | -- | 86.0 (5.5) | 97.4 (7.4) |
| Tonalide | 98.4 (10) | 98.0 (0.83) | 81.0 (2.6) | 84.0 (2.2) | n.c | n.c |
| Moskene | 93.0 (12) | 89.1 (11) | 109 (11) | 103 (15) | 98.2 (8.1) | 103 (8.6) |
| Tibetene | 107 (10) | 105 (4.4) | 111 (11) | 106 (7.8) | 104 (7.1) | 109 (7.9) |
| Ambrettolide | 114 (13) | 101 (0.98) | 94.4 (10) | 86.2 (4.8) | 80.1 (10) | 93.2 (3.5) |
| Ketone | 109 (11) | 99.0 (7.4) | 109 (13) | 114 (3.7) | 108 (9.5) | 115 (10) |
3.3. Application to Real Samples
3.3.1. Fragrance Allergens
| Fragrance Allergens | Rinse-off Samples b | Leave-on Samples b | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | S11 | S12 | S13 | S14 | S15 | S16 | S17 | S18 | |
| Pinene | - | 0.777 | - | 1.18 | - | - | 2.24 | - | - | - | 1.51 | - | 0.972 | 0.396 | 1.40 | 0.881 | 0.426 | 0.0800 |
| Limonene | 266 | 148 | - | 283 | - | 0.446 | 199 | - | 152 | - | 11.0 | 1.31 | 163 | 222 | 53.2 | 14.3 | 2.73 | 6.38 |
| Benzyl alcohol | 4.00 | - | - | - | 3.32 | 3.06 | 7.55 | - | 2.50 | 2.93 | 31.2 | 3.76 | 17.9 | - | - | 6.14 | 0.698 | 27.5 |
| Linalool | 666 | 84.6 | - | 172 | - | 27.3 | 358 | 17.2 | 369 | 101 | 161 | 252 | 178 | 1024 | 255 | 228 | - | 26.4 |
| Methyl-2-octynoate | 60.4 | - | - | - | - | - | - | 105 | 24.7 | - | - | - | 72.0 | - | - | - | - | - |
| Citronellol | 25.0 | - | - | - | - | - | 79.6 | - | - | 1.45 | 45.3 | 234 | - | 63.1 | - | 58.0 | 7.75 | 13.2 |
| Citral | - | - | - | 13.6 | - | - | 8.84 | - | 10.5 | 6.28 | - | - | - | - | 6.50 | - | - | - |
| Geraniol | 90.2 | 19.1 | - | 44.5 | - | - | 167 | - | - | 2.83 | 23.1 | 10.3 | - | - | 37.2 | 41.1 | - | 17.3 |
| Cinnamal | - | - | - | - | - | - | - | - | - | - | - | - | - | 4.73 | - | 1.14 | - | - |
| Hydroxycitronellal | - | - | 2.08 | - | - | 0.212 | 33.1 | - | - | - | 57.4 | - | - | 1.29 | - | 18.3 | 52.0 | 1.00 |
| Anise alcohol | - | - | - | - | - | 0.155 | - | 23.6 | - | - | - | - | - | - | - | - | - | - |
| Cinnamyl alcohol | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 12.4 | 9.23 | 6.42 |
| Eugenol | - | - | - | - | - | 2.67 | - | - | - | - | - | 1.66 | 31.8 | 40.5 | - | - | 3.63 | 0.114 |
| Coumarin | 30.8 | 3.44 | 6.89 | 9.55 | - | 0.460 | 11.8 | - | 16.6 | - | - | 113 | 21.0 | 557 | 3.25 | - | 1.79 | 20.3 |
| α-isomethyl ionone | 3.32 | - | - | - | - | 0.297 | 31.5 | - | 48.5 | - | 36.5 | - | - | 136 | - | 89.9 | 167 | 31.8 |
| Lilial® | - | 7.83 | 1.36 | - | - | 0.843 | 30.8 | - | 172 | - | 14.0 | 331 | 491 | 2.79 | 233 | 82.4 | - | - |
| Amyl cinnamal | - | 116 | - | - | - | - | - | - | - | 7.34 | - | - | - | - | - | - | - | - |
| Lyral® | - | - | - | - | - | - | 108 | - | - | - | 211 | - | - | - | - | 58.1 | 41.1 | 10.7 |
| Farnesol | - | - | - | - | - | - | - | - | - | - | - | 2924 | 2188 | - | - | - | - | - |
| Hexylcinnamal | - | - | - | - | - | 12.9 | 29.6 | 27.4 | 115 | 393 | 885 | 4.37 | 0.475 | 60.2 | - | 74.5 | ||
| Benzyl benzoate | - | 0.316 | - | 5.88 | 0.380 | 0.452 | 5.66 | - | - | - | 7.63 | 1.53 | - | 0.789 | 1.28 | 4.21 | 14.1 | 8.28 |
| Benzyl salicylate | - | 2.48 | - | 524 | 0.355 | 12.4 | 4.29 | - | 217 | - | - | 111 | 770 | 0.642 | 1.70 | 486 | 19.9 | 10.3 |
| Preservatives | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | S11 | S12 | S13 | S14 | S15 | S16 | S17 | S18 |
| PhEtOH | 14.2 | - | 15.3 | 1.30 | 2009 | 1395 | 2455 | 3609 | 4017 | 1.45 | 15530 | - | 0.383 | - | 17.3 | 1993 | 1877 | 1849 |
| MePc | - | 4.65 | 3.22 | - | 284 | 1244 | 654 | 789 | 887 | - | 1484 | - | - | - | 2.74 | 540 | - | 1350 |
| BHA | - | - | - | - | - | 2.78 | - | - | - | - | - | - | - | - | - | - | - | - |
| BHT | 0.544 | 1.98 | - | 0.105 | 0.0430 | 12.1 | 186 | - | - | 2996 | - | 20.3 | 9.93 | 5.01 | 625 | 0.237 | 25.2 | |
| Preservatives | Rinse-off Samples b | Leave-on Samples b | ||||||||||||||||
| S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | S11 | S12 | S13 | S14 | S15 | S16 | S17 | S18 | |
| EtP c | - | - | - | - | 89.7 | 135 | 202 | 203 | - | - | - | - | - | 5.19 | 119 | - | 15.46 | |
| iPrP c | - | - | - | - | 144 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| PrP c | - | 1.61 | 0.374 | - | 60.1 | 470 | 1.45 | 364 | 262 | 1146 | 7660 | - | - | - | 1.76 | 62.4 | - | 588 |
| IPBC | - | - | 0.504 | 40.2 | - | - | - | - | - | - | - | 5.94 | - | - | - | - | - | - |
| iBuP c | - | - | 0.258 | - | 46.7 | 57.3 | - | - | - | - | - | - | - | - | - | 64.5 | - | - |
| BuP c | - | - | 1.17 | - | 91.0 | 146 | - | - | - | - | - | - | - | - | 0.280 | 122 | - | - |
| Triclosan | - | - | - | - | - | - | - | - | - | - | - | - | 2794 | 1.21 | - | - | - | - |
| Plasticizers | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | S11 | S12 | S13 | S14 | S15 | S16 | S17 | S18 |
| DEP | 432 | 153 | 0.888 | 0.190 | 0.316 | 109 | 39.4 | 46.0 | 0.118 | 0.378 | 20.4 | 0.737 | 1.88 | 1539 | 0.254 | 40.5 | 303 | 0.300 |
| DIBP | - | 0.138 | - | - | - | - | - | - | 0.337 | 0.420 | 26.4 | - | 0.292 | 0.546 | - | - | - | - |
| DBP | 0.409 | 0.0722 | 0.0511 | - | - | 0.509 | 0.194 | 0.340 | 0.299 | 0.549 | 1434 | - | 2.24 | 0.575 | 1.37 | - | - | 1.24 |
| DEHA | - | 10.4 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 16387 | - |
| DEHP | 0.640 | 0.731 | 0.168 | 0.553 | 0.800 | - | 0.986 | - | - | - | 4.78 | 1.24 | 1.02 | 0.818 | - | 1.78 | - | - |
| DCHP | - | - | - | - | - | - | - | - | - | - | - | - | 0.196 | - | - | - | - | - |
| DPhP | - | - | - | - | - | - | - | - | - | 8.48 | - | - | - | - | - | - | - | - |
| DNOP | - | - | - | - | - | - | - | - | - | - | - | - | 0.444 | 0.0932 | - | - | - | - |
| Musks | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | S11 | S12 | S13 | S14 | S15 | S16 | S17 | S18 |
| Celestolide | 1.23 | - | - | - | - | - | 0.598 | 0.110 | - | - | - | - | - | - | - | - | - | - |
| Phantolide | 0.320 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Galaxolide | 625 | 0.379 | - | 0.0820 | - | 114 | 93.2 | 128 | - | 0.119 | 3.90 | 199 | 0.120 | 0.844 | - | 86.8 | 211 | - |
| Xylene | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 3.14 | - | - |
| Tonalide | 244 | - | - | - | - | - | - | 20.8 | - | - | 3.54 | - | - | 0.153 | - | 26.3 | - | - |
| Ambrettolide | - | - | 16.0 | 34.7 | - | - | - | - | - | - | 10.8 | - | - | - | - | - | - | - |
| Ketone | - | - | - | - | - | - | 33.4 | - | - | - | - | - | - | - | - | 1368 | - | - |
3.3.2. Preservatives
3.3.3. Plasticizers
3.3.4. Musks
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- European Union. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products (recast). Off. J. Eur. Union 2009, L342, 59–209. [Google Scholar]
- Kirchhof, M.G.; de Gannes, G.C. The health controversies of parabens. Skin Ther. Lett. 2013, 18, 5–7. [Google Scholar]
- Polati, S.; Gosetti, S.; Gennaro, M.C. Preservatives in Cosmetics. Analytical Methods. In Analysis of Cosmetic Products; Salvador, A., Chisvert, A., Eds.; Elsevier: Amsterdam, The Netherland, 2007. [Google Scholar]
- Darbre, P.D.; Harvey, P.W. Paraben esters: Review of recent studies of endocrine toxicity, absorption, esterase and human exposure, and discussion of potential human health risks. J. Appl. Toxicol. 2008, 28, 561–578. [Google Scholar] [CrossRef]
- Nohynek, G.J.; Borgert, C.J.; Dietrich, D.; Rozman, K.K. Endocrine disruption: Fact or urban legend? Toxicol. Lett. 2013, 223, 295–305. [Google Scholar] [CrossRef]
- Boberg, J.; Taxvig, C.; Christiansen, S.; Hass, U. Possible endocrine disrupting effects of parabens and their metabolites. Reprod. Toxicol. 2010, 30, 301–312. [Google Scholar] [CrossRef]
- ANSM. L’ANSM Recommande de Restreindre la Concentration de Phénoxyéthanol dans les Produits Cosmétiques Destinés aux Enfants de Moins de 3 ans—Point D’information. Available online: http://ansm.sante.fr/S-informer/Points-d-information-Points-d-information/L-ANSM-recommande-de-restreindre-la-concentration-de-phenoxyethanol-dans-les-produits-cosmetiques-destines-aux-enfants-de-moins-de-3-ans-Point-d-information (accessed on 22 May 2014).
- Roosens, L.; Covaci, A.; Neels, H. Concentrations of synthetic musk compounds in personal care and sanitation products and human exposure profiles through dermal application. Chemosphere 2007, 69, 1540–1547. [Google Scholar] [CrossRef]
- Petersen, G.; Rasmussen, D.; Gustavson, K. Study on Enhancing the Endocrine Disrupter Priority List with a Focus on Low Production Volume Chemiclas; DHI Water and Environment: Hørsholm, Denmark, 2007. [Google Scholar]
- Chisvert, A.; Salvador, A. Perfumes in Cosmetics. Analytical Methods. In Analysis of Cosmetic Products; Salvador, A., Chisvert, A., Eds.; Elsevier: Amsterdam, The Netherland, 2007. [Google Scholar]
- Cabaleiro, N.; de la Calle, I.; Bendicho, C.; Lavilla, I. Current trends in liquid-liquid and solid-liquid extraction for cosmetic analysis: A review. Anal. Methods 2013, 5, 323–340. [Google Scholar] [CrossRef]
- Cabaleiro, N.; de la Calle, I.; Bendicho, C.; Lavilla, I. An overview of sample preparation for the determination of parabens in cosmetics. TrAC Trends Anal. Chem. 2014, 57, 34–46. [Google Scholar]
- Wang, L.-H. Fragrances: from essential oils to the human body and atmospheric aerosols. Anal. Methods 2013, 5, 316–322. [Google Scholar] [CrossRef]
- Capriotti, A.L.; Cavaliere, C.; Lagana, A.; Piovesana, S.; Samperi, R. Recent trends in matrix solid-phase dispersion. TrAC Trends Anal. Chem. 2013, 43, 53–66. [Google Scholar] [CrossRef]
- Kristenson, E.M.; Brinkman, U.A.T.; Ramos, L. Recent advances in matrix solid-phase dispersion. TrAC Trends Anal. Chem. 2006, 25, 96–111. [Google Scholar] [CrossRef]
- Alvarez-Rivera, G.; Dagnac, T.; Lores, M.; Garcia-Jares, C.; Sanchez-Prado, L.; Lamas, J.P.; Llompart, M. Determination of isothiazolinone preservatives in cosmetics and household products by matrix solid-phase dispersion followed by high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2012, 1270, 41–50. [Google Scholar] [CrossRef]
- Sanchez-Prado, L.; Alvarez-Rivera, G.; Lamas, J.P.; Llompart, M.; Lores, M.; Garcia-Jares, C. Content of suspected allergens and preservatives in marketed baby and child care products. Anal. Methods 2013, 5, 416–427. [Google Scholar]
- Sanchez-Prado, L.; Alvarez-Rivera, G.; Lamas, J.P.; Lores, M.; Garcia-Jares, C.; Llompart, M. Analysis of multi-class preservatives in leave-on and rinse-off cosmetics by matrix solid-phase dispersion. Anal. Bioanal. Chem. 2011, 401, 3293–3304. [Google Scholar] [CrossRef]
- Llompart, M.; Celeiro, M.; Lamas, P.J.; Sanchez-Prado, L.; Lores, M.; Garcia-Jares, C. Analysis of plasticizers and synthetic musks in cosmetic and personal care products by matrix solid-phase dispersion gas chromatography-mass spectrometry. J. Chromatogr. A 2013, 1293, 10–19. [Google Scholar] [CrossRef]
- Celeiro, M.; Guerra, E.; Lamas, J.P.; Lores, M.; Garcia-Jares, C.; Llompart, M. Development of a multianalyte method based on micro-matrix-solid-phase dispersion for the analysis of fragrance allergens and preservatives in personal care products. J. Chromatogr. A 2014, 1344, 1–14. [Google Scholar]
- Sanchez-Prado, L.; Lamas, J.P.; Alvarez-Rivera, G.; Lores, M.; Garcia-Jares, C.; Llompart, M. Determination of suspected fragrance allergens in cosmetics by matrix solid-phase dispersion gas chromatography-mass spectrometry analysis. J. Chromatogr. A 2011, 1218, 5055–5062. [Google Scholar]
- Lamas, J.P.; Sanchez-Prado, L.; Garcia-Jares, C.; Lores, M.; Llompart, M. Development of a solid phase dispersion-pressurized liquid extraction method for the analysis of suspected fragrance allergens in leave-on cosmetics. J. Chromatogr. A 2010, 1217, 8087–8094. [Google Scholar] [CrossRef]
- Becerril-Bravo, E.; Lamas, P.J.; Sanchez-Prado, L.; Lores, M.; Garcia-Jares, C.; Jimenez, B.; Llompart, M. Ultrasound-assisted emulsification-microextraction of fragrance allergens in water. Chemosphere 2010, 81, 1378–1385. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Celeiro, M.; Lamas, J.P.; Llompart, M.; Garcia-Jares, C. In-Vial Micro-Matrix-Solid Phase Dispersion for the Analysis of Fragrance Allergens, Preservatives, Plasticizers, and Musks in Cosmetics. Cosmetics 2014, 1, 171-201. https://doi.org/10.3390/cosmetics1030171
Celeiro M, Lamas JP, Llompart M, Garcia-Jares C. In-Vial Micro-Matrix-Solid Phase Dispersion for the Analysis of Fragrance Allergens, Preservatives, Plasticizers, and Musks in Cosmetics. Cosmetics. 2014; 1(3):171-201. https://doi.org/10.3390/cosmetics1030171
Chicago/Turabian StyleCeleiro, Maria, Juan Pablo Lamas, Maria Llompart, and Carmen Garcia-Jares. 2014. "In-Vial Micro-Matrix-Solid Phase Dispersion for the Analysis of Fragrance Allergens, Preservatives, Plasticizers, and Musks in Cosmetics" Cosmetics 1, no. 3: 171-201. https://doi.org/10.3390/cosmetics1030171
APA StyleCeleiro, M., Lamas, J. P., Llompart, M., & Garcia-Jares, C. (2014). In-Vial Micro-Matrix-Solid Phase Dispersion for the Analysis of Fragrance Allergens, Preservatives, Plasticizers, and Musks in Cosmetics. Cosmetics, 1(3), 171-201. https://doi.org/10.3390/cosmetics1030171