In Vitro Cytotoxicity and Phototoxicity Assessment of Acylglutamate Surfactants Using a Human Keratinocyte Cell Line
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Reagents
2.2. Surfactants Tested
| Surfactant | Average MW | Base amino acid | Charge | Fatty acid chain length (C % by weight) | Foaming power a (After 5 min) mm |
|---|---|---|---|---|---|
| Amisoft CS 11 | 359 | l-glutamic acid | Anionic | C8–14 Mixture (86.9) b | 203 |
| Amisoft LS 11 | 356 | l-glutamic acid | Anionic | C12 (97.3) c | 219 |
| Amisoft MS 11 | 384 | l-glutamic acid | Anionic | C14 (97.8) c | 212 |
2.3. Cell Culture
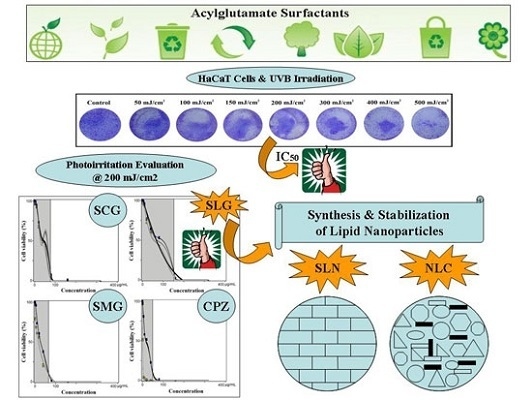
2.4. UVB-Irradiation and Cell Viability
2.5. Surfactant Treatment
2.6. MTS Assay
2.7. Photoirritation Evaluation
2.8. Data Analysis
2.9. Statistical Analysis
3. Results
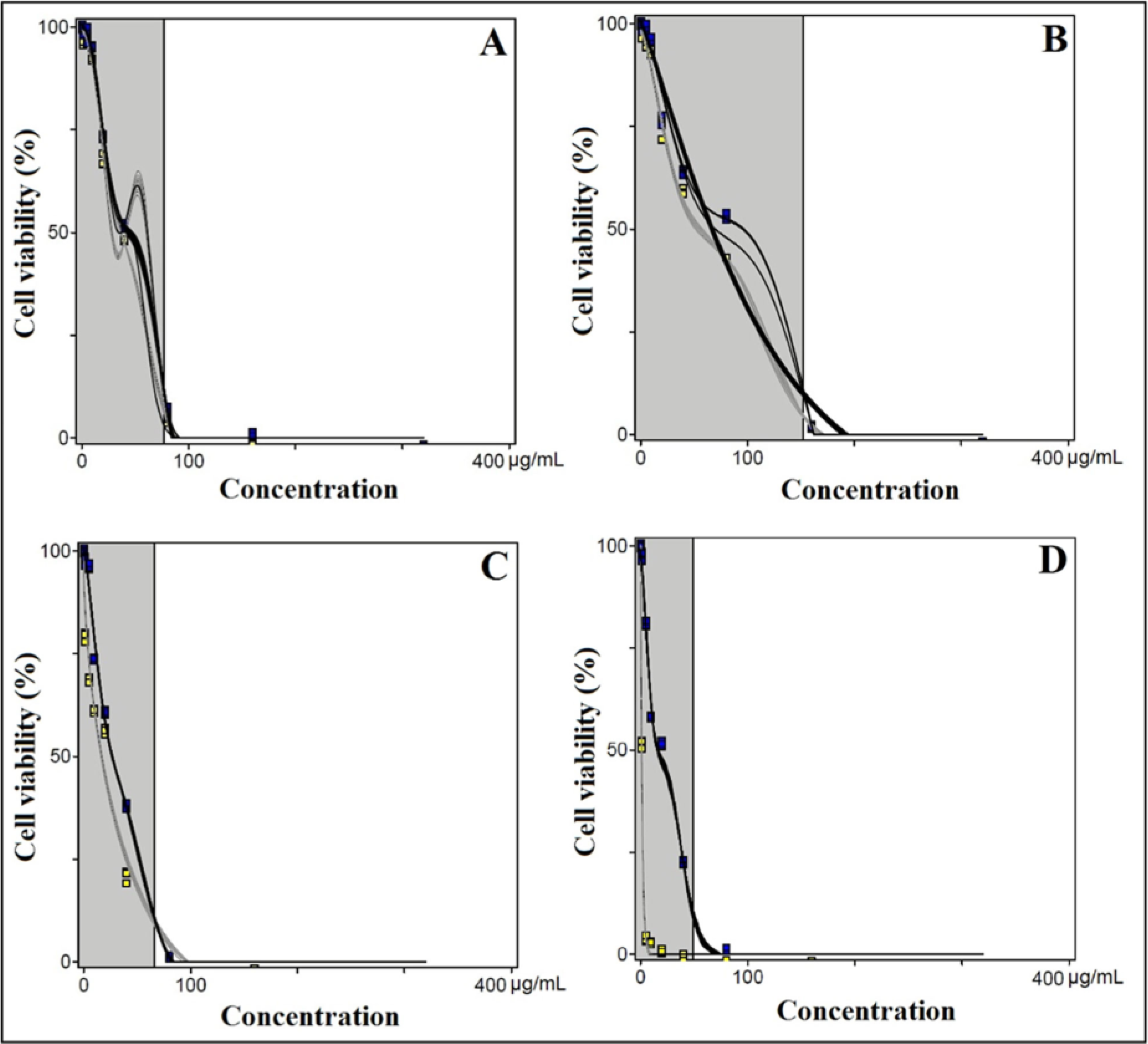
| Surfactant | UVB light | IC50 a ± SD (μg/mL) |
|---|---|---|
| Amisoft CS 11 | − | 43.48 ± 1.09 |
| Amisoft CS 11 | + | 30.62 ± 2.72 |
| Amisoft LS 11 | − | 69.97 ± 1.66 |
| Amisoft LS 11 | + | 54.22 ± 1.81 |
| Amisoft MS 11 | − | 26.48 ± 1.47 |
| Amisoft MS 11 | + | 16.72 ± 0.92 |
| CPZ | − | 16.00 ± 0.89 |
| CPZ | + | 1.054 ± 0.23 |
| Surfactant | PIF mean a | Toxicity probability | MPE mean a | Toxicity probability | Phototoxic potential |
|---|---|---|---|---|---|
| Amisoft CS 11 | 1.292 | 0 | 0.019 | 0 | No |
| Amisoft LS 11 | 1.442 | 0 | 0.007 | 0 | No |
| Amisoft MS11 | 1.585 | 0 | 0.092 | 0.055 | No |
| CPZ | 15.186 | 1.0 | 0.435 | 1.0 | Yes |
4. Discussion
5. Conclusion
Acknowledgements
Author Contributions
Conflicts of interest
References
- Liu, J.; Hu, W.; Chen, H.; Ni, Q.; Xu, H.; Yang, X. Isotretinoin-loaded solid lipid nanoparticles with skin targeting for topical delivery. Int. J. Pharm. 2007, 328, 91–195. [Google Scholar]
- Kovacevic, A.; Savic, S.; Vuleta, G.; Müller, R.H.; Keck, C.M. Polyhydroxy surfactants for the formulation of lipid nanoparticles (SLN and NLC): Effects on size, physical stability and particle matrix structure. Int. J. Pharm. 2011, 406, 163–172. [Google Scholar]
- Mitri, K.; Shegokar, R.; Gohla, S.; Anselmi, C.; Müller, R.H. Lipid nanocarriers for dermal delivery of lutein: Preparation, characterization, stability and performance. Int. J. Pharm. 2011, 414, 267–275. [Google Scholar] [CrossRef]
- Somasundaran, P.; Soma, C.; Puspendu, D.; Namita, D.; Somasundaran, T. Contribution of surfactants to personal care products. In Surfactant in Personal Care Products and Decorative Cosmetics, 3rd ed.; Rhein, L.D., Olenick, A., Schlossman, M., Somasundaran, P., Eds.; CRC Press: Florida, FL, USA, 2007; pp. 121–135. [Google Scholar]
- Infante, M.R.; Molinero, J.; Erra, P.; Juliá, M.R.; García Domínguez, J.J.; Robert, M. The influence of steric configuration of some Nα-lauroyl amino-acid derivatives on their antimicrobial activity. Fette Seifen Anstrichm. 1986, 88, 108–110. [Google Scholar] [CrossRef]
- Okahata, Y.; Tanamachi, S.; Nagai, M.; Kunitake, T. Synthetic bilayer membranes prepared from dialkyl amphiphiles with nonionic and zwitterionic head groups. J. Colloid Interface Sci. 1981, 82, 401–417. [Google Scholar]
- Kida, T.; Morishima, N.; Masuyama, A.; Nakatsuji, Y. New cleavable surfactants derived from glucono-1,5-lactone. J. Am. Oil Chem. Soc. 1994, 71, 705–710. [Google Scholar] [CrossRef]
- Heutrich, W.; Keppler, H.; Hintzmann, K. Detergents, Wetting, Dispersing and Leveling Agents. German Patent 635522, 18 September 1936. [Google Scholar]
- Amisoft® CS 22. Amino Acid Based Anionic Surfactant. Available online: www.cosmesi.it/portals/7/documenti/amisoft%20CS-22_brochure.pdf (accessed on 15 April 2014).
- Kawasaki, Y.; Quan, D.; Sakamoto, K.; Cooke, R.; Maibach, H.I. Influence of surfactant mixtures on intercellular lipid fluidity and skin barrier function. Skin Res. Technol. 1999, 5, 96–101. [Google Scholar] [CrossRef]
- Lee, C.H.; Kawasaki, Y.; Maibach, H. Effect of surfactant mixtures on irritant contact dermatitis potential in man: Sodium lauroyl glutamate and sodium lauryl sulphate. Contact Dermat. 1994, 30, 205–209. [Google Scholar] [CrossRef]
- Takehara, M. Properties and applications of amino acid based surfactants. Colloids Surf. 1989, 38, 149–167. [Google Scholar]
- Cosmetic Directive. Available online: http://ec.europa.eu/consumers/archive/sectors/cosmetics/documents/directive/index_en.htm (accessed on 8 July 2014).
- Full EU Ban on Animal Testing for Cosmetics Enters into Force. Available online: http://europa.eu/rapid/press-release_IP-13-210_en.htm (accessed on 15 April 2014).
- Botham, P.A.; Earl, L.K.; Fentem, J.H.; Roguet, R.; van de Sandt, J.J.M. Alternative Methods for Skin Irritation Testing: The Current Status. Altern. Lab. Anim. 1998, 26, 195–211. [Google Scholar]
- Wilhelm, K.P.; Böttjer, B.; Siegers, C.-P. Quantitative assessment of primary skin irritants in vitro in a cytotoxicity model: Comparison with in vivo human irritation tests. Br. J. Dermatol. 2001, 145, 709–715. [Google Scholar]
- Benavides, T.; Martínez, V.; Mitjans, M.; Infante, M.R.; Moran, C.; Clapés, P.; Clothier, R.; Vinardell, M.P. Assessment of the potential irritation and photoirritation of novel amino acid-based surfactants by in vitro methods as alternative to the animal tests. Toxicology 2004, 201, 87–93. [Google Scholar]
- Sanchez, L.; Mitjans, M.; Infante, M.R.; Vinardell, M.P. Potential irritation of lysine derivative surfactants by hemolysis and HaCaT cell viability. Toxicol. Lett. 2006, 161, 53–60. [Google Scholar]
- Wilheilm, K.P.; Samblebe, M.; Siegers, C.P. Quantitative in vitro assessment of N-alkylsulphate-induced cytotoxicity in human keratinocytes (HaCaT). Comparison with in vivo human irritation tests. Br. J. Dermatol. 1994, 130, 18–23. [Google Scholar] [CrossRef]
- Lohézic-Le Dévéhat, F.; Legouin, B.; Couteau, C.; Boustie, J.; Coiffard, L. Lichenic extracts and metabolites as UV filters. J. Photochem. Photobiol. B 2013, 120, 17–28. [Google Scholar]
- Maupas, C.; Moulari, B.; Beduneau, A.; Lamprecht, A.; Pellequer, Y. Surfactant dependent toxicity of lipid nanocapsules in HaCaT cells. Int. J. Pharm. 2011, 411, 136–141. [Google Scholar] [CrossRef]
- Nichols, J.A.; Katiyar, S.K. Skin photoprotection by natural polyphenols: Anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef]
- Kim, J.K.; Kim, Y.; Na, K.M.; Surh, Y.J.; Kim, T.Y. [6]-Gingerol prevents UVB-induced ROS production and COX-2 expression in vitro and in vivo. Free Radic. Res. 2007, 41, 603–614. [Google Scholar] [CrossRef]
- Yoshida, R.; Yoshimurra, I.; Usuba, Y.; Shibue, A. Surface active N-acylglutamate: V. Application of N-acylglutamates to detergent bars. J. Am. Oil Chem. Soc. 1976, 53, 113–117. [Google Scholar] [CrossRef]
- Takehara, M.; Moriyuki, H.; Yoshimura, I.; Yoshida, R. Surface active N-acylglutamate: II. Physicochemical properties of long chain N-acylglutamic acids and their sodium salts. J. Am. Oil Chem. Soc. 1972, 49, 143–150. [Google Scholar]
- Park, K.; Lee, J.H. Protective effects of resveratrol on UVB-irradiated HaCaT cells through attenuation of the caspase pathway. Oncol. Rep. 2008, 19, 413–417. [Google Scholar]
- Cory, A.H.; Owen, T.C.; Barltrop, J.A.; Cory, J.G. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 1991, 3, 207–212. [Google Scholar]
- Yin, J.J.; Liu, J.; Ehrenshaft, M.; Roberts, J.E.; Fu, P.P.; Mason, R.P.; Zhao, B. Phototoxicity of nano titanium dioxides in HaCaT keratinocytes--generation of reactive oxygen species and cell damage. Toxicol. Appl. Pharm. 2012, 263, 81–88. [Google Scholar]
- Vinardell, M.P.; Benavides, T.; Mitjans, M.; Infante, M.R.; Clapés, P.; Clothier, R. Comparative evaluation of cytotoxicity and phototoxicity of mono and diacylglycerol amino acid-based surfactants. Food Chem. Toxicol. 2008, 46, 3837–3841. [Google Scholar]
- Test No. 432: In vitro 3T3 NRU phototoxicity test. In OECD Guidelines for the Testing of Chemicals Section 4: Health Effects; Organisation for Economic Cooperation and Development (OECD) iLibrary: Paris, France, 2004; pp. 1–15.
- OECD. Available online: http://www.oecd.org/document/55/0,2340,en_2649_34377_2349687_1_1_1_1,00.html (accessed on 1 April 2014).
- Peters, B.; Holzhutter, H.G. In vitro phototoxicity testing: development and validation of a new concentration response analysis software and biostatistical analyses related to the use of various prediction models. Altern. Lab. Anim. 2002, 30, 415–432. [Google Scholar]
- Safety Assessment of Amino Acid Alkyl Amides as Used in Cosmetics. Available online: www.cir-safety.org/sites/default/files/alkyl_amides_0.pdf (accessed on 4 April 2014).
- Berardesca, E.; Distante, F. The modulation of skin irritation. Contact Dermat. 1994, 31, 281–287. [Google Scholar] [CrossRef]
- Athar, M.; Kim, A.L.; Ahmad, N.; Mukhtar, H.; Gautier, J.; Bickers, D.R. Mechanism of ultraviolet B-induced cell cycle arrest in G2/M phase in immortalized skin keratinocytes with defective p53. Biochem. Biophys. Res. Commun. 2000, 277, 107–111. [Google Scholar] [CrossRef]
- Kudish, A.I.; Lyubansky, V.; Evseev, E.G.; Ianetz, A. Statistical analysis and inter-comparison of the solar UVB, UVA and global radiation for Beer Sheva and Neve Zohar (Dead Sea), Israel. Theor. Appl. Climatol. 2005, 80, 1–15. [Google Scholar]
- Suh, Y.-W. An Investigation of the Phototoxicity of Decabromodiphenyl Ether and Triclosan. Ph.D. Thesis, University of Iowa, Iowa City, IA, USA, 2010. [Google Scholar]
- Chignell, C.F.; Motten, A.G.; Buettner, G.R. Photoinduced free radicals from chlorpromazine and related phenothiazines: Relationship to phenothiazine-induced photosensitization. Environ. Health Perspect. 1985, 64, 103–110. [Google Scholar] [CrossRef]
- Kanari, M.; Kawasaki, Y.; Sakamoto, K. Acylglutamate as an anti-irritant for mild detergent system. J. Soc. Cosmet. Chem. Jpn. 1993, 27, 498–505. [Google Scholar]
- Osborne, R.; Perkins, M.A. An approach for development of alternative test methods based on mechanisms of skin irritation. Food Chem. Toxicol. 1994, 32, 133–142. [Google Scholar]
- De Brugerolle de Fraissinette, A.; Picarles, V.; Chibout, S.; Kolopp, M.; Medina, J.; Burtin, P.; Ebelin, M.E.; Osborne, S.; Mayer, F.K.; Spake, A.; et al. Predictivity of an in vitro model for acute and chronic skin irritation (SkinEthic) applied to the testing of topical vehicles. Cell Biol. Toxicol. 1999, 15, 121–135. [Google Scholar] [CrossRef]
- Afaq, F.; Mukhtar, H. Effects of solar radiation on cutaneous detoxification pathways. J. Photochem. Photobiol. B 2001, 63, 61–69. [Google Scholar] [CrossRef]
- Leccia, M.T.; Richard, M.J.; Joanny-Crisci, F.; Beani, J.C. UV-A1 cytotoxicity and antioxidant defence in keratinocytes and fibroblasts. Eur. J. Dermatol. 1998, 8, 478–482. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kyadarkunte, A.; Patole, M.; Pokharkar, V. In Vitro Cytotoxicity and Phototoxicity Assessment of Acylglutamate Surfactants Using a Human Keratinocyte Cell Line. Cosmetics 2014, 1, 159-170. https://doi.org/10.3390/cosmetics1030159
Kyadarkunte A, Patole M, Pokharkar V. In Vitro Cytotoxicity and Phototoxicity Assessment of Acylglutamate Surfactants Using a Human Keratinocyte Cell Line. Cosmetics. 2014; 1(3):159-170. https://doi.org/10.3390/cosmetics1030159
Chicago/Turabian StyleKyadarkunte, Abhay, Milind Patole, and Varsha Pokharkar. 2014. "In Vitro Cytotoxicity and Phototoxicity Assessment of Acylglutamate Surfactants Using a Human Keratinocyte Cell Line" Cosmetics 1, no. 3: 159-170. https://doi.org/10.3390/cosmetics1030159
APA StyleKyadarkunte, A., Patole, M., & Pokharkar, V. (2014). In Vitro Cytotoxicity and Phototoxicity Assessment of Acylglutamate Surfactants Using a Human Keratinocyte Cell Line. Cosmetics, 1(3), 159-170. https://doi.org/10.3390/cosmetics1030159