Ultra-Performance Liquid Chromatography Tandem Mass Spectrometry Measurement of Caffeine in Caffeine-Laced Pants and in Urine and Skin of a Pants User
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Reagents
2.2. Cosmetic Caffeine-Laced Pants
2.3. Study Design
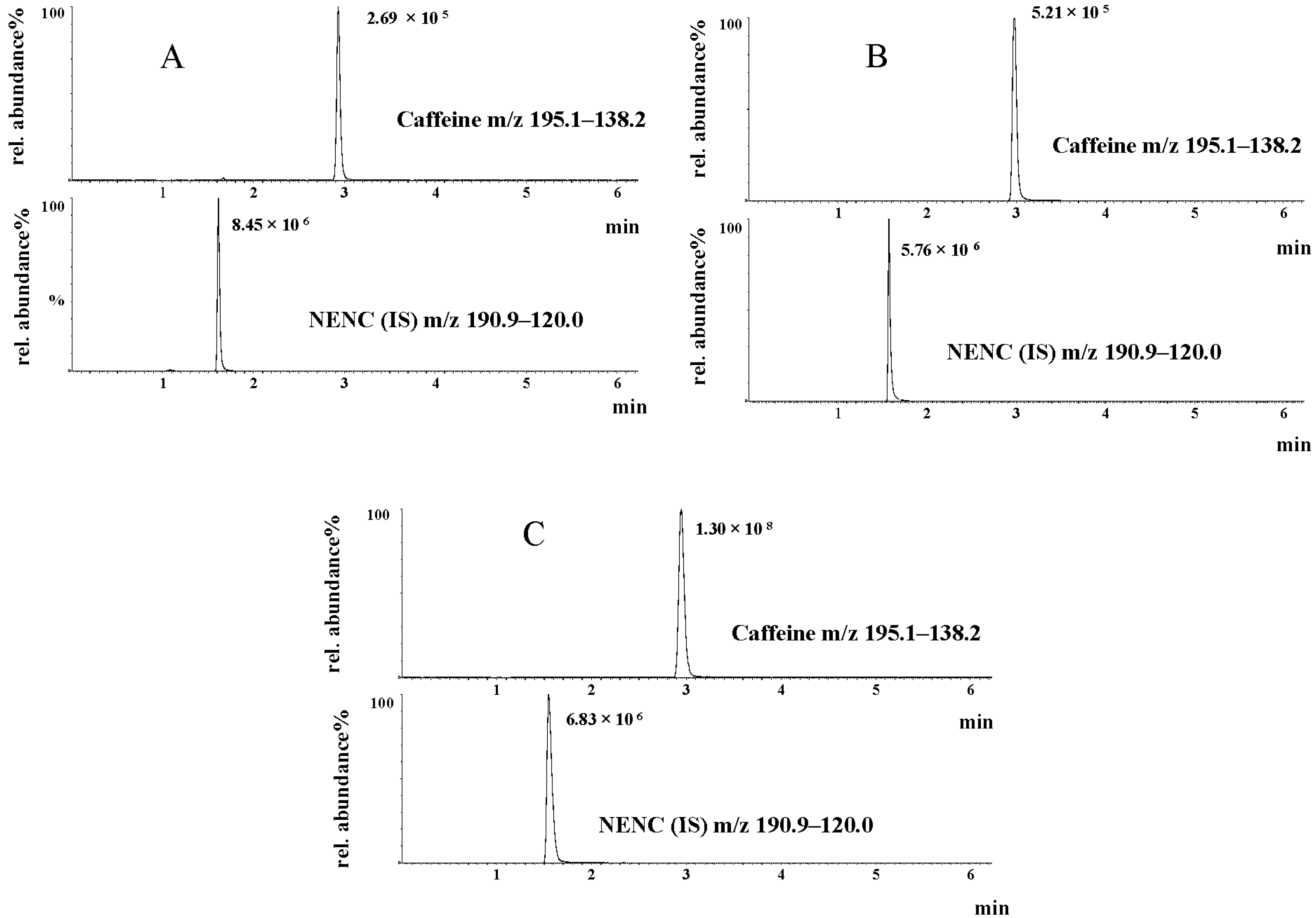
2.4. Ultra-Performance Liquid Chromatography Tandem Mass Spectrometry (UPLC-MS/MS) for Caffeine Determination
2.5. Calibration Standards and Quality Control Samples
2.6. Analysis Validation Protocol
2.7. Urine Sample Extraction
2.8. Cotton Swab Extraction
2.9. Textiles Sample Extraction
3. Results and Discussion
3.1. UPLC-MS/MS for Caffeine Determination and Validation Parameters
| Sample | Determination of coefficient (r2) a | LOD | LOQ | Mean recovery (%) b | ||
|---|---|---|---|---|---|---|
| Low QC sample | Medium QC sample | High QC sample | ||||
| Urine caffeine (ng/mL) | 0.999 ° 0.004 | 1.4 | 4.5 | (7 ng/mL) 85.2 ° 1.2 | (40 ng/mL) 89.2 ° 2.1 | (85 ng/mL) 87.3 ° 1.8 |
| Cotton swabs caffeine (ng/swab) | 0.995 ° 0.003 | 1.5 | 5.0 | (7 ng/swab) 85.8 ° 2.2 | (40 ng/swab) 86.6 ° 1.8 | (85 ng/swab) 87.3 ° 2.5 |
| Textiles sample caffeine (µg/sample) | 0.996 ° 0.005 | 1.3 | 4.0 | (5 µg/sample) 85.3 ° 3.2 | (40 µg/sample) 86.4 ° 2.2 | (85 µg/sample) 86.1 ° 1.2 |
| Sample | Intra-assay | Inter-assay | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Precision (CV%) | Accuracy (% error) | Precision (CV%) | Accuracy (% error) | |||||||||
| Low QC sample | Medium QC sample | High QC sample | Low QC sample | Medium QC sample | High QC sample | Low QC sample | Medium QC sample | High QC sample | Low QC sample | Medium QC sample | High QC sample | |
| Urine (ng/mL) | 7 | 40 | 85 | 7 | 40 | 85 | 7 | 40 | 85 | 7 | 40 | 85 |
| CV% or % error (e) | 8.0 | 8.8 | 5.7 | 3.2 | 3.1 | 2.8 | 4.8 | 1.3 | 2.4 | 9.9 | 8.3 | 5.6 |
| Cotton swabs (ng/swab) | 7 | 40 | 85 | 7 | 40 | 85 | 7 | 40 | 85 | 7 | 40 | 85 |
| CV% or % error (e) | 7.8 | 9.4 | 7.6 | 3.9 | 9.4 | 1.2 | 1.3 | 9.8 | 6.2 | 5.3 | 4.1 | 4.5 |
| Textiles sample (µg/sample) | 5 | 40 | 85 | 5 | 40 | 85 | 5 | 40 | 85 | 5 | 40 | 85 |
| CV% or % error (e) | 5.2 | e 3.2 | e 3.9 | 3.8 | 4.0 | 4.4 | 7.7 | 8.6 | 9.1 | 4.7 | 9.1 | 5.3 |
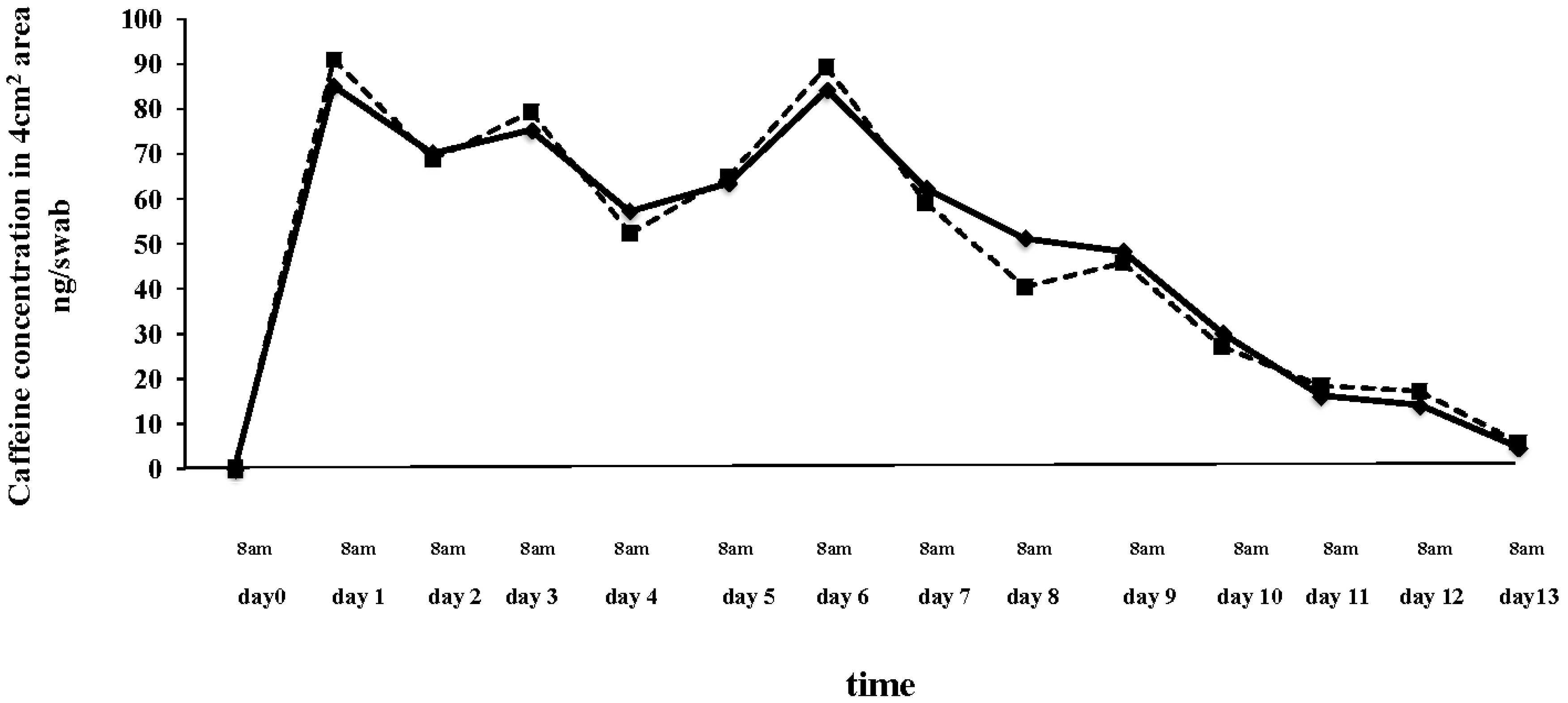
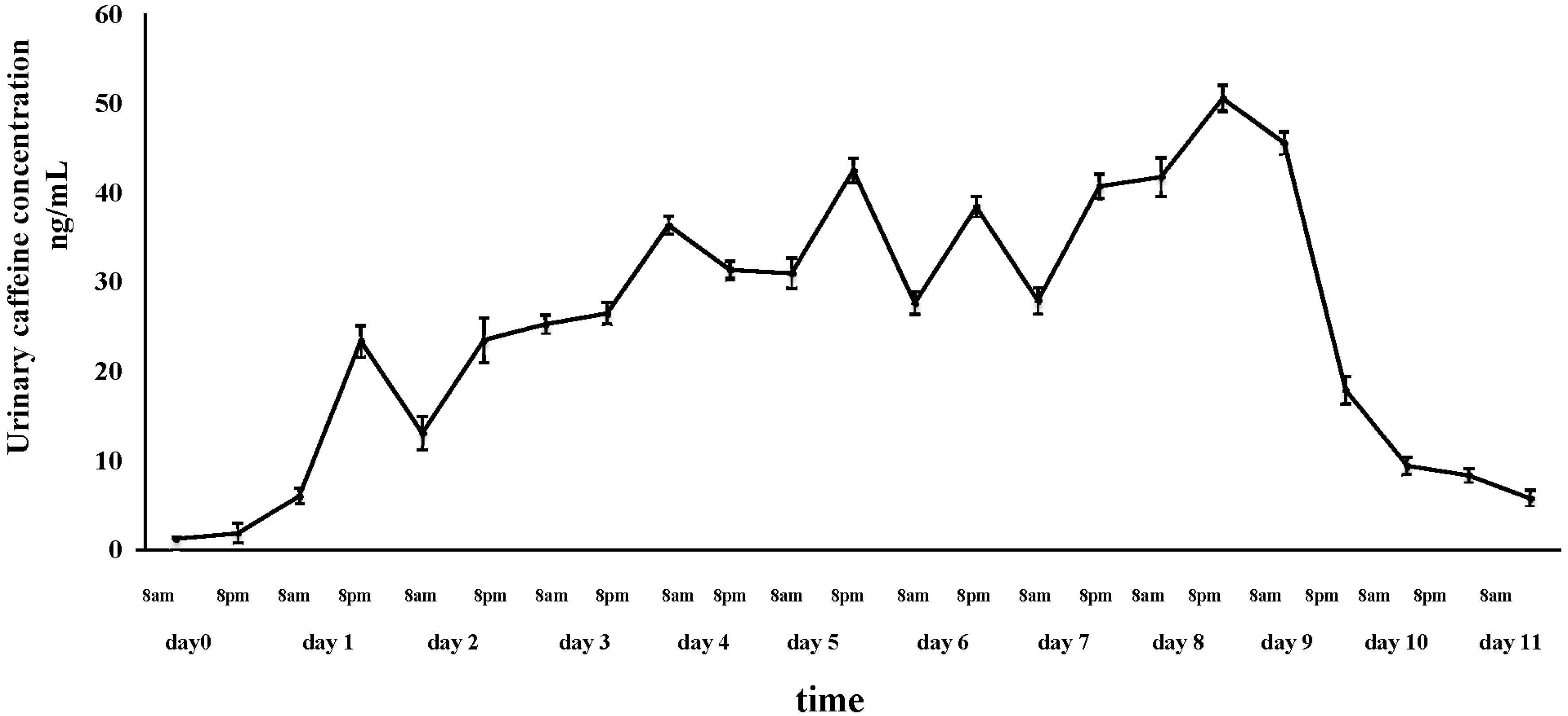
3.2. Caffeine Content in Laced Pants, on the Skin and into the Urine of a Pants-User
| Matrices where caffeine was measured | Caffeine amount |
|---|---|
| Caffeine laced pants before using | Total amount: 36 mg. |
| Urine samples | Baseline: 1.2 ± 0.1 ng/mL; First day after night wearing: 6.0 ± 0.9 ng/mL; Maximum value at day 8: 50.5 ± 1.4 ng/mL; First day after the end of treatment: 5.7 ± 0.9 ng/mL. |
| Skin swabs | Baseline: Not detected in the right and left thigh; First day after night wearing: 91.0 ng/swab on right thigh; 85. 2 ng/swab on the left thigh; Value at day 7: 89.3 ng/swab on right thigh; 84.3 ng/swab on the left thigh; First day after the end of treatment: 27.0 ng/swab on right thigh; 30.2/swab on the left thigh; Total amount of caffeine in the skin area: 708 ng for right thigh and 735 ng for left thigh. |
| Caffeine laced pants after 10 days using | Total amount: 10.7 mg. |
4. Conclusion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Al-Bader, T.; Byrne, A.; Gillbro, J.; Mitarotonda, A.; Metois, A.; Vial, F.; Rawlings, A.V.; Laloeuf, A. Effect of cosmetic ingredients as anticellulite agents: Synergistic action of actives with in vitro and in vivo efficacy. J. Cosmet. Dermatol. 2012, 11, 17–26. [Google Scholar] [CrossRef]
- Khan, M.H.; Victor, F.; Rao, B.; Sadick, N.S. Treatment of cellulite: Part I. Pathophysiology. J. Am. Acad. Dermatol. 2010, 62, 361–370. [Google Scholar] [CrossRef]
- Hexsel, D.; Orlandi, C.; Zechmeister do Prado, D. Botanical extracts used in the treatment of cellulite. Dermatol. Surg. 2005, 31, 866–872. [Google Scholar]
- Rawlings, A.V. Cellulite and its treatment. Int. J. Cosmet. Sci. 2006, 28, 175–190. [Google Scholar] [CrossRef]
- Roure, R.; Oddos, T.; Rossi, A.; Vial, F.; Bertin, C. Evaluation of the efficacy of a topical cosmetic slimming product combining tetrahydroxypropyl ethylenediamine, caffeine, carnitine, forskolin and retinol, in vitro, ex vivo and in vivo studies. Int. J. Cosmet. Sci. 2011, 33, 519–526. [Google Scholar] [CrossRef]
- Barel, A.O.; Paye, M.; Maibach, H.I. Handbook of Cosmetic Science and Technology; CRC Press: New York, NY, USA, 2001. [Google Scholar]
- Lupi, O.; Semenovitch, I.J.; Treu, C.; Bottino, D.; Bouskela, E. Evaluation of the effects of caffeine in the microcirculation and edema on thighs and buttocks using the orthogonal polarization spectral imaging and clinical parameters. J. Cosmet. Dermatol. 2007, 6, 102–107. [Google Scholar] [CrossRef]
- Velasco, M.V.; Tano, C.T.; Machado-Santelli, G.M.; Consiglieri, V.O.; Kaneko, T.M.; Baby, A.R. Effects of caffeine and siloxanetriol alginate caffeine, as anticellulite agents, on fatty tissue: Histological evaluation. J. Cosmet. Dermatol. 2008, 7, 23–29. [Google Scholar] [CrossRef]
- Pires-de-Campos, M.S.; Leonardi, G.R.; Chorilli, M.; Spadari-Bratfisch, R.C.; Polacow, M.L.; Grassi-Kassisse, D.M. The effect of topical caffeine on the morphology of swine hypodermis as measured by ultrasound. J. Cosmet. Dermatol. 2008, 7, 232–237. [Google Scholar] [CrossRef]
- Buschmann, H.J.; Schollmeyer, E. Applications of cyclodextrins in cosmetic products: A review. J. Cosmet. Sci. 2002, 53, 185–191. [Google Scholar]
- Bhaskara-Amrit, U.R.; Agrawal, P.B.; Warmoeskerken, M.M.C.G. Applications of β-cyclodextrins in textiles. AUTEX Res. J. 2011, 11, 94–101. [Google Scholar]
- European Committee for Standardization. Available online: http://www.cen.eu/CEN/Sectors/TechnicalCommitteesWorkshops/CENTechnicalCommittees/Pages/Standards.aspx?param=6229&title=CEN%2FTC+248 (accessed on 10 April 2014).
- Regulation n. (CE) 1223/2009 of the European Parliament. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32009R1223:it:NOT (accessed on 30 November 2009).
- Regulation n. (CE) 1007/2011 of the European Parliament. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32011R1007:IT:NOT (accessed on 27 September 2011).
- ICH Topic Q 2 (R1) Validation of Analytical Procedures: Text and Methodology. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002662.pdf (accessed on 10 April 2014).
- ICH Technical Coordination. Guidance for Industry, Bioanalytical Method Validation, US Department of Health and Human Services, Food and Drug Administration. Available online: http://www.fda.gov/downloads/Drugs/Guidances/ucm070107.pdf (accessed on 10 April 2014).
- Marchei, E.; Escuder, D.; Pallas, C.R.; Garcia-Algar, O.; Gómez, A.; Friguls, B.; Pellegrini, M.; Pichini, S. Simultaneous analysis of frequently used licit and illicit psychoactive drugs in breast milk by liquid chromatography tandem mass spectrometry. J. Pharm. Biomed. Anal. 2011, 55, 309–316. [Google Scholar] [CrossRef]
- Marchei, E.; de Orsi, D.; Guarino, C.; Dorato, S.; Pacifici, R.; Pichini, S. Measurement of iodide and caffeine content in cellulite reduction cosmetic products sold in the European market. Anal. Methods 2013, 5, 376–383. [Google Scholar] [CrossRef]
- Rybak, M.E.; Pao, C.I.; Pfeiffer, C.M. Determination of urine caffeine and its metabolites by use of high-performance liquid chromatography-tandem mass spectrometry: Estimating dietary caffeine exposure and metabolic phenotyping in population studies. Anal. Bioanal. Chem. 2014, 406, 771–784. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pellegrini, M.; De Orsi, D.; Guarino, C.; Rotolo, M.C.; Di Giovannandrea, R.; Pacifici, R.; Pichini, S. Ultra-Performance Liquid Chromatography Tandem Mass Spectrometry Measurement of Caffeine in Caffeine-Laced Pants and in Urine and Skin of a Pants User. Cosmetics 2014, 1, 82-93. https://doi.org/10.3390/cosmetics1020082
Pellegrini M, De Orsi D, Guarino C, Rotolo MC, Di Giovannandrea R, Pacifici R, Pichini S. Ultra-Performance Liquid Chromatography Tandem Mass Spectrometry Measurement of Caffeine in Caffeine-Laced Pants and in Urine and Skin of a Pants User. Cosmetics. 2014; 1(2):82-93. https://doi.org/10.3390/cosmetics1020082
Chicago/Turabian StylePellegrini, Manuela, Daniela De Orsi, Carmine Guarino, Maria Concetta Rotolo, Rita Di Giovannandrea, Roberta Pacifici, and Simona Pichini. 2014. "Ultra-Performance Liquid Chromatography Tandem Mass Spectrometry Measurement of Caffeine in Caffeine-Laced Pants and in Urine and Skin of a Pants User" Cosmetics 1, no. 2: 82-93. https://doi.org/10.3390/cosmetics1020082
APA StylePellegrini, M., De Orsi, D., Guarino, C., Rotolo, M. C., Di Giovannandrea, R., Pacifici, R., & Pichini, S. (2014). Ultra-Performance Liquid Chromatography Tandem Mass Spectrometry Measurement of Caffeine in Caffeine-Laced Pants and in Urine and Skin of a Pants User. Cosmetics, 1(2), 82-93. https://doi.org/10.3390/cosmetics1020082